| 1 |
吴谦, 王栋, 孙瑾. PBO纤维研究进展[J]. 辽宁化工, 2013, 42(11): 1323-1324.
|
|
WU Q, WANG D, SUN J. Progress in PBO fiber research[J]. Liaoning Chemical Industry, 2013, 42(11): 1323-1324.
|
| 2 |
HSIAO S H, HUANG Y H. A new class of aromatic polybenzoxazoles containing ortho-phenylenedioxy groups[J]. European Polymer Journal, 2004, 40: 1127-1135.
|
| 3 |
KOLHE N B, ASHA S K, SENANAVAK S P, et al. n-Type field effect transistors based on rigid rod and liquid crystalline alternating copoly(benzobisoxazole) imides containing perylene and/or naphthalene[J]. Phys. Chem., 2010, 114: 16694-16704.
|
| 4 |
肖立伟, 高红杰, 孔洁, 等. 2-取代苯并 唑类化合物合成研究进展[J]. 有机化学, 2014, 34(6): 1048-1060. 唑类化合物合成研究进展[J]. 有机化学, 2014, 34(6): 1048-1060.
|
|
XIAO L W, GAO H J, KONG J, et al. Progress in the synthesis of 2-substituted benzooxazoles[J]. Organic Chemistry, 2014, 34(6): 1048-1060.
|
| 5 |
XU X H, LIU X Y, ZHUANG Q X, et al. Rigid-rod polybenzoxazoles containing perylene bisimide: synthesis, structures and photophysical properties[J]. Polymer Science, 2009, 116: 455-460.
|
| 6 |
XU Y F, FEI F, ZHAO J X, et al. Preparation and characterization of novel polyimides with hydroxyl groups[J]. Journal of Macromolecular Science, 2011, 50: 2090-2102.
|
| 7 |
赵德明, 吴锋, 陈中海, 等. 单氨基改性PBO的AB型新单体的合成[J]. 化工进展, 2016, 35(4): 1197-1202.
|
|
ZHAO D M, WU F, CHEN Z H, et al. Synthesis of new AB monomers of amino modified PBO[J]. Chemical Industry and Engineering Progress, 2016, 35(4): 1197-1202.
|
| 8 |
BIBIANA C G, CALLE M, JO H J, et al. Thermally rearranged polybenzoxazoles membranes with biphenyl moieties: monomer isomeric effect[J]. Journal of Membrane Science, 2013, 450: 369-379.
|
| 9 |
费斐, 徐永芬, 傅菊荪, 等. 3,3′-二氨基-4,4′-二羟基联苯及其聚酰亚胺的合成与性能研究[J]. 绝缘材料, 2009, 42(6): 12-22.
|
|
FEI P, XU Y F, FU J S, et al. Synthesis and properties of 3,3′-diamino-4,4′-dihydroxybiphenyl and its polyimide[J]. Insulation Materials, 2009, 42(6): 12-22.
|
| 10 |
BORJIGIN H, LIU Q, ZHANG W, et al. Synthesis and characterization of thermally rearranged (TR) polybenzoxazoles: influence of isomeric structure on gas transport properties[J]. Polymer, 2015, 75: 199-210.
|
| 11 |
KUO H M, HSU Y T, WANG Y W, et al. The π-π interactions enhanced in salicylaldimines and salicylaldazines[J]. Tetrahedron, 2015, 71: 7729-7738.
|
| 12 |
金宁人, 李晨, 胡晓峰, 等. AB型羟基改性PBO单体及其合成新技术[J]. 化工学报, 2015, 66(5): 1955-1963.
|
|
JIN N R, LI C, HU X F, et al. AB monomers of hydroxy modified PBO and its novel synthesis technology[J]. CIESC Jorunal, 2015, 66(5): 1955-1963.
|
| 13 |
LIU Q, BORJIGIN H, PAUL D R, et al. Gas permeation properties of thermally rearranged (TR) isomers and their aromatic polyimide precursors[J]. Journal of Membrane Science, 2016, 518: 88-99.
|
| 14 |
陶立明, 杨海霞, 刘金刚, 等. 芳杂环聚苯并噁唑材料的合成研究进展[J]. 高分子通报, 2010(11): 10-25.
|
|
TAO L M, YANG H X, LIU J G, et al. Progress in the synthesis of aromatic heterocyclic polybenzoxazole materials[J]. Polymer Bulletin, 2010(11): 10-25.
|
| 15 |
李云雁. 实验设计与数据处理[M]. 3版. 北京: 化学工业出版社, 2015: 50-208.
|
|
LI Y Y. Design of experiments and data analysis[M]. 3rd ed. Beijing: Chemical Industry Press, 2015: 50-208.
|
 ),Yuxi CHANG,Dichao CEHN,Jianting ZHANG,Ningren JIN,Deming ZHAO(
),Yuxi CHANG,Dichao CEHN,Jianting ZHANG,Ningren JIN,Deming ZHAO( )
)
 唑(PBO)纤维复合黏结性能差等缺点,在苯环上引入极性基团羟基进行化学分子结构改性,3,3′-二氨基-4,4′-二羟基联苯盐酸盐(DADHBP·2HCl)是羟基改性PBO的关键单体。以4,4′-二羟基联苯(DHBP)为原料经硝化、还原反应合成得到中间体3,3′-二硝基-4,4′-二羟基联苯(DNDHBP)和羟基改性PBO的单体DADHBP·2HCl,并对其反应条件进行了优化。结果表明:对于硝化反应,以甲苯、冰乙酸的混合液为反应溶剂,n(DHBP)∶n(HNO3)=1∶2,反应温度5℃,反应时间2.5h,收率81.39%,高效液相色谱(HPLC)纯度(质量分数)92.43%;还原反应,n(DNDHBP)∶n(FeSO4·7H2O)=1∶9,乙醇为溶剂,七水合硫酸亚铁为助剂,n(DNDHBP)∶n(N2H4·H2O)=1∶5.5,反应温度78℃,反应时间9h,热过滤,滤饼盐酸精制后得到DADHBP·2HCl,收率67.16%,HPLC纯度(质量分数)98.20%。中间体和产物结构经红外光谱、核磁氢谱和电喷雾质谱表征确认。
唑(PBO)纤维复合黏结性能差等缺点,在苯环上引入极性基团羟基进行化学分子结构改性,3,3′-二氨基-4,4′-二羟基联苯盐酸盐(DADHBP·2HCl)是羟基改性PBO的关键单体。以4,4′-二羟基联苯(DHBP)为原料经硝化、还原反应合成得到中间体3,3′-二硝基-4,4′-二羟基联苯(DNDHBP)和羟基改性PBO的单体DADHBP·2HCl,并对其反应条件进行了优化。结果表明:对于硝化反应,以甲苯、冰乙酸的混合液为反应溶剂,n(DHBP)∶n(HNO3)=1∶2,反应温度5℃,反应时间2.5h,收率81.39%,高效液相色谱(HPLC)纯度(质量分数)92.43%;还原反应,n(DNDHBP)∶n(FeSO4·7H2O)=1∶9,乙醇为溶剂,七水合硫酸亚铁为助剂,n(DNDHBP)∶n(N2H4·H2O)=1∶5.5,反应温度78℃,反应时间9h,热过滤,滤饼盐酸精制后得到DADHBP·2HCl,收率67.16%,HPLC纯度(质量分数)98.20%。中间体和产物结构经红外光谱、核磁氢谱和电喷雾质谱表征确认。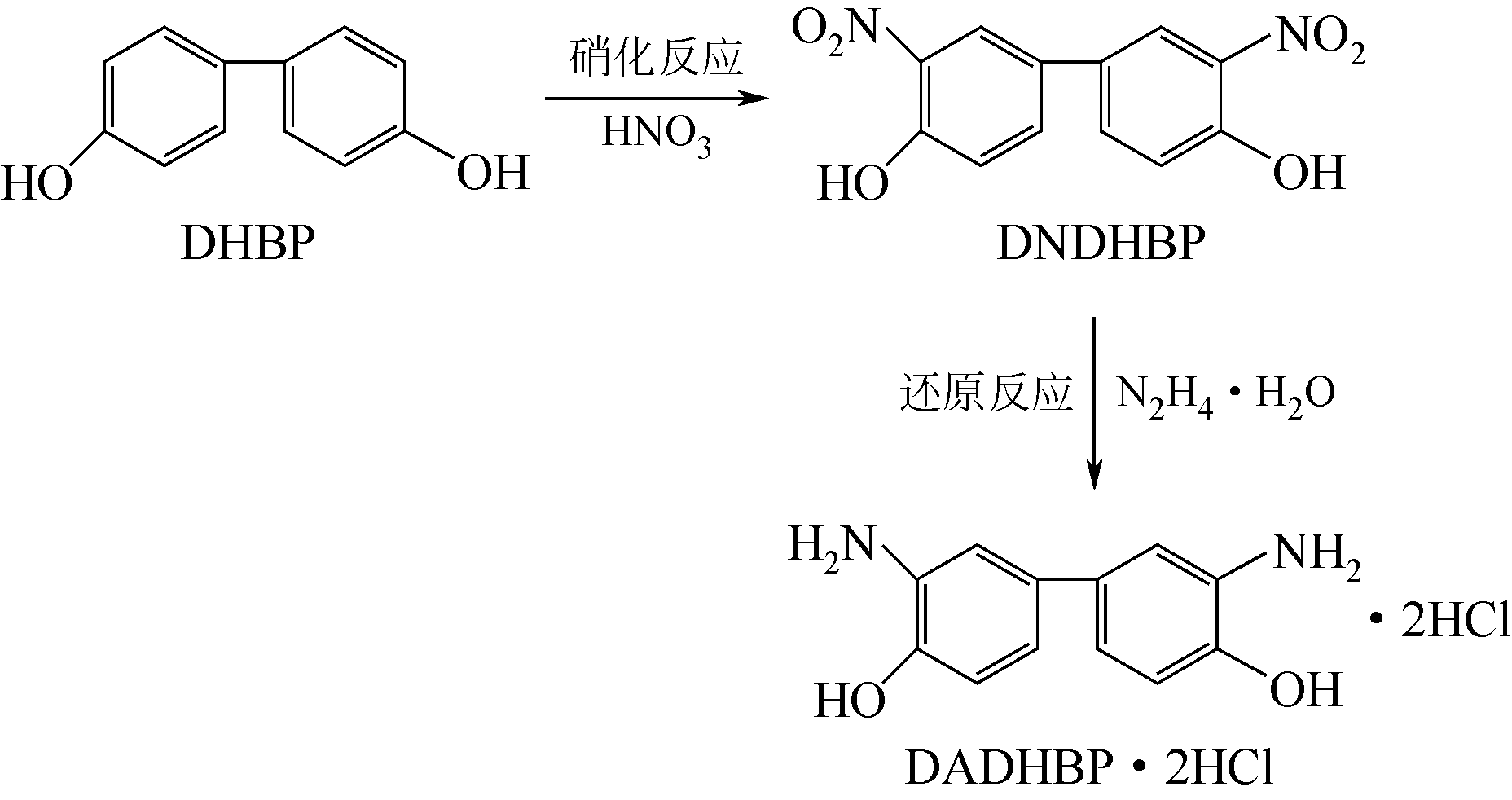
 唑类化合物合成研究进展[J]. 有机化学, 2014, 34(6): 1048-1060.
唑类化合物合成研究进展[J]. 有机化学, 2014, 34(6): 1048-1060.