化工进展 ›› 2020, Vol. 39 ›› Issue (1): 402-412.DOI: 10.16085/j.issn.1000-6613.2019-0686
低温等离子体降解苯的工艺参数优化
- 1. 北京工业大学环境与能源工程学院,北京 100124
2. 北京石油化工学院机械工程学院,北京 102617
-
收稿日期:2019-04-26出版日期:2020-01-05发布日期:2020-01-14 -
通讯作者:朱玲 -
作者简介:王春雨(1992—),女,硕士研究生,研究方向为有机废气净化。E-mail:2542421572@qq.com 。 -
基金资助:北京市长城学者培养计划(CIT&TCD20190314)
Process parameters optimization for degradation of benzene by non-thermal plasma
Chunyu WANG1,2( ),Ling ZHU2(
),Ling ZHU2( ),Danyun XU2,Qingyue LUO2
),Danyun XU2,Qingyue LUO2
- 1. College of Environmental and Energy Engineering, Beijing University of Technology, Beijing 100124, China
2. School of Mechanical Engineering, Beijing Institute of Petrochemical Technology, Beijing 102617, China
-
Received:2019-04-26Online:2020-01-05Published:2020-01-14 -
Contact:Ling ZHU
摘要:
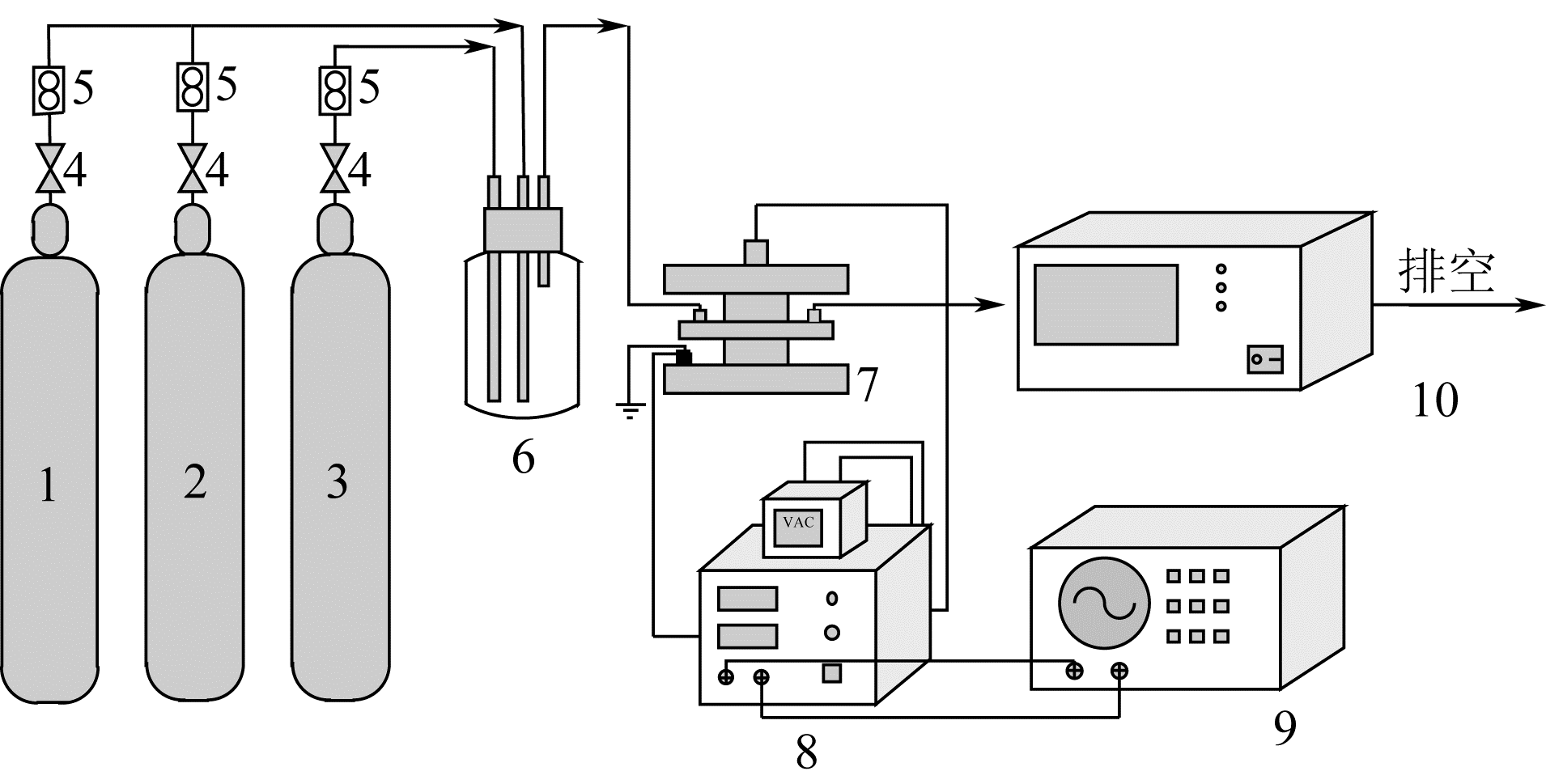
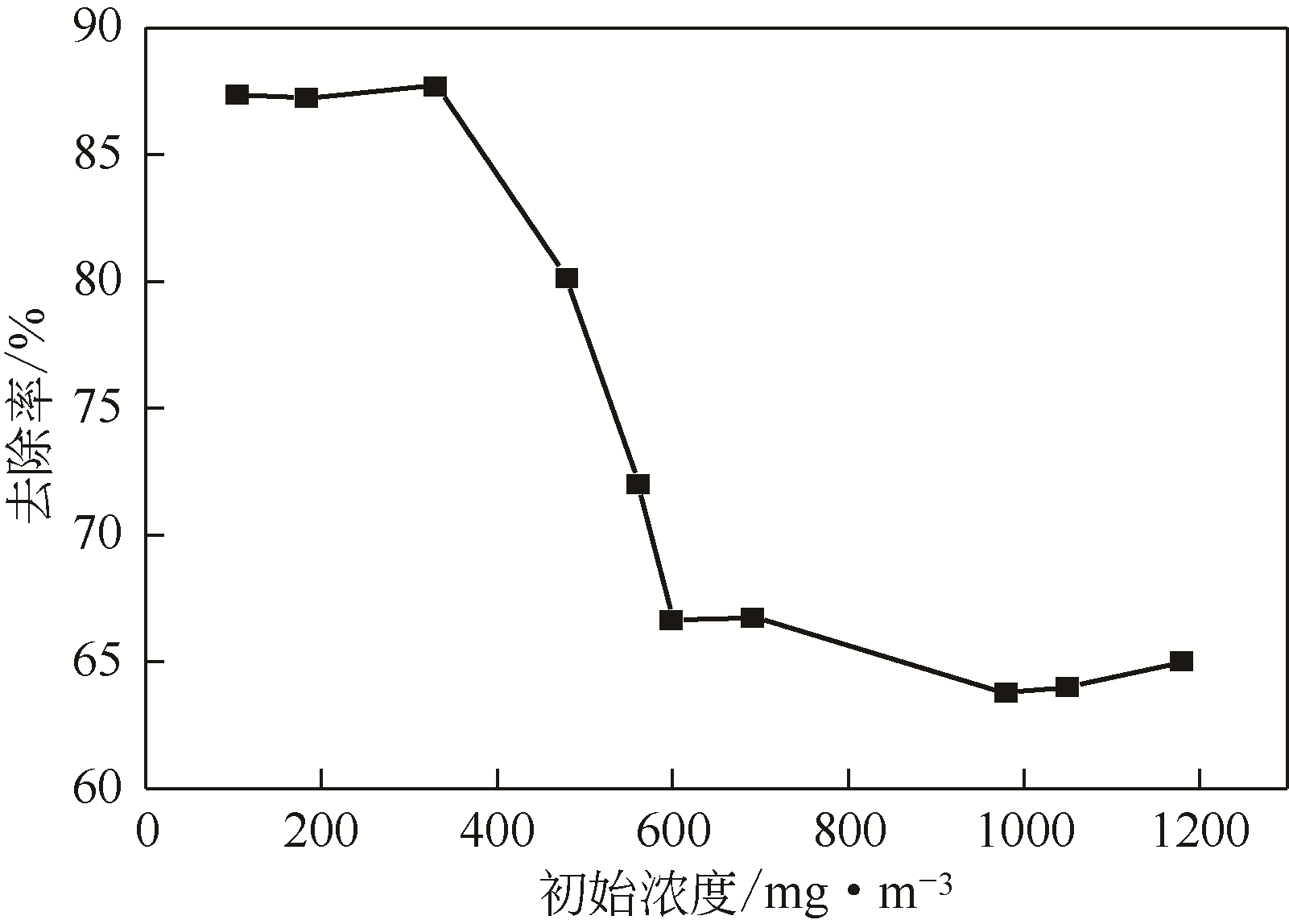
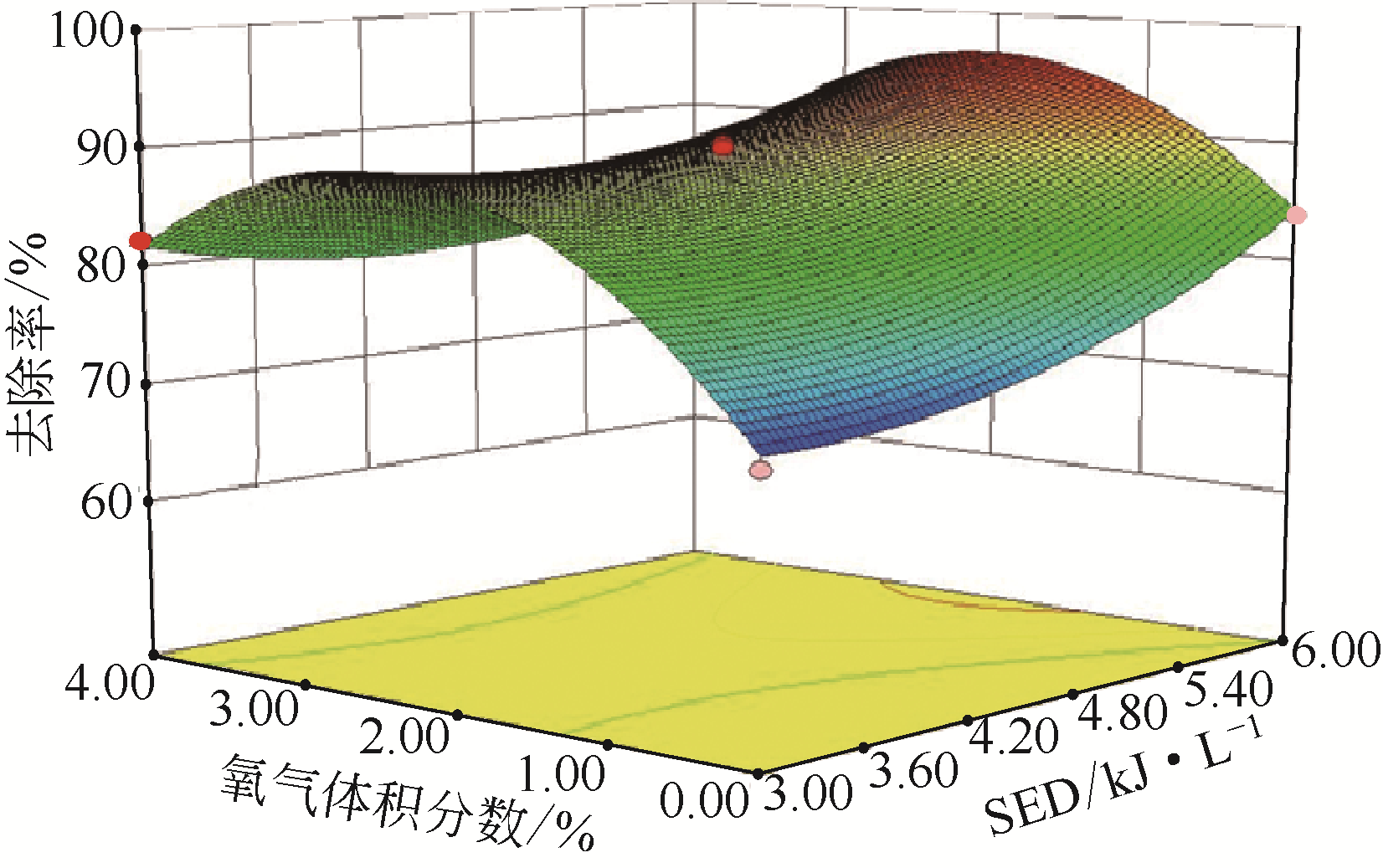
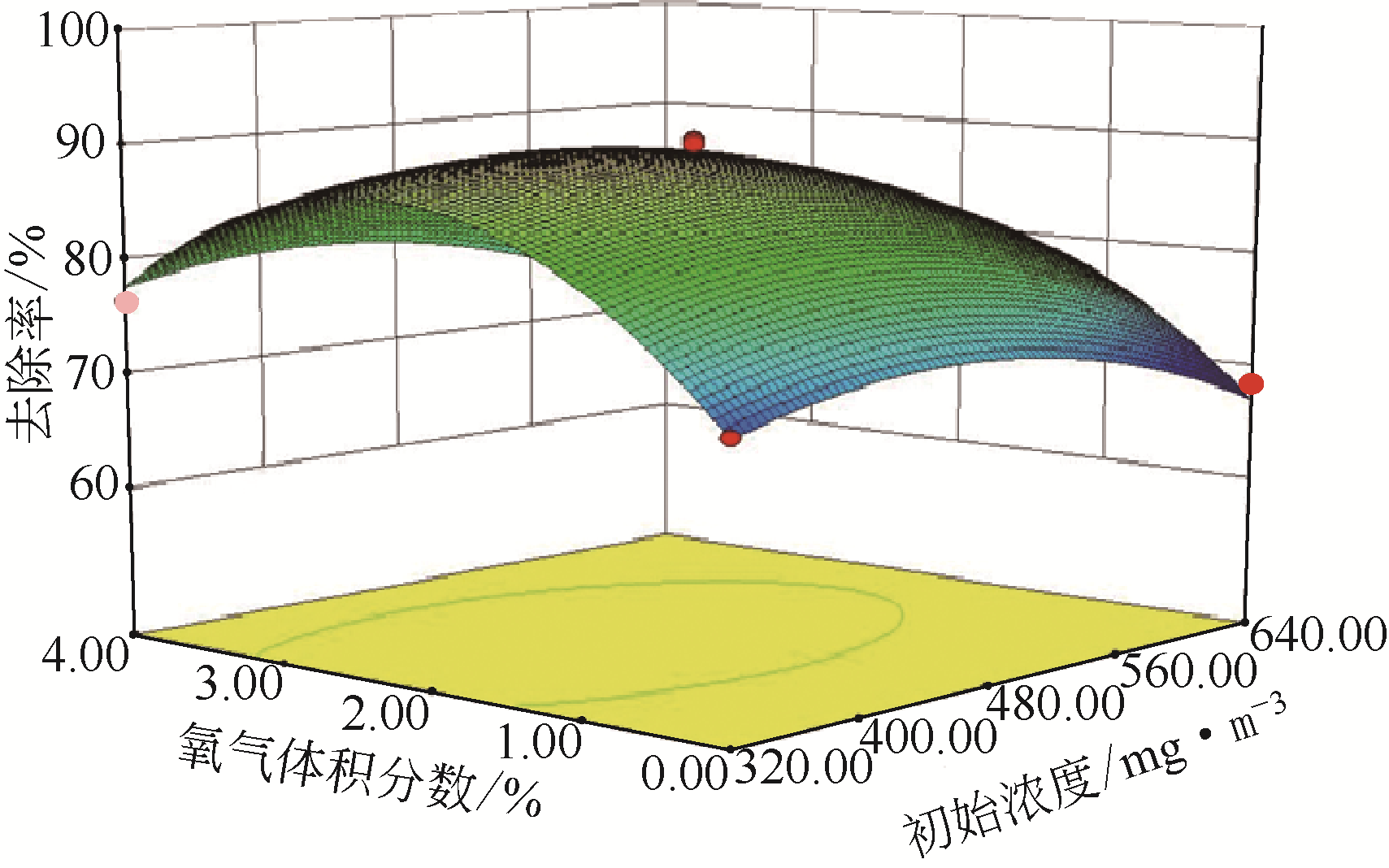
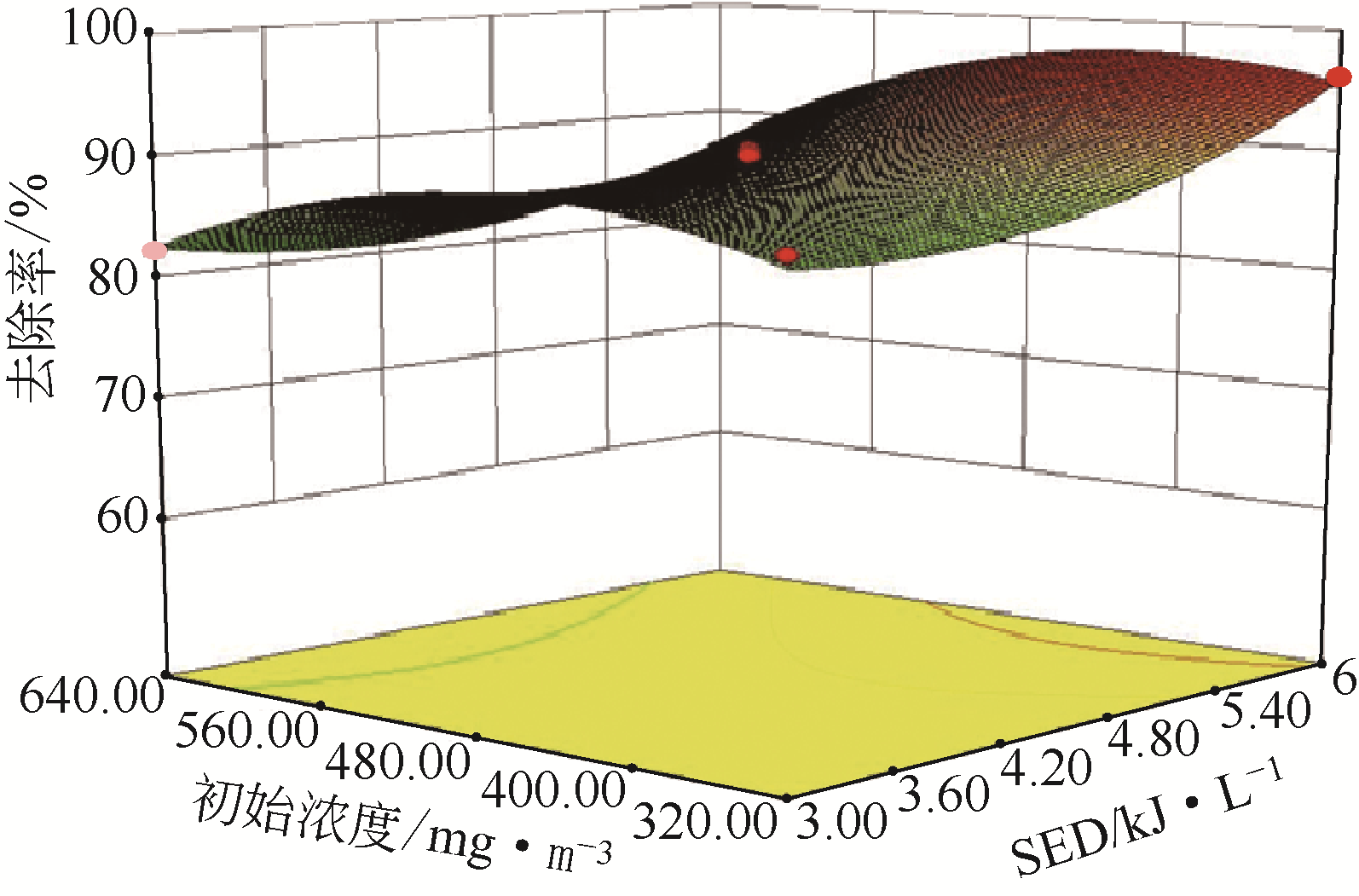
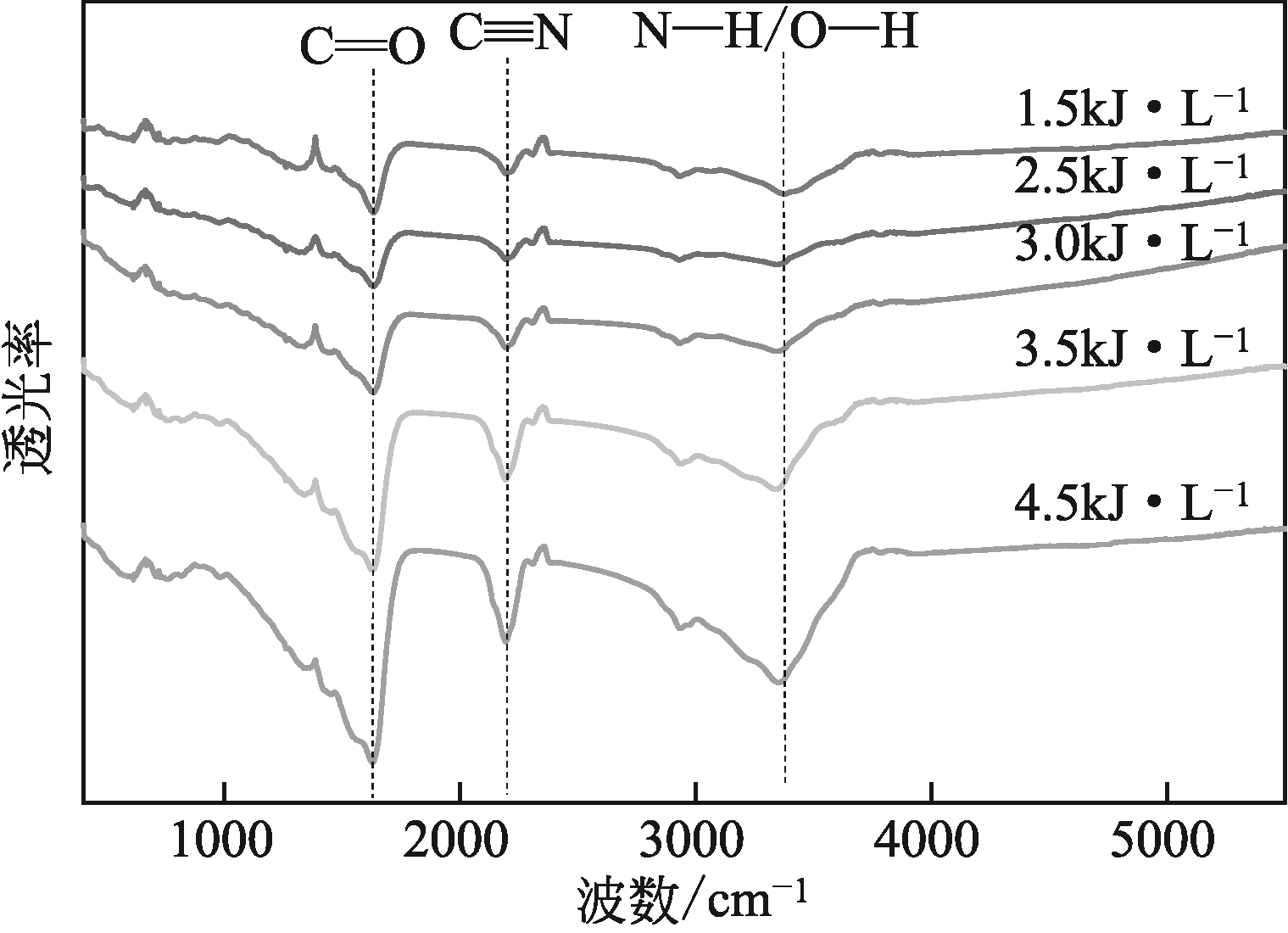
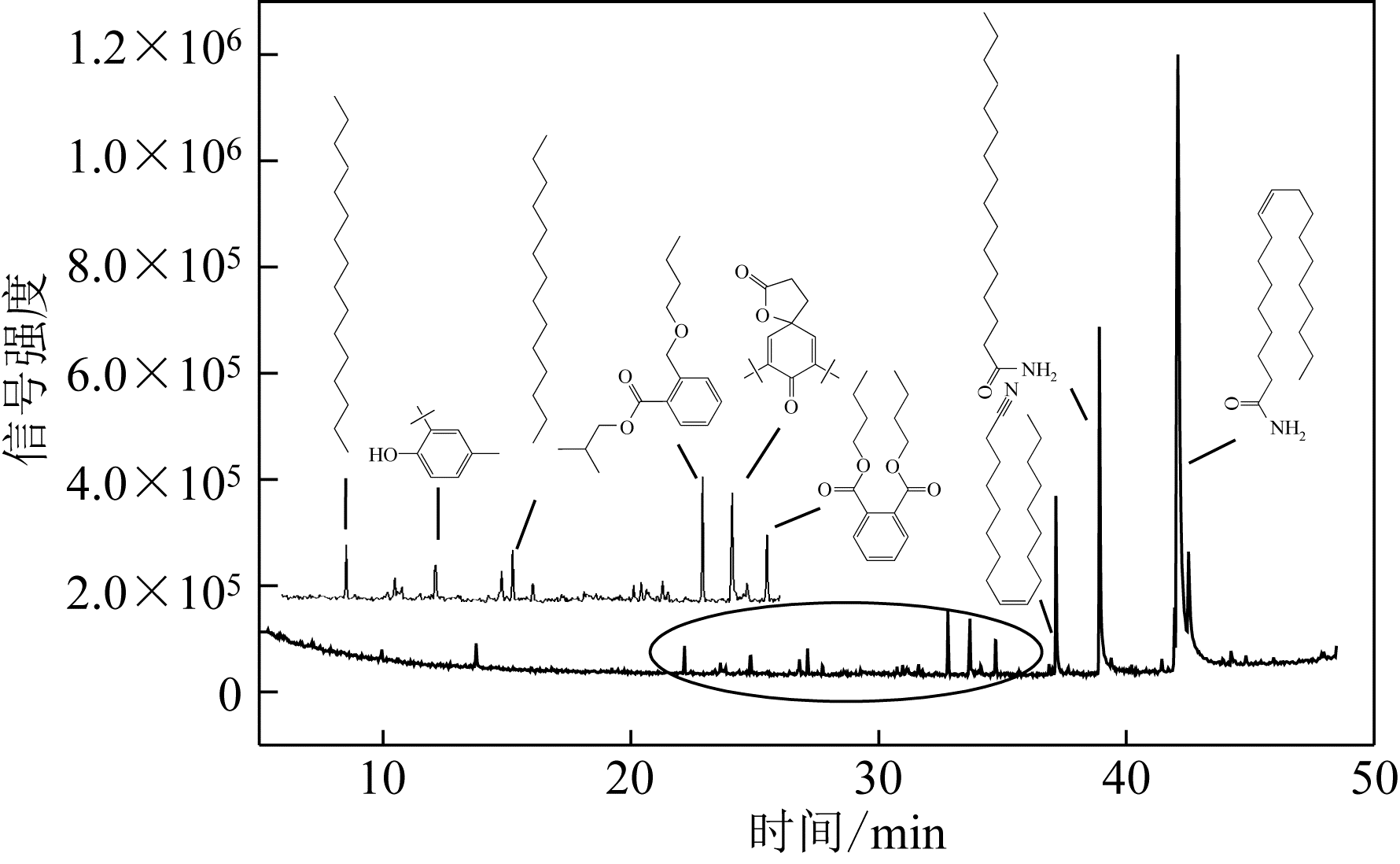
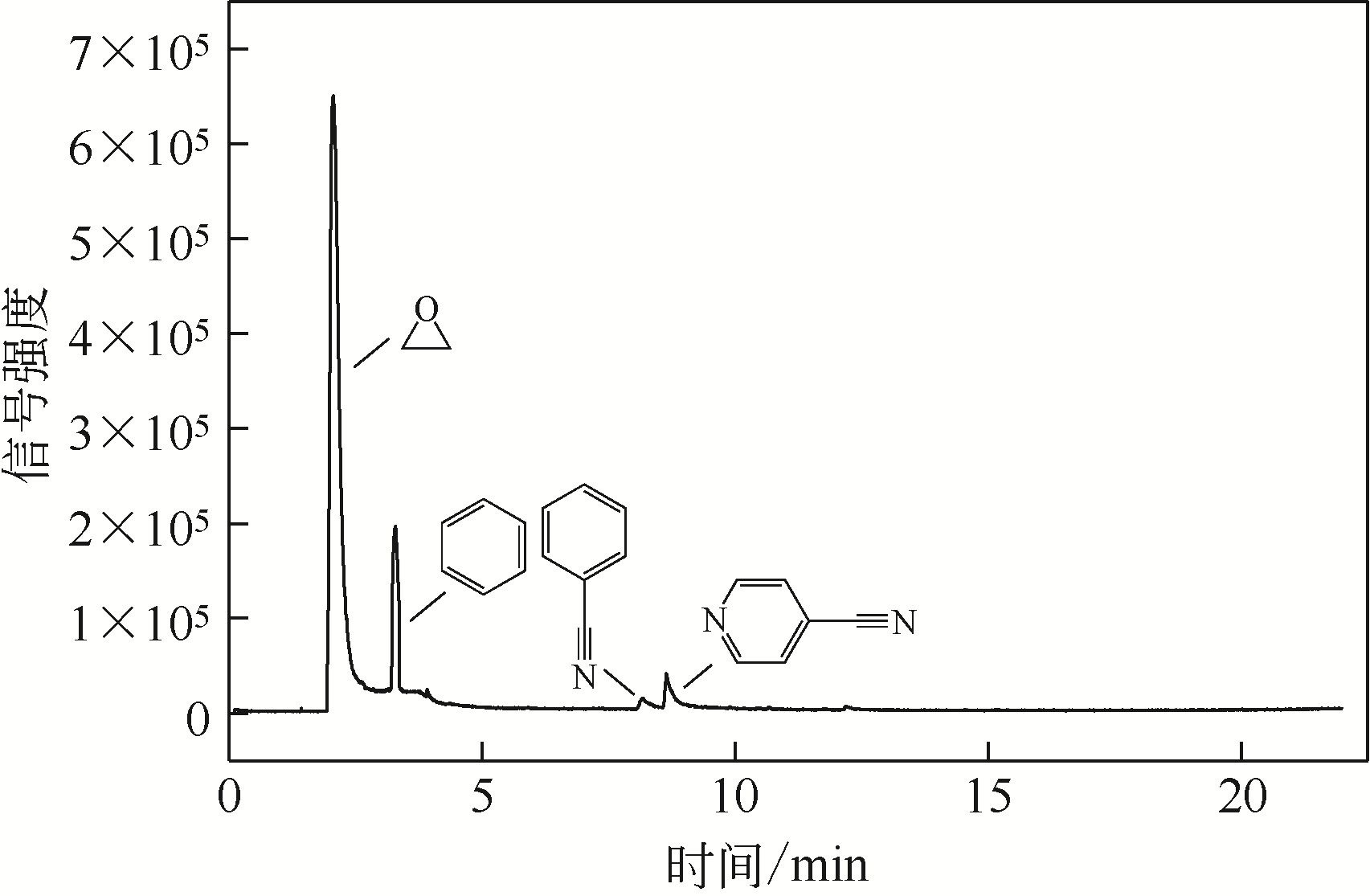
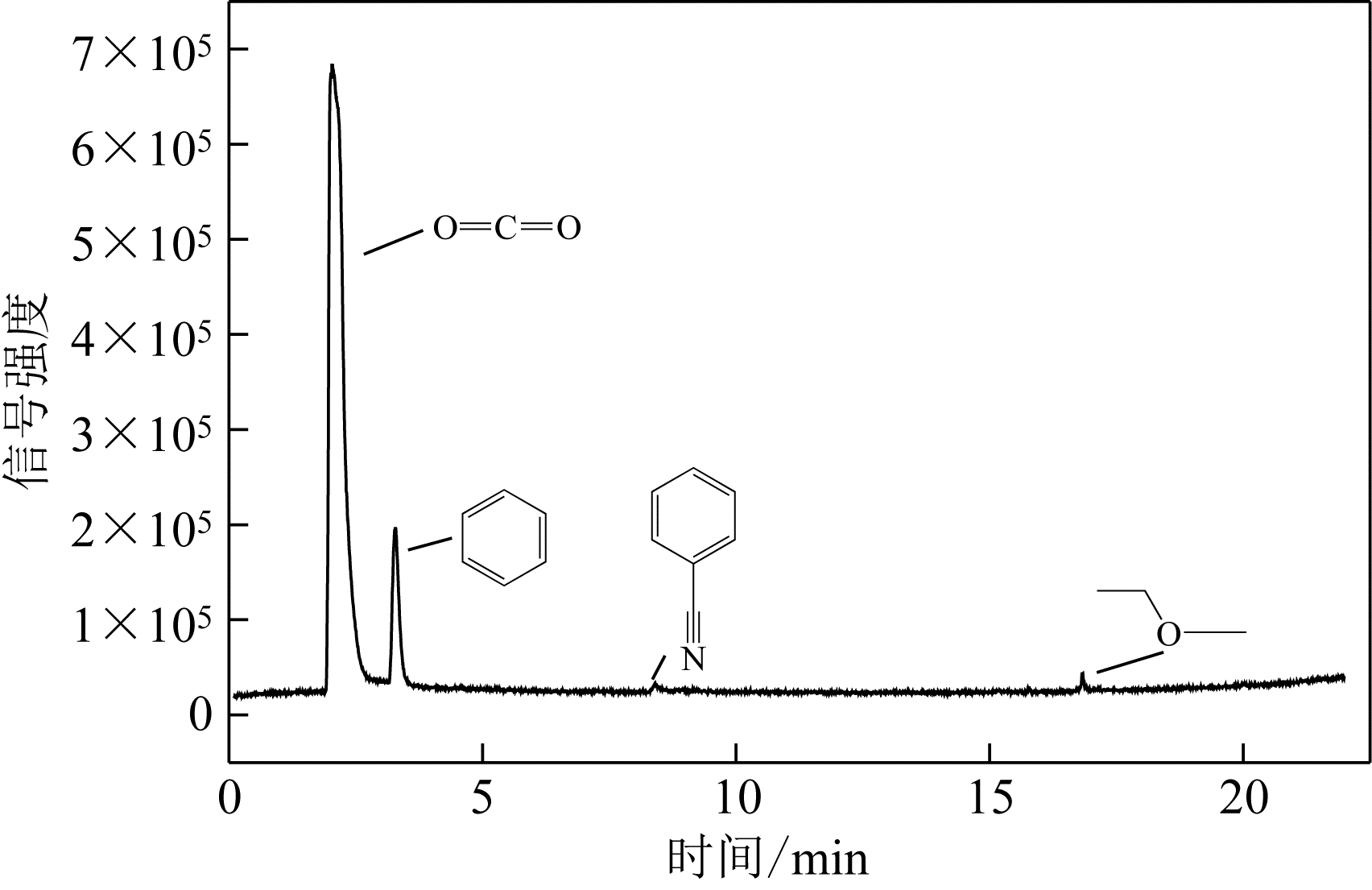
采用低温等离子体技术净化苯,以降解后的苯去除效率为评价指标。根据单因素实验,确定能量密度、初始浓度、氧气含量的取值范围;采用Design-Expert响应曲面法,考察单独变量作用及交互作用对苯去除率的影响;通过傅里叶变换红外光谱仪(FIIR)、气相色谱-质谱联用仪(GC-MS)及扫描电子显微镜(SEM)对反应产物组成进行分析。根据二次多项式模型模拟可知,单因素变量、能量密度和氧气含量的交互项均对苯去除率具有显著影响;优化结果显示,低温等离子体降解苯的最佳工艺条件为能量密度5.98kJ/L,初始浓度452.08mg/m3,氧气体积分数1.66%,模型预测苯去除效率为96.63%,实验验证平均值为95.23%,测定值与预测值之间相对误差为1.40%,证明该模型具有可靠性。固相副产物中主要含长链烷烃、长链烯烃、酚类、酯类、酮类、酰胺类,整体形貌属于团簇状,拥有较明显的球形形貌;液相产物中检测到未降解的苯、环氧乙烷、苯腈、4-氰基吡啶;气相产物除了含有矿化生成的CO2和未降解的苯之外,还含有苯腈和酯。
中图分类号:
引用本文
王春雨,朱玲,许丹芸,罗清月. 低温等离子体降解苯的工艺参数优化[J]. 化工进展, 2020, 39(1): 402-412.
Chunyu WANG,Ling ZHU,Danyun XU,Qingyue LUO. Process parameters optimization for degradation of benzene by non-thermal plasma[J]. Chemical Industry and Engineering Progress, 2020, 39(1): 402-412.
| 项目 | 影响因素 | 水平 | ||
|---|---|---|---|---|
| -1 | 0 | 1 | ||
| 能量密度(X1) | 能量密度/kJ?L-1 | 3 | 4.5 | 6 |
| 初始浓度(X2) | 初始浓度/mg?m-3 | 320 | 480 | 640 |
| 氧气体积分数(X3) | 氧气体积分数/% | 0 | 2 | 4 |
表1 实验设计因素与水平
| 项目 | 影响因素 | 水平 | ||
|---|---|---|---|---|
| -1 | 0 | 1 | ||
| 能量密度(X1) | 能量密度/kJ?L-1 | 3 | 4.5 | 6 |
| 初始浓度(X2) | 初始浓度/mg?m-3 | 320 | 480 | 640 |
| 氧气体积分数(X3) | 氧气体积分数/% | 0 | 2 | 4 |
| 序号 | 编码 | 去除率/% | ||||
|---|---|---|---|---|---|---|
| X1 | X2 | X3 | 实验值 | 预测值 | ||
| 1 | -1 | -1 | 0 | 86.65 | 85.69 | |
| 2 | 1 | -1 | 0 | 96.00 | 95.74 | |
| 3 | -1 | 1 | 0 | 82.30 | 82.56 | |
| 4 | 1 | 1 | 0 | 86.35 | 87.31 | |
| 5 | -1 | 0 | -1 | 69.89 | 70.98 | |
| 6 | 1 | 0 | -1 | 83.95 | 84.34 | |
| 7 | -1 | 0 | 1 | 82.23 | 81.85 | |
| 8 | 1 | 0 | 1 | 84.39 | 83.30 | |
| 9 | 0 | -1 | -1 | 71.32 | 71.20 | |
| 10 | 0 | 1 | -1 | 68.39 | 67.04 | |
| 11 | 0 | -1 | 1 | 76.38 | 77.73 | |
| 12 | 0 | 1 | 1 | 70.21 | 70.33 | |
| 13 | 0 | 0 | 0 | 90.37 | 89.23 | |
| 14 | 0 | 0 | 0 | 90.25 | 89.23 | |
| 15 | 0 | 0 | 0 | 89.88 | 89.23 | |
| 16 | 0 | 0 | 0 | 88.31 | 89.23 | |
| 17 | 0 | 0 | 0 | 87.36 | 89.23 | |
表2 响应曲面法实验设计及结果
| 序号 | 编码 | 去除率/% | ||||
|---|---|---|---|---|---|---|
| X1 | X2 | X3 | 实验值 | 预测值 | ||
| 1 | -1 | -1 | 0 | 86.65 | 85.69 | |
| 2 | 1 | -1 | 0 | 96.00 | 95.74 | |
| 3 | -1 | 1 | 0 | 82.30 | 82.56 | |
| 4 | 1 | 1 | 0 | 86.35 | 87.31 | |
| 5 | -1 | 0 | -1 | 69.89 | 70.98 | |
| 6 | 1 | 0 | -1 | 83.95 | 84.34 | |
| 7 | -1 | 0 | 1 | 82.23 | 81.85 | |
| 8 | 1 | 0 | 1 | 84.39 | 83.30 | |
| 9 | 0 | -1 | -1 | 71.32 | 71.20 | |
| 10 | 0 | 1 | -1 | 68.39 | 67.04 | |
| 11 | 0 | -1 | 1 | 76.38 | 77.73 | |
| 12 | 0 | 1 | 1 | 70.21 | 70.33 | |
| 13 | 0 | 0 | 0 | 90.37 | 89.23 | |
| 14 | 0 | 0 | 0 | 90.25 | 89.23 | |
| 15 | 0 | 0 | 0 | 89.88 | 89.23 | |
| 16 | 0 | 0 | 0 | 88.31 | 89.23 | |
| 17 | 0 | 0 | 0 | 87.36 | 89.23 | |
| 方差来源 | 平方和 | 自由度 | 均方 | F | P | 显著性 |
|---|---|---|---|---|---|---|
| 模型 | 1109.89 | 9 | 123.32 | 55.87 | <0.0001 | 显著 |
| X1 | 109.67 | 1 | 109.67 | 49.68 | 0.0002 | 显著 |
| X2 | 66.70 | 1 | 66.70 | 30.22 | 0.0009 | 显著 |
| X3 | 48.31 | 1 | 48.31 | 21.89 | 0.0023 | 显著 |
| X1X2 | 7.02 | 1 | 7.02 | 3.18 | 0.1177 | 不显著 |
| X1X3 | 35.40 | 1 | 35.40 | 16.04 | 0.0052 | 显著 |
| X2X3 | 2.62 | 1 | 2.62 | 1.19 | 0.3117 | 不显著 |
| X12 | 53.53 | 1 | 53.53 | 24.25 | 0.0017 | 显著 |
| X22 | 104.19 | 1 | 104.19 | 47.20 | 0.0002 | 显著 |
| X32 | 677.46 | 1 | 677.46 | 306.89 | <0.0001 | 显著 |
| 残差 | 15.45 | 7 | 2.21 | — | — | — |
| 失拟项 | 8.35 | 3 | 2.78 | 1.57 | 0.3292 | 不显著 |
| 误差 | 7.11 | 4 | 1.78 | — | — | — |
| 总和 | 1125.34 | 16 | — | — | — | — |
| R2值 | 0.9863 | — | — | — | — | — |
| R2Adj值 | 0.9686 | — | — | — | — | — |
| R2Pred值 | 0.8751 | — | — | — | — | — |
表3 回归方程的方差分析和显著性检验
| 方差来源 | 平方和 | 自由度 | 均方 | F | P | 显著性 |
|---|---|---|---|---|---|---|
| 模型 | 1109.89 | 9 | 123.32 | 55.87 | <0.0001 | 显著 |
| X1 | 109.67 | 1 | 109.67 | 49.68 | 0.0002 | 显著 |
| X2 | 66.70 | 1 | 66.70 | 30.22 | 0.0009 | 显著 |
| X3 | 48.31 | 1 | 48.31 | 21.89 | 0.0023 | 显著 |
| X1X2 | 7.02 | 1 | 7.02 | 3.18 | 0.1177 | 不显著 |
| X1X3 | 35.40 | 1 | 35.40 | 16.04 | 0.0052 | 显著 |
| X2X3 | 2.62 | 1 | 2.62 | 1.19 | 0.3117 | 不显著 |
| X12 | 53.53 | 1 | 53.53 | 24.25 | 0.0017 | 显著 |
| X22 | 104.19 | 1 | 104.19 | 47.20 | 0.0002 | 显著 |
| X32 | 677.46 | 1 | 677.46 | 306.89 | <0.0001 | 显著 |
| 残差 | 15.45 | 7 | 2.21 | — | — | — |
| 失拟项 | 8.35 | 3 | 2.78 | 1.57 | 0.3292 | 不显著 |
| 误差 | 7.11 | 4 | 1.78 | — | — | — |
| 总和 | 1125.34 | 16 | — | — | — | — |
| R2值 | 0.9863 | — | — | — | — | — |
| R2Adj值 | 0.9686 | — | — | — | — | — |
| R2Pred值 | 0.8751 | — | — | — | — | — |
| 名称 | 出峰时间/min | 分子量 | 分子式 | 结构式 | 匹配度/% |
|---|---|---|---|---|---|
| 十四烷 | 22.164 | 198.24 | C14H30 | 98 | |
| 2,4叔丁基苯酚 | 24.846 | 206.17 | C14H22O | 96 | |
| 十六烷 | 27.152 | 226.27 | C16H34 | 96 | |
| 1,2-苯二甲酸,丁基2-甲基丙酯 | 32.819 | 278.15 | C16H22O4 | 86 | |
| 7,9-二叔丁基-1-氧杂螺(4,5)癸-6,9-二烯-2,8-二酮 | 33.686 | 276.17 | C17H24O3 | 99 | |
| 邻苯二甲酸二丁酯 | 34.736 | 278.15 | C16H22O4 | 95 | |
| 9-十八烯腈 | 37.117 | 263.26 | C18H33N | 99 | |
| 十六碳酰胺 | 38.934 | 255.26 | C16H33NO | 91 | |
| 9-十八碳烯酰胺 | 42.119 | 281.27 | C18H35NO | 96 |
表4 固相副产物组成信息表
| 名称 | 出峰时间/min | 分子量 | 分子式 | 结构式 | 匹配度/% |
|---|---|---|---|---|---|
| 十四烷 | 22.164 | 198.24 | C14H30 | 98 | |
| 2,4叔丁基苯酚 | 24.846 | 206.17 | C14H22O | 96 | |
| 十六烷 | 27.152 | 226.27 | C16H34 | 96 | |
| 1,2-苯二甲酸,丁基2-甲基丙酯 | 32.819 | 278.15 | C16H22O4 | 86 | |
| 7,9-二叔丁基-1-氧杂螺(4,5)癸-6,9-二烯-2,8-二酮 | 33.686 | 276.17 | C17H24O3 | 99 | |
| 邻苯二甲酸二丁酯 | 34.736 | 278.15 | C16H22O4 | 95 | |
| 9-十八烯腈 | 37.117 | 263.26 | C18H33N | 99 | |
| 十六碳酰胺 | 38.934 | 255.26 | C16H33NO | 91 | |
| 9-十八碳烯酰胺 | 42.119 | 281.27 | C18H35NO | 96 |
| 名称 | 出峰时间 /min | 分子量 | 分子式 | 结构式 | 匹配度/% |
|---|---|---|---|---|---|
| 环氧乙烷 | 2.211 | 44.03 | C2H4O | 98 | |
| 苯 | 3.053 | 78.05 | C6H6 | 96 | |
| 苯腈 | 8.406 | 103.04 | C7H5N | 72 | |
| 4-氰基吡啶 | 8.774 | 104.04 | C6H4N2 | 75 |
表5 液相产物组成信息表
| 名称 | 出峰时间 /min | 分子量 | 分子式 | 结构式 | 匹配度/% |
|---|---|---|---|---|---|
| 环氧乙烷 | 2.211 | 44.03 | C2H4O | 98 | |
| 苯 | 3.053 | 78.05 | C6H6 | 96 | |
| 苯腈 | 8.406 | 103.04 | C7H5N | 72 | |
| 4-氰基吡啶 | 8.774 | 104.04 | C6H4N2 | 75 |
| 名称 | 出峰时间/min | 分子量 | 分子式 | 结构式 | 匹配度/% |
|---|---|---|---|---|---|
| 二氧化碳 | 2.126 | 43.99 | CO2 | 98 | |
| 苯 | 3.053 | 78.05 | C6H6 | 94 | |
| 苯腈 | 8.406 | 103.04 | C7H5N | 68 | |
| 邻苯二甲酸二乙酯 | 16.828 | 222.09 | C12H14O4 | 83 |
表6 气相产物组成信息表
| 名称 | 出峰时间/min | 分子量 | 分子式 | 结构式 | 匹配度/% |
|---|---|---|---|---|---|
| 二氧化碳 | 2.126 | 43.99 | CO2 | 98 | |
| 苯 | 3.053 | 78.05 | C6H6 | 94 | |
| 苯腈 | 8.406 | 103.04 | C7H5N | 68 | |
| 邻苯二甲酸二乙酯 | 16.828 | 222.09 | C12H14O4 | 83 |
| 化学键 | 能量/eV |
|---|---|
| C—C | 3.6 |
| C—H(环内) | 4.3 |
| C | 5.5 |
| C—O | 3.7 |
| C | 8.3 |
| O | 5.12 |
| N | 9.82 |
表7 主要化学键及键能
| 化学键 | 能量/eV |
|---|---|
| C—C | 3.6 |
| C—H(环内) | 4.3 |
| C | 5.5 |
| C—O | 3.7 |
| C | 8.3 |
| O | 5.12 |
| N | 9.82 |
| 1 | 陈文泰, 邵敏, 袁斌, 等. 大气中挥发性有机物(VOCs)对二次有机气溶胶(SOA)生成贡献的参数化估算[J]. 环境科学学报, 2013, 33(1): 163-172. |
| CHEN W T, SHAO M, YUAN B, et al. Parameterization of contribution to secondary organic aerosol(SOA) formation from ambient volatile organic compounds[J]. Acta Scientiae Circumstantiae, 2013, 33(1): 163-172. | |
| 2 | 姜理英, 张迪, 郭海倩, 等. 低温等离子体对复合CVOCs的降解特性[J]. 环境科学, 2017, 38(5): 1792-1798. |
| JIANG L Y, ZHANG D, GUO H Q, et al. Degradation characteristics of composite CVOCs by non-thermal plasma[J]. Environmental Science, 2017, 38(5): 1792-1798. | |
| 3 | KIM K H, SZULEJKO J E, KUMAR P, et al. Air ionization as a control technology for off-gas emissions of volatile organic compounds[J]. Environment Pollution, 2017, 225: 729-743. |
| 4 | VELLINGIRI K, KIM K H, KWON E E, et al. Insights into the adsorption capacity and breakthrough properties of a synthetic zeolite against a mixture of various sulfur species at low ppb levels[J]. Journal of Environmental Management, 2016, 166(5): 484-492. |
| 5 | 曹晓晓. 介质阻挡放电低温等离子体脱除烟气NOx及VOCs实验研究[D]. 济南: 山东大学, 2016. |
| CAO X X. Experimental study on removal of NOx and VOCs from flue gas by barrier discharge plasma process[D]. Jinan: Shandong University, 2016. | |
| 6 | 王志伟, 裴多斐, 于丽平. VOCs控制与处理技术综述[J]. 环境与发展, 2017, 29(1): 1-4. |
| WANG Z W, PEI D F, YU L P. A review of the control and treatment techniques of volatile organic compounds[J]. Environment and Development, 2017, 29(1): 1-4. | |
| 7 | 高根煜. 废气中烃类的排放控制和回收利用技术[J]. 工业安全与环保, 2004, 30(3): 11-13. |
| GAO G Y. Discharge control and recovery technologies for hydrocarbon in waste gas[J]. Industrial Safety and Environmental Protection, 2004, 30(3): 11-13. | |
| 8 | 刘程, 张贵新, 王强, 等. 大气压微波等离子体处理有机废气的实验研究[J]. 高电压技术, 2017, 43(8): 247-253. |
| LIU C, ZHNAG G X, WANG Q, et al. Experimental study of organic waste gas treatment by microwave plasma generated at atmospheric[J]. Pressure High Voltage Engineering, 2017, 43(8): 247-253. | |
| 9 | 陈海红, 骆永明, 滕应, 等. 重度滴滴涕污染土壤低温等离子体修复条件优化研究[J]. 环境科学, 2013, 34(1): 302-307. |
| CHEN H H, LUO Y M, TENG Y, et al. Optimizing remediation conditions of non-thermal plasma for DDTs heavily contaminated soil[J]. Environmental Science, 2013, 34(1): 302-307. | |
| 10 | AN H T Q, HUU T P, VAN T L, et al. Application of atmospheric non thermal plasma-catalysis hybrid system for air pollution control: toluene removal[J]. Catalysis Today, 2011, 176(1): 474-477. |
| 11 | 李华琴, 何觉聪, 陈洲洋, 等. 低温等离子体-生物法处理硫化氢气体研究[J]. 环境科学, 2014, 35(4): 1256-1262. |
| LI H Q, HE J C, CHEN Z Y, et al. Hydrogen sulfide removal by the combination of non-thermal plasma and biological process[J]. Environmental Science, 2014, 35(4): 1256-1262. | |
| 12 | WANG B, CHI C, XU M, et al. Plasma-catalytic removal of toluene over CeO2-MnOx catalysts in an atmosphere dielectric barrier discharge[J]. Chemical Engineering Journal, 2017, 322: 679-692. |
| 13 | 甘蓉丽, 罗光前, 许洋, 等. 低温等离子体协同铜铈催化剂脱除甲苯[J]. 化工进展, 2018, 37(9): 3416-3423. |
| GAN R L, LUO G Q, XU Y, et al. Removal of toluene by non-thermal plasma coupled with Cu-Ce catalysts[J]. Chemical Industry and Engineering Progress, 2018, 37(9): 3416-3423. | |
| 14 | YE Z, ZHANG Y, PING L, et al. Feasibility of destruction of gaseous benzene with dielectric barrier discharge[J]. Journal of Hazardous Materials, 2008, 156(1): 356-364. |
| 15 | 梁文俊, 李坚, 李依丽, 等. 低温等离子体法去除苯和甲苯废气性能研究[J]. 环境工程学报, 2005, 6(5): 51-55. |
| LIANG W J, LI J, LI Y L, et al. Degradation of benzene and toluene with non-thermal plasma[J]. Techniques and Equipment for Environmental Pollution Control, 2005, 6(5): 51-55. | |
| 16 | JIANG N, LU N, SHANG K, et al. Effects of electrode geometry on the performance of dielectric barrier/packed-bed discharge plasmas in benzene degradation[J]. Journal of Hazardous Materials, 2013, 262(22): 387-393. |
| 17 | ZHANG J, LIU J, ZHANG R, et al. Destruction of gaseous styrene with a low-temperature plasma induced by a tubular multilayer dielectric barrier discharge[J].Plasma Science and Technology, 2015, 17(1): 50-55. |
| 18 | 刘丹, 张连成, 黄逸凡, 等. 双杆介质阻挡放电降解酸性红73废水[J]. 化工进展, 2018, 37(9): 3640-3648. |
| LIU D, ZHANG L C, HUANG Y F, et al. Degradation of Brilliant Crocein wastewater by double-rod dielectric barrier discharge[J]. Chemical Industry and Engineering Progress, 2018, 37(9): 3640-3648. | |
| 19 | LI Y, FAN Z, SHI J, et al. Removal of volatile organic compounds (VOCs) at room temperature using dielectric barrier discharge and plasma-catalysis[J]. Plasma Chemistry & Plasma Processing, 2014, 34(4): 801-810. |
| 20 | KARATUM O, DESHUSSES M A. A comparative study of dilute VOCs treatment in a non-thermal plasma reactor[J]. Chemical Engineering Journal, 2016, 294: 308-315. |
| 21 | WANG B, YAO S, PENG Y, et al. Toluene removal over TiO2-BaTiO3 catalysts in an atmospheric dielectric barrier discharge[J]. Journal of Environmental Chemical Engineering, 2018, 6(4): 3819-3826. |
| 22 | 王梅. 介质阻挡空气放电等离子体(DBD)处理挥发性有机物苯的效果及其影响因素研究[D]. 苏州: 苏州大学, 2016. |
| WANG M. Study on the influencing factors of the degradation of volatile organic compounds benzene by air dielectric barrier discharge(DBD) plasma[D]. Suzhou: Soochow University, 2016. | |
| 23 | RUBIO S J, QUINTERO M C, RODERO A, et al. Assessment of a new carbon tetrachloride destruction system based on a microwave plasma torch operating at atmospheric pressure[J]. Journal of Hazardous Materials, 2007, 148(1): 419-427. |
| 24 | LIANG P, JIANG W, ZHANG L, et al. Experimental studies of removing typical VOCs by dielectric barrier discharge reactor of different sizes[J]. Process Safety and Environmental Protection, 2015, 94: 380-384. |
| 25 | 朱伊娜, 徐耀东, 伍斌. 低温等离子体降解污染土壤热脱附尾气中DDTs[J]. 环境科学研究, 2018, 31(12): 2140-2145. |
| ZHU Y N, XU Y D, W B. Degradation of DDTs in thermal desorption off-gas by non-thermal plasma[J]. Research of Environmental Sciences, 2018, 31(12): 2140-2145. | |
| 26 | 区瑞锟, 陈砺, 严宗诚, 等. 低温等离子体-催化协同降解挥发性有机废气[J]. 环境科学与技术, 2011, 34(1): 79-84. |
| QU R K, CHEN L, YAN Z C, et al. Decomposition of volatile organic compounds by non-thermal plasma-catalyst hybrid technology[J]. Environmental Science & Technology, 2011, 34(1): 79-84. | |
| 27 | LU W, ABBAS Y, MUSTAFA M F, et al. A review on application of dielectric barrier discharge plasma technology on the abatement of volatile organic compounds[J]. Frontiers of Environmental Science & Engineering, 2019, 13(2): 30. |
| 28 | 廖晓晓. 低温等离子体——催化净化有机废气反应自由基及机理研究[D]. 广州: 华南理工大学, 2010. |
| LIAO X X. Free radicals formation and mechanism for volatile organic compouds decomposition with non-thermal plasma-catalysis technology[D]. Guangzhou: South China University of Technology, 2010. | |
| 29 | 翁诗甫. 傅里叶变换红外光谱分析[M]. 北京: 化学工业出版社, 2010: 308-312. |
| WENG S F. Fourier transform infrared spectroscopy[M]. Beijing: Chemical Industry Press, 2010: 308-312. | |
| 30 | LIANG W J, LIN M, HUAN L, et al. Toluene degradation by non-thermal plasma combined with a ferroelectric catalyst[J]. Chemosphere, 2013, 92(10): 1390-1395. |
| 31 | DANG X, QIN C, HUANG J, et al. Adsorbed benzene/toluene oxidation using plasma driven catalysis with gas circulation: elimination of the byproducts[J]. Journal of Industrial & Engineering Chemistry, 2016, 37: 366-371. |
| 32 | LEE B Y, PARK S H, LEE S C, et al. Decomposition of benzene by using a discharge plasma-photocatalyst hybrid system[J]. Catalysis Today, 2004, 93(9): 769-776. |
| 33 | 竹涛, 万艳东, 李坚, 等. 低温等离子体-催化耦合降解甲苯的研究及机理探讨[J]. 高校化学工程学报, 2011, 25(1): 161-167. |
| ZHU T, WAN Y D, LI J, et al. Study on decomposition mechanism of toluene by non-thermal plasma coupled with catalysis[J]. Journal of Chemical Engineering of Chinese Universities, 2011, 25(1): 161-167. | |
| 34 | 王洪昌. 介质阻挡放电去除气态混合VOCs的研究[D]. 大连: 大连理工大学, 2010. |
| WANG H C. Study on removal of mixed VOCs in air by dielectric barrier discharge[D]. Dalian: Dalian University of Technology, 2010. | |
| 35 | 郑光云, 侯健, 蒋洁敏, 等. 非平稳态等离子体降解流动态低浓度甲苯气体的研究[J]. 复旦学报(自然科学版), 2001, 40(4): 364-367. |
| ZHENG G Y, HOU J, JIANG J M, et al. Degradation of flowing low-density gaseous toluene by non-equilibrium plasma[J]. Journal of Fudan University (Natural Science), 2001, 40(4): 364-367. | |
| 36 | 马竞涛, 周则飞, 吴祖成, 等. 低温等离子体-工业废气处理系统说明[J]. 炼油技术与工程, 2007, 37(4): 50-54. |
| MA J T, ZHOU Z F, WU Z C, et al. Study on commercial application of low-thermal plasma in treating fouling waste gas[J]. Petroleum Refinery Engineering, 2007, 37(4): 50-54. | |
| 37 | XU N, FU W, HE C, et al. Benzene removal using non-thermal plasma with CuO/AC catalyst: reaction condition optimization and decomposition mechanism[J]. Plasma Chem Plasma Process, 2014, 34: 1387-1402. |
| 38 | 叶招莲, 宋潇潇, 何锦丛. 介质阻挡放电脱除模拟工业苯系物的可行性[J]. 环境科学学报, 2008, 28(12): 2480-2486. |
| YE Z L, SONG X X, HE J C. Feasibility of benzene series waste gas destruction with DBD technology[J]. Acta Scientiae Circumstantiae, 2008, 28(12): 2480-2486. | |
| 39 | JIANG B, WEN Y, LI Z, et al. Theoretical analysis on the removal of cyclic volatile organic compounds by non-thermal plasma[J]. Water Air Soil Pollution, 2018, 229(2): 35. |
| 40 | JINGTING W, XU C, RENXI Z, et al. Effect of water vaporon toluene removal in catalysis-DBD plasma reactors[J]. Plasma Science & Technology, 2016, 18(4): 370-375. |
| 41 | OGNIER S, CAVADIAS S, AMOUROUX J. Aromatic VOC removal by formation of microparticles in pure nitrogen discharge barrier discharge[J]. Plasma Processes & Polymers, 2010, 4(5): 528-536. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 郑谦, 官修帅, 靳山彪, 张长明, 张小超. 铈锆固溶体Ce0.25Zr0.75O2光热协同催化CO2与甲醇合成DMC[J]. 化工进展, 2023, 42(S1): 319-327. |
| [6] | 戴欢涛, 曹苓玉, 游新秀, 徐浩亮, 汪涛, 项玮, 张学杨. 木质素浸渍柚子皮生物炭吸附CO2特性[J]. 化工进展, 2023, 42(S1): 356-363. |
| [7] | 高雨飞, 鲁金凤. 非均相催化臭氧氧化作用机理研究进展[J]. 化工进展, 2023, 42(S1): 430-438. |
| [8] | 王莹, 韩云平, 李琳, 李衍博, 李慧丽, 颜昌仁, 李彩侠. 城市污水厂病毒气溶胶逸散特征研究现状与未来展望[J]. 化工进展, 2023, 42(S1): 439-446. |
| [9] | 顾永正, 张永生. HBr改性飞灰对Hg0的动态吸附及动力学模型[J]. 化工进展, 2023, 42(S1): 498-509. |
| [10] | 孙玉玉, 蔡鑫磊, 汤吉海, 黄晶晶, 黄益平, 刘杰. 反应精馏合成甲基丙烯酸甲酯工艺优化及节能[J]. 化工进展, 2023, 42(S1): 56-63. |
| [11] | 李宁, 李金科, 董金善. 乙烯裂解炉多孔介质燃烧器的研究与开发[J]. 化工进展, 2023, 42(S1): 73-83. |
| [12] | 杨寒月, 孔令真, 陈家庆, 孙欢, 宋家恺, 王思诚, 孔标. 微气泡型下向流管式气液接触器脱碳性能[J]. 化工进展, 2023, 42(S1): 197-204. |
| [13] | 杨建平. 降低HPPO装置反应系统原料消耗的PSE[J]. 化工进展, 2023, 42(S1): 21-32. |
| [14] | 王福安. 300kt/a环氧丙烷工艺反应器降耗减排分析[J]. 化工进展, 2023, 42(S1): 213-218. |
| [15] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||