化工进展 ›› 2019, Vol. 38 ›› Issue (12): 5603-5611.DOI: 10.16085/j.issn.1000-6613.2019-0541
• 资源与环境化工 • 上一篇
N-杂环离子液体液固相变吸收SO2
- 昆明理工大学化学工程学院,云南 昆明 650500
-
收稿日期:2019-04-09出版日期:2019-12-05发布日期:2019-12-05 -
通讯作者:赵文波 -
作者简介:谢旭豪(1995—),男,硕士研究生,研究方向为酸性废气的捕集与利用。E-mail:18487214508@163.com 。 -
基金资助:国家自然科学基金(21306071)
Liquid-solid phase change absorption of SO2 by N-heterocyclic ionic liquid
Xuhao XIE( ),Ling CHEN,Shengchao XU,Wenbo ZHAO(
),Ling CHEN,Shengchao XU,Wenbo ZHAO( )
)
- Faculty of Chemical Engineering,Kunming University of Science and Technology,Kunming 650500,Yunnan,China
-
Received:2019-04-09Online:2019-12-05Published:2019-12-05 -
Contact:Wenbo ZHAO
摘要:
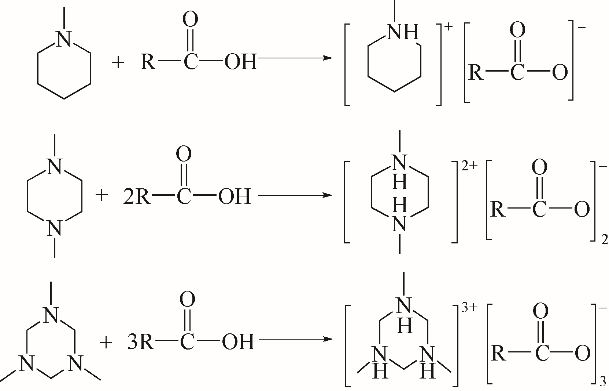
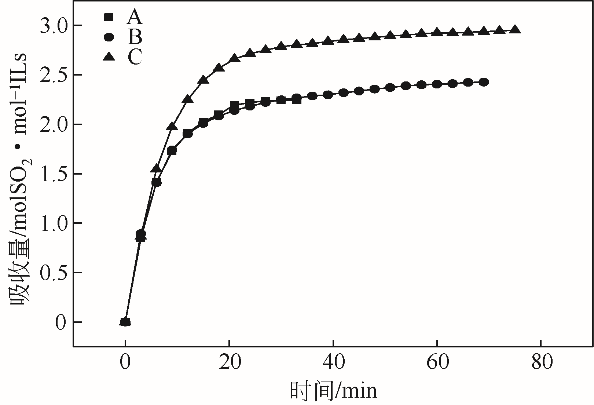
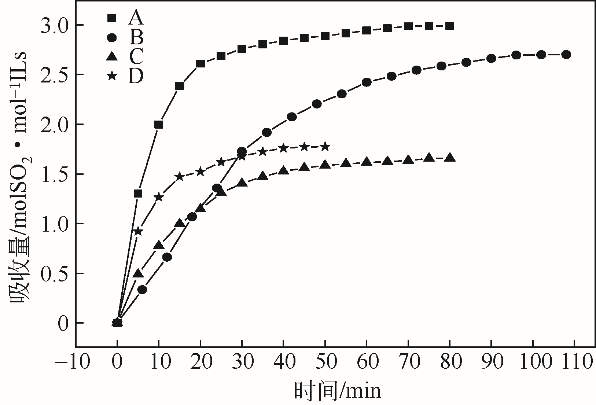
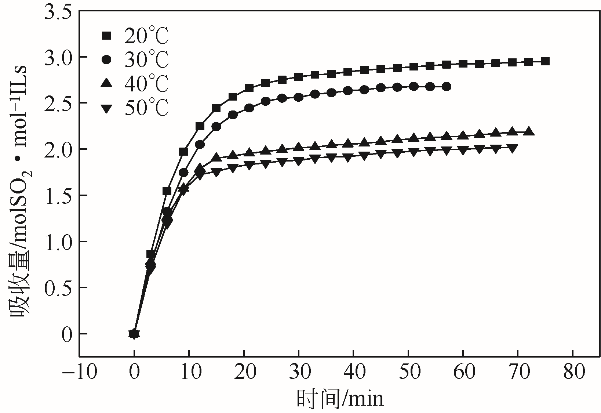
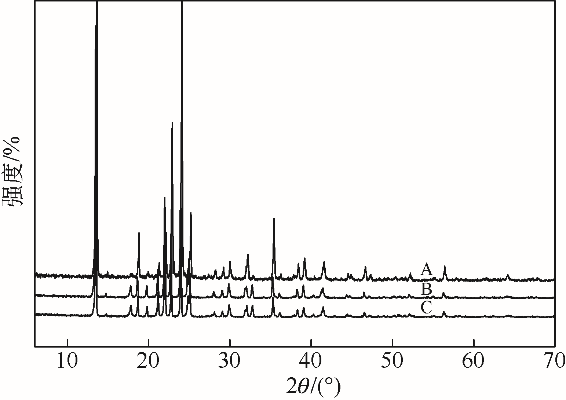
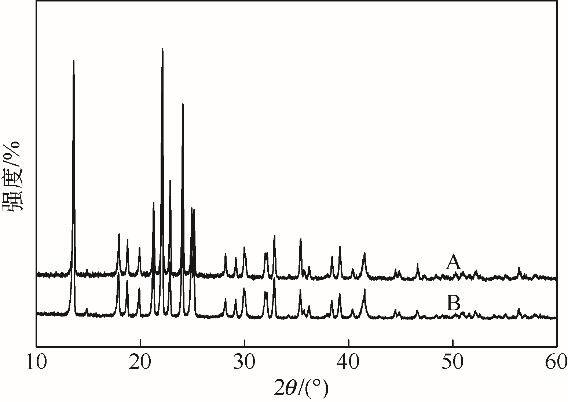
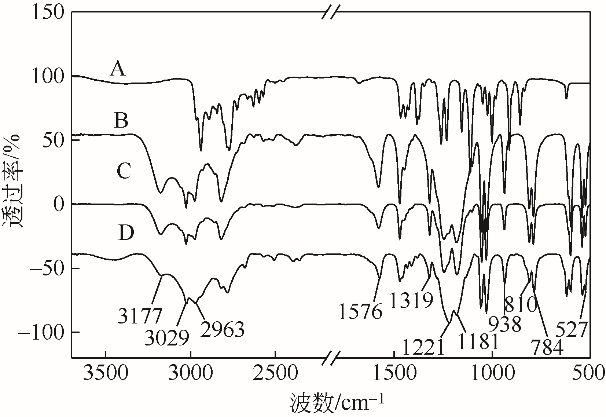
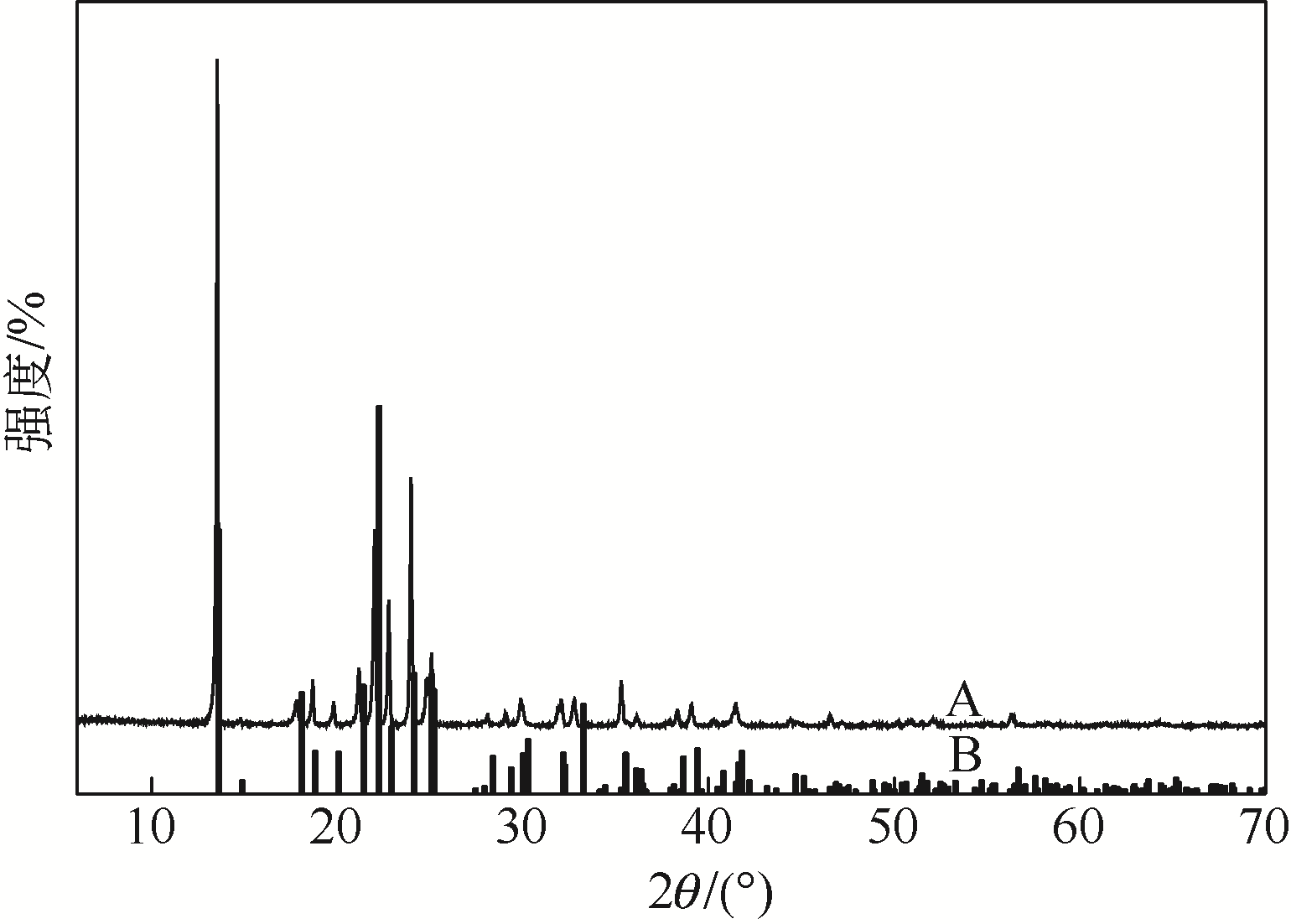
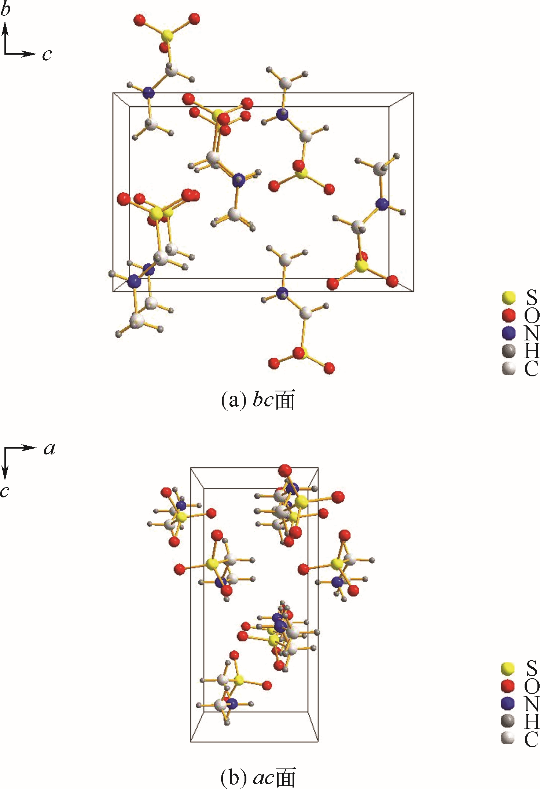
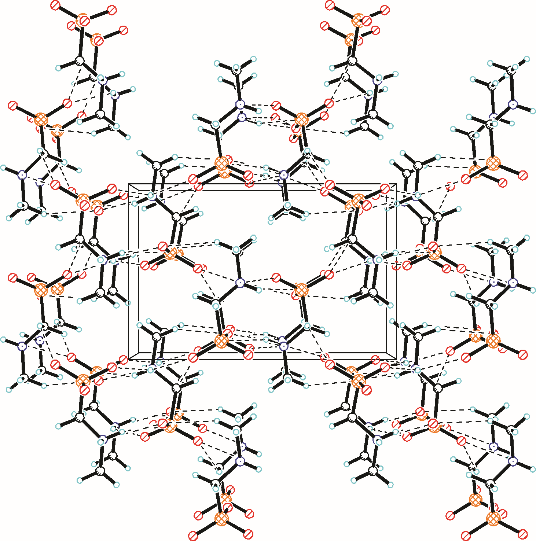
采用不同N含量及碱性的六元N-杂环三级胺与不同酸性的有机羧酸直接中和制备了一系列离子液体,并将其用于SO2的捕集,发现制得的部分离子液体可液固相变吸收SO2。通过探究不同N-杂环离子液体对SO2的吸收负载量,发现离子液体的SO2吸收性能受阳离子N含量以及阴、阳离子的pKa共同影响。采用XRD、FTIR、元素分析、单晶衍射等表征手段对1,3,5-三甲基六氢-1,3,5-三嗪乙酸盐离子液体相变吸收SO2的产物进行分析,单晶解析发现固体吸收产物为甲胺基甲磺酸,并进一步推测了该离子液体吸收SO2的反应过程与相变机理,反应过程先是1,3,5-三甲基六氢-1,3,5-三嗪在酸性条件下分解成亚甲基甲胺,然后由于SO2的亲核作用,导致亚甲基甲胺碳氮双键的断裂和产物碳硫单键的形成。
中图分类号:
引用本文
谢旭豪,陈玲,许胜超,赵文波. N-杂环离子液体液固相变吸收SO2[J]. 化工进展, 2019, 38(12): 5603-5611.
Xuhao XIE,Ling CHEN,Shengchao XU,Wenbo ZHAO. Liquid-solid phase change absorption of SO2 by N-heterocyclic ionic liquid[J]. Chemical Industry and Engineering Progress, 2019, 38(12): 5603-5611.
| 有机胺 | pKa |
|---|---|
| 1-甲基哌啶 | 9.59 |
| 1,4-二甲基哌嗪 | 7.99 |
| 1,3,5-三嗪 | 6.5 |
表1 阳离子供体胺pKa
| 有机胺 | pKa |
|---|---|
| 1-甲基哌啶 | 9.59 |
| 1,4-二甲基哌嗪 | 7.99 |
| 1,3,5-三嗪 | 6.5 |
| 吸收体系 | 结果 | C/% | H/% | N/% | S/% |
|---|---|---|---|---|---|
| [tmtz][Ac]+3SO2 | 实验值1 | 20.1 | 6.61 | 11.26 | 26.25 |
| 实验值2 | 20.02 | 6.19 | 10.98 | 25.91 | |
| [tmtz][Ac]+H2O+3SO2 | 实验值1 | 19.91 | 6.38 | 11.18 | 24.85 |
| 实验值2 | 20 | 6.99 | 11.48 | 23.31 | |
| [tmtz][Ac]+3SO2 | 理论值 | 22.41 | 4.66 | 13.07 | 29.88 |
| [tmtz][Ac]+H2O+3SO2 | 理论值 | 21.22 | 5.00 | 12.38 | 28.29 |
| [tmtz][Ac]+2H2O+3SO2 | 理论值 | 20.15 | 5.32 | 11.75 | 26.86 |
| [tmtz][Ac]+3H2O+3SO2 | 理论值 | 19.18 | 5.59 | 11.19 | 25.58 |
表3 [tmtz][Ac]吸收SO2产物中C、H、N、S的质量分数
| 吸收体系 | 结果 | C/% | H/% | N/% | S/% |
|---|---|---|---|---|---|
| [tmtz][Ac]+3SO2 | 实验值1 | 20.1 | 6.61 | 11.26 | 26.25 |
| 实验值2 | 20.02 | 6.19 | 10.98 | 25.91 | |
| [tmtz][Ac]+H2O+3SO2 | 实验值1 | 19.91 | 6.38 | 11.18 | 24.85 |
| 实验值2 | 20 | 6.99 | 11.48 | 23.31 | |
| [tmtz][Ac]+3SO2 | 理论值 | 22.41 | 4.66 | 13.07 | 29.88 |
| [tmtz][Ac]+H2O+3SO2 | 理论值 | 21.22 | 5.00 | 12.38 | 28.29 |
| [tmtz][Ac]+2H2O+3SO2 | 理论值 | 20.15 | 5.32 | 11.75 | 26.86 |
| [tmtz][Ac]+3H2O+3SO2 | 理论值 | 19.18 | 5.59 | 11.19 | 25.58 |
| 参数 | C2H7NO3S |
|---|---|
| 分子质量 | 125.15 |
| 测试温度 | (100±2)K |
| 辐射波长 | 0.71073nm |
| 晶系 | 正交 |
| 空间群 | P212121 |
| 晶胞参数 | a=(5.3814±0.0007)? |
| b=(7.7464±0.0009)? | |
| c=(11.8913±0.0015)? | |
| 晶胞体积 | (495.71±0.11)?3 |
| Z值 | 4 |
| 晶体密度 | 1.677mg/m3 |
| 吸收系数 | 0.546mm-1 |
| 单胞中电子数目F(000) | 264 |
| 晶体尺寸 | 0.570×0.130×0.100mm3 |
| 扫描范围 | 3.139°~30.673° |
| 极限指数 | -7≤h≤7 -10≤k≤10 -14≤l≤16 |
| 衍射点数目 | 5323 |
| 独立衍射点数目 | 1434 [R(int)=0.0204] |
| 至25.242°的扫描完整度 | 99.5% |
| 吸收校正方法 | 半经验 |
| 精修方法 | 基于F2的全矩阵最小二乘法 |
| 精修衍射点/限制数据/参数 | 1434/0/65 |
| 拟合优度 | 1.098 |
| 最终偏离因子[>2(I)] | R1=0.0199, wR2=0.0519 |
| 偏离因子(全部数据) | R1=0.0211, wR2=0.0527 |
| 绝对结构参数 | 0.36±0.03 |
| 消光系数 | n/a |
| 最高峰与最低峰 | 0.358e·?与-0.229e·? |
表4 [tmtz][Ac]吸收SO2产物具体单晶结构数据
| 参数 | C2H7NO3S |
|---|---|
| 分子质量 | 125.15 |
| 测试温度 | (100±2)K |
| 辐射波长 | 0.71073nm |
| 晶系 | 正交 |
| 空间群 | P212121 |
| 晶胞参数 | a=(5.3814±0.0007)? |
| b=(7.7464±0.0009)? | |
| c=(11.8913±0.0015)? | |
| 晶胞体积 | (495.71±0.11)?3 |
| Z值 | 4 |
| 晶体密度 | 1.677mg/m3 |
| 吸收系数 | 0.546mm-1 |
| 单胞中电子数目F(000) | 264 |
| 晶体尺寸 | 0.570×0.130×0.100mm3 |
| 扫描范围 | 3.139°~30.673° |
| 极限指数 | -7≤h≤7 -10≤k≤10 -14≤l≤16 |
| 衍射点数目 | 5323 |
| 独立衍射点数目 | 1434 [R(int)=0.0204] |
| 至25.242°的扫描完整度 | 99.5% |
| 吸收校正方法 | 半经验 |
| 精修方法 | 基于F2的全矩阵最小二乘法 |
| 精修衍射点/限制数据/参数 | 1434/0/65 |
| 拟合优度 | 1.098 |
| 最终偏离因子[>2(I)] | R1=0.0199, wR2=0.0519 |
| 偏离因子(全部数据) | R1=0.0211, wR2=0.0527 |
| 绝对结构参数 | 0.36±0.03 |
| 消光系数 | n/a |
| 最高峰与最低峰 | 0.358e·?与-0.229e·? |
| 1 | SRIVASTAVA R K, JOZEWICZ W. Flue gas desulfurization: the state of the art[J]. Journal of the Air & Waste Management Association, 2011, 51(12): 1676-1688. |
| 2 | ORTIZ F J G, VIDAL F, OLLERO P, et al. Pilot-plant technical assessment of wet flue gas desulfurization using limestone[J]. Industrial & Engineering Chemistry Research, 2013, 45(4): 1466-1477. |
| 3 | RODRÍGUEZ-SEVILLA J, ÁLVAREZ M, DÍAZ M C, et al. Absorption equilibria of dilute SO2 in seawater[J]. Journal of Chemical & Engineering Data, 2004, 49(6): 1710-1716. |
| 4 | XIANG G, DING H, ZHEN D, et al. Gas-liquid absorption reaction between (NH4)2SO3 solution and SO2 for ammonia-based wet flue gas desulfurization[J]. Applied Energy, 2010, 87(8): 2647-2651. |
| 5 | BRENNECKE J F, MAGINN E J. Ionic liquids: innovative fluids for chemical processing[J]. AIChE Journal, 2010, 47(11): 2384-2389. |
| 6 | BLANCHARD L A, DAN H, BECKMAN E J, et al. Green processing using ionic liquids and CO2[J]. Nature, 1999, 399(6731): 28-29. |
| 7 | WELTON T. Room-temperature ionic liquids solvents for synthesis and catalysis[J]. Cheminform, 2011, 111(5): 2071-2084. |
| 8 | ARMAND M, ENDRES F, MACFARLANE D R, et al. Ionic-liquid materials for the electrochemical challenges of the future[J]. Nature Materials, 2009, 8(8): 621. |
| 9 | KARADAS F, ATILHAN M, APARICIO S. Review on the use of ionic liquids (ILs) as alternative fluids for CO2 capture and natural gas sweetening[J]. Energy & Fuels, 2010, 24(11): 5817-5828. |
| 10 | WU W Z, HAN B X, GAO H X, et al. Desulfurization of flue gas: SO2 absorption by an ionic liquid[J]. Angewandte Chemie, 2004, 43(18): 2415-2417. |
| 11 | ZHANG X, ZHANG X, DONG H, et al. Carbon capture with ionic liquids: overview and progress[J]. Energy & Environmentalence, 2012, 5(5): 6668-6681. |
| 12 | SCOVAZZO P, KIEFT J, FINAN D A, et al. Gas separations using non-hexafluorophosphate [PF6]-anion supported ionic liquid membranes[J]. Journal of Membrane Science, 2004, 238(1): 57-63. |
| 13 | NOBLE R D, GIN D L. Perspective on ionic liquids and ionic liquid membranes[J]. Journal of Membrane Science, 2011, 369(1): 1-4. |
| 14 | HUDDLESTON J G,WILLAUER H D, SWATLOSKI R P, et al. Room temperature ionic liquids as novel media for ‘clean’ liquid-liquid extraction[J]. Chem. Commun., 1998(16): 1765-1766. |
| 15 | HUANG K, CHEN Y L, ZHANG X M, et al. SO2 absorption in acid salt ionic liquids/sulfolane binary mixtures: experimental study and thermodynamic analysis[J]. Chemical Engineering Journal, 2014, 237(1): 478-486. |
| 16 | HONG S Y, IM J, PALGUNADI J, et al. Ether-functionalized ionic liquids as highly efficient SO2 absorbents[J]. Energy & Environmental Science, 2011, 4(5): 1802-1806. |
| 17 | HUANG K, WANG G N, DAI Y, et al. Dicarboxylic acid salts as task-specific ionic liquids for reversible absorption of SO2 with a low enthalpy change[J]. RSC Advances, 2013, 3(37): 16264-16269. |
| 18 | CUI G K, ZHAO N, LI Y N, et al. Limited number of active sites strategy for improving SO2 capture by ionic liquids with fluorinated acetylacetonate anion[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(9): 7985-7992. |
| 19 | MENG X C, WANG J Y, XIE P T, et al. Structure and SO2 absorption properties of guanidinium-based dicarboxylic acid ionic liquids[J]. Energy & Fuels, 2018, 32(2): 1956-1962. |
| 20 | ZHAO T X, LI Y F, ZHANG Y T, et al. Efficient SO2 capture and fixation to cyclic sulfites by dual ether-functionalized protic ionic liquids without any additives[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(8): 10886-10895. |
| 21 | ZHAI L, QIN Z, HE C, et al. Hydroxyl ammonium ionic liquids synthesized by water-bath microwave: synthesis and desulfurization[J]. Journal of Hazardous Materials, 2010, 177(1): 807-813. |
| 22 | JIAN W, ZENG S, LU B, et al. Novel ether-functionalized pyridinium chloride ionic liquids for efficient SO2 capture[J]. Industrial & Engineering Chemistry Research, 2014, 53(43): 16832-16839. |
| 23 | ZENG S, GAO H, ZHANG X, et al. Efficient and reversible capture of SO2 by pyridinium-based ionic liquids[J]. Chemical Engineering Journal, 2014, 251(1): 248-256. |
| 24 | TAYLOR S F R, MCCLUNG M, MCREYNOLDS C, et al. Understanding the competitive gas absorption of CO2 and SO2 in superbase ionic liquids[J]. Industrial & Engineering Chemistry Research, 2018, 57(50): 17033-17042. |
| 25 | CUI G K, LI Y N, LIU J X, et al. Tuning environmentally friendly chelate-based ionic liquids for highly efficient and reversible SO2 chemisorption[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(11): 15292-15300. |
| 26 | LI W, LIU Y, WANG L H, et al. Using ionic liquid mixtures to improve the SO2 absorption performance in flue gas[J]. Energy & Fuels, 2017, 31(2): 1771-1777. |
| 27 | HASIB-UR-RAHMAN M, SIAJ M, LARACHI F. CO2 capture in alkanolamine/room-temperature ionic liquid emulsions: a viable approach with carbamate crystallization and curbed corrosion behavior[J]. International Journal of Greenhouse Gas Control, 2012, 6(3): 246-252. |
| 28 | EISINGER R S, KELLER G E. Process for CO2 capture using ionic liquid that exhibits phase change[J]. Energy & Fuels, 2014, 28(11): 7070-7078. |
| 29 | SEO S, SIMONI L D, MA M T, et al. Phase-change ionic liquids for postcombustion CO2 capture[J]. Energy & Fuels, 2015, 28(9): 5968-5977. |
| 30 | WANG Y, ZHAO W, CHAI M, et al. Phase-change absorption of SO2 by imidazole in organic solvents and conversion of the absorption product in the presence of water and oxygen[J]. Energy & Fuels, 2017, 31(12): 13999-14006. |
| 31 | ZHAO Q, ZHAO W, CHAI M, et al. Phase-change absorption of SO2 by N,N,N′,N′-tetramethyl-p-phenylenediamine in organic solvents and utilization of absorption product[J]. Energy & Fuels, 2018, 32(2): 2073-2080. |
| 32 | LI G M, ZHAO W B, CHAI M Y, et al. Liquid-liquid phase-change absorption of SO2 using N,N-dimethylcyclohexylamine as absorbent and liquid paraffin as solvent[J]. Journal of Hazardous Materials, 2018, 360: 89-96. |
| 33 | CHAI M, ZHAO W, LI G, et al. Novel SO2 phase-change absorbent: mixture of N,N-dimethylaniline and liquid paraffin[J]. Industrial & Engineering Chemistry Research, 2018, 57(37): 12502-12510. |
| 34 | XIAO L Y, SUO J Z, XING M L. Hydroxyl ammonium ionic liquids: synthesis, properties, and solubility of SO2[J]. Journal of Chemical & Engineering Data, 2007, 2(52): 596-599. |
| 35 | 刑其毅. 基础有机化学[M]. 3版. 北京: 高等教育出版社, 2005. |
| XING Q Y. Basic organic chemistry[M]. 3rd ed. Beijing: Higher Education Press, 2005. | |
| 36 | MENG X C, WANG J Y, JIANG H C, et al. Guanidinium-based dicarboxylic acid ionic liquids for SO2 capture[J]. Journal of Chemical Technology & Biotechnology, 2017, 92(4): 767-774. |
| 37 | SHANG Y, LI H P, ZHANG S J, et al. Guanidinium-based ionic liquids for sulfur dioxide sorption[J]. Chemical Engineering Journal, 2011, 175: 324-329. |
| 38 | REN S, HOU Y, TIAN S, et al. What are functional ionic liquids for the absorption of acidic gases?[J]. Journal of Physical Chemistry B, 2013, 117(8): 2482-2486. |
| 39 | 李绛, 谢代前, 鄢国森. 呋喃与HCl和CHCl3构成的分子间氢键的理论研究[J]. 中国科学, 2003, 33(1): 21-25. |
| LI J, XIE D Q, YAN G S. Theoretical study on intermolecular hydrogen bonds between furan and HCl and CHCl3[J]. Science in China, 2003, 33(1): 21-25. | |
| 40 | HOBZA P, HAVLAS Z. Blue-shifting hydrogen bonds[J]. Chemical Reviews, 2000, 100(11): 4253-4264. |
| 41 | ZHAO W, ZHAO Q, ZHANG Z, et al. Liquid-solid phase-change absorption of acidic gas by polyamine in nonaqueous organic solvent[J]. Fuel, 2017, 209: 69-75. |
| 42 | GIVAN A, LOEWENSCHUSS A, NIELSEN C J. Infrared spectrum and ab initio calculations of matrix isolated methanesulfonic acid species and its 1∶1 water complex[J]. Journal of Molecular Structure, 2005, 748(1): 77-90. |
| 43 | HUANG L, SONG T, YONG F, et al. Synthesis and characterization of a new chiral open-framework indium phosphite with intertwined host and guest helices[J]. Microporous & Mesoporous Materials, 2012, 149(1): 95-100. |
| 44 |
GOWENLOCK B G, THOMAS K E. The gaseous equilibrium of 1,3,5-trimethylhexahydro-1,3,5-triazine and N-methylenernethylamhe[J]. Journal of the Chemical Society B: Physical Organic, 1966, 409/410.DOI:10.1039/J29ll0000409.
DOI |
| 45 | FRANKEL M, MOSES P. Syntheses of amino alkyl sulphonic acids and their peptide analogues[J]. Tetrahedron, 1960, 9(3/4): 289–294. |
| 46 | 大连理工大学无机化学教研室. 无机化学[M]. 5版. 北京: 高等教育出版社, 2006: 494. |
| Department of Inorganic Chemistry, Dalian University of Technology. Inorganic chemistry[M]. 5nd ed. Beijing: Higher Education Press, 2006: 494. | |
| 47 | GRYAZNOV P I, KATAEVA O N, NAUMOVA O E, et al. Reaction of β-iminoalcohols with sulfur dioxide. Synthesis of (±) -(2-hydroxyalkylamino) phenyl (isopropyl)-methanesulfonic acids[J]. Russian Journal of General Chemistry, 2010, 80(4): 761-764. |
| 48 | GRYGORENKO O O, BIITSEVA A V, ZHERSH S. Amino sulfonic acids, peptidosulfonamides and other related compounds[J]. Tetrahedron, 2018, 74(13): 1355-1421. |
| 49 | HELDEBRANT D J, KOECH P K, YONKER C R. A reversible zwitterionic SO2-binding organic liquid[J]. Energy Environ. Sci., 2010, 3(1): 111-113. |
| 50 | YANG D, HOU M, NING H, et al. Efficient SO2 capture by amine functionalized PEG[J]. Physical Chemistry Chemical Physics, 2013, 15(41): 18123-18127. |
| 51 | LONG R D, HILLIARD N P, CHHATRE S A, et al. Comparison of zwitterionic N-alkylaminomethanesulfonic acids to related compounds in the good buffer series[J]. Beilstein Journal of Organic Chemistry, 2010, 6(31): 1-7. |
| 52 | KHOMA R E, SHESTAKA A A, SHISHKIN O V, et al. Features of interaction in the sulfur(IV) oxide-hexamethylenetetramine-water system: a first example of identification of the product with a sulfur-carbon bond[J]. Russian Journal of General Chemistry, 2011, 81(3): 620-621. |
| 53 | KHOMA R E, GEL’MBOL’DT V O, ENNAN A A, et al. Synthesis, crystal structure, and spectral characteristics of N-(tert-butyl)aminomethanesulfonic acid[J]. Russian Journal of General Chemistry, 2015, 85(10): 2282-2284. |
| [1] | 李季桐, 王刚, 熊亚选, 徐钱. 不同工质单效吸收式制冷系统的能量和㶲分析[J]. 化工进展, 2023, 42(S1): 104-112. |
| [2] | 杨寒月, 孔令真, 陈家庆, 孙欢, 宋家恺, 王思诚, 孔标. 微气泡型下向流管式气液接触器脱碳性能[J]. 化工进展, 2023, 42(S1): 197-204. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 张凤岐, 崔成东, 鲍学伟, 朱炜玄, 董宏光. 胺液吸收-分步解吸脱硫工艺的设计与评价[J]. 化工进展, 2023, 42(S1): 518-528. |
| [5] | 时雨, 赵运超, 樊智轩, 蒋达华. 夏热冬冷地区相变屋面最佳相变温度的实验研究[J]. 化工进展, 2023, 42(9): 4828-4836. |
| [6] | 卜治丞, 焦波, 林海花, 孙洪源. 脉动热管计算流体力学模型与研究进展[J]. 化工进展, 2023, 42(8): 4167-4181. |
| [7] | 张超, 杨鹏, 刘广林, 赵伟, 杨绪飞, 张伟, 宇波. 表面微结构对阵列微射流沸腾换热的影响[J]. 化工进展, 2023, 42(8): 4193-4203. |
| [8] | 汤磊, 曾德森, 凌子夜, 张正国, 方晓明. 相变蓄冷材料及系统应用研究进展[J]. 化工进展, 2023, 42(8): 4322-4339. |
| [9] | 吴亚, 赵丹, 方荣苗, 李婧瑶, 常娜娜, 杜春保, 王文珍, 史俊. 用于复杂原油乳液的高效破乳剂开发及应用研究进展[J]. 化工进展, 2023, 42(8): 4398-4413. |
| [10] | 张雪伟, 黄亚继, 许月阳, 程好强, 朱志成, 李金壘, 丁雪宇, 王圣, 张荣初. 碱性吸附剂对燃煤烟气中SO3的吸附特性[J]. 化工进展, 2023, 42(7): 3855-3864. |
| [11] | 董晓珊, 王建, 林法伟, 颜蓓蓓, 陈冠益. 基于钙钛矿氧化物的金属纳米粒子溶出策略:溶出过程、驱动力及控制策略[J]. 化工进展, 2023, 42(6): 3049-3065. |
| [12] | 杨许召, 李庆, 袁康康, 张盈盈, 韩敬莉, 吴诗德. 含Gemini离子液体低共熔溶剂热力学性质[J]. 化工进展, 2023, 42(6): 3123-3129. |
| [13] | 吕超, 张习文, 金理健, 杨林军. 新型两相吸收剂-离子液体系统高效捕获CO2[J]. 化工进展, 2023, 42(6): 3226-3232. |
| [14] | 刘厚励, 顾中浩, 阳康, 张莉. 3D打印槽道结构槽宽对池沸腾传热特性的影响[J]. 化工进展, 2023, 42(5): 2282-2288. |
| [15] | 张晨宇, 王宁, 徐洪涛, 罗祝清. 纳米颗粒强化传热的多级潜热储热器性能评价[J]. 化工进展, 2023, 42(5): 2332-2342. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||