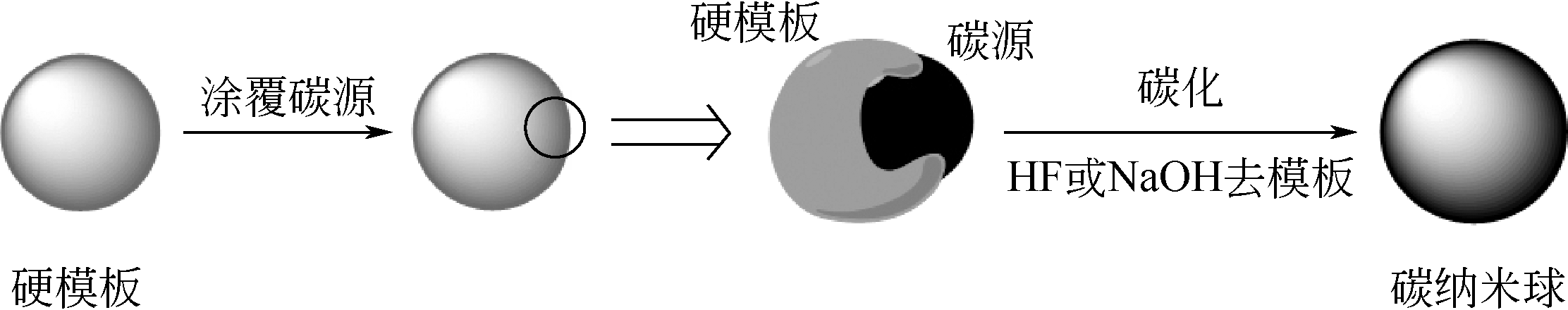
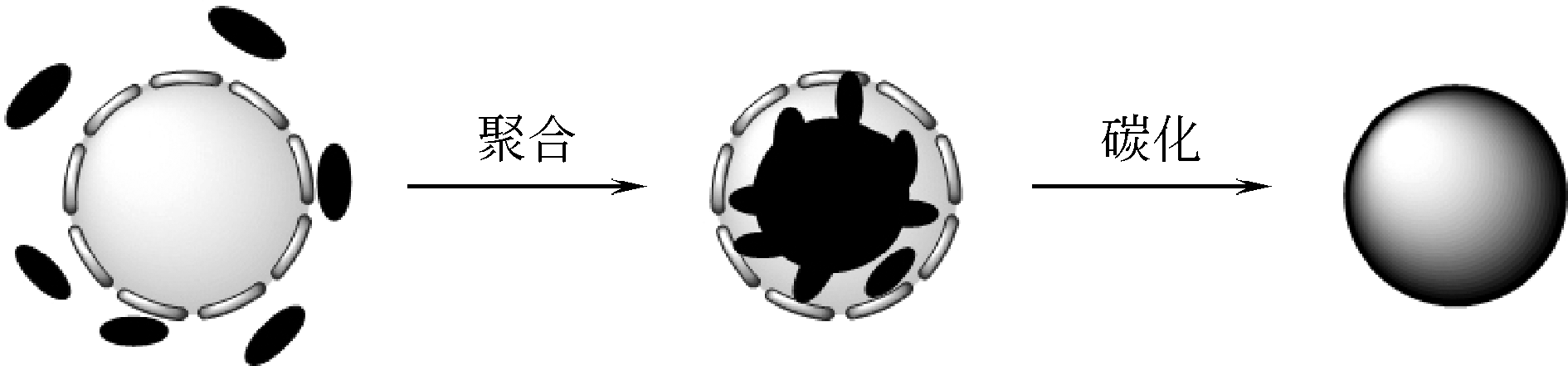
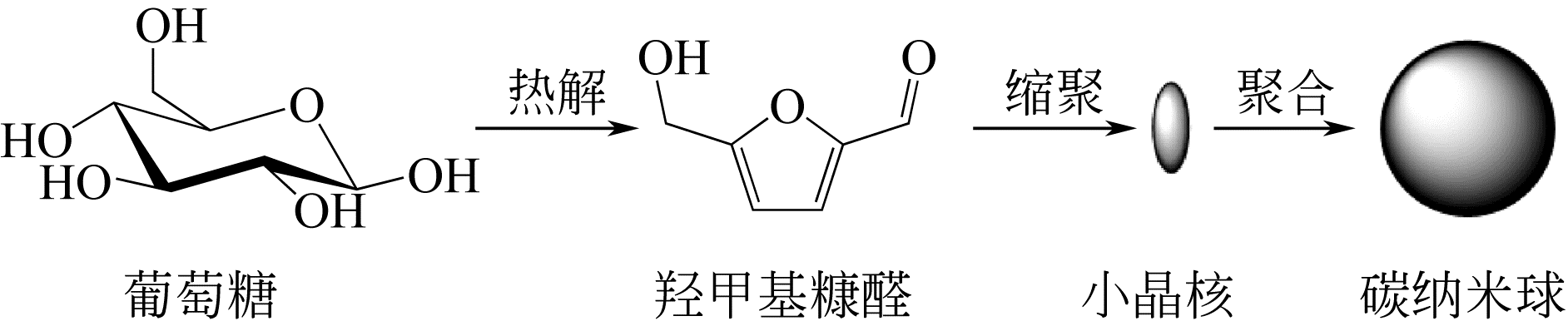
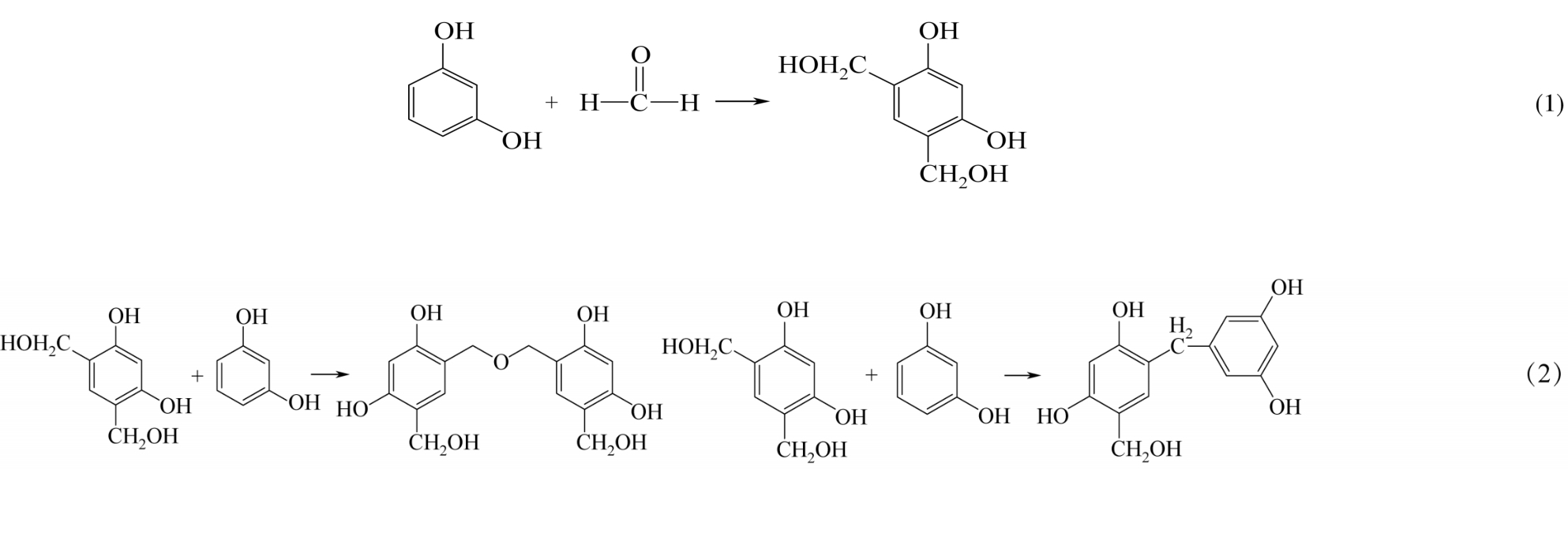
化工进展 ›› 2019, Vol. 38 ›› Issue (07): 3143-3152.DOI: 10.16085/j.issn.1000-6613.2018-1911
多孔碳纳米球及其负载金属催化剂的研究进展
季豪克( ),张雪洁,王昊,朱倩文,周烨彬,卢春山(
),张雪洁,王昊,朱倩文,周烨彬,卢春山( ),李小年
),李小年
- 浙江工业大学化学工程学院,浙江 杭州 310014
-
收稿日期:2018-09-21出版日期:2019-07-05发布日期:2019-07-05 -
通讯作者:卢春山 -
作者简介:季豪克(1993—),男,硕士研究生,研究方向为炭材料及金属催化剂和催化工程应用。E-mail:<email>jhkyx0@163.com</email>。 -
基金资助:国家自然科学基金(21476208);浙江省自然科学基金(LY17B060008)
Research progress of the porous carbon nanospheres and their supported metal catalysts
Haoke JI( ),Xuejie ZHANG,Hao WANG,Qianwen ZHU,Yebin ZHOU,Chunshan LU(
),Xuejie ZHANG,Hao WANG,Qianwen ZHU,Yebin ZHOU,Chunshan LU( ),Xiaonian LI
),Xiaonian LI
- College of Chemical Engineering, Zhejiang University of Technology, Hangzhou 310014, Zhejiang, China
-
Received:2018-09-21Online:2019-07-05Published:2019-07-05 -
Contact:Chunshan LU
摘要:
多孔碳纳米球由于可实现尺寸、形貌、孔结构以及表面基团等的可控合成制备,其负载/镶嵌的金属粒子又兼具高活性和高热稳定性等,在多相催化领域中受到越来越多的关注。本文追溯了多孔碳纳米球形貌调控的发展历程及其负载金属催化剂在催化反应领域中的应用。归纳了不同形貌的多孔碳纳米球及其制备方法和原理,详细对比了各个方法的优缺点;阐述了多孔碳纳米球负载金属催化剂的性能和碳球结构与形貌之间的构效关系;总结了目前碳球作为催化剂载体亟需解决的问题是碳球的多孔结构及其负载尺寸可控和空间匀称分布的金属粒子的可控合成,并展望了其发展方向是进一步研究和探索结构可调、经济可行的碳纳米球制备方法,真正实现工业化应用。
中图分类号:
引用本文
季豪克, 张雪洁, 王昊, 朱倩文, 周烨彬, 卢春山, 李小年. 多孔碳纳米球及其负载金属催化剂的研究进展[J]. 化工进展, 2019, 38(07): 3143-3152.
Haoke JI, Xuejie ZHANG, Hao WANG, Qianwen ZHU, Yebin ZHOU, Chunshan LU, Xiaonian LI. Research progress of the porous carbon nanospheres and their supported metal catalysts[J]. Chemical Industry and Engineering Progress, 2019, 38(07): 3143-3152.
| 催化剂结构 | 催化剂名称 | 催化剂组成 | 催化性能 | 参考文献 |
|---|---|---|---|---|
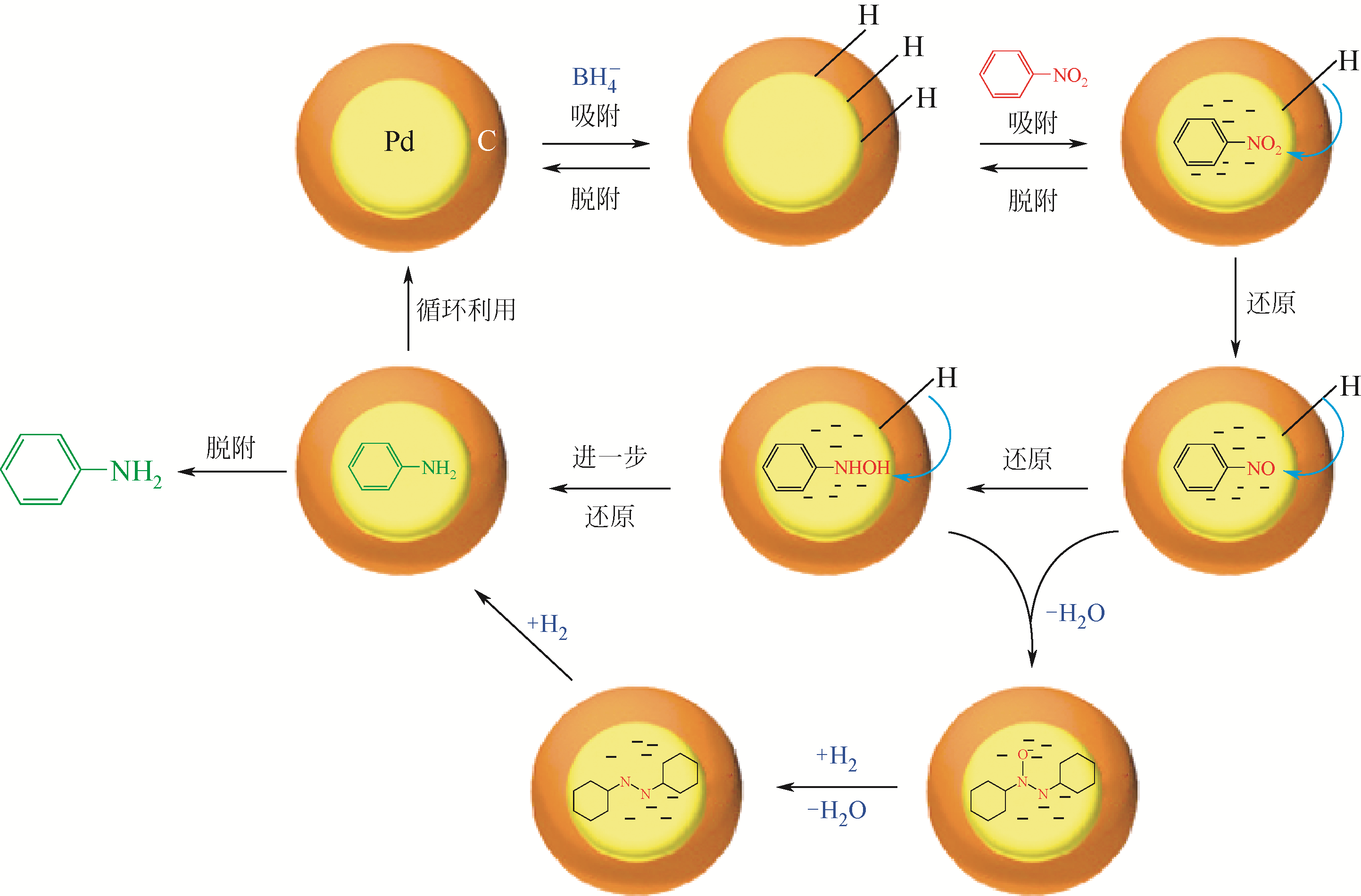
| 多孔实心碳纳米球载金属催化剂 | Pd/CSs | Pd颗粒(粒径约5.4nm)均匀分布在碳球(约120nm)外表面 | 温和反应条件下催化硝基芳香化合物加氢反应的转化率以及选择性均为100% | [ |
| M/N-MCNSs | 金属颗粒M(Au、 Pt、Rh、Ru、 Ag、 Pd 和 Ir)均匀分布在氮掺杂碳球的表面和孔道 | 室温或较高的温度下催化苯甲醛加氢反应中,羰基还原的选择性为100% | [ | |
| 多孔中空碳纳米球载金属催化剂 | Pd/CSs | Pd颗粒(粒径4~15nm)均匀分布在碳球外表面 | 在硝基苯液相催化还原反应中表现出高活性 | [ |
| Pt/N-HCNSs | Pt颗粒(粒径约2.8 nm)均匀分布在中空碳球的内和外表面,碳壳厚度约为50nm | 肉桂醛催化加氢的选择性为99.9%,转化率在催化剂循环套用12次后未下降 | [ | |
| Pd/B-HCSs | Pd颗粒(粒径约4.7 nm)均匀分布在中空碳球的内和外表面 | 催化苯甲醇无溶剂氧化反应,转化率为100%,选择性为99% | [ | |
| 多孔核壳碳纳米球载金属催化剂 | Pd@C | Pd颗粒(粒径约10.6nm)表面包裹碳层的实心核壳结构 | 室温下催化硝基苯液相还原反应中表现高转化率 | [ |
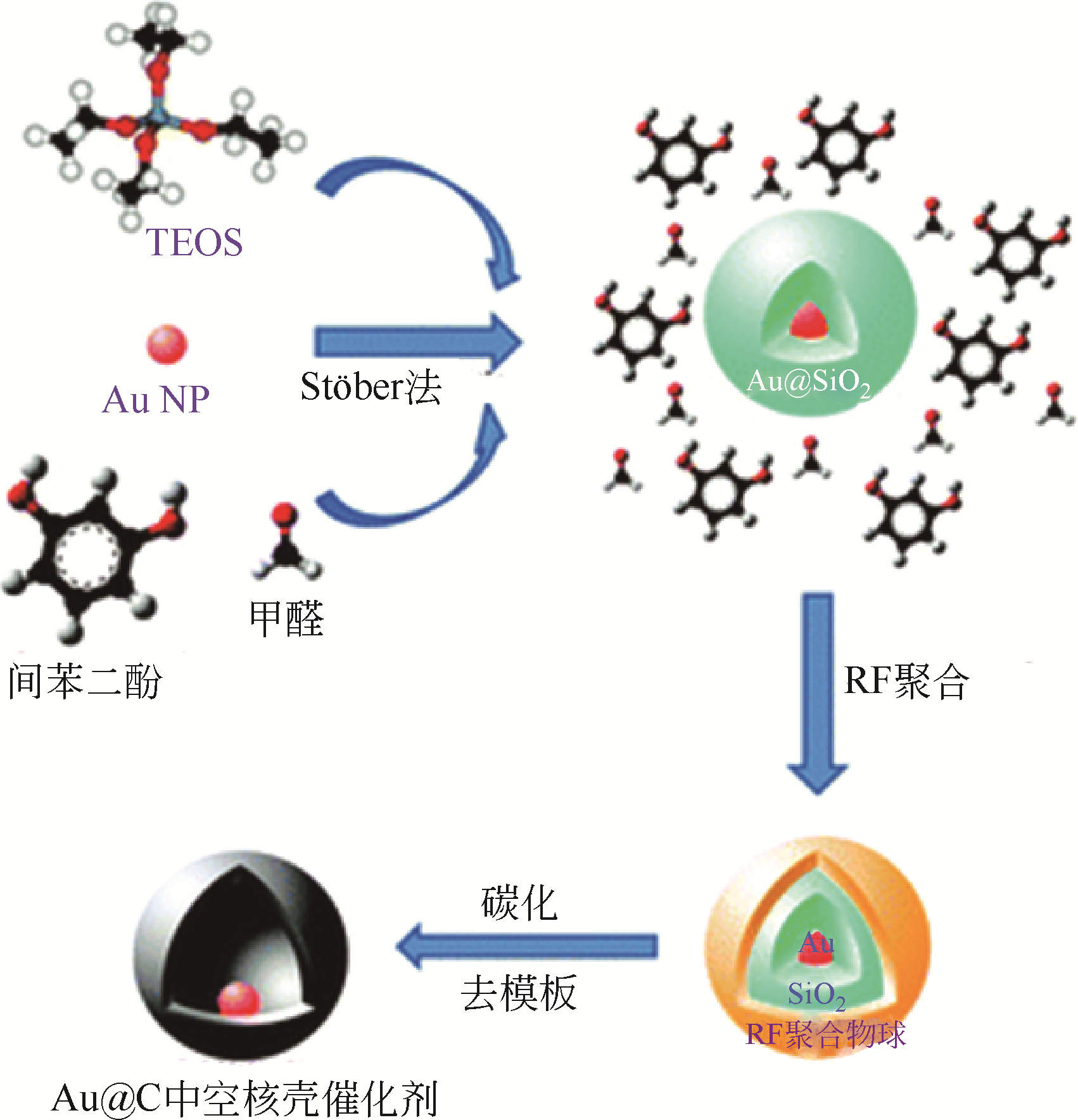
| Au@C | Au颗粒(粒径约15nm)在中空碳球(约为150nm)内部自由活动,碳壳厚度约为18nm | 在2-硝基苯酚液相催化还原反应中表现出高活性和高稳定性 | [ |
表1 多孔碳纳米球负载金属催化剂的应用文献汇总
| 催化剂结构 | 催化剂名称 | 催化剂组成 | 催化性能 | 参考文献 |
|---|---|---|---|---|
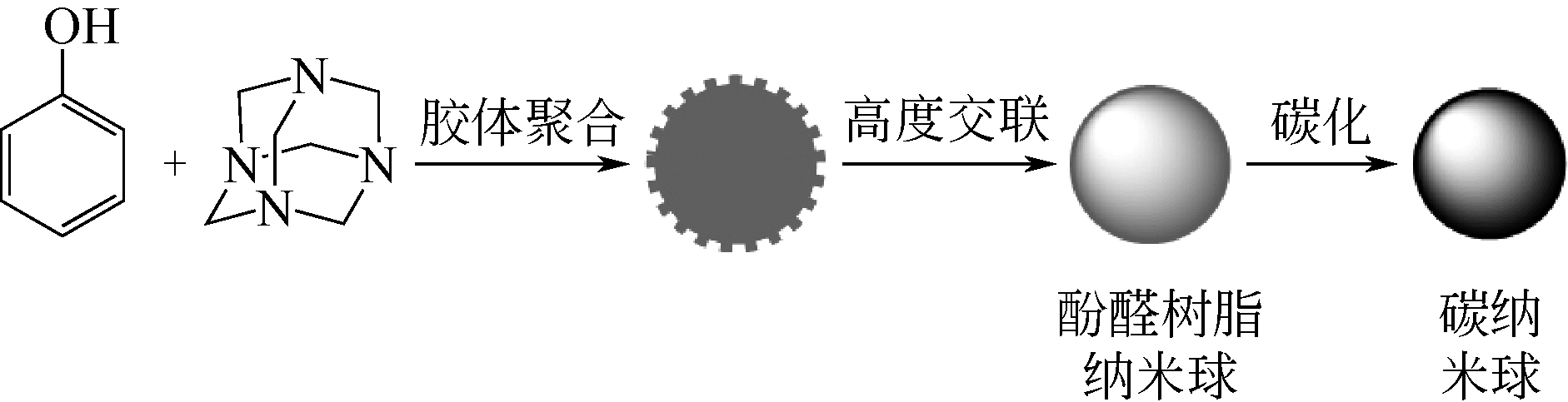
| 多孔实心碳纳米球载金属催化剂 | Pd/CSs | Pd颗粒(粒径约5.4nm)均匀分布在碳球(约120nm)外表面 | 温和反应条件下催化硝基芳香化合物加氢反应的转化率以及选择性均为100% | [ |
| M/N-MCNSs | 金属颗粒M(Au、 Pt、Rh、Ru、 Ag、 Pd 和 Ir)均匀分布在氮掺杂碳球的表面和孔道 | 室温或较高的温度下催化苯甲醛加氢反应中,羰基还原的选择性为100% | [ | |
| 多孔中空碳纳米球载金属催化剂 | Pd/CSs | Pd颗粒(粒径4~15nm)均匀分布在碳球外表面 | 在硝基苯液相催化还原反应中表现出高活性 | [ |
| Pt/N-HCNSs | Pt颗粒(粒径约2.8 nm)均匀分布在中空碳球的内和外表面,碳壳厚度约为50nm | 肉桂醛催化加氢的选择性为99.9%,转化率在催化剂循环套用12次后未下降 | [ | |
| Pd/B-HCSs | Pd颗粒(粒径约4.7 nm)均匀分布在中空碳球的内和外表面 | 催化苯甲醇无溶剂氧化反应,转化率为100%,选择性为99% | [ | |
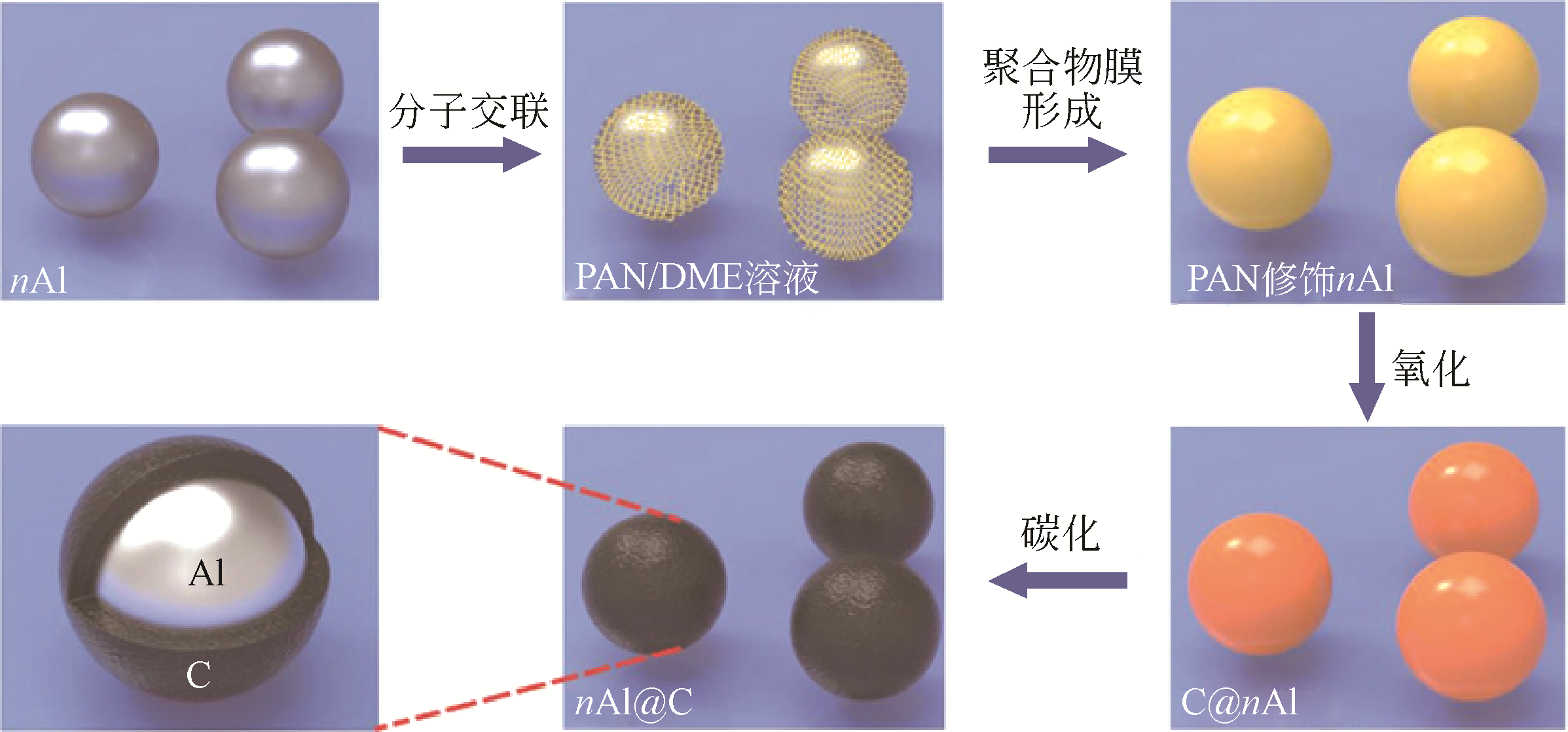
| 多孔核壳碳纳米球载金属催化剂 | Pd@C | Pd颗粒(粒径约10.6nm)表面包裹碳层的实心核壳结构 | 室温下催化硝基苯液相还原反应中表现高转化率 | [ |
| Au@C | Au颗粒(粒径约15nm)在中空碳球(约为150nm)内部自由活动,碳壳厚度约为18nm | 在2-硝基苯酚液相催化还原反应中表现出高活性和高稳定性 | [ |
| 1 | RADZUAN N A M , ZAKARIA M Y , SULONG A B , et al . The effect of milled carbon fibre filler on electrical conductivity in highly conductive polymer composites[J]. Composites Part B: Engineering, 2017, 110: 153-160. |
| 2 | GHOLINEJAD M , BAHRAMI M , NÁJERA C . A fluorescence active catalyst support comprising carbon quantum dots and magnesium oxide doping for stabilization of palladium nanoparticles: application as a recoverable catalyst for Suzuki reaction in water[J]. Molecular Catalysis, 2017, 433: 12-19. |
| 3 | YANG N J , FOORD J S , JIANG X . Diamond electrochemistry at the nanoscale: a review[J]. Carbon, 2016, 99: 90-110. |
| 4 | ABRAHAM J , VASU K S , WILLIAMS C D , et al . Tunable sieving of ions using graphene oxide membranes[J]. Nature Nanotechnology, 2017, 12(6): 546-550. |
| 5 | GU W T , YUSHIN G . Review of nanostructured carbon materials for electrochemical capacitor applications: advantages and limitations of activated carbon, carbide-derived carbon, zeolite-templated carbon, carbon aerogels, carbon nanotubes, onion-like carbon, and graphene[J]. Wiley Interdisciplinary Reviews: Energy and Environment, 2014, 3(5): 424-473. |
| 6 | HOU S , WANG M , XU X , et al . Nitrogen-doped carbon spheres: a new high-energy-density and long-life pseudo-capacitive electrode material for electrochemical flow capacitor[J]. Journal of Colloid and Interface Science, 2017, 491: 161-166. |
| 7 | MAI W, SUN B , CHEN L , et al . Water-dispersible, responsive, and carbonizable hairy microporous polymeric nanospheres[J]. Journal of the American Chemical Society, 2015, 137(41): 13256-13259. |
| 8 | LONG Y , LIU Y S , ZHAO Z M , et al . Distinctive morphology effects of porous-spherical / yolk-shell / hollow Pd-nitrogen-doped-carbon spheres catalyst for catalytic reduction of 4-nitrophenol[J]. Journal of Colloid and Interface Science, 2017, 496: 465-473. |
| 9 | LIU M X , WANG X , ZHU D Z , et al . Encapsulation of NiO nanoparticles in mesoporous carbon nanospheres for advanced energy storage[J]. Chemical Engineering Journal, 2017, 308: 240-247. |
| 10 | WANG G H , CHEN K , ENGELHARDT J , et al . Scalable one-pot synthesis of yolk-shell carbon nanospheres with yolk-supported Pd nanoparticles for size-selective catalysis[J]. Chemistry of Materials, 2018, 30: 2483-2487. |
| 11 | YANG Q , ZHANG J , ZHANG L , et al . Ruthenium nanoparticles on colloidal carbon spheres: an efficient catalyst for hydrogenation of ethyl lactate in aqueous phase[J]. Catalysis Communications, 2013, 40: 37-41. |
| 12 | NONGWE I , RIVAT V , MEIJBOOM R , et al . Pt supported nitrogen doped hollow carbon spheres for the catalysed reduction of cinnamaldehyde[J]. Applied Catalysis A: General, 2016, 517: 30-38. |
| 13 | LIU R , MAHURIN S M , LI C , et al . Dopamine as a carbon source: the controlled synthesis of hollow carbon spheres and yolk-structured carbon nanocomposites[J]. Angewandte Chemie: International Edition, 2011, 50: 6799-6802. |
| 14 | WEN G , WANG B , WANG C , et al . Hydrothermal carbon enriched with oxygenated groups from biomass glucose as an efficient carbocatalyst[J]. Angewandte Chemie: International Edition, 2017, 56(2): 600-604. |
| 15 | WANG X , FENG J , BAI Y , et al . Synthesis, properties, and applications of hollow micro-/nanostructures[J]. Chemical Reviews, 2016, 116: 10983-11060. |
| 16 | EL-TONI A M , HABILA M A , LABIS J P , et al . Design, synthesis and applications of core-shell, hollow core, and nanorattle multifunctional nanostructures[J]. Nanoscale, 2016, 8(5): 2510-2531. |
| 17 | YOU Z , WEI Y , RUI Y L , et al . Preparation of carbon nanospheres by non-catalytic chemical vapor deposition and their formation mechanism[J]. New Carbon Materials, 2016, 31: 467-474. |
| 18 | SUN Z , LIU Y , LI B , et al . General synthesis of discrete mesoporous carbon microspheres through a confined self-assembly process in inverse opals[J]. ACS Nano, 2013, 7(10): 8706-8714. |
| 19 | TAE W, KIM P W C, IGOR I , et al . Structurally ordered mesoporous carbon nanoparticles as transmembrane delivery vehicle in human cancer cells[J]. Nano Letters, 2008, 8(11): 3724-3727. |
| 20 | LEI Z , CHRISTOV N , ZHANG L L , et al . Mesoporous carbon nanospheres with an excellent electrocapacitive performance[J]. Journal of Materials Chemistry, 2011, 21(7): 2274-2281. |
| 21 | LIANG C D , HONG K L , GUIOCHON G A , et al . Synthesis of a large-scale highly ordered porous carbon film by self-assembly of block copolymers[J]. Angewandte Chemie, 2004, 116: 5909-5913. |
| 22 | AI K , LIU Y , RUAN C , et al . Sp2 C-dominant N-doped carbon sub-micrometer spheres with a tunable size: a versatile platform for highly efficient oxygen-reduction catalysts[J]. Advanced Materials, 2013, 25: 998-1003. |
| 23 | ZHANG P , GONG Y , WEI Z , et al . Updating biomass into functional carbon material in ionothermal manner[J]. ACS Applied Materials & Interfaces, 2014, 6(15):12515. |
| 24 | ZHANG P , YUAN J , FELLINGER T P , et al . Improving hydrothermal carbonization by using poly(ionic liquid)s[J]. Angewandte Chemie: International Edition, 2013, 52: 6028-6032. |
| 25 | BACCILE N , ANTONIETTI M , TITIRICI M M . One-step hydrothermal synthesis of nitrogen-doped nanocarbons: albumine directing the carbonization of glucose[J]. ChemSusChem, 2010, 3(2): 246-253. |
| 26 | CHEN T Q , PAN L K , LU T , et al . Fast synthesis of carbon microspheres via a microwave-assisted reaction for sodium ion batteries[J]. Journal of Materials Chemistry A, 2014, 2(5): 1263-1267. |
| 27 | STÖBER W , ARTHUR F , ERNST B . Controlled growth of monodisperse silica spheres in the micron size range[J]. Journal of Colloid and Interface Science, 1968,26(1): 62-69. |
| 28 | LIU J , QIAO S Z , LIU H , et al . Extension of the Stöber method to the preparation of monodisperse resorcinol-formaldehyde resin polymer and carbon spheres[J]. Angewandte Chemie:International Edition, 2011, 50: 5947-5951. |
| 29 | ZHAO J M , NIU W X , ZHANG L , et al . A template-free and surfactant-free method for high-yield synthesis of highly monodisperse 3-aminophenol-formaldehyde resin and carbon nano/microspheres[J]. Macromolecules, 2013, 46(1): 140-145. |
| 30 | ZHANG C F , HATZELL K B , BOOTA M , et al . Highly porous carbon spheres for electrochemical capacitors and capacitive flowable suspension electrodes[J]. Carbon, 2014, 77: 155-164. |
| 31 | LIU J , WICKRAMARATNE N P , QIAO S Z , et al . Molecular-based design and emerging applications of nanoporous carbon spheres[J]. Nature Materials, 2015, 14(8): 763-774. |
| 32 | CHOMA J , JAMIOLA D , AUGUSTYNEK K , et al . New opportunities in Stöber synthesis: preparation of microporous and mesoporous carbon spheres[J]. Journal of Materials Chemistry, 2012, 22(25): 12636-12642. |
| 33 | JIANG J G , BAO L , QIANG Y W , et al . Sol-gel process-derived rich nitrogen-doped porous carbon through KOH activation for supercapacitors[J]. Electrochimica Acta, 2015, 158: 229-236. |
| 34 | LUDWINOWICZ J , JARONIEC M . Potassium salt-assisted synthesis of highly microporous carbon spheres for CO2 adsorption[J]. Carbon, 2015, 82: 297-303. |
| 35 | YANG T Y , LIU J , ZHOU R F , et al . N-doped mesoporous carbon spheres as the oxygen reduction reaction catalysts[J]. Journal of Materials Chemistry A, 2014, 2(42): 18139-18146. |
| 36 | WICKRAMARATNE N P , JARONIEC M . Tailoring microporosity and nitrogen content in carbons for achieving high uptake of CO2 at ambient conditions[J]. Adsorption, 2013, 20: 287-293. |
| 37 | XU Z G , GUO Q P . A simple method to prepare monodisperse and size-tunable carbon nanospheres from phenolic resin[J]. Carbon, 2013, 52(2): 464-467. |
| 38 | XU S , LUO Y , TAN B . Recent development of hypercrosslinked microporous organic polymers[J]. Macromolecular Rapid Communications, 2013, 34: 471-484. |
| 39 | YONG Z Z , LI X D , LIANG Z , et al . N-doped hollow carbon nanospheres as sulfur hosts for high performance Li-S batteries[J]. New Carbon Materials, 2017, 32: 297-303. |
| 40 | BIN D S, CHI Z X , LI Y , et al . Controlling the compositional chemistry in single nanoparticles for functional hollow carbon nanospheres[J]. Journal of the American Chemical Society, 2017, 139(38): 13492-13498. |
| 41 | FENG S S , LI W , SHI Q , et al . Synthesis of nitrogen-doped hollow carbon nanospheres for CO2 capture[J]. Chemistry Communications, 2014, 50(3): 329-331. |
| 42 | ZHANG W , WANG F S , LI X L , et al . Fabrication of hollow carbon nanospheres introduced with Fe and N species immobilized palladium nanoparticles as catalysts for the semihydrogenation of phenylacetylene under mild reaction conditions[J]. Applied Surface Science, 2017, 404: 398-408. |
| 43 | YANG Z C , ZHANG Y , KONG J H , et al . Hollow carbon nanoparticles of tunable size and wall thickness by hydrothermal treatment of α-cyclodextrin templated by F127 block copolymers[J]. Chemistry of Materials, 2013, 25: 704-710. |
| 44 | WU J , JIN C , YANG Z R , et al . Synthesis of phosphorus-doped carbon hollow spheres as efficient metal-free electrocatalysts for oxygen reduction[J]. Carbon, 2015, 82: 562-571. |
| 45 | FANG Y , ZHENG G , YANG J , et al . Dual-pore mesoporous carbon@silica composite core-shell nanospheres for multidrug delivery[J]. Angewandte Chemie, 2014, 53(21): 5366-5370. |
| 46 | FANG X , LIU S , ZANG J , et al . Precisely controlled resorcinol-formaldehyde resin coating for fabricating core-shell, hollow, and yolk-shell carbon nanostructures[J]. Nanoscale, 2013, 5: 6908-6916. |
| 47 | LU Z Y , LI B , YANG D J , et al . A self-assembled silicon/phenolic resin-based carbon core-shell nanocomposite as an anode material for lithium-ion batteries[J]. RSC Advances, 2018, 8(7): 3477-3482. |
| 48 | CHEN J , ZHANG F , ZHAO Y L , et al . Facile synthesis of CdS/C core-shell nanospheres with ultrathin carbon layer for enhanced photocatalytic properties and stability[J]. Applied Surface Science, 2016, 362: 126-131. |
| 49 | ZHANG Z , SHI X D , YANG X . Synthesis of core-shell NiSe/C nanospheres as anodes for lithium and sodium storage[J]. Electrochimica Acta, 2016, 208: 238-243. |
| 50 | TONG X F , ZHANG F , CHEN G G , et al . Core-shell aluminum@carbon nanospheres for dual-ion batteries with excellent cycling performance under high rates[J]. Advanced Energy Materials, 2018, 8: 1701967. |
| 51 | WANG D H , ZHAO B , JIANG Y , et al . Self-etching preparation of yolk-shell Ag@carbon nanostructures for highly effective reduction of 4-nitrophenol[J]. Catalysis Communications, 2017, 102: 114-117. |
| 52 | LIANG H W , WEI W , WU Z S , et al . Mesoporous metal-nitrogen-doped carbon electrocatalysts for highly efficient oxygen reduction reaction[J]. Journal of the American Chemical Society, 2013, 135: 16002-16005. |
| 53 | PRIETO G , TUYSUZ H , DUYCKAERTS N , et al . Hollow nano- and microstructures as catalysts[J]. Chemical Reviews, 2016, 116(22): 14056-14119. |
| 54 | ZHANG P , QIAO Z A , DAI S . Recent advances in carbon nanospheres: synthetic routes and applications[J]. Chem. Commun. (Camb.), 2015, 51(45): 9246-9256. |
| 55 | LU Y M , ZHU H Z , LI W G , et al . Size-controllable palladium nanoparticles immobilized on carbon nanospheres for nitroaromatic hydrogenation[J]. Journal of Materials Chemistry A, 2013, 1: 3783. |
| 56 | YANG T Y , LING H J , LAMONIER J F , et al . A synthetic strategy for carbon nanospheres impregnated with highly monodispersed metal nanoparticles[J]. NPG Asia Materials, 2016, 8(2): e240. |
| 57 | HUANG X H , MOON B K , BYEON S J , et al . Palladium nanoparticles decorated mesoporous carbon spheres as catalyst for reduction of 4-nitrophenol[J]. Journal of Nanoscience and Nanotechnology, 2014, 14(11): 8771-8776. |
| 58 | RAVAT V , NONGWE I , COVILLE N J . Palladium-supported boron-doped hollow carbon spheres as catalysts for the solvent-free aerobic oxidation of alcohols[J]. ChemCatChem, 2012, 4(12): 1930-1934. |
| 59 | KIM Y J, MA R, REDDY D A , et al . Liquid-phase pulsed laser ablation synthesis of graphitized carbon-encapsulated palladium core-shell nanospheres for catalytic reduction of nitrobenzene to aniline[J]. Applied Surface Science, 2015, 357: 2112-2120. |
| 60 | LIU R , QU F L , GUO Y L , et al . Au@carbon yolk-shell nanostructures via one-step core-hell-shell template[J]. Chemistry Communications, 2014, 50(4): 478-480. |
| 61 | DOU J , ZENG H C . Targeted synthesis of silicomolybdic acid (Keggin acid) inside mesoporous silica hollow spheres for Friedel-Crafts alkylation[J]. Journal of the American Chemical Society, 2012, 134: 16235-16246. |
| [1] | 胡喜, 王明珊, 李恩智, 黄思鸣, 陈俊臣, 郭秉淑, 于博, 马志远, 李星. 二硫化钨复合材料制备与储钠性能研究进展[J]. 化工进展, 2023, 42(S1): 344-355. |
| [2] | 许春树, 姚庆达, 梁永贤, 周华龙. 共价有机框架材料功能化策略及其对Hg(Ⅱ)和Cr(Ⅵ)的吸附性能研究进展[J]. 化工进展, 2023, 42(S1): 461-478. |
| [3] | 杨莹, 侯豪杰, 黄瑞, 崔煜, 王兵, 刘健, 鲍卫仁, 常丽萍, 王建成, 韩丽娜. 利用煤焦油中酚类物质Stöber法制备碳纳米球用于CO2吸附[J]. 化工进展, 2023, 42(9): 5011-5018. |
| [4] | 刘毅, 房强, 钟达忠, 赵强, 李晋平. Ag/Cu耦合催化剂的Cu晶面调控用于电催化二氧化碳还原[J]. 化工进展, 2023, 42(8): 4136-4142. |
| [5] | 张凯, 吕秋楠, 李刚, 李小森, 莫家媚. 南海海泥中甲烷水合物的形貌及赋存特性[J]. 化工进展, 2023, 42(7): 3865-3874. |
| [6] | 于志庆, 黄文斌, 王晓晗, 邓开鑫, 魏强, 周亚松, 姜鹏. B掺杂Al2O3@C负载CoMo型加氢脱硫催化剂性能[J]. 化工进展, 2023, 42(7): 3550-3560. |
| [7] | 龚鹏程, 严群, 陈锦富, 温俊宇, 苏晓洁. 铁酸钴复合碳纳米管活化过硫酸盐降解铬黑T的性能及机理[J]. 化工进展, 2023, 42(7): 3572-3581. |
| [8] | 陈怡欣, 甄摇摇, 陈瑞浩, 吴继伟, 潘丽美, 姚翀, 罗杰, 卢春山, 丰枫, 王清涛, 张群峰, 李小年. 铂基纳米催化剂的制备及在加氢领域的进展[J]. 化工进展, 2023, 42(6): 2904-2915. |
| [9] | 张巍, 秦川, 谢康, 周运河, 董梦瑶, 李婕, 汤云灏, 马英, 宋健. H2-SCR改性铂系催化剂低温脱硝的应用及性能强化挑战[J]. 化工进展, 2023, 42(6): 2954-2962. |
| [10] | 符淑瑢, 王丽娜, 王东伟, 刘蕊, 张晓慧, 马占伟. 析氧助催化剂增强光阳极光电催化分解水性能研究进展[J]. 化工进展, 2023, 42(5): 2353-2370. |
| [11] | 马源, 肖晴月, 岳君容, 崔彦斌, 刘姣, 许光文. CeO2-Al2O3复合载体负载Ni基催化剂催化CO x 共甲烷化性能[J]. 化工进展, 2023, 42(5): 2421-2428. |
| [12] | 张宁, 吴海滨, 李钰, 李剑锋, 程芳琴. 漂浮型光催化材料的制备及其在水处理领域的应用研究进展[J]. 化工进展, 2023, 42(5): 2475-2485. |
| [13] | 王嘉, 彭冲, 唐磊, 陆安慧. 渣油加氢催化剂活性相结构调控及对反应性能影响[J]. 化工进展, 2023, 42(4): 1811-1821. |
| [14] | 阮鹏, 杨润农, 林梓荣, 孙永明. 甲烷催化部分氧化制合成气催化剂的研究进展[J]. 化工进展, 2023, 42(4): 1832-1846. |
| [15] | 张孟旭, 王红琴, 李金, 安霓虹, 戴云生, 钱颖, 沈亚峰. PtSn/MgAl2O4-sheet催化剂的制备及其PDH反应性能[J]. 化工进展, 2023, 42(3): 1365-1372. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||