| 1 |
钛石膏资源化是钛白粉行业可持续发展的关键[J]. 中国粉体工业, 2012(3): 32-33.
|
|
Titanium gypsum resource is the key to the sustainable development of titanium dioxide industry[J]. China Powder Industry, 2012(3): 32-33.
|
| 2 |
AZDARPOUR A , ASADULLAH M , JUNIN R , et al . Direct carbonation of red gypsum to produce solid carbonates[J]. Fuel Processing Technology, 2014, 126(1): 429-434.
|
| 3 |
刘长春, 李荣军, 刘磊, 钛石膏作水泥缓凝剂的试验研究 [J]. 水泥, 2006(11): 4-6.
|
|
LIU C C , LI R J , LIU L . Study on application of titanium gypsum as cement retarder[J]. Cement, 2006(11): 4-6..
|
| 4 |
QU D Y , WANG J . Research on titanium gypsum for light-quality wall material[J]. Bulletin of the Chinese Ceramic Society, 2009, 28(5): 1064-1070.
|
| 5 |
AZDARPOUR A , ASADULLAH M , MOHAMMADIAN E , et al . Mineral carbonation of red gypsum via pH-swing process:effect of CO2 pressure on the efficiency and products characteristics[J]. Chemical Engineering Journal, 2015, 264:425-436.
|
| 6 |
彭志辉, 刘巧玲, 彭家惠, 等 . 钛石膏作水泥缓凝剂研究[J]. 重庆建筑大学学报, 2004(1): 93-96.
|
|
PENG Zhihui , LIU Qiaoling , PENG Jiahui , et al . Study on titanium gypsum as set retarder for cement[J]. Journal of Chongqing Jianzhu University, 2004(1): 93-96.
|
| 7 |
杜传伟, 李国忠, 陈娟 . 利用天然石膏形态组成模拟钛石膏及其性能研究[J]. 建筑材料学报, 2014, 17(3): 511-516.
|
|
DU Chuanwei , LI Guozhong , CHEN Juan . Simulation of tianium gypsum with morphology and composition of natural gypsum and its property[J]. Journal of Building Materials, 2014, 17(3): 511-516.
|
| 8 |
丁明, 黄俊俊, 王凌云,等 . 一种钛石膏中铁元素的分离去除工艺:CN105502464A[P]. 2016.
|
|
DING Ming, HUANG Junjun, WANG Lingyun, Titanium gypsum iron element seperation and remocval process:CN105502464A[P]. 2016.
|
| 9 |
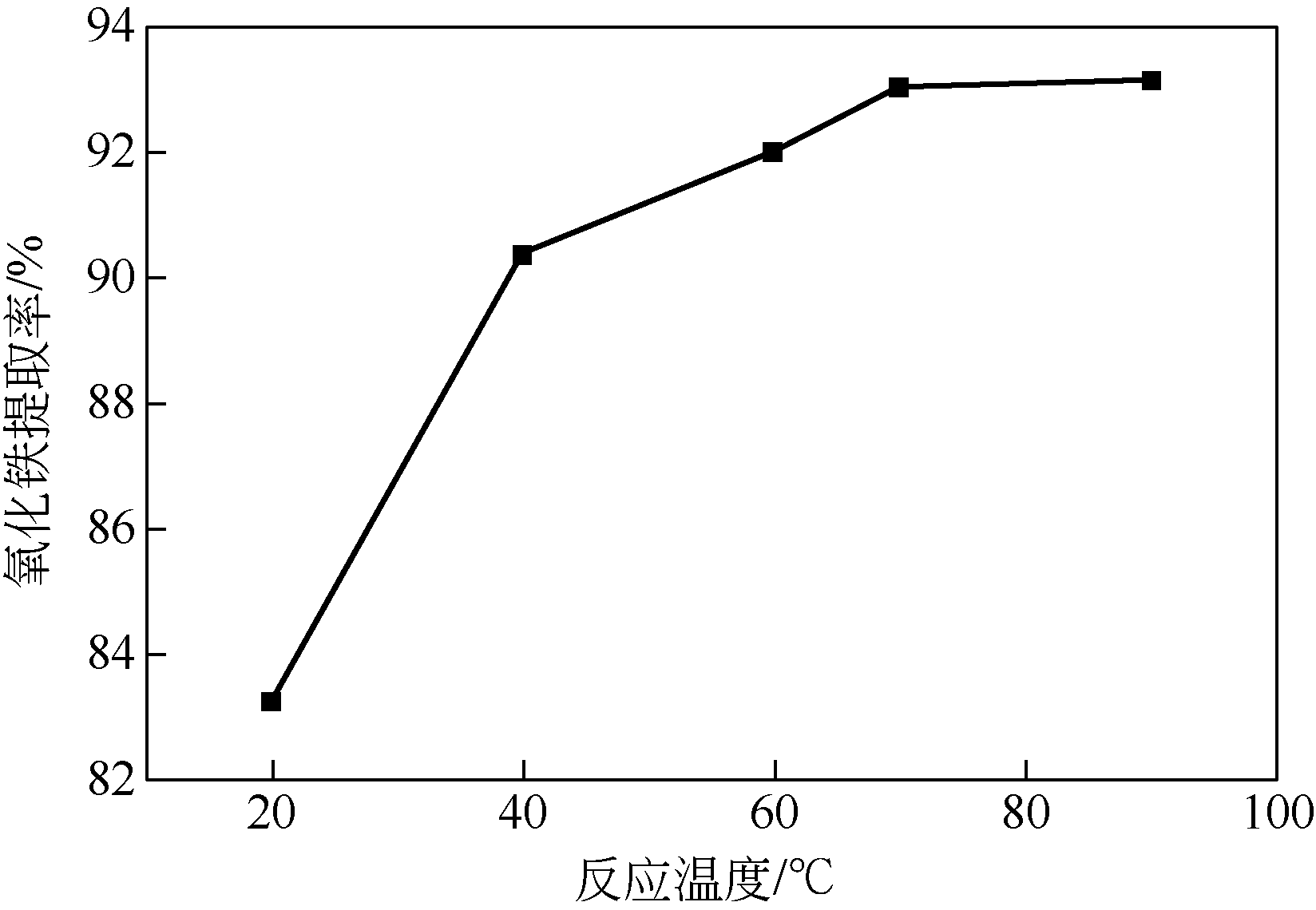
江莹 . 钛石膏除铁试验研究[J]. 化工管理, 2015(34): 199-200.
|
|
JIANG Ying . Experimental study on iron removal from titanium gypsum[J]. Chemical Enterprise Management, 2015(34): 199-200.
|
| 10 |
PÉREZ-MORENO S M , GÁZQUEZ M J , BOLÍVAR J P . CO2 sequestration by indirect carbonation of artificial gypsum generated in the manufacture of titanium dioxide pigments[J]. Chemical Engineering Journal, 2015, 262:737-746.
|
| 11 |
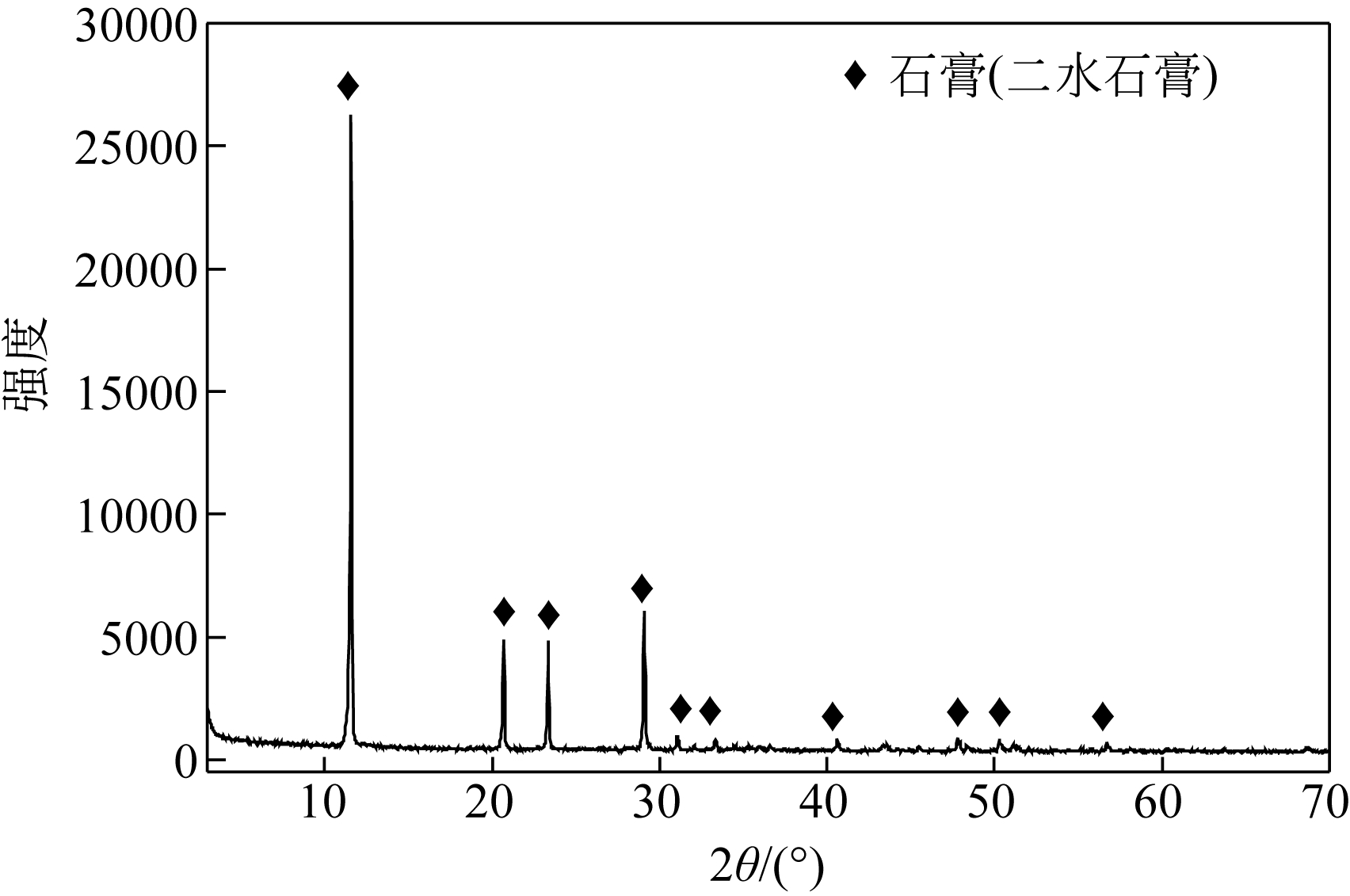
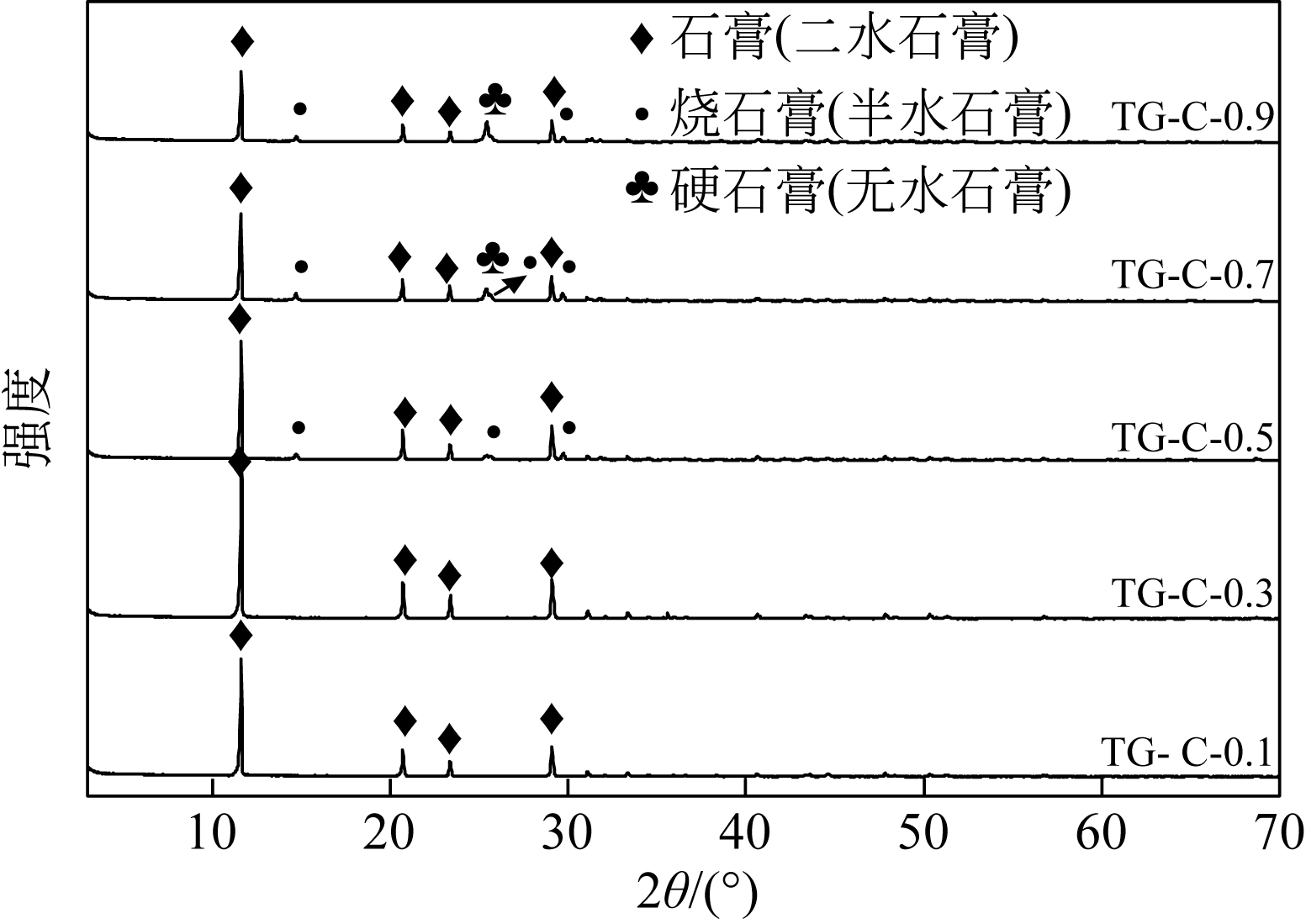
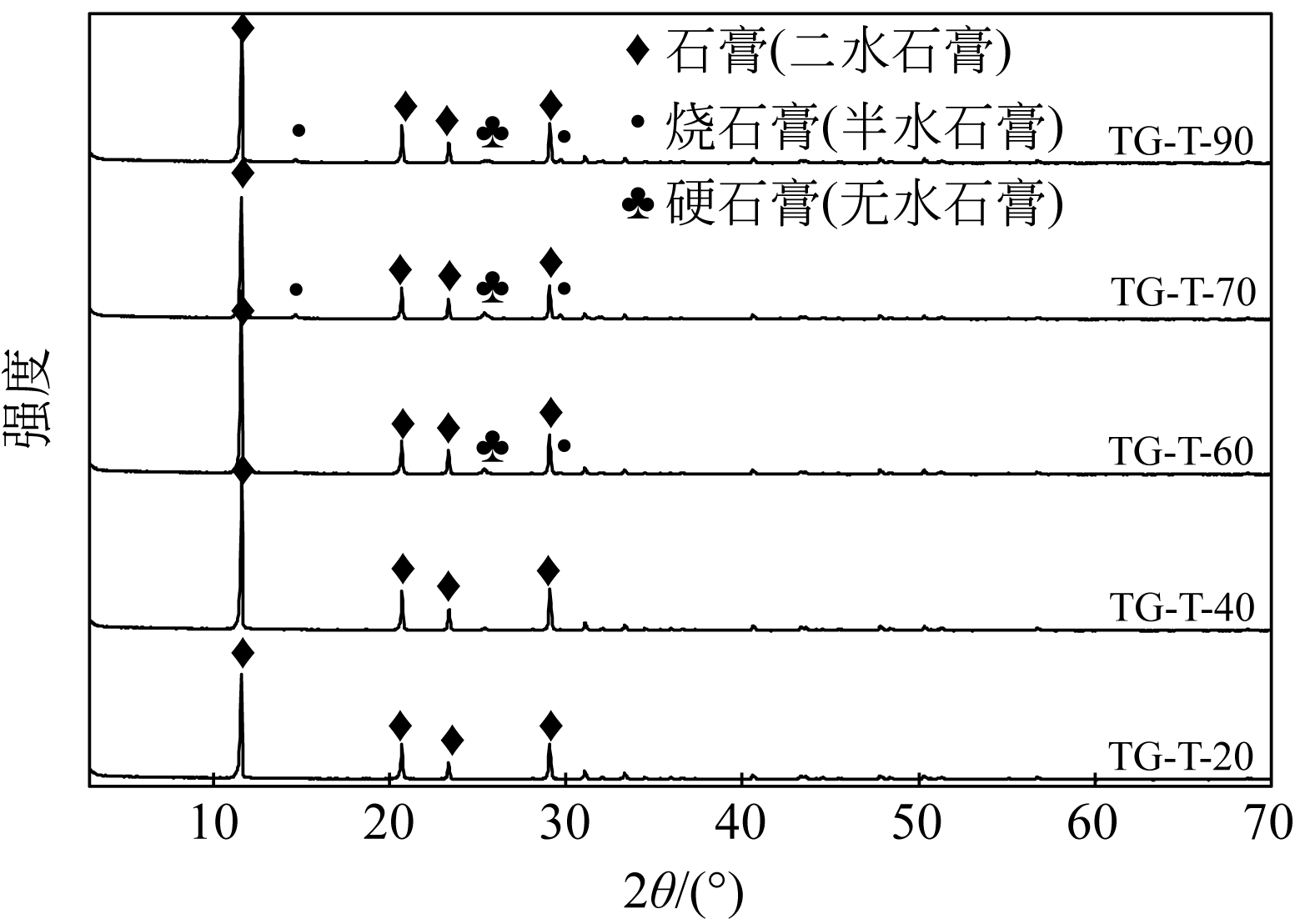
汪潇, 刘强, 杨留栓, 等 . 钛石膏颗粒物特性及其热重分析[J]. 硅酸盐通报, 2014, 33(1): 212-215.
|
|
WANG Xiao , LIU Qiang , YANG Liushuan , et al . Analysis of particle characteristics and TG of titanium gypsum[J]. Bulletin of the Chinese Ceramic Society, 2014, 33(1): 212-215.
|
| 12 |
AZDARPOUR A , ASADULLAH M , JUNIN R , et al . Extraction of calcium from red gypsum for calcium carbonate production[J]. Fuel Processing Technology, 2015, 130: 12-19.
|
| 13 |
刘民荣, 田颖, 赵帅 . 碱性激发剂对钛石膏-粉煤灰复合胶结材料性能的影响[J]. 粉煤灰, 2008, 20(6): 15-17.
|
|
LIU Mingrong , TIAN Ying , ZHAO Shuai , et al . Effect of alkali activator on properties of titanium gypsum/fly ash compound cementitious material[J]. Fly Ash, 2008, 20(6): 68-74.
|
| 14 |
FAUZIAH I , ZAUYAH S , JAMAL T . Characterization and land application of red gypsum: a waste product from the titanium dioxide industry[J]. Science of the Total Environment, 1996, 188(2/3): 243-251.
|
| 15 |
WANG W L , ZENG D W , YIN X , et al . Prediction and measurement of gypsum solubility in the systems CaSO4 + HMSO4 + H2SO4 + H2O (HM=Cu, Zn, Ni, Mn) at 298.15K[J]. Industrial & Engineering Chemistry Research, 2012, 51(14): 5124-5134.
|
| 16 |
WANG W L , ZENG D W , CHEN Q Y , et al . Experimental determination and modeling of gypsum and insoluble anhydrite solubility in the system CaSO4-H2SO4-H2O[J]. Chemical Engineering Science, 2013, 101(14): 120-129.
|
| 17 |
倪丽娜, 李沪萍, 罗康碧, 等 . 磷石膏在低浓度硫酸中的溶解及相态变化[J]. 化工进展, 2014, 33(3): 769-772.
|
|
NI Lina , LI Huping , LUO Kangbi , et al . Research of the dissolution and phase behavior change on the phosphogypsum in the low concentration of sulfuric acid[J]. Chemical Industry and Engineering Progress, 2014, 33(3): 769-772.
|
 ),Hongjuan SUN1,2,Tongjiang PENG1,2,3(
),Hongjuan SUN1,2,Tongjiang PENG1,2,3( )
)