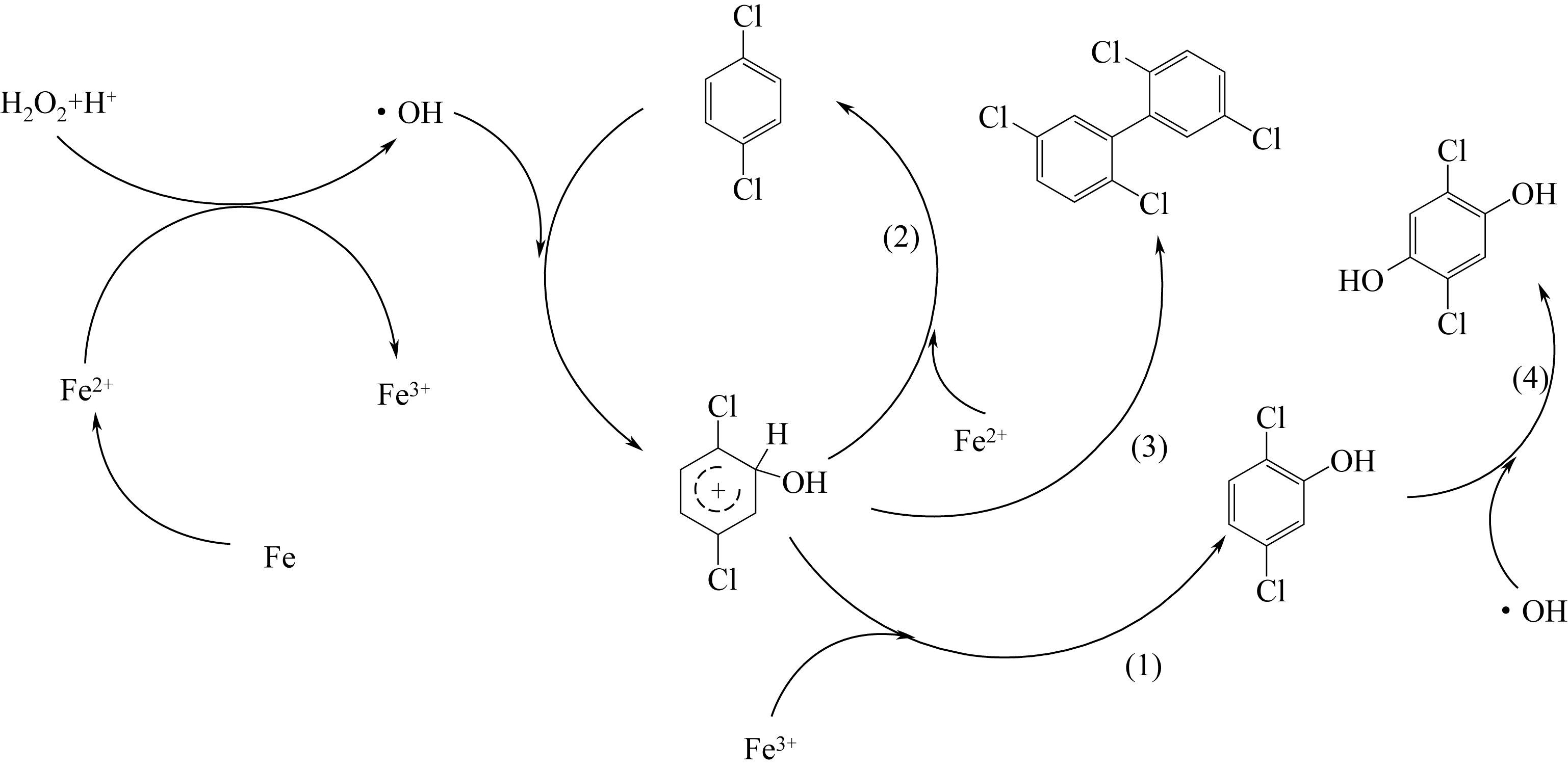
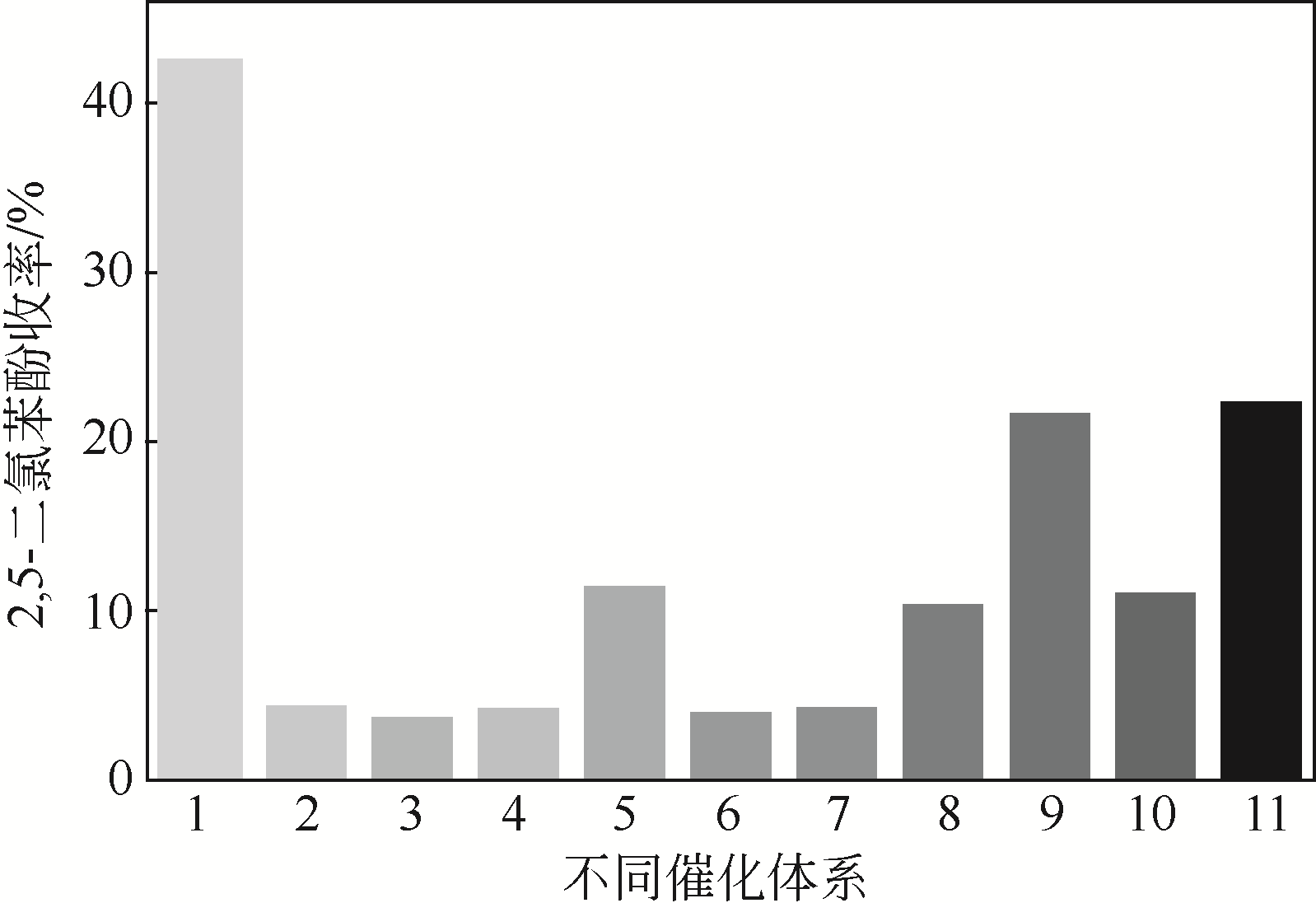
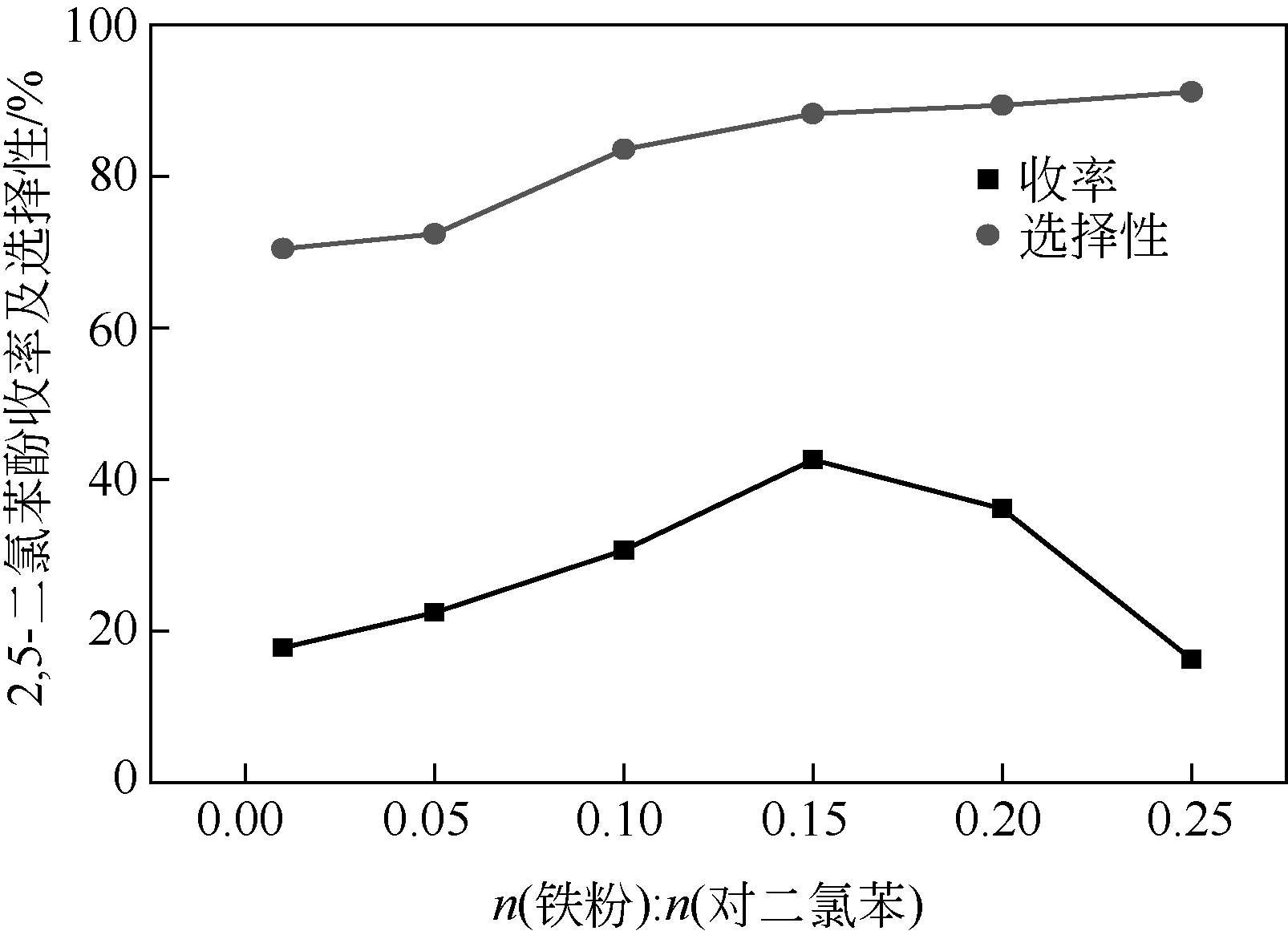
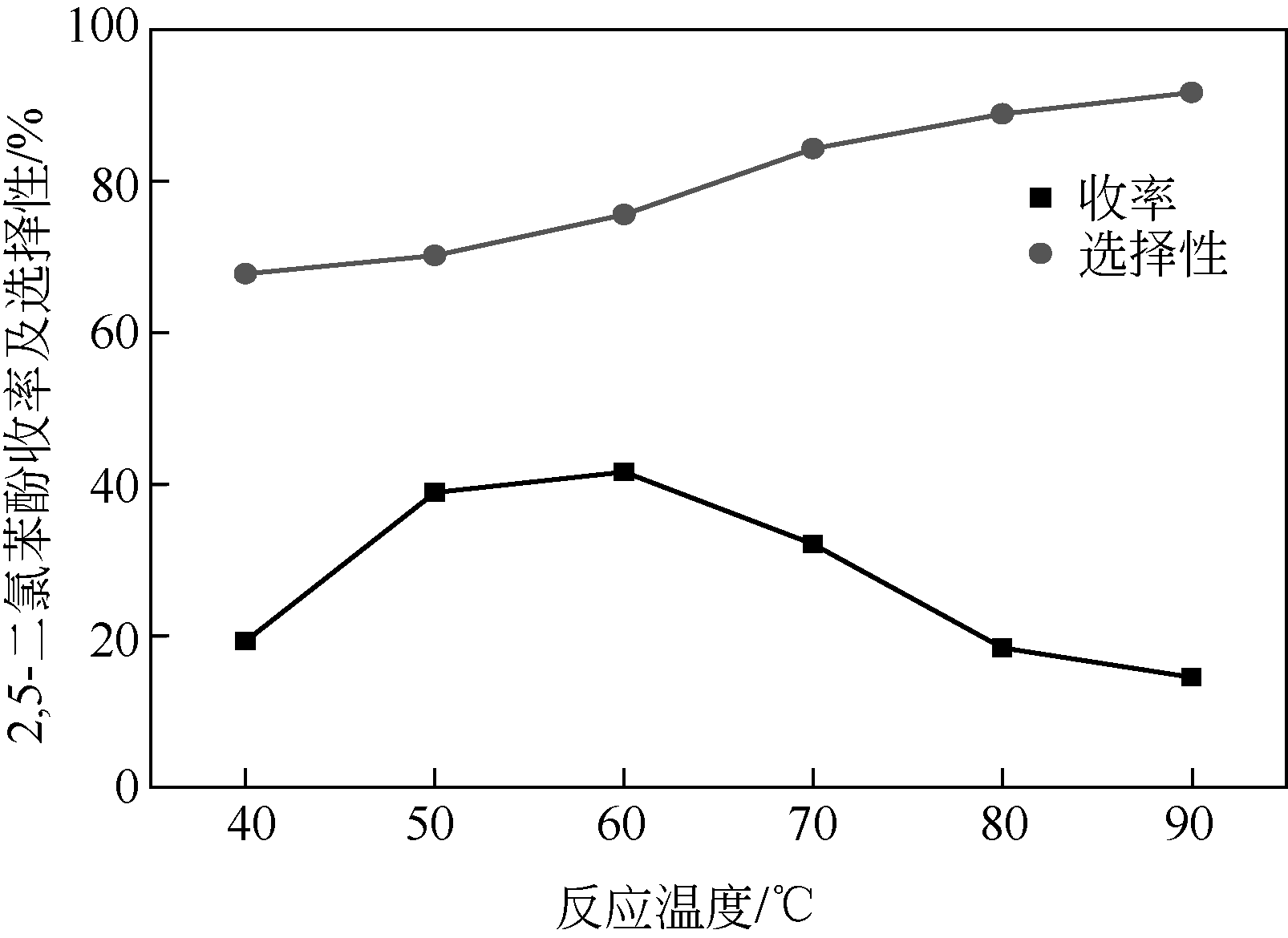
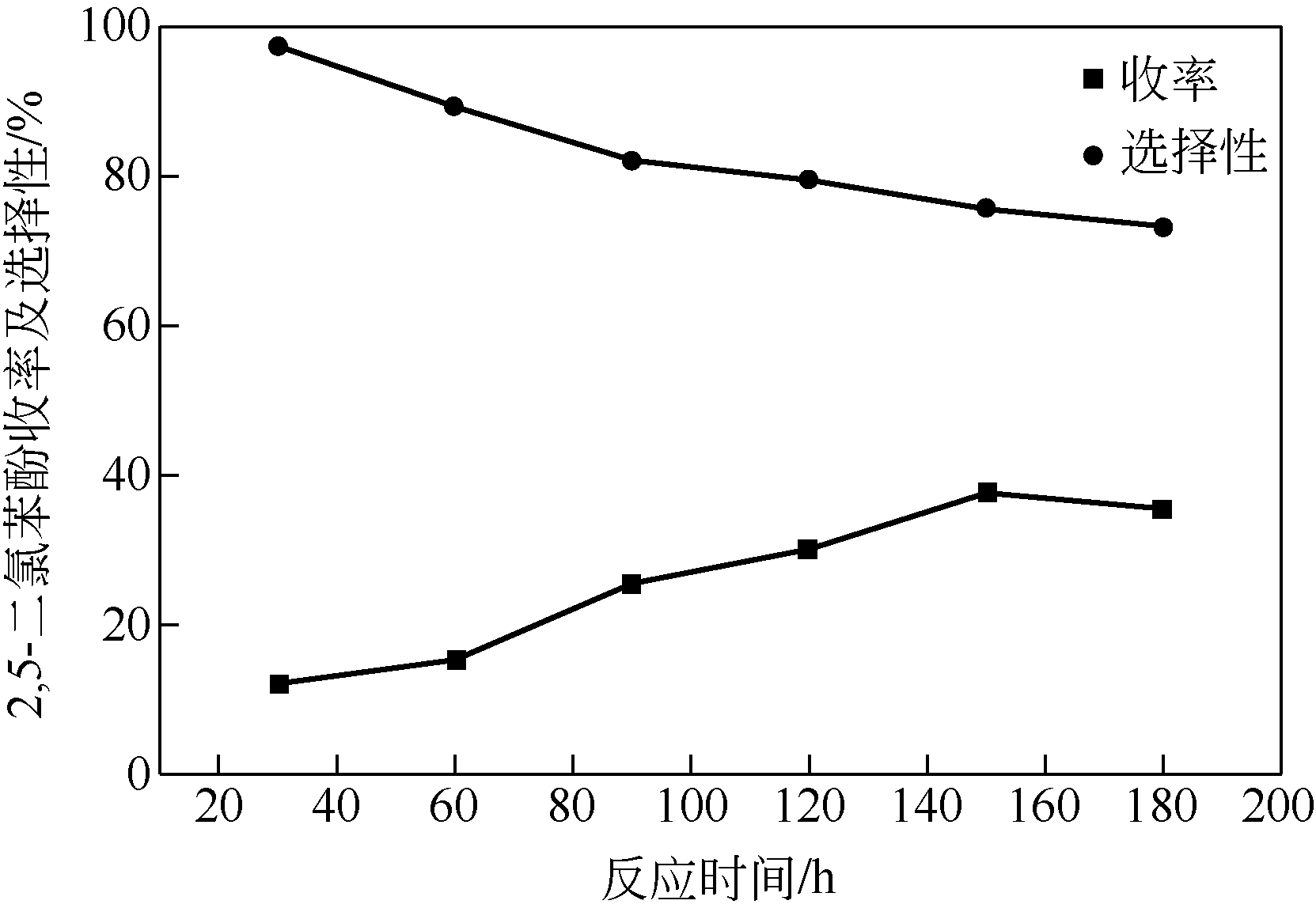
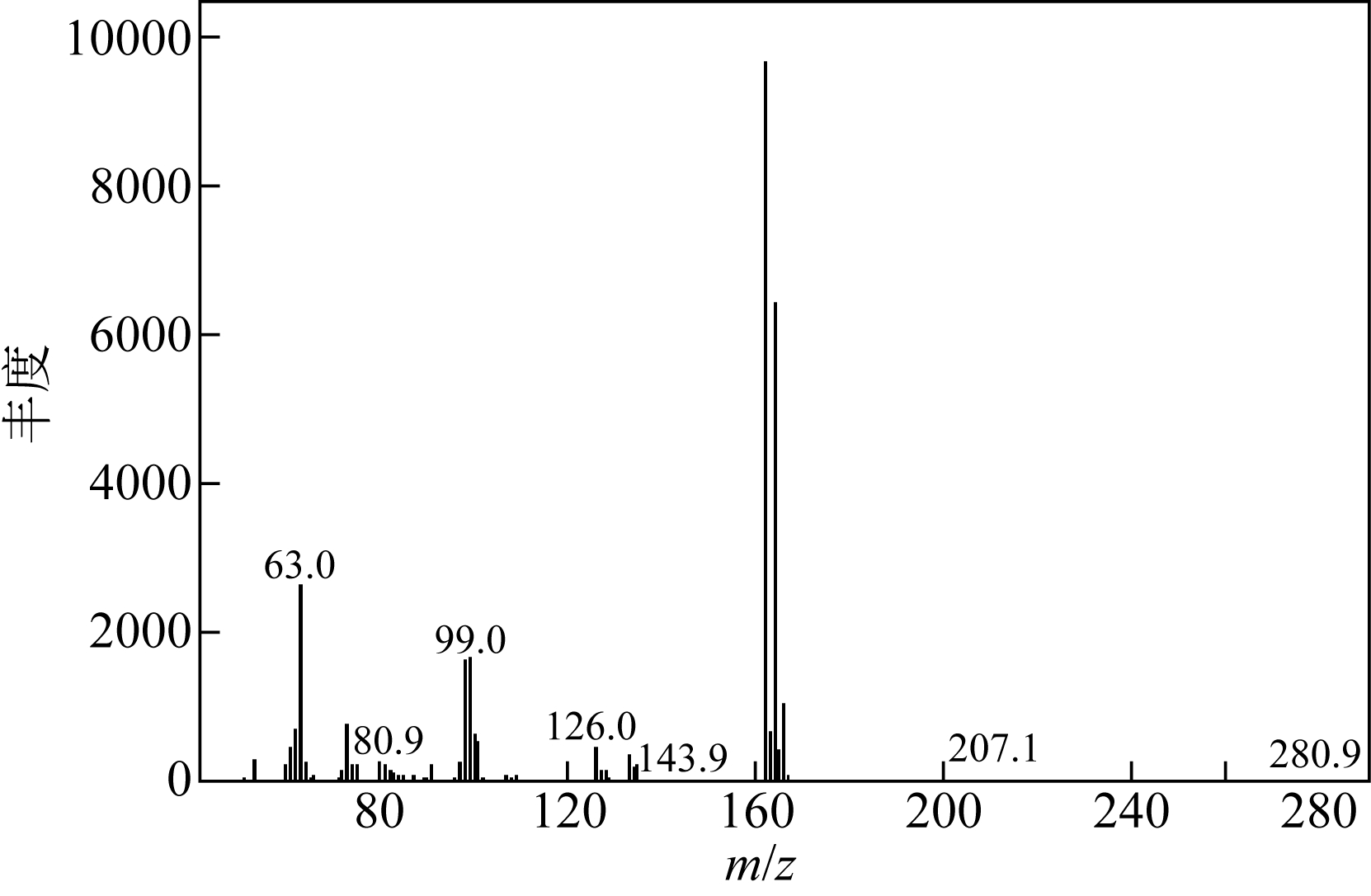
| 1 |
蔡春, 吕春绪, 苗丽, 等 . 2,5-二氯苯酚的合成新方法[J]. 化学试剂, 2000, 22(5): 315-318.
|
|
CAI Chun , Chunxu LÜ , MIAO Li , et al . A new method for the synthesis of 2,5-dichlorophenol[J]. Chemical Reagents, 2000, 22 (5): 315-318.
|
| 2 |
王玉灿, 马瑛, 孟明扬, 等 . 2,5-二氯苯酚的制备研究[J]. 染料与染色, 2012, 49(3): 15-16.
|
|
WANG Yucan , Ying MA , MENG Mingyang , et al . Preparation of 2,5-dichlorophenol[J]. Dyes & Dyeing, 2012, 49(3): 15-16.
|
| 3 |
曹广宏 . 2,5-二氯苯酚生产工艺述评[J]. 化学与生物工程, 1991(2): 30-32.
|
|
CAO Guanghong . Review on the production process of 2,5-dichlorophenol[J]. Chemistry and Bioengineering, 1991(2): 30-32.
|
| 4 |
杜晓宇 . 3,6-二氯水杨酸的合成工艺研究[D]. 天津: 河北工业大学, 2015.
|
|
DU Xiaoyu . Study on the synthesis of 3,6-dichlorosalicylic acid[D]. Tianjin: Hebei University of Technology, 2015.
|
| 5 |
陈晓晖, 许锡恩, 陈宪, 等 . H2O2在绿色化工生产中的应用进展[J]. 化工进展, 1999, 18(2): 30-32.
|
|
CHEN Xiaohui , XU Xien , CHEN Xian , et al . Application progress of H2O2 in green chemical production[J]. Chemical Industry and Engineering Progress, 1999, 18(2): 30-32.
|
| 6 |
BAILEY C L , MURPHY C L , CLARY J W , et al . Reaction of grignard reagents with diisopropylaminoborane. Synthesis of alkyl, aryl, heteroaryl and allyl boronic acids from organo(diisopropyl)aminoborane by a simple hydrolysis[J]. Cheminform, 2013, 44(17). DOI:10.1002/chin.201317186.
DOI
URL
|
| 7 |
BORAH R , SAIKIA E , BORA S J , et al . On-water synthesis of phenols using biogenic Cu2O nanoparticles without using H2O2 [J]. RSC Advances, 2016, 6(102): 100443-100447.
|
| 8 |
JIANG L , CHAN T H . Regioselective acylation of hexopyranosides with pivaloyl chloride[J]. Journal of Organic Chemistry, 1998, 63(17): 6035-6038.
|
| 9 |
纪传武, 陈高部, 申剑冰, 等 . 一种用于量产化的2,5-二氯苯酚的制备工艺: CN104876805A[P]. 2015-09-02.
|
|
JI Chuanwu , CHEN Gaobu , SHEN Jianbing , et al . A preparation process for mass production of 2,5-dichlorophenol: CN104876805A[P]. 2015-09-02.
|
| 10 |
张湘宁, 刘宇, 陈高部, 等 . 一种麦草畏关键中间体2,5-二氯苯酚的工业合成方法: CN104909993A[P]. 2015-09-16.
|
|
ZHANG Xiangning , LIU Yu , CHEN Gaobu , et al . An industrial synthesis method of 2,5-dichlorophenol of a key intermediate of dicamba: CN104909993A[P]. 2015-09-16.
|
| 11 |
潘飞, 张伟, 鲁墨弘, 等 . 麦草畏中间体3,6-二氯水杨酸的合成[J]. 高校化学工程学报, 2017, 31(1): 142-147.
|
|
PAN Fei , ZHANG Wei , LU Mohong , et al . Synthesis of dicamba intermediate 3,6-dichlorosalicylic acid[J]. Journal of Chemical Engineering of Chinese Universities, 2017, 31(1): 142-147.
|
| 12 |
宋少飞, 胡道道, 沈淑坤, 等 . 过氧化氢催化氧化绿色化学研究进展[J]. 世界科技研究与发展, 2006, 28(2): 20-29.
|
|
SONG Shaofei , HU Daodao , SHEN Shukun , et al . Research progress in catalytic oxidation of green chemistry by hydrogen peroxide [J]. World Science and Technology Research and Development, 2006, 28(2): 20-29.
|
| 13 |
SCHEUERMAN R A , HENRICK C A . Oxidation process: US6323377[P]. 2001-11-27.
|
| 14 |
ULLRICH R , HOFRICHTER M . Enzymatic hydroxylation of aromatic compounds[J]. Cellular & Molecular Life Sciences, 2007, 64(3): 271.
|
| 15 |
ALSABAGH A M , YEHIA F Z , ESHAQ G , et al . Eclectic hydroxylation of benzene to phenol using ferrites of Fe and Zn as durable and magnetically retrievable catalysts[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(6): 4811-4819.
|
| 16 |
HU L , WANG C , YUE B , et al . Direct hydroxylation of benzene to phenol using H2O2 as an oxidant over vanadium-containing nitrogen doped mesoporous carbon catalysts[J]. RSC Advances, 2016, 6(90): 87656-87664.
|
| 17 |
MONFARED H H , GHADIMI M . Catalytic epoxidation with hydrogen peroxide mediated by iron(Ⅱ) ions immobilized on γ-alumina[J]. Journal of Chemical Research, 2004, 35(2): 313-314.
|
| 18 |
FAPONLE A SBANSE F , VISSER S P , et al . Arene activation by a nonheme iron(Ⅲ)–hydroperoxo complex: pathways leading to phenol and ketone products[J]. Journal of Biological Inorganic Chemistry, 2016, 21(4): 453-462.
|
 ),张跃2(
),张跃2( ),严生虎1,2,刘建武1,马晓明1,2,辜顺林1,沈介发1,2,陈代祥1,2
),严生虎1,2,刘建武1,马晓明1,2,辜顺林1,沈介发1,2,陈代祥1,2
 ),Yue ZHANG2(
),Yue ZHANG2( ),Shenghu YAN1,2,Jianwu LIU1,Xiaoming MA1,2,Shunlin GU1,Jiefa SHEN1,2,Daixiang CHEN1,2
),Shenghu YAN1,2,Jianwu LIU1,Xiaoming MA1,2,Shunlin GU1,Jiefa SHEN1,2,Daixiang CHEN1,2