化工进展 ›› 2019, Vol. 38 ›› Issue (04): 1611-1623.DOI: 10.16085/j.issn.1000-6613.2018-1195
选择催化还原(SCR)反应机理研究进展
张道军1( ),马子然2,孙琦2,徐文强2,李永龙2,竹涛3,王宝冬2(
),马子然2,孙琦2,徐文强2,李永龙2,竹涛3,王宝冬2( )
)
- 1. 北京化工大学化工资源有效利用国家重点实验室,北京100029
2. 北京低碳清洁能源研究所,北京 102211
3. 中国矿业大学(北京)化学与环境工程学院,北京 100083
-
收稿日期:2018-06-07修回日期:2018-12-03出版日期:2019-04-05发布日期:2019-04-05 -
通讯作者:王宝冬 -
作者简介:张道军(1989—),男,博士,主要研究方向为大气污染控制。E-mail: <email>zhangdaojun@nicenergy.com</email>。|王宝冬,教授级高级工程师,青年千人计划专家,主要研究方向为大气污染控制。E-mail: <email>wangbaodong@nicenergy.com</email>。 -
基金资助:中组部青年千人启动经费-洁净煤(燃煤电厂)污染物控制(GB9300120001);北京低碳清洁能源研究所科技项目(CF9300171821)
Progress in the mechanism of selective catalytic reduction (SCR) reaction
Daojun ZHANG1( ),Ziran MA2,Qi SUN2,Wenqiang XU2,Yonglong LI2,Tao ZHU3,Baodong WANG2(
),Ziran MA2,Qi SUN2,Wenqiang XU2,Yonglong LI2,Tao ZHU3,Baodong WANG2( )
)
- 1. State Key Laboratory of Chemical Resource Engineering, Beijing University of Chemical Technology, Beijing 100029, China
2. National Institute of Clean-and-Low-Carbon Energy,Beijing 102211,China
3. School of Chemical & Environmental Engineering,China University of Mining & Technology Beijing, Beijing 100083,China
-
Received:2018-06-07Revised:2018-12-03Online:2019-04-05Published:2019-04-05 -
Contact:Baodong WANG
摘要:
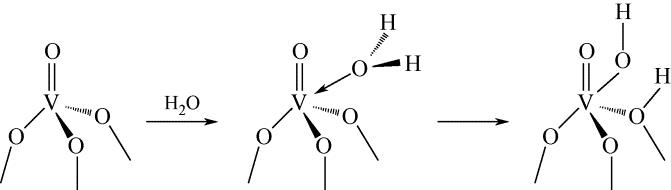
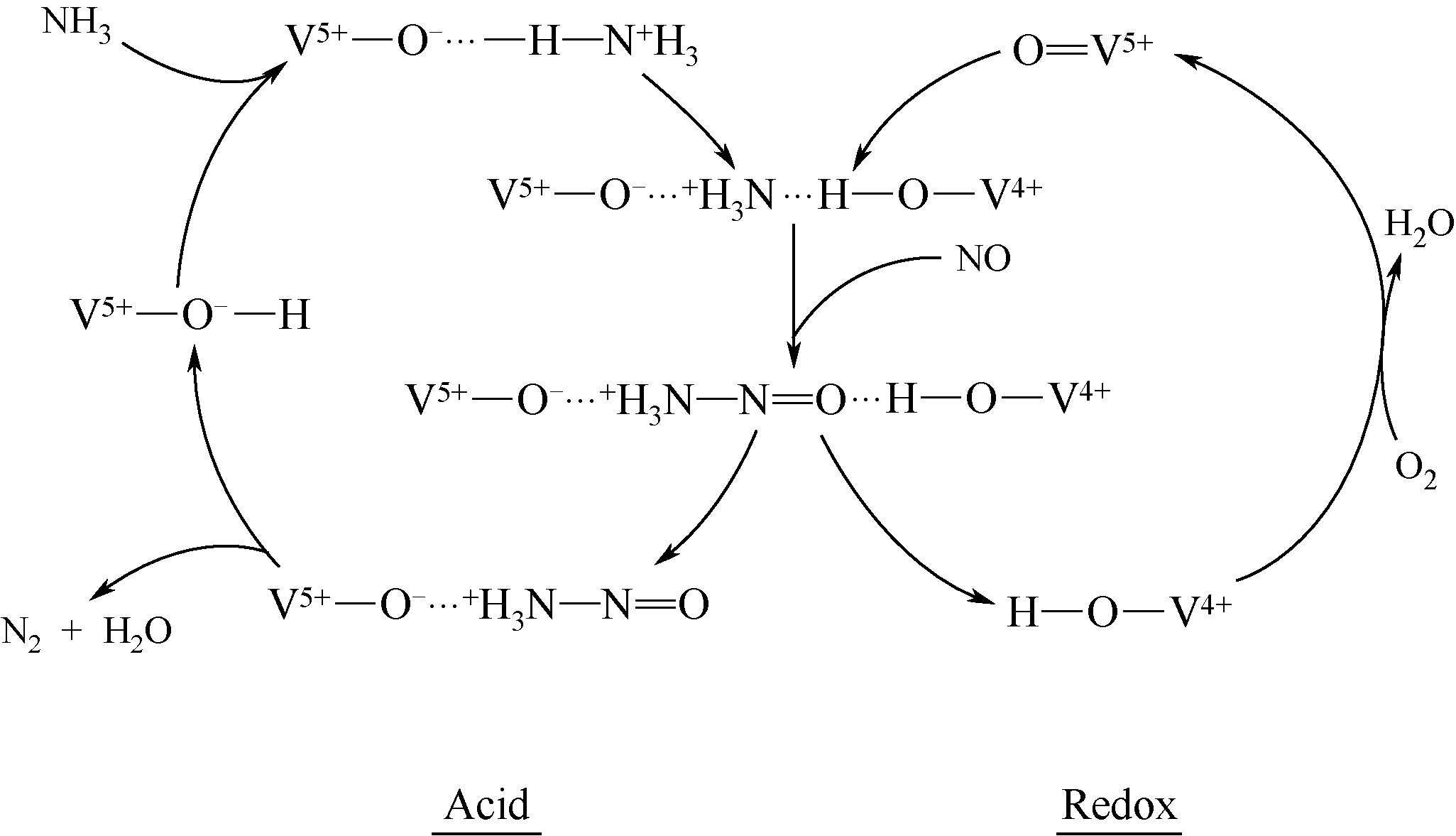
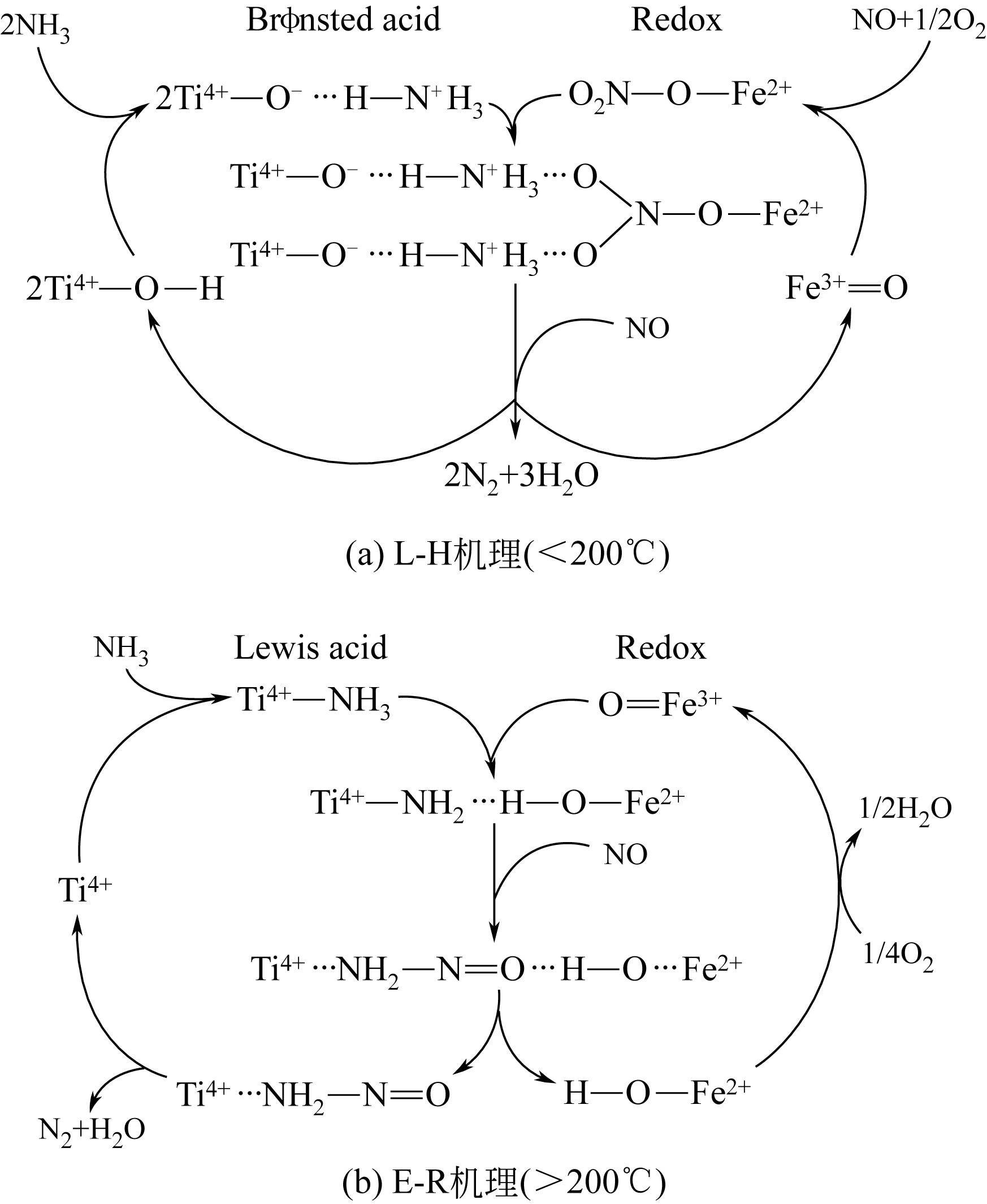
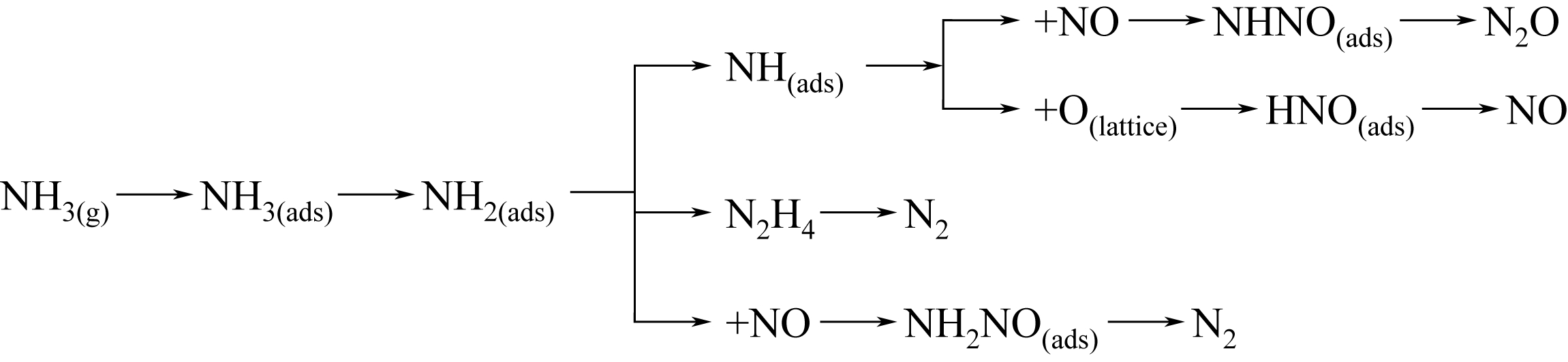
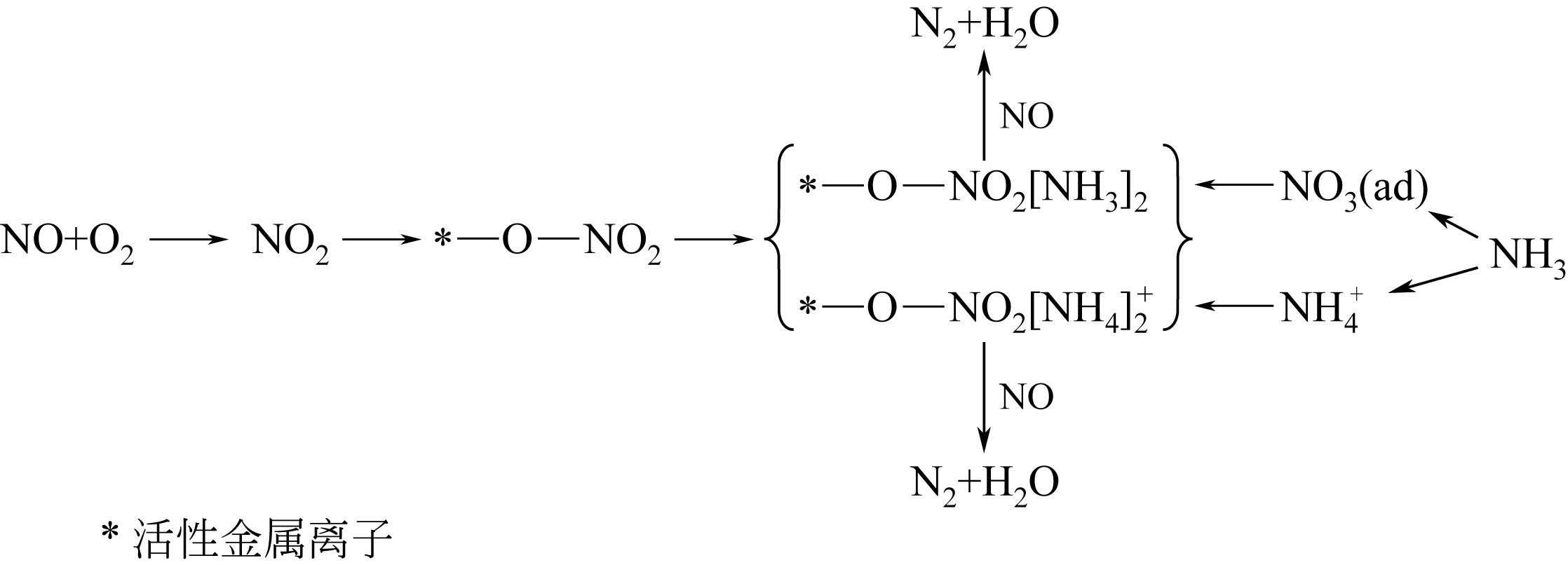
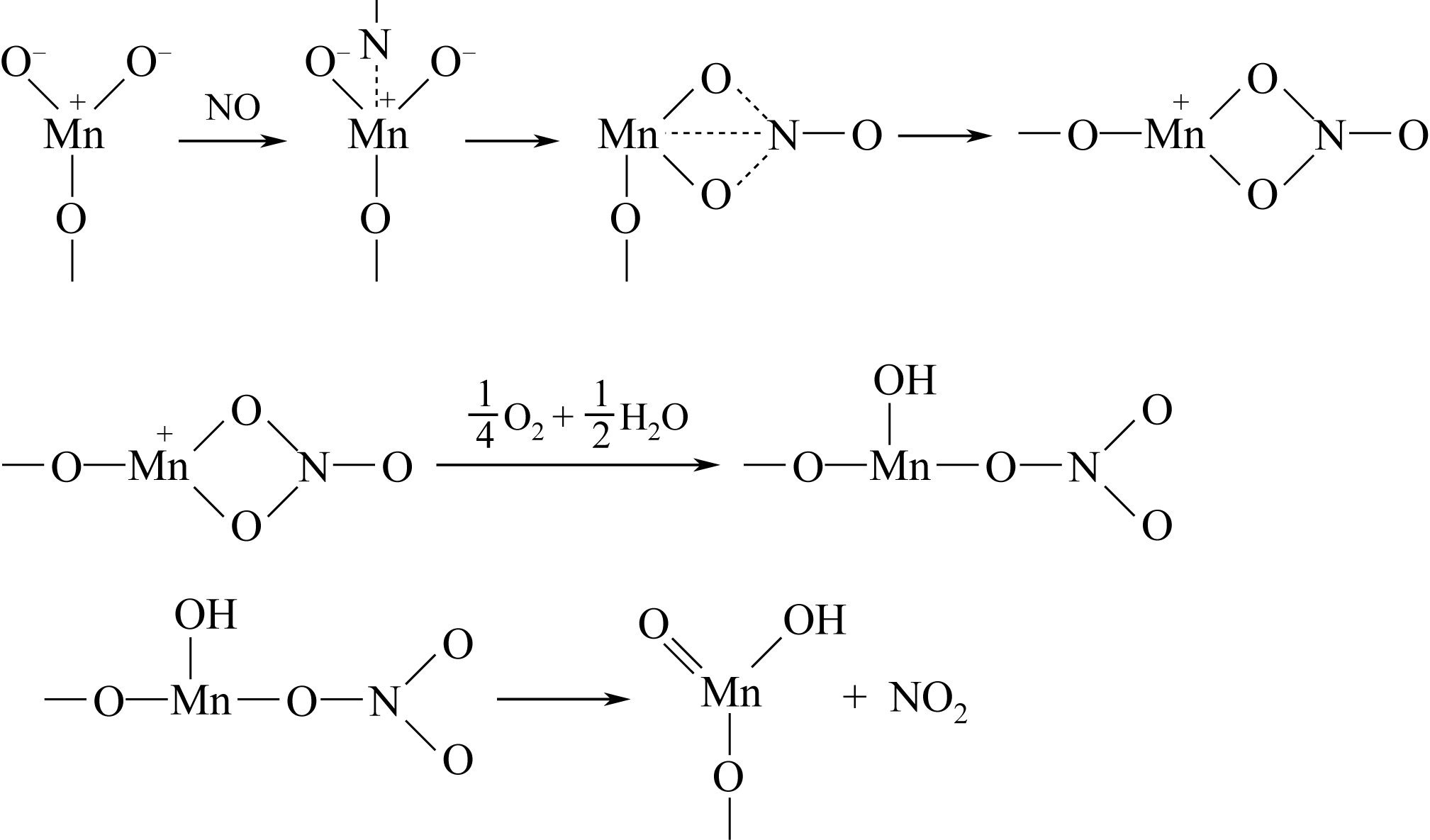
简述了NH3和NO在催化剂表面吸附、转化活化和反应历程及H2O和SO2对以上反应行为的影响。分析表明,NH3氧化脱氢进而与NO反应是决定NH3反应性和最终产物的关键。NO以气态(Eley-Rideal机理)或硝基类物质等吸附态(Langmuir-Hinshelwood机理)形式参与选择催化还原(SCR)反应。提高催化剂酸性和氧化还原循环性能,利于NH3和NO吸附和转化及相互间反应。高温时,H2O影响轻微,而SO2增强催化剂酸性,提高脱硝活性。低温时,H2O和SO2抑制NO吸附和转化活化,导致硫铵盐累积和活性位转变为硫酸盐使催化剂失活。因此,提高抗H2O、抗SO2性能是低温脱硝催化剂研发的重要方向。而发展在线升温等再生工艺以解决硝酸盐或含硫化合物导致的失活问题,对保障低温脱硝系统长期稳定运行具有重要意义。
中图分类号:
引用本文
张道军, 马子然, 孙琦, 徐文强, 李永龙, 竹涛, 王宝冬. 选择催化还原(SCR)反应机理研究进展[J]. 化工进展, 2019, 38(04): 1611-1623.
Daojun ZHANG, Ziran MA, Qi SUN, Wenqiang XU, Yonglong LI, Tao ZHU, Baodong WANG. Progress in the mechanism of selective catalytic reduction (SCR) reaction[J]. Chemical Industry and Engineering Progress, 2019, 38(04): 1611-1623.
| 吸附物种 | 峰位置/cm-1 | 结构式 | 脱附或分解温度/K |
|---|---|---|---|
| 亚硝酰基 | 1835 | Mn n +—N | 323 |
| 桥联状硝基 | 1620/1220 |  | 423~573 |
| “Ⅰ型”双齿状硝基类 | 1580/1220 |  | 473~573 |
| “Ⅱ型”双齿状硝基类 | 1555/1290 |  | 573~698 |
| 线状亚硝基 | 1466/1075 | M n +—O—N | 323~473 |
| 单齿状亚硝基 | 1415/1322 |  | 323~473 |
| 桥联线状亚硝基 | 1230 |  | 323~523 |
表1 催化剂表面吸附态NO存在形式及热稳定性[20]
| 吸附物种 | 峰位置/cm-1 | 结构式 | 脱附或分解温度/K |
|---|---|---|---|
| 亚硝酰基 | 1835 | Mn n +—N | 323 |
| 桥联状硝基 | 1620/1220 |  | 423~573 |
| “Ⅰ型”双齿状硝基类 | 1580/1220 |  | 473~573 |
| “Ⅱ型”双齿状硝基类 | 1555/1290 |  | 573~698 |
| 线状亚硝基 | 1466/1075 | M n +—O—N | 323~473 |
| 单齿状亚硝基 | 1415/1322 |  | 323~473 |
| 桥联线状亚硝基 | 1230 |  | 323~523 |
| 1 | LIU Y , ZHAO J , LEE J . Conventional and new materials for selective catalytic reduction (SCR) of NO x [J]. Chem. Cat. Chem., 2018, 10 (7): 1499-1511. |
| 2 | GAN L , LEI S , YU J , et al . Development of highly active coated monolith SCR catalyst with strong abrasion resistance for low-temperature application[J]. Frontiers of Environmental Science & Engineering, 2015, 9(6): 979-987. |
| 3 | ZHU M , LAI J , WACHS I . Formation of N2O greenhouse gas during SCR of NO with NH3 by supported vanadium oxide catalysts[J]. Applied Catalysis B: Environmental, 2018, 224: 836-840. |
| 4 | ARFAOUI J , GHORBEL A , PETITTO C , et al . Novel V2O5-CeO2-TiO2-SO4 2- nanostructured aerogel catalyst for the low temperature selective catalytic reduction of NO by NH3 in excess O2 [J]. Applied Catalysis B: Environmental, 2018, 224: 264-275. |
| 5 | 曲瑞阳 . 新型宽温度窗口催化剂选择性催化还原NO x 的机理研究[D]. 杭州: 浙江大学, 2017. |
| QU R Y . Study on the mechanism of novel SCR catalysts with broad temperature window[D]. Hangzhou: Zhejiang University, 2017. | |
| 6 | 高凤雨 . Mn基低温NH3-SCR催化剂的抗水抗硫性能及反应机理研究[D]. 北京: 北京科技大学, 2017. |
| GAO F Y . Study on the H2O and SO2 resistance ability and reaction mechanism of Mn based low temperature NH3-SCR catalyst[D]. Beijing: University of Science & Technology Beijing, 2017. | |
| 7 | BUSCA G , SAUSSEY H , SAUR O , et al . FT-IR characterization of the surface acidity of different titanium dioxide anatase preparations[J]. Applied Catalysis, 1985, 14: 245-260. |
| 8 | RAMIS G , BUSCA G , LORENZELLI V , et al . Fourier transform infrared study of the adsorption and coadsorption of nitric oxide, nitrogen dioxide and ammonia on TiO2 anatase[J]. Applied Catalysis, 1990, 64: 243-257. |
| 9 | TOPSØE N Y , DUMESIC J A , TOPSØE H . Vanadia/titania catalysts for selective catalytic reduction of nitric oxide by ammonia: Ⅱ. Studies of active sites and formulation of catalytic cycles[J]. Journal of Catalysis, 1995, 151(1): 241-252. |
| 10 | ZHU M , LAI J K , TUMULURI U , et al . Nature of active sites and surface intermediates during SCR of NO with NH3 by supported V2O5-WO3/TiO2 catalysts[J]. Journal of the American Chemical Society, 2017, 139(44): 15624-15627. |
| 11 | LIU Q , LIU Z , LI C . Adsorption and activation of NH3 during selective catalytic reduction of NO by NH3 [J]. Chinese Journal of Catalysis, 2006, 27(7): 636-646. |
| 12 | ZHANG Q , FAN J , NING P , et al . In situ DRIFTS investigation of NH3-SCR reaction over CeO2/zirconium phosphate catalyst [J]. Applied Surface Science, 2018, 435: 1037-1045. |
| 13 | ZHA K , CAI S , HU H , et al . In situ DRIFTs investigation of promotional effects of tungsten on MnO x -CeO2/meso-TiO2 catalysts for NO x reduction[J]. Journal of Physical Chemistry C, 2017, 121(45): 25243-25254. |
| 14 | ZHANG L , SUN J , XIONG Y , et al . Catalytic performance of highly dispersed WO3 loaded on CeO2 in the selective catalytic reduction of NO by NH3 [J]. Chinese Journal of Catalysis, 2017, 38(10): 1749-1758. |
| 15 | PENG Y , LI K , LI J . Identification of the active sites on CeO2-WO3 catalysts for SCR of NO x with NH3: an in situ IR and Raman spectroscopy study[J]. Applied Catalysis B: Environmental, 2013, 140-141(2): 483-492. |
| 16 | GAO F , WASHTON N M , WANG Y , et al . Effects of Si/Al ratio on Cu/SSZ-13 NH3-SCR catalysts: implications for the active Cu species and the roles of Brønsted acidity[J]. Journal of Catalysis, 2015, 331: 25-38. |
| 17 | PEÑA D A , UPHADE B S , REDDY E P , et al . Identification of surface species on titania-supported manganese, chromium, and copper oxide low-temperature SCR catalysts[J]. Journal of Physical Chemistry B, 2004, 108(28): 9927-9936. |
| 18 | LIU F , HE H , ZHANG C , et al . Mechanism of the selective catalytic reduction of NO x with NH3 over environmental-friendly iron titanate catalyst[J]. Catalysis Today, 2011, 175(1): 18-25. |
| 19 | KIJLSTRA W S , BRANDS D S , SMIT H I , et al . Mechanism of the selective catalytic reduction of NO with NH3 over MnO x /Al2O3:Ⅱ. Reactivity of adsorbed NH3 and NO complexes[J]. Journal of Catalysis, 1997, 171(1): 219-230. |
| 20 | KIJLSTRA W S , BRANDS D S , POELS E K , et al . Mechanism of the selective catalytic reduction of NO by NH3 over MnO x /Al2O3: Ⅱ. Adsorption and desorption of the single reaction components[J]. Journal of Catalysis, 1997, 171(1): 208-218. |
| 21 | TOPSØE N Y , TOPSØE H , DUMESIC J A . Vanadia/titania catalysts for selective catalytic reduction(SCR) of nitric-oxide by ammonia: Ⅱ. Combined temperature-programmed in-situ FTIR and on-line mass spectroscopy studies[J]. Journal of Catalysis, 1995, 151(1): 226-240. |
| 22 | SCHNEIDER H , TSCHUDIN S , SCHNEIDER M , et al . In situ diffuse reflectance FTIR study of the selective catalytic reduction of NO by NH3 over vanadia-titania aerogels[J]. Journal of Catalysis, 1994,147(1): 5-14. |
| 23 | RAMIS G , BUSCA G , BREGANI F , et al . Fourier transform-infrared study of the adsorption and coadsorption of nitric oxide, nitrogen dioxide and ammonia on vanadia-titania and mechanism of selective catalytic reduction[J]. Applied Catalysis, 1990, 64(1/2): 259-278. |
| 24 | ZHU M , LAI J K , TUMULURI U , et al . Reaction pathways and kinetics for selective catalytic reduction(SCR) of acidic NO x emissions from power plants with NH3 [J]. ACS Catalysis, 2017, 7(12): 8358-8361. |
| 25 | LIETTI L , SVACHULA J , FORZATTI P , et al . Surface and catalytic properties of vanadia-titania and tungsta-titania systems in the selective catalytic reduction of nitrogen oxides[J].Catalysis Today, 1993, 17(1/2): 131-140. |
| 26 | RAMIS G , YI L , BUSCA G , et al . Adsorption, activation, and oxidation of ammonia over SCR catalysts[J]. Journal of Catalysis, 1995, 157(2): 523-535. |
| 27 | 刘清雅 . 蜂窝状堇青石基Cu-Al2O3催化剂的制备及其烟气脱硫脱硝的研究[D]. 太原:中国科学院山西煤炭化学研究所,2004. |
| LIU Q Y . Study on the preparation of honeycomb cordierite based Cu-Al2O3 catalyst and its application in flue gas desulfurization and denitrification[D]. Taiyuan: Shanxi Institute of Coal Chemistry, Chinese Academy of Sciences, 2004. | |
| 28 | QI G , YANG R T . Characterization and FTIR studies of MnO x -CeO2 catalyst for low-temperature selective catalytic reduction of NO with NH3 [J]. Journal of Physical Chemistry B, 2004, 108(40): 15738-15747. |
| 29 | QI G , YANG R T , CHANG R . MnO x -CeO mixed oxides prepared by co-precipitation for selective catalytic reduction of NO with NH3 at low temperatures[J]. Applied Catalysis B: Environmental, 2004, 51(2): 93-106. |
| 30 | LIU F , SHAN W , LIAN Z , et al . Novel MnWO x catalyst with remarkable performance for low temperature NH3-SCR of NO x [J]. Catalysis Science & Technology, 2013, 3(10): 2699-2707. |
| 31 | LIU F , HE H . Structure-activity relationship of iron titanate catalysts in the selective catalytic reduction of NO x with NH3 [J]. Journal of Physical Chemistry C, 2010, 114(40): 16929-16936. |
| 32 | MARBÁN G , FUERTES A B . Kinetics of the low-temperature selective catalytic reduction of NO with NH3 over activated carbon fiber composite-supported iron oxides[J]. Catalysis Letters, 2002, 84(1-2): 13-19. |
| 33 | LONG R Q , YANG R T . FTIR and kinetic studies of the mechanism of Fe3+-exchanged TiO2-pillared clay catalyst for selective catalytic reduction of NO with ammonia[J]. Journal of Catalysis, 2000, 190(1): 22-31. |
| 34 | CHEN T , GUAN B , LIN H , et al . In situ DRIFTS study of the mechanism of low temperature selective catalytic reduction over manganese-iron oxides[J]. Chinese Journal of Catalysis, 2014, 35(3): 294-301. |
| 35 | JIANG B , LI Z , LEE S C . Mechanism study of the promotional effect of O2 on low-temperature SCR reaction on Fe-Mn/TiO2 by DRIFT[J]. Chemical Engineering Journal, 2013, 225(3): 52-58. |
| 36 | CHEN L , LI J , GE M . The poisoning effect of alkali metals doping over nano V₂O₅-WO₃/TiO₂ catalysts on selective catalytic reduction of NO x by NH₃[J]. Chemical Engineering Journal, 2011, 170(2-3): 531-537. |
| 37 | LI X , LI X , YANG R T , et al . The poisoning effects of calcium on V2O5-WO3/TiO2 catalyst for the SCR reaction: comparison of different forms of calcium[J]. Molecular Catalysis, 2017, 434: 16-24. |
| 38 | LI X , LI X , LI J , et al . High calcium resistance of CeO2-WO3 SCR catalysts: structure investigation and deactivation analysis[J]. Chemical Engineering Journal, 2017, 317: 70-79. |
| 39 | GU T , JIN R , LIU Y , et al . Promoting effect of calcium doping on the performances of MnO x /TiO2 catalysts for NO reduction with NH3 at low temperature[J]. Applied Catalysis B: Environmental, 2013, 129(3): 30-38. |
| 40 | SUN W , LI X , ZHAO Q , et al . Fe-Mn mixed oxide catalysts synthesized by one-step urea-precipitation method for the selective catalytic reduction of NO x with NH3 at low temperatures[J]. Catalysis Letters, 2018, 148(1): 227-234. |
| 41 | KOEBEL M , ELSENER M , Madia G . Reaction pathways in the selective catalytic reduction process with NO and NO2 at low temperatures[J]. Industrial & Engineering Chemistry Research, 2001, 40(1): 52-59. |
| 42 | IWASAKI M , SHINJOH H . A comparative study of “standard”, “fast” and “NO2” SCR reactions over Fe/zeolite catalyst[J]. Applied Catalysis A: General, 2010, 390(1): 71-77. |
| 43 | NOVA I , CIARDELLI C , TRONCONI E , et al . NH3-NO/NO2 chemistry over V-based catalysts and its role in the mechanism of the fast SCR reaction[J]. Catalysis Today, 2006, 114(1): 3-12. |
| 44 | RUGGERI M P , GROSSALE A , NOVA I , et al . FTIR in situ mechanistic study of the NH3, NO/NO2, “fast SCR” reaction over a commercial Fe-ZSM-5 catalyst[J]. Catalysis Today, 2012, 184(1): 107-114. |
| 45 | LONG R Q , YANG R T . Superior Fe-ZSM-5 catalyst for selective catalytic reduction of nitric oxide by ammonia[J]. Journal of the American Chemical Society, 1999, 121(23): 5595-5596. |
| 46 | LONG R Q , YANG R T . The promoting role of rare earth oxides on Fe-exchanged TiO2-pillared clay for selective catalytic reduction of nitric oxide by ammonia[J]. Applied Catalysis B: Environmental, 2000, 27(2): 87-95. |
| 47 | FORZATTI P , NOVA I , TRONCONI E . Enhanced NH3 selective catalytic reduction for NO x abatement[J]. Angewandte Chemie International Edition, 2009, 121(44): 8516-8518. |
| 48 | LI W , ZHANG C , LI X , et al . Ho-modified Mn-Ce/TiO2 for low-temperature SCR of NO x with NH3: evaluation and characterization[J]. Chinese Journal of Catalysis, 2018, 39(10): 1653-1663. |
| 49 | SHEN B , LIU T , ZHAO N , et al . Iron-doped Mn-Ce/TiO2 catalyst for low temperature selective catalytic reduction of NO with NH3 [J]. Journal of Environmental Sciences, 2010, 22(9): 1447-1454. |
| 50 | LIU Z , YANG Y , ZHANG S , et al . Selective catalytic reduction of NO x with NH3 over Mn-Ce mixed oxide catalyst at low temperatures[J]. Catalysis Today, 2013, 216: 76-81. |
| 51 | BONINGARI T , ETTIREDDY P R , SOMOGYVARI A , et al . Influence of elevated surface texture hydrated titania on Ce-doped Mn/TiO2 catalysts for the low-temperature SCR of NO x under oxygen-rich conditions[J]. Journal of Catalysis, 2015, 325: 145-155. |
| 52 | 刘建东,黄张根,李哲,等 . Ce对Mn/TiO2/堇青石整体低温脱硝选择性催化还原催化剂的改性[J].高等学校化学学报,2014,35(3): 589-595. |
| LIU Jiandong , HUANG Zhanggen , LI Zhe , et al . Ce modification on Mn/TiO2/cordierite monolithic catalyst for low-temperature NO x reduction[J]. Chemical Journal of Chinese Universities, 2014, 35(3): 589-595. | |
| 53 | SVACHULA J , FERLAZZO N , FORZATTI P , et al . Selective reduction of NO x by ammonia over honeycomb DeNO x ing catalysts[J]. Industrial & Engineering Chemistry Research, 1993, 32(6): 1053-1060. |
| 54 | TURCO M , LISI L , PIRONE R , et al . Effect of water on the kinetics of nitric oxide reduction over a high-surface-area V2O5/TiO2 catalyst[J]. Applied Catalysis B: Environmental, 1994, 3(2/3): 133-149. |
| 55 | AMIRIDIS M D , WACHS I E , DEO G, et al . Reactivity of V2O5 catalysts for the selective catalytic reduction of NO by NH3: influence of vanadia loading, H2O, and SO2 [J]. Journal of Catalysis, 1996, 161(1): 247-253. |
| 56 | WU S , LI H , LI L , et al . Effects of flue-gas parameters on low temperature NO reduction over a Cu-promoted CeO2-TiO2 catalyst[J]. Fuel, 2015, 159: 876-882. |
| 57 | LIU F , HE H . Selective catalytic reduction of NO with NH3 over manganese substituted iron titanate catalyst: reaction mechanism and H2O/SO2 inhibition mechanism study[J]. Catalysis Today, 2010, 153(3): 70-76. |
| 58 | PAN S , LUO H , LI L , et al . H2O and SO2 deactivation mechanism of MnO x /MWCNTs for low-temperature SCR of NO x with NH3 [J]. Journal of Molecular Catalysis A: Chemical, 2013, 377: 154-161. |
| 59 | YOUN S , SONG I , LEE H, et al . Effect of pore structure of TiO2 on the SO2 poisoning over V2O5/TiO2 catalysts for selective catalytic reduction of NO x with NH3 [J]. Catalysis Today, 2018, 303: 19-24. |
| 60 | TOPSØE N Y , SLABIAK T , CLAUSEN B S , et al . Influence of water on the reactivity of vanadia-titania for catalytic reduction of NO x [J]. Journal of Catalysis, 1992, 134: 742-746. |
| 61 | 张道军, 马子然, 孙琦, 等 . 硫酸氢铵在钒基选择性催化还原催化剂表面的生成、作用及防治[J]. 化工进展, 2018, 37(7): 2635-2643. |
| ZHANG Daojun , Ziran MA , SUN Qi , et al . Formation mechanism, effects and prevention of NH4HSO4 formed on the surface of V2O5 based catalysts[J]. Chemical Industry and Engineering Progress, 2018, 37(7): 2635-2643. | |
| 62 | ZHANG L , WANG D , LIU Y , et al . SO2 poisoning impact on the NH3-SCR reaction over a commercial Cu-SAPO-34 SCR catalyst[J]. Applied Catalysis B: Environmental, 2014, 156/157(9): 371-377. |
| 63 | GUO X , BARTHOLOMEW C , HECKER W , et al . Effects of sulfate species on V2O5/TiO2 SCR catalysts in coal and biomass-fired systems[J]. Applied Catalysis B: Environmental, 2009, 92(1): 30-40. |
| 64 | YANG S , GUO Y , CHANG H , et al . Novel effect of SO2 on the SCR reaction over CeO2: mechanism and significance[J]. Applied Catalysis B: Environmental, 2013, 136/137(12): 19-28. |
| 65 | YAN Q , CHEN S , ZHANG C , et al . Synthesis and catalytic performance of Cu1Mn0.5Ti0.5O x mixed oxide as low-temperature NH3-SCR catalyst with enhanced SO2 resistance[J]. Applied Catalysis B: Environmental, 2018, 238: 236-247. |
| 66 | LIU F , ASAKURA K , He H , et al . Influence of sulfation on iron titanate catalyst for the selective catalytic reduction of NO x with NH3 [J]. Applied Catalysis B: Environmental, 2011, 103(3): 369-377. |
| 67 | WIJAYANTI K , LEISTNER K , CHAND S , et al . Deactivation of Cu-SSZ-13 by SO2 exposure under SCR conditions[J]. Catalysis Science & Technology, 2015, 2016, 6(8): 2565-2579. |
| [1] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [2] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [3] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [4] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [5] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [6] | 崔守成, 徐洪波, 彭楠. 两种MOFs材料用于O2/He吸附分离的模拟分析[J]. 化工进展, 2023, 42(S1): 382-390. |
| [7] | 陈崇明, 陈秋, 宫云茜, 车凯, 郁金星, 孙楠楠. 分子筛基CO2吸附剂研究进展[J]. 化工进展, 2023, 42(S1): 411-419. |
| [8] | 高雨飞, 鲁金凤. 非均相催化臭氧氧化作用机理研究进展[J]. 化工进展, 2023, 42(S1): 430-438. |
| [9] | 许春树, 姚庆达, 梁永贤, 周华龙. 共价有机框架材料功能化策略及其对Hg(Ⅱ)和Cr(Ⅵ)的吸附性能研究进展[J]. 化工进展, 2023, 42(S1): 461-478. |
| [10] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [11] | 顾永正, 张永生. HBr改性飞灰对Hg0的动态吸附及动力学模型[J]. 化工进展, 2023, 42(S1): 498-509. |
| [12] | 赵景超, 谭明. 表面活性剂对电渗析减量化工业含盐废水的影响[J]. 化工进展, 2023, 42(S1): 529-535. |
| [13] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [14] | 郭强, 赵文凯, 肖永厚. 增强流体扰动强化变压吸附甲硫醚/氮气分离的数值模拟[J]. 化工进展, 2023, 42(S1): 64-72. |
| [15] | 王谨航, 何勇, 史伶俐, 龙臻, 梁德青. 气体水合物阻聚剂研究进展[J]. 化工进展, 2023, 42(9): 4587-4602. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||