化工进展 ›› 2025, Vol. 44 ›› Issue (S1): 222-231.DOI: 10.16085/j.issn.1000-6613.2024-2116
• 工业催化 • 上一篇
Cu/SiO2-Al2O3催化1,2-丁二醇加氢脱氧制备1-丁醇
- 河北工业大学化工学院,天津 300130
-
收稿日期:2024-12-30修回日期:2025-03-11出版日期:2025-10-25发布日期:2025-11-24 -
通讯作者:孙道来 -
作者简介:李军良(2000—),男,硕士研究生,研究方向为生物质催化转化。E-mail:707316813@qq.com。 -
基金资助:河北省自然科学基金(B2023202017)
Hydrodeoxygenation of 1,2-butanediol to 1-butanol over Cu/SiO2-Al2O3 catalyst
LI Junliang( ), LI Yue, SUN Daolai(
), LI Yue, SUN Daolai( )
)
- College of Chemical Engineering, Hebei University of Technology, Tianjin 300130, China
-
Received:2024-12-30Revised:2025-03-11Online:2025-10-25Published:2025-11-24 -
Contact:SUN Daolai
摘要:
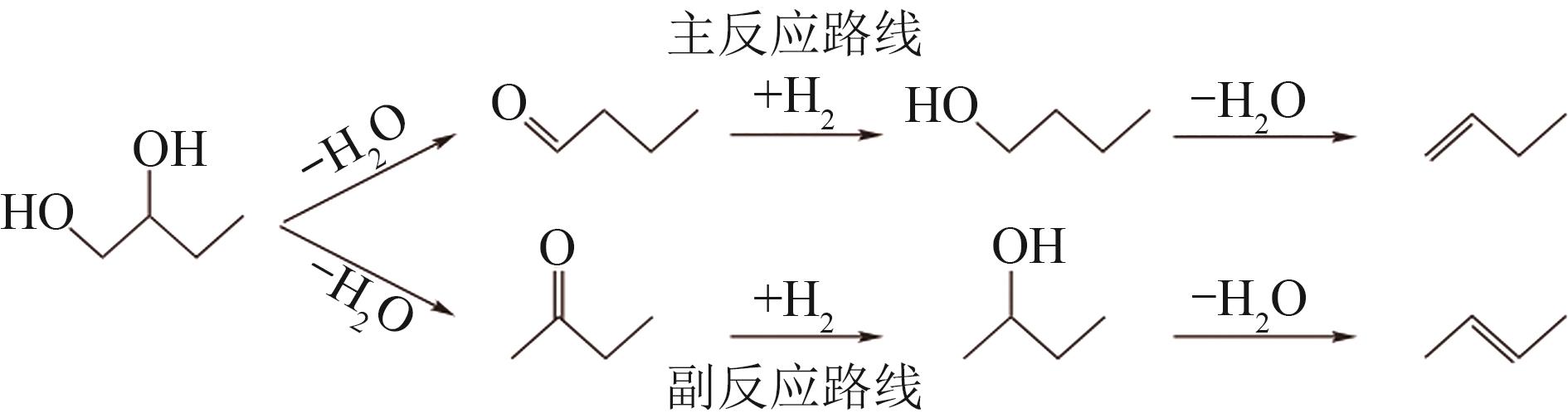
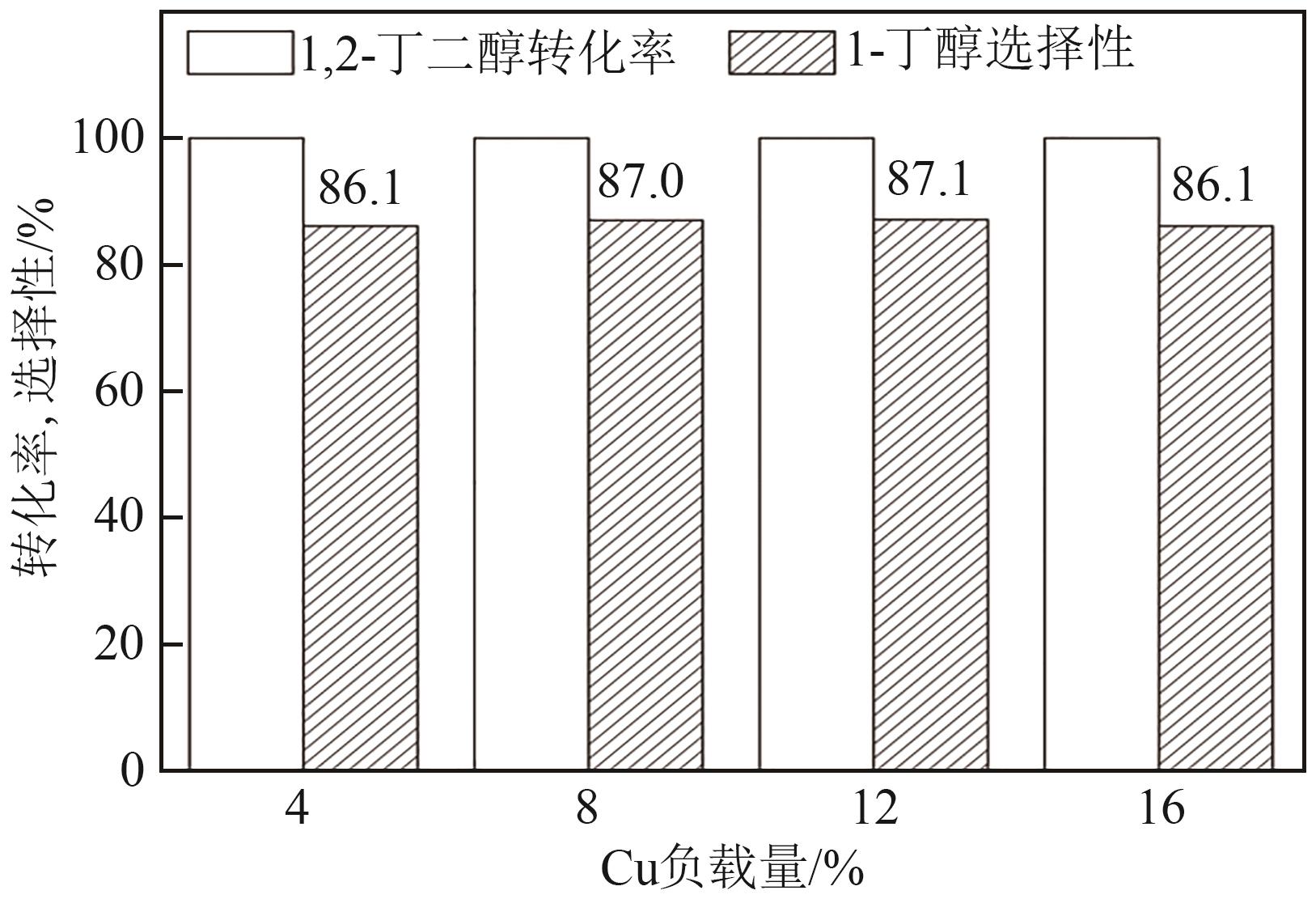
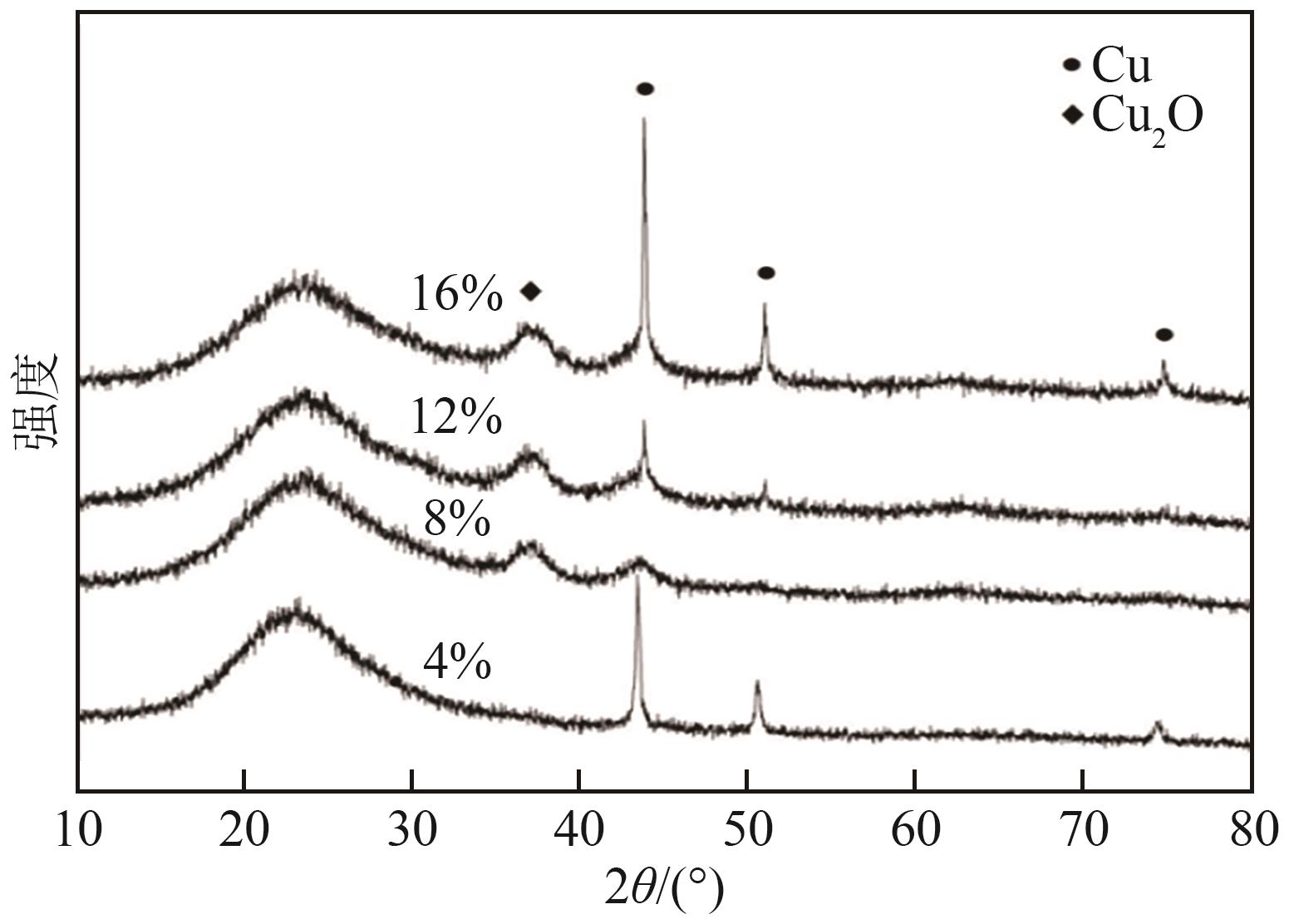
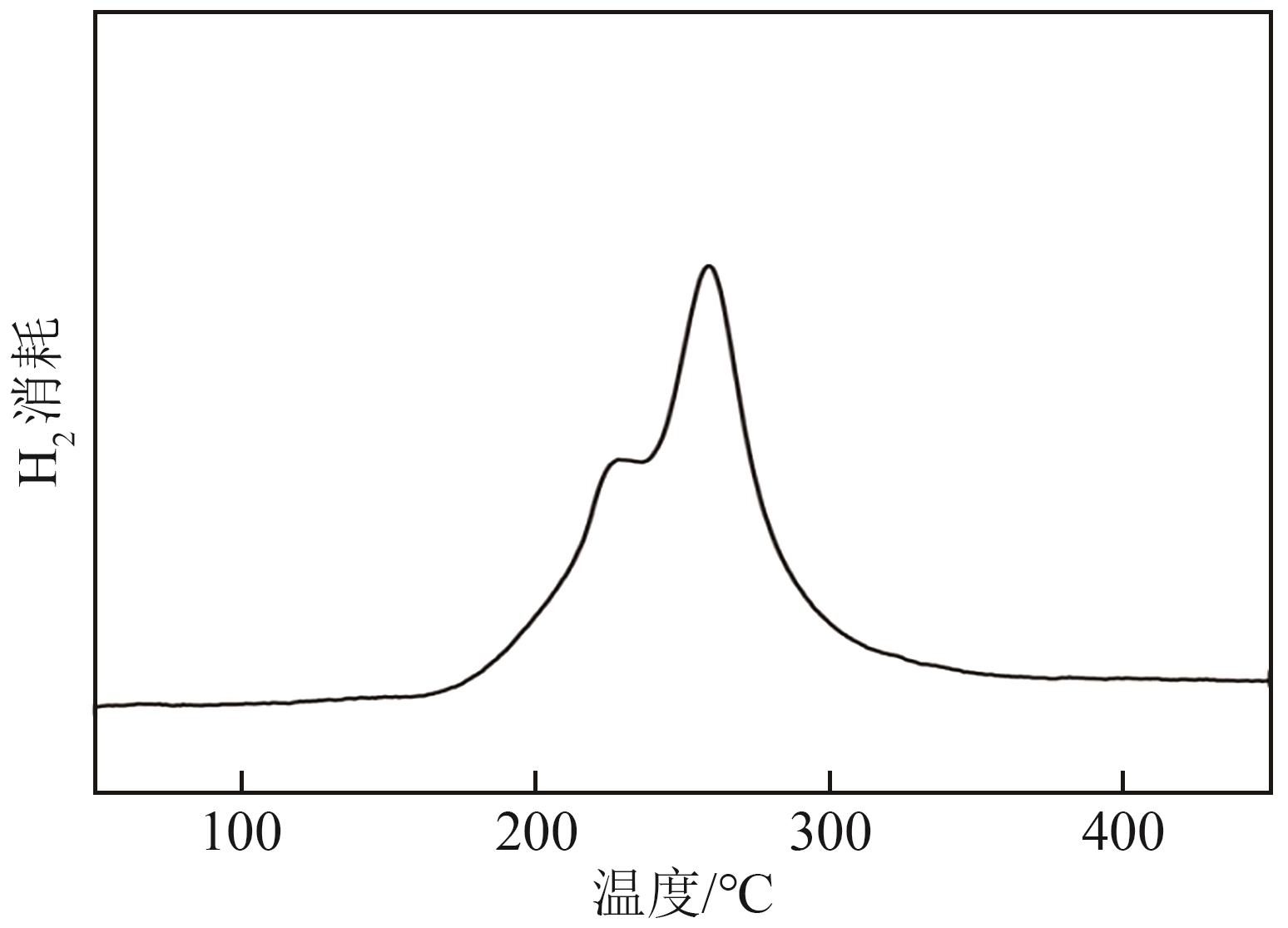
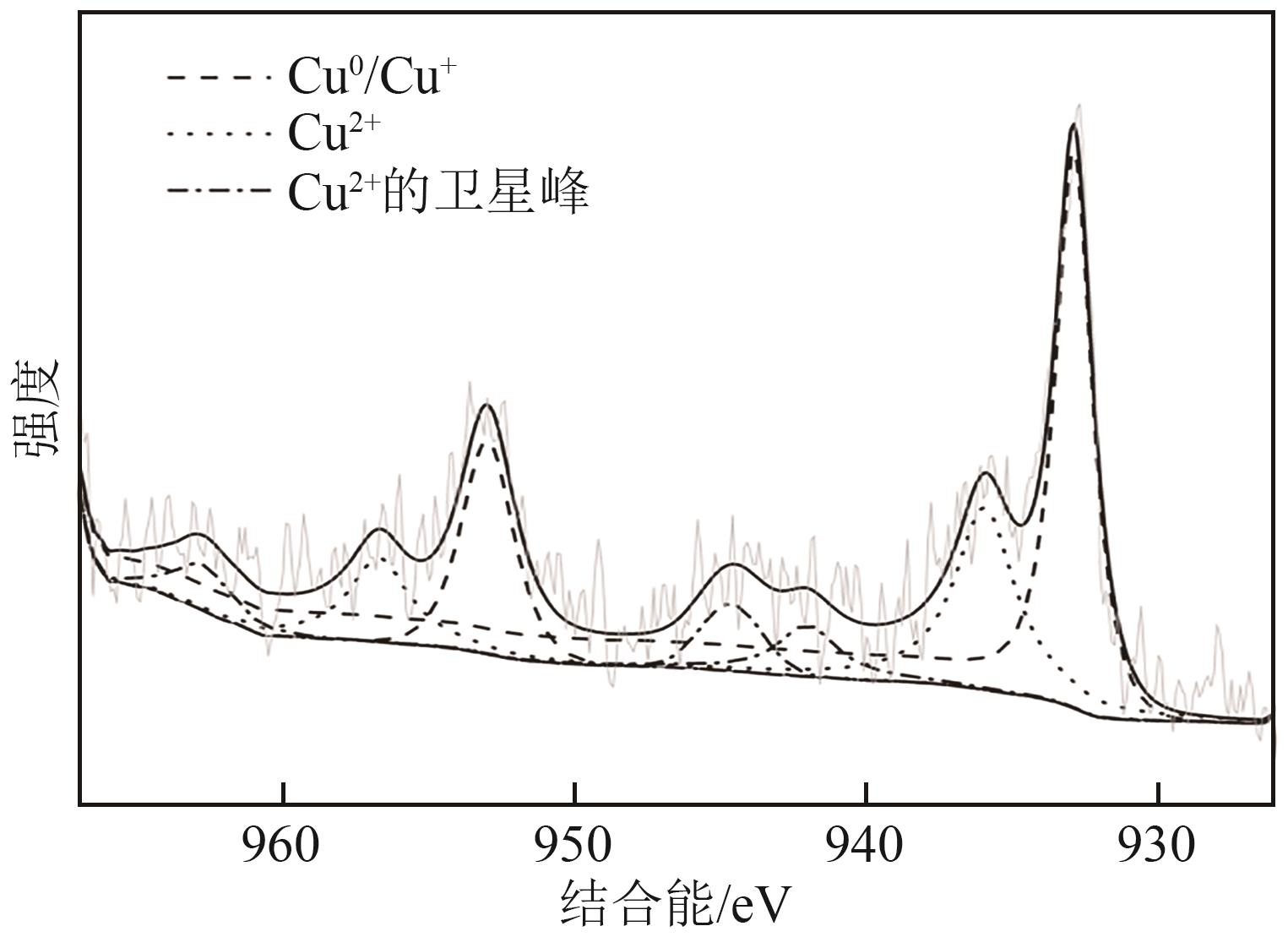
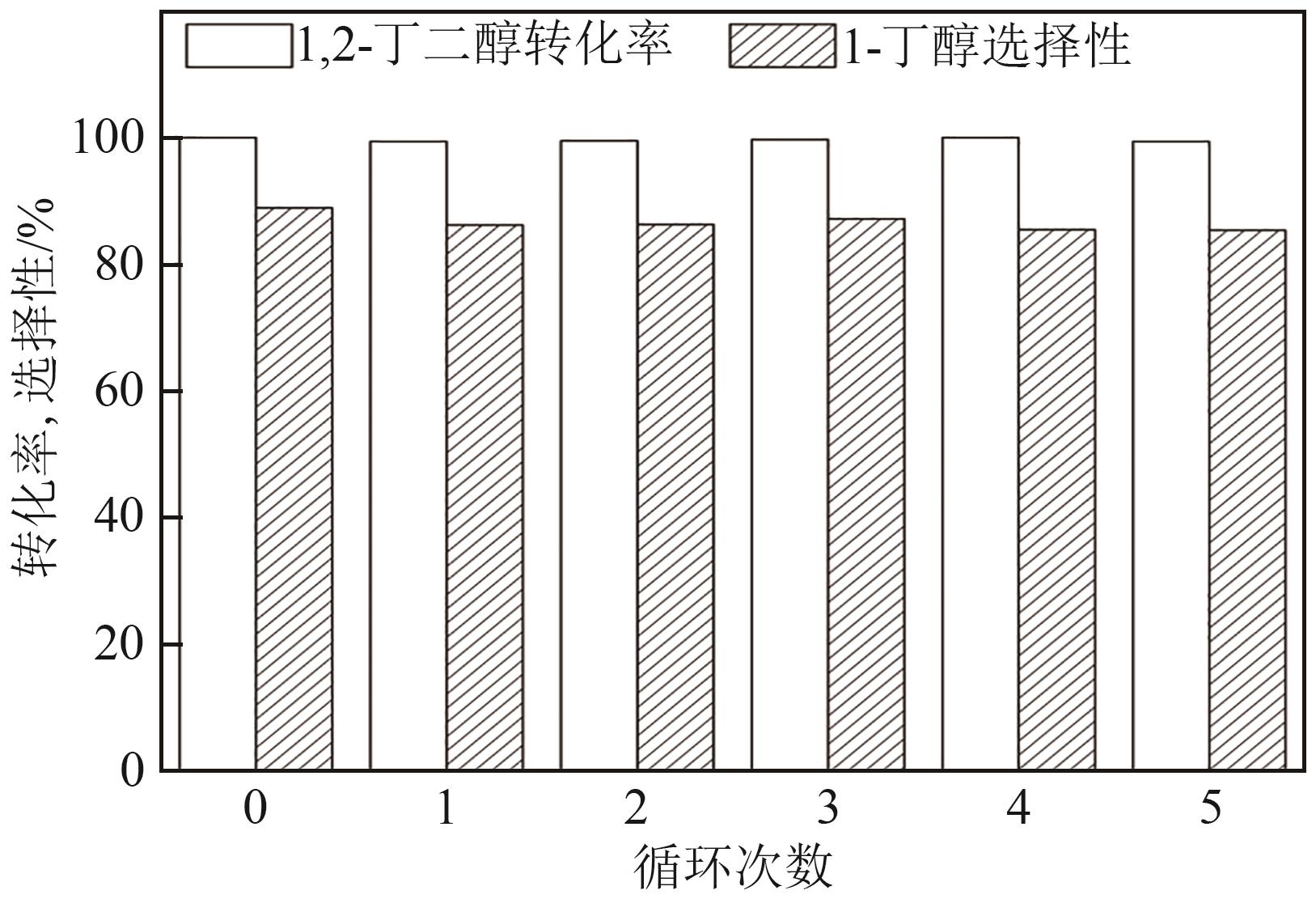
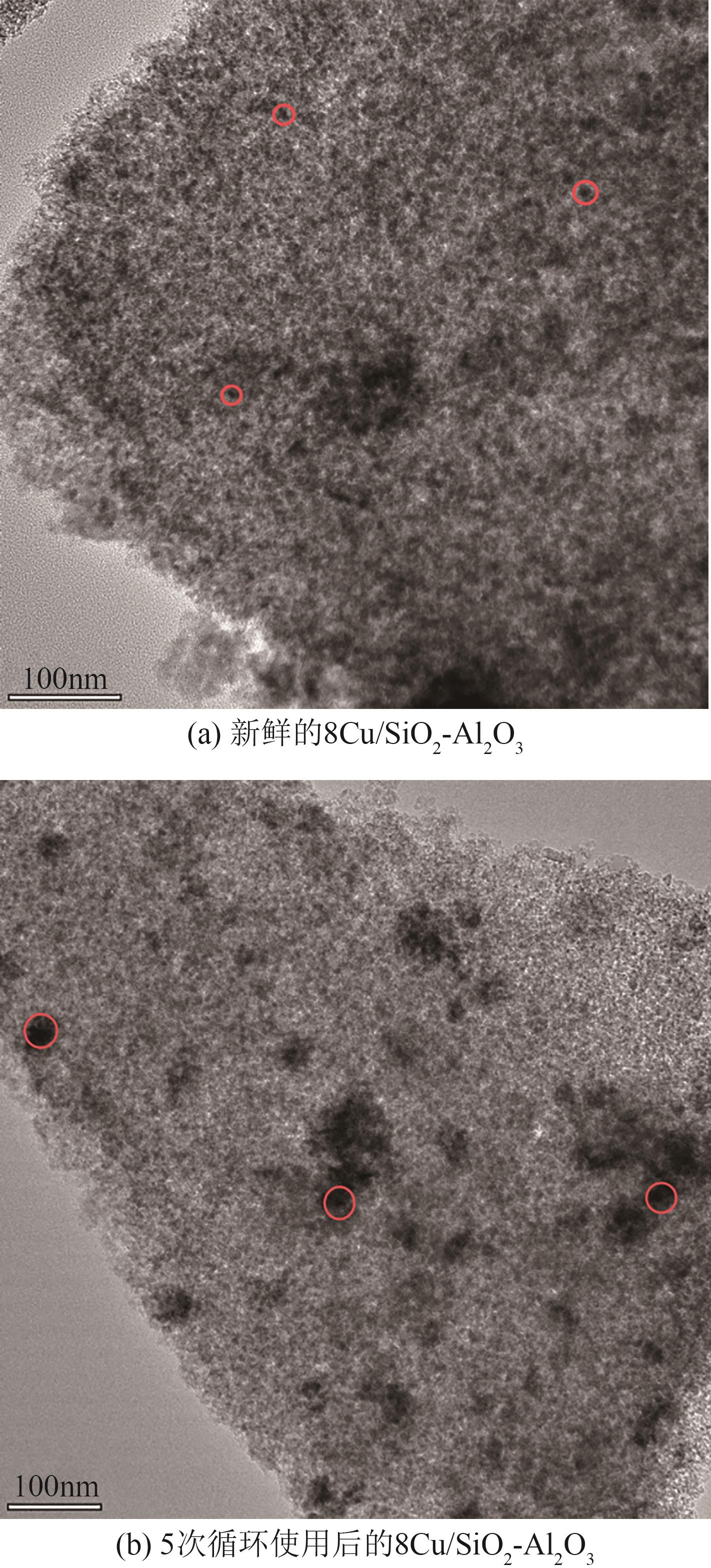
通过浸渍法制备了一系列负载型金属催化剂,并在高压反应釜中考察了催化剂对1,2-丁二醇加氢脱氧制备1-丁醇的催化活性。Cu/SiO2-Al2O3催化剂对1,2-丁二醇加氢脱氧制备1-丁醇具有较高的催化活性,可以同时获得较高的1,2-丁二醇转化率和1-丁醇选择性。在此基础上,研究了金属负载量、反应温度、反应压力、反应时间等对反应的影响。在反应温度为250℃、反应压力为5MPa的条件下,使用Cu/SiO2-Al2O3催化剂进行反应仅需15min,1,2-丁二醇的转化率便达到50.7%,1-丁醇的选择性达到75.9%。经过3h的反应后1,2-丁二醇完全转化,1-丁醇的选择性提高至88.9%。此外,Cu/SiO2-Al2O3催化剂的活性稳定,在5次循环实验中无明显失活现象。
中图分类号:
引用本文
李军良, 李悦, 孙道来. Cu/SiO2-Al2O3催化1,2-丁二醇加氢脱氧制备1-丁醇[J]. 化工进展, 2025, 44(S1): 222-231.
LI Junliang, LI Yue, SUN Daolai. Hydrodeoxygenation of 1,2-butanediol to 1-butanol over Cu/SiO2-Al2O3 catalyst[J]. Chemical Industry and Engineering Progress, 2025, 44(S1): 222-231.
| 催化剂 | 转化率/% | 选择性/% | ||||||
|---|---|---|---|---|---|---|---|---|
| 丁烯① | 丁醛 | 2-丁酮 | 四氢呋喃 | 1-丁醇 | 2-丁醇 | 其他② | ||
| 12Cu/SiO2-Al2O3 | 100.0 | 0.4 | 0.2 | 0.5 | 0.1 | 87.0 | 0.4 | 11.4 |
| 12Ni/SiO2-Al2O3 | 100.0 | 0.2 | 0.0 | 0.0 | 0.8 | 74.7 | 0.1 | 24.2 |
| 12Cu/H-Beta | 100.0 | 3.6 | 0.7 | 1.8 | 0.2 | 0.8 | 3.2 | 89.7 |
| 12Cu/MCM-41 | 100.0 | 1.7 | 1.5 | 2.3 | 0.2 | 79.6 | 0.3 | 14.4 |
| 12Cu/γ-Al2O3 | 70.8 | 0.4 | 0.2 | 0.5 | 0.1 | 35.8 | 12.4 | 50.6 |
| 12Cu/H-USY | 92.3 | 0.1 | 0.0 | 0.0 | 4.3 | 0.2 | 0.0 | 95.4 |
表1 不同金属-固体酸催化剂对1,2-丁二醇加氢脱氧制备1-丁醇反应的影响
| 催化剂 | 转化率/% | 选择性/% | ||||||
|---|---|---|---|---|---|---|---|---|
| 丁烯① | 丁醛 | 2-丁酮 | 四氢呋喃 | 1-丁醇 | 2-丁醇 | 其他② | ||
| 12Cu/SiO2-Al2O3 | 100.0 | 0.4 | 0.2 | 0.5 | 0.1 | 87.0 | 0.4 | 11.4 |
| 12Ni/SiO2-Al2O3 | 100.0 | 0.2 | 0.0 | 0.0 | 0.8 | 74.7 | 0.1 | 24.2 |
| 12Cu/H-Beta | 100.0 | 3.6 | 0.7 | 1.8 | 0.2 | 0.8 | 3.2 | 89.7 |
| 12Cu/MCM-41 | 100.0 | 1.7 | 1.5 | 2.3 | 0.2 | 79.6 | 0.3 | 14.4 |
| 12Cu/γ-Al2O3 | 70.8 | 0.4 | 0.2 | 0.5 | 0.1 | 35.8 | 12.4 | 50.6 |
| 12Cu/H-USY | 92.3 | 0.1 | 0.0 | 0.0 | 4.3 | 0.2 | 0.0 | 95.4 |
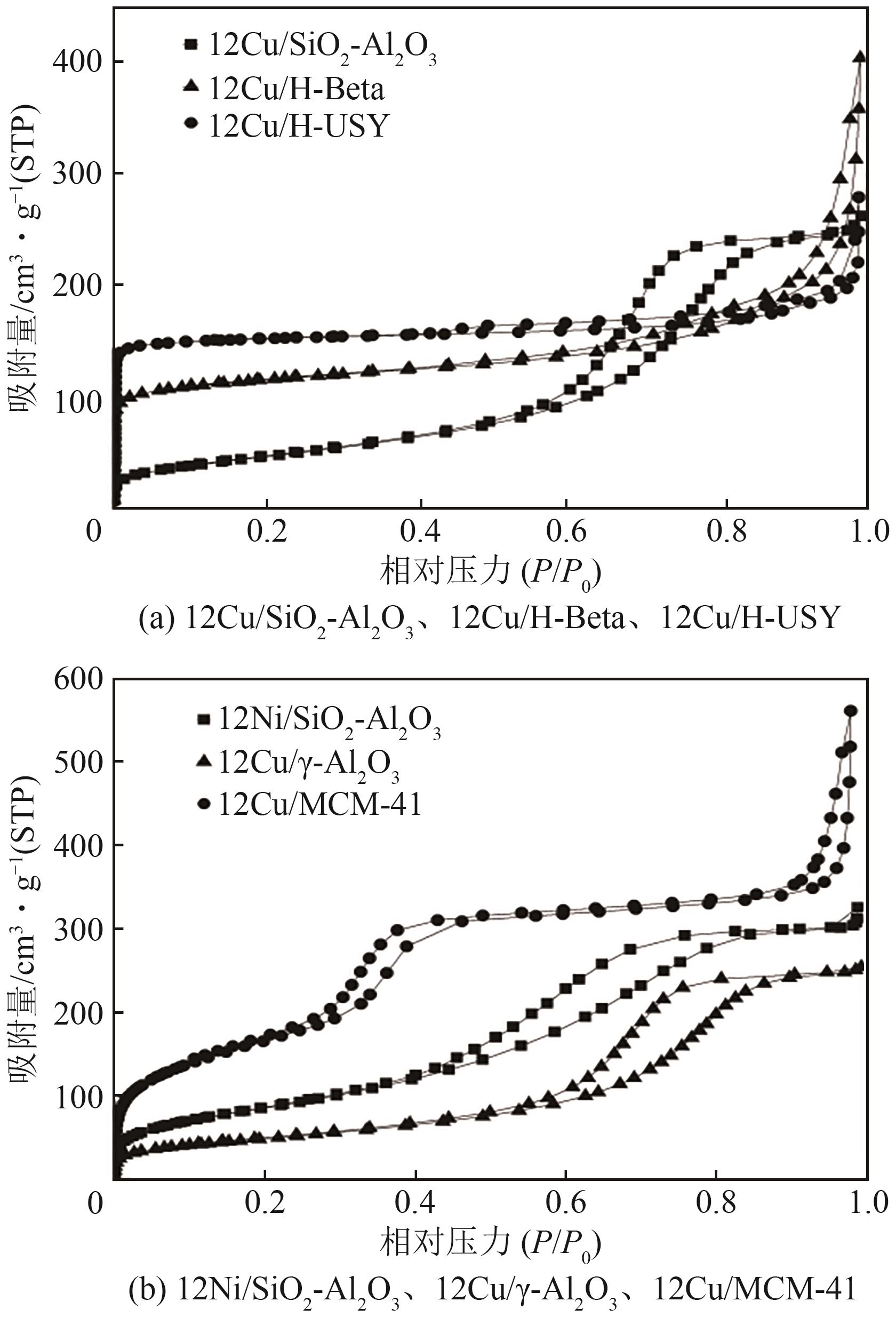
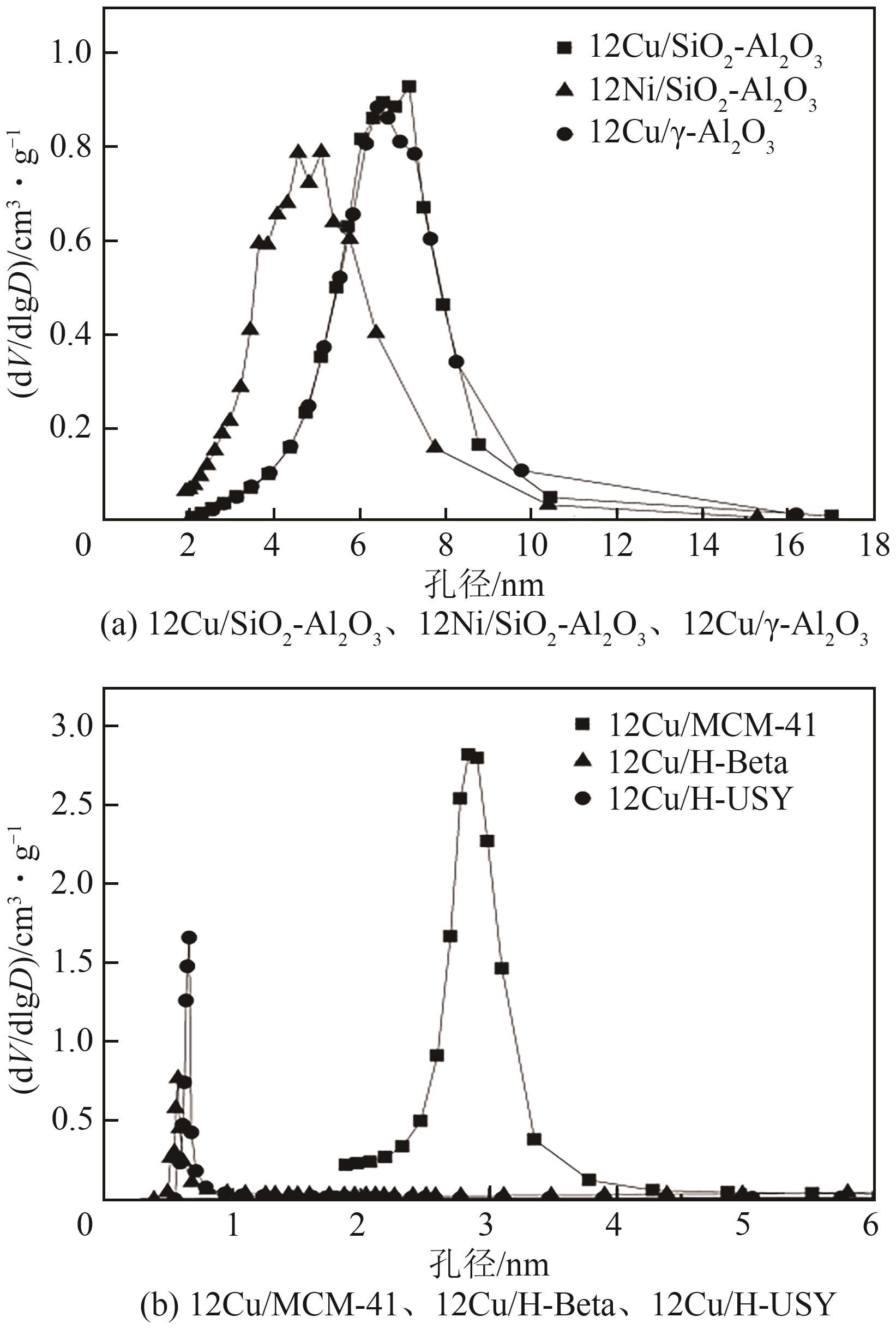
| 催化剂 | 比表面积/m2·g-1 | 孔容/cm3·g-1 | 平均孔径/nm |
|---|---|---|---|
| 12Cu/SiO2-Al2O3 | 171(450) | 0.40(0.71) | 6.2(6.3) |
| 12Ni/SiO2-Al2O3 | 319(450) | 0.50(0.71) | 6.3(6.3) |
| 12Cu/H-Beta | 360 | 0.42 | 4.4 |
| 12Cu/MCM-41 | 609 | 0.72 | 5.7 |
| 12Cu/γ-Al2O3 | 177 | 0.39 | 8.8 |
| 12Cu/H-USY | 457 | 0.34 | 2.8 |
表2 不同催化剂的织构性质
| 催化剂 | 比表面积/m2·g-1 | 孔容/cm3·g-1 | 平均孔径/nm |
|---|---|---|---|
| 12Cu/SiO2-Al2O3 | 171(450) | 0.40(0.71) | 6.2(6.3) |
| 12Ni/SiO2-Al2O3 | 319(450) | 0.50(0.71) | 6.3(6.3) |
| 12Cu/H-Beta | 360 | 0.42 | 4.4 |
| 12Cu/MCM-41 | 609 | 0.72 | 5.7 |
| 12Cu/γ-Al2O3 | 177 | 0.39 | 8.8 |
| 12Cu/H-USY | 457 | 0.34 | 2.8 |
| 反应时间 | 转化率/% | 选择性/% | ||||||
|---|---|---|---|---|---|---|---|---|
| 丁烯① | 丁醛 | 2-丁酮 | 四氢呋喃 | 1-丁醇 | 2-丁醇 | 其他② | ||
| 5min | 9.3 | 0.7 | 0.3 | 0.9 | 0.2 | 58.4 | 2.8 | 36.7 |
| 15min | 50.7 | 0.5 | 0.5 | 0.9 | 0.3 | 75.9 | 3.1 | 17.7 |
| 30min | 65.3 | 0.5 | 0.5 | 1.0 | 0.2 | 78.8 | 2.5 | 16.5 |
| 1h | 74.2 | 0.6 | 0.4 | 1.2 | 0.2 | 81.6 | 2.1 | 14.0 |
| 2h | 100.0 | 2.0 | 0.4 | 0.8 | 0.1 | 88.3 | 0.5 | 9.9 |
| 3h | 100.0 | 0.8 | 0.3 | 0.7 | 0.1 | 88.9 | 0.4 | 8.8 |
| 4h | 100.0 | 0.7 | 0.3 | 0.6 | 0.1 | 87.1 | 0.5 | 10.7 |
| 8h | 100.0 | 3.4 | 0.3 | 0.7 | 0.2 | 79.0 | 0.4 | 16.3 |
表3 反应时间对8Cu/SiO2-Al2O3催化1,2-丁二醇加氢脱氧制备1-丁醇反应的影响
| 反应时间 | 转化率/% | 选择性/% | ||||||
|---|---|---|---|---|---|---|---|---|
| 丁烯① | 丁醛 | 2-丁酮 | 四氢呋喃 | 1-丁醇 | 2-丁醇 | 其他② | ||
| 5min | 9.3 | 0.7 | 0.3 | 0.9 | 0.2 | 58.4 | 2.8 | 36.7 |
| 15min | 50.7 | 0.5 | 0.5 | 0.9 | 0.3 | 75.9 | 3.1 | 17.7 |
| 30min | 65.3 | 0.5 | 0.5 | 1.0 | 0.2 | 78.8 | 2.5 | 16.5 |
| 1h | 74.2 | 0.6 | 0.4 | 1.2 | 0.2 | 81.6 | 2.1 | 14.0 |
| 2h | 100.0 | 2.0 | 0.4 | 0.8 | 0.1 | 88.3 | 0.5 | 9.9 |
| 3h | 100.0 | 0.8 | 0.3 | 0.7 | 0.1 | 88.9 | 0.4 | 8.8 |
| 4h | 100.0 | 0.7 | 0.3 | 0.6 | 0.1 | 87.1 | 0.5 | 10.7 |
| 8h | 100.0 | 3.4 | 0.3 | 0.7 | 0.2 | 79.0 | 0.4 | 16.3 |
| 反应压力/MPa | 转化率/% | 选择性/% | ||||||
|---|---|---|---|---|---|---|---|---|
| 丁烯① | 丁醛 | 2-丁酮 | 四氢呋喃 | 1-丁醇 | 2-丁醇 | 其他② | ||
| 4 | 100.0 | 1.1 | 0.7 | 1.4 | 0.1 | 72.1 | 1.0 | 23.6 |
| 5 | 100.0 | 0.8 | 0.3 | 0.7 | 0.1 | 88.9 | 0.4 | 8.8 |
| 6 | 100.0 | 1.2 | 0.2 | 0.5 | 0.1 | 86.1 | 1.0 | 10.9 |
| 7 | 100.0 | 1.4 | 0.3 | 0.4 | 0.1 | 85.3 | 0.7 | 11.8 |
表4 反应压力对8Cu/SiO2-Al2O3催化1,2-丁二醇加氢脱氧制备1-丁醇反应的影响
| 反应压力/MPa | 转化率/% | 选择性/% | ||||||
|---|---|---|---|---|---|---|---|---|
| 丁烯① | 丁醛 | 2-丁酮 | 四氢呋喃 | 1-丁醇 | 2-丁醇 | 其他② | ||
| 4 | 100.0 | 1.1 | 0.7 | 1.4 | 0.1 | 72.1 | 1.0 | 23.6 |
| 5 | 100.0 | 0.8 | 0.3 | 0.7 | 0.1 | 88.9 | 0.4 | 8.8 |
| 6 | 100.0 | 1.2 | 0.2 | 0.5 | 0.1 | 86.1 | 1.0 | 10.9 |
| 7 | 100.0 | 1.4 | 0.3 | 0.4 | 0.1 | 85.3 | 0.7 | 11.8 |
| 反应温度/℃ | 转化率/% | 选择性/% | ||||||
|---|---|---|---|---|---|---|---|---|
| 丁烯① | 丁醛 | 2-丁酮 | 四氢呋喃 | 1-丁醇 | 2-丁醇 | 其他② | ||
| 200 | 16.6 | 0.0 | 0.1 | 0.3 | 0.0 | 70.3 | 3.8 | 25.5 |
| 230 | 72.5 | 0.4 | 0.2 | 0.4 | 0.0 | 82.0 | 3.0 | 14.0 |
| 250 | 100.0 | 0.8 | 0.3 | 0.7 | 0.1 | 88.9 | 0.4 | 8.8 |
| 280 | 100.0 | 5.0 | 0.9 | 1.7 | 0.6 | 49.6 | 0.5 | 41.7 |
表5 反应温度对8Cu/SiO2-Al2O3催化1,2-丁二醇加氢脱氧制备1-丁醇反应的影响
| 反应温度/℃ | 转化率/% | 选择性/% | ||||||
|---|---|---|---|---|---|---|---|---|
| 丁烯① | 丁醛 | 2-丁酮 | 四氢呋喃 | 1-丁醇 | 2-丁醇 | 其他② | ||
| 200 | 16.6 | 0.0 | 0.1 | 0.3 | 0.0 | 70.3 | 3.8 | 25.5 |
| 230 | 72.5 | 0.4 | 0.2 | 0.4 | 0.0 | 82.0 | 3.0 | 14.0 |
| 250 | 100.0 | 0.8 | 0.3 | 0.7 | 0.1 | 88.9 | 0.4 | 8.8 |
| 280 | 100.0 | 5.0 | 0.9 | 1.7 | 0.6 | 49.6 | 0.5 | 41.7 |
| [1] | WU Jing, LIU Hongjuan, YAN Xiang, et al. Efficient catalytic dehydration of high-Concentration 1-butanol with Zn-Mn-Co modified γ-Al2O3 in jet fuel production[J]. Catalysts, 2019, 9(1): 93. |
| [2] | PÉREZ-MACIÁ María Ángeles, Roger BRINGUÉ, IBORRA Montserrat, et al. Thermodynamic equilibrium for the dehydration of 1-butanol to di-n-butyl ether[J]. Chemical Engineering Research and Design, 2015, 102: 186-195. |
| [3] | 付友思, 吴又多, 陈丽杰. Zn2+、Ca2+和Mn2+对丙酮丁醇发酵的协同影响[J]. 化工进展, 2015, 34(10): 3719-3724. |
| FU Yousi, WU Youduo, CHEN Lijie. Synergistic effect of Zn2+, Ca2+, and Mn2+ on acetone-butanol fermentation[J]. Chemical Industry and Engineering Progress, 2015, 34(10): 3719-3724. | |
| [4] | KELLA Tatinaidu, VENNATHAN Anjana Anandan, DUTTA Saikat, et al. Selective dehydration of 1-butanol to butenes over silica supported heteropolyacid catalysts: Mechanistic aspect[J]. Molecular Catalysis, 2021, 516: 111975. |
| [5] | 柯啸宇. 邻苯二甲酸二丁酯的合成工艺: CN103030563A[P]. 2013-04-10. |
| KE Xiaoyu. Synthesis process of di-n-butyl phthalate (DBP): CN103030563A[P]. 2013-04-10. | |
| [6] | PALLA Venkata Chandra Sekhar, SHEE Debaprasad, MAITY Sunil K. Conversion of n-butanol to gasoline range hydrocarbons, butylenes and aromatics[J]. Applied Catalysis A: General, 2016, 526: 28-36. |
| [7] | SU Jiantao, YANG Xiaohui, SHI Hui, et al. Heteropolyacid promoted lignin-MOF derived spherical catalyst for catalytic hydrogen transfer of 5-hydroxymethylfurfural[J]. Journal of Colloid and Interface Science, 2024, 669: 336-348. |
| [8] | CONESA J M, MORALES M V, GARCÍA-BOSCH N, et al. Graphite supported heteropolyacid as a regenerable catalyst in the dehydration of 1-butanol to butenes[J]. Catalysis Today, 2023, 420: 114017. |
| [9] | JOHN Mathew, ALEXOPOULOS Konstantinos, REYNIERS Marie-Francoise, et al. First-principles kinetic study on the effect of the zeolite framework on 1-butanol dehydration[J]. ACS Catalysis, 2016, 6(7): 4081-4094. |
| [10] | 叶梅芳, 魏怡林, 刘媛媛, 等. 基于HPLC指纹图谱与多成分含量测定的凹叶景天正丁醇部位差异性研究[J]. 医药导报, 2023, 42(6): 904-911. |
| YE Meifang, WEI Yilin, LIU Yuanyuan, et al. Study on the regional differences of Sedum aizoon based on HPLC fingerprint and multi-component content determination of n-butanol extracts[J]. Herald of Medicine, 2023, 42(6): 904-911. | |
| [11] | TOMISHIGE Keiichi, FURIKADO Ippei, YAMAGISHI Takashi, et al. Promoting effect of Mo on alcohol formation in hydroformylation of propylene and ethylene on Mo-Rh/SiO2 [J]. Catalysis Letters, 2005, 103(1): 15-21. |
| [12] | Shuhei OGO, ONDA Ayumu, YANAGISAWA Kazumichi. Selective synthesis of 1-butanol from ethanol over strontium phosphate hydroxyapatite catalysts[J]. Applied Catalysis A: General, 2011, 402(1/2): 188-195. |
| [13] | OSMANBEGOVIC Nahla, CHANDGUDE Vijaya, BANKAR Sandip, et al. 1-Butanol separation from aqueous acetone-butanol-ethanol(ABE) solutions by freeze concentration[J]. Crystal Growth & Design, 2023, 23(6): 4147-4153. |
| [14] | CUI Yuyang, LI Shuaiqi, AN Hualiang, et al. Improvement of ethanol guerbet condensation by acetal hydrolysis[J]. Industrial & Engineering Chemistry Research, 2022, 61(34): 12392-12404. |
| [15] | SUN Daolai, YAMADA Yasuhiro, SATO Satoshi, et al. Production of aldehydes from 1,2-alkanediols over silica-supported WO3 catalyst[J]. Applied Catalysis A: General, 2016, 526: 164-171. |
| [16] | RAJU Suresh, MORET Marc‐Etienne, KLEIN GEBBINK Robertus J M . et al. ChemInform abstract: Rhenium-catalyzed dehydration and deoxydehydration of alcohols and polyols: Opportunities for the formation of olefins from biomass[J]. ChemInform, 2015, 46(11): 281-300. |
| [17] | ZHANG Liangqing, HUANG Suchang, QIU Jiarong, et al. Selective transformation of biomass-derived substrates to 1,2-butanediol: A comprehensive review and new insights[J]. Industrial Crops and Products, 2023, 202: 116984. |
| [18] | 练彩霞, 李凝, 蒋武, 等. 生物质油催化加氢脱氧(HDO)反应机理及催化剂研究进展[J]. 化工进展, 2020, 39(S1): 153-162. |
| LIAN Caixia, LI Ning, JIANG Wu, et al. Research progress on reaction mechanism and catalysts for catalytic hydrodeoxygenation(HDO) of biomass oil[J]. Chemical Industry and Engineering Progress, 2020, 39(S1): 153-162. | |
| [19] | LIU Ben, NAKAGAWA Yoshinao, LI Congcong, et al. Selective C—O hydrogenolysis of terminal C—OH bond in 1,2-diols over rutile-titania-supported iridium-iron catalysts[J]. ACS Catalysis, 2022, 12(24): 15431-15450. |
| [20] | CHIA Mei, PAGÁN-TORRES Yomaira J, HIBBITTS David, et al. Selective hydrogenolysis of polyols and cyclic ethers over bifunctional surface sites on rhodium-rhenium catalysts[J]. Journal of the American Chemical Society, 2011, 133(32): 12675-12689. |
| [21] | 李帅哲, 聂懿宸, PHIDSAVARD Keomeesay, 等. 非贵金属催化生物质加氢脱氧制备烃基生物燃料的研究进展[J]. 化工进展, 2024, 43(S1): 225-242. |
| LI Shuaizhe, NIE Yichen, PHIDSAVARD Keomeesay, et al. Research progress on non-precious metal-catalyzed hydrogenation and deoxygenation of biomass to produce hydrocarbon-based biofuels[J]. Chemical Industry and Engineering Progress, 2024, 43(S1): 225-242. | |
| [22] | TOMISHIGE Keiichi, TAMURA Masazumi, NAKAGAWA Yoshinao. Role of Re species and acid cocatalyst on Ir-ReO x /SiO2 in the C—O hydrogenolysis of biomass-derived substrates[J]. The Chemical Record, 2014, 14(6): 1041-1054. |
| [23] | AMADA Yasushi, KOSO Shuichi, NAKAGAWA Yoshinao, et al. Hydrogenolysis of 1,2-propanediol for the production of biopropanols from glycerol[J]. ChemSusChem, 2010, 3(6): 728-736. |
| [24] | SRIFA Atthapon, Nawin VIRIYA-EMPIKUL, ASSABUMRUNGRAT Suttichai, et al. Catalytic behaviors of Ni/γ-Al2O3 and Co/γ-Al2O3 during the hydrodeoxygenation of palm oil[J]. Catalysis Science & Technology, 2015, 5(7): 3693-3705. |
| [25] | UEDA Naoyuki, NAKAGAWA Yoshinao, TOMISHIGE Keiichi. Conversion of glycerol to ethylene glycol over Pt-modified Ni catalyst[J]. Chemistry Letters, 2010, 39(5): 506-507. |
| [26] | HAO Fang, ZHONG Jun, LIU Pingle, et al. Preparation of mesoporous SiO2-Al2O3 supported Co or Mn catalysts and their catalytic properties in cyclohexane nitrosation to ɛ-caprolactam[J]. Journal of Molecular Catalysis A: Chemical, 2011, 351: 210-216. |
| [27] | LI Chunyu, WU Haihong, CEN Ziyu, et al. Conversion of polyethylene to gasoline: Influence of porosity and acidity of zeolites[J]. Frontiers in Energy, 2023, 17(6): 763-774. |
| [28] | SUN Daolai, MISU Takuya, YAMADA Yasuhiro, et al. Advantages of using Cu/SiO2 catalyst for vapor-phase dehydrogenation of 1-decanol into decanal[J]. Applied Catalysis A: General, 2019, 582: 117109. |
| [29] | 何迈, 方萍, 谢冠群, 等. CuO/CeO2-Al2O3催化剂中CuO物种的原位XRD、Raman和TPR表征[J]. 物理化学学报, 2005, 21(9): 997-1000. |
| HE Mai, FANG Ping, XIE Guanqun, et al. In situ XRD, Raman and TPR characterization of CuO species in CuO/CeO2-Al2O3 catalysts[J]. Acta Physico-Chimica Sinica, 2005, 21(9): 997-1000. | |
| [30] | SUN Daolai, SAITO Takeshi, YAMADA Yasuhiro, et al. Hydrogenation of γ-valerolactone to 1,4-pentanediol in a continuous flow reactor[J]. Applied Catalysis A: General, 2017, 542: 289-295. |
| [31] | CHEN Shin-Fu, YEH Chen-Sheng. Influence of alkanethiols adsorption on oxidized copper nanoparticles[J]. Journal of the Chinese Chemical Society, 2002, 49(5): 895-898. |
| [32] | GHODSELAHI T, VESAGHI M A, SHAFIEKHANI A, et al. XPS study of the Cu@Cu2O core-shell nanoparticles[J]. Applied Surface Science, 2008, 255(5): 2730-2734. |
| [33] | ZHAO Binran, LIU Yajun, ZHU Zijun, et al. Highly selective conversion of CO2 into ethanol on Cu/ZnO/Al2O3 catalyst with the assistance of plasma[J]. Journal of CO2 Utilization, 2018, 24: 34-39. |
| [1] | 刘哲, 周顺利, 李永祥, 张成喜, 刘宜鹏. 烷基萘合成催化剂研究进展[J]. 化工进展, 2025, 44(S1): 144-158. |
| [2] | 林已杰, 乔鹏, 李心睿, 张宏斌, 王雪芹. TiO2纳米光催化剂的异质结构建策略与应用研究进展[J]. 化工进展, 2025, 44(S1): 159-177. |
| [3] | 王涛, 张雪冰, 张琪, 陈强, 张魁, 门卓武. 还原碳化温度和CO浓度对工业级费托合成沉淀铁催化剂性能的影响[J]. 化工进展, 2025, 44(S1): 178-184. |
| [4] | 包新德, 刘必烨, 黄仁伟, 洪宇豪, 关鑫, 林金国. 生物质基@CuNiOS复合催化剂的制备及其在有机染料还原中的应用[J]. 化工进展, 2025, 44(S1): 185-196. |
| [5] | 赵思阳, 李陈冉, 刘洋. 副产C4预积炭调控MTO再生催化剂双烯选择性的工艺优化[J]. 化工进展, 2025, 44(S1): 205-212. |
| [6] | 赵雨龙, 蔡凯, 于善青. 氧化铝孔结构对催化裂化烃类分子吸附扩散及反应性能的影响[J]. 化工进展, 2025, 44(S1): 213-221. |
| [7] | 蒋春喜, 林定标, 卞耀, 周威, 陆海峰, 郭晓镭, 刘海峰. 用于气流床气化的稻壳原料特性及其影响[J]. 化工进展, 2025, 44(9): 4937-4944. |
| [8] | 陈子朝, 何方书, 胡强, 杨扬, 陈汉平, 杨海平. 甲烷干重整抗积炭Ni基催化剂研究进展[J]. 化工进展, 2025, 44(9): 4968-4978. |
| [9] | 王振, 张耀远, 吴芹, 史大昕, 陈康成, 黎汉生. 甲烷干重整用Ni/Al2O3基催化剂研究进展[J]. 化工进展, 2025, 44(9): 4979-4998. |
| [10] | 张海鹏, 秦珊珊, 王俣萱, 于海彪. 3.0F-Ag x Co催化剂的制备及其催化分解N2O[J]. 化工进展, 2025, 44(9): 4999-5005. |
| [11] | 张文静, 黄致新, 李士腾, 邓帅, 李双俊. 生物质碳气凝胶CO2吸附剂研究进展[J]. 化工进展, 2025, 44(9): 5018-5032. |
| [12] | 孙梦圆, 陆诗建, 刘玲, 薛艳阳, 张云蓉, 董琦, 康国俊. 金属有机框架及衍生物在碳捕集领域的研究进展[J]. 化工进展, 2025, 44(9): 5339-5350. |
| [13] | 王文君, 刘瑞鑫, 王军, 张庆磊, 侯立安. 浅析二氧化钛材料可见光降解室内VOCs的研究进展[J]. 化工进展, 2025, 44(9): 5351-5362. |
| [14] | 曾金, 高艳, 王赵鹏, 谢雨芸, 刘俊, 梁旗, 王春英. NaYF4:Yb,Tm复合TiO2/Bi2WO6光催化降解2,4-二氯苯氧乙酸机制及产物毒性评价[J]. 化工进展, 2025, 44(9): 5416-5431. |
| [15] | 赵用明, 卜亿峰, 王涛, 杜冰, 门卓武. 费托合成催化剂动态置换与稳态工艺的集成优化[J]. 化工进展, 2025, 44(8): 4536-4544. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||