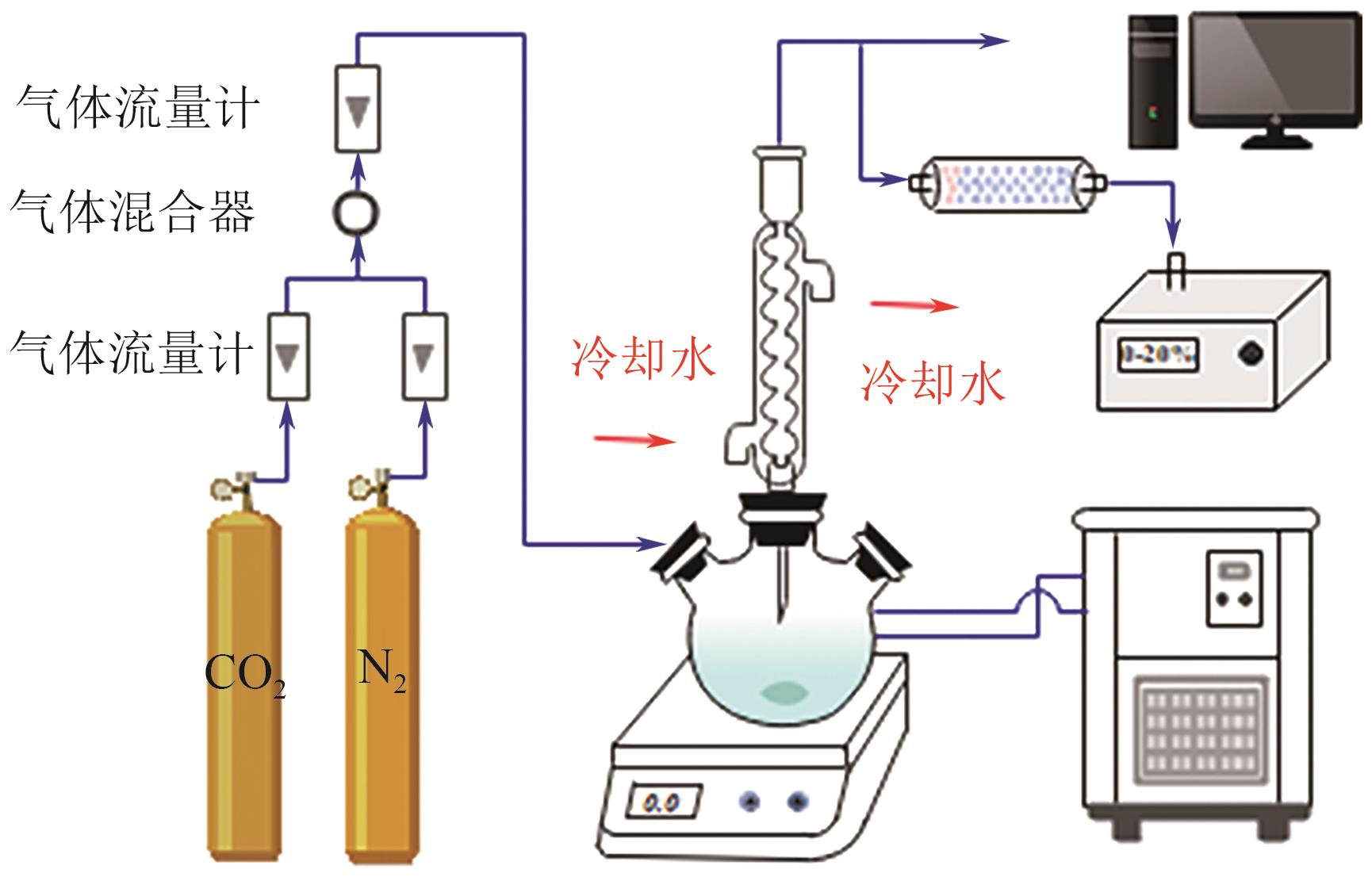
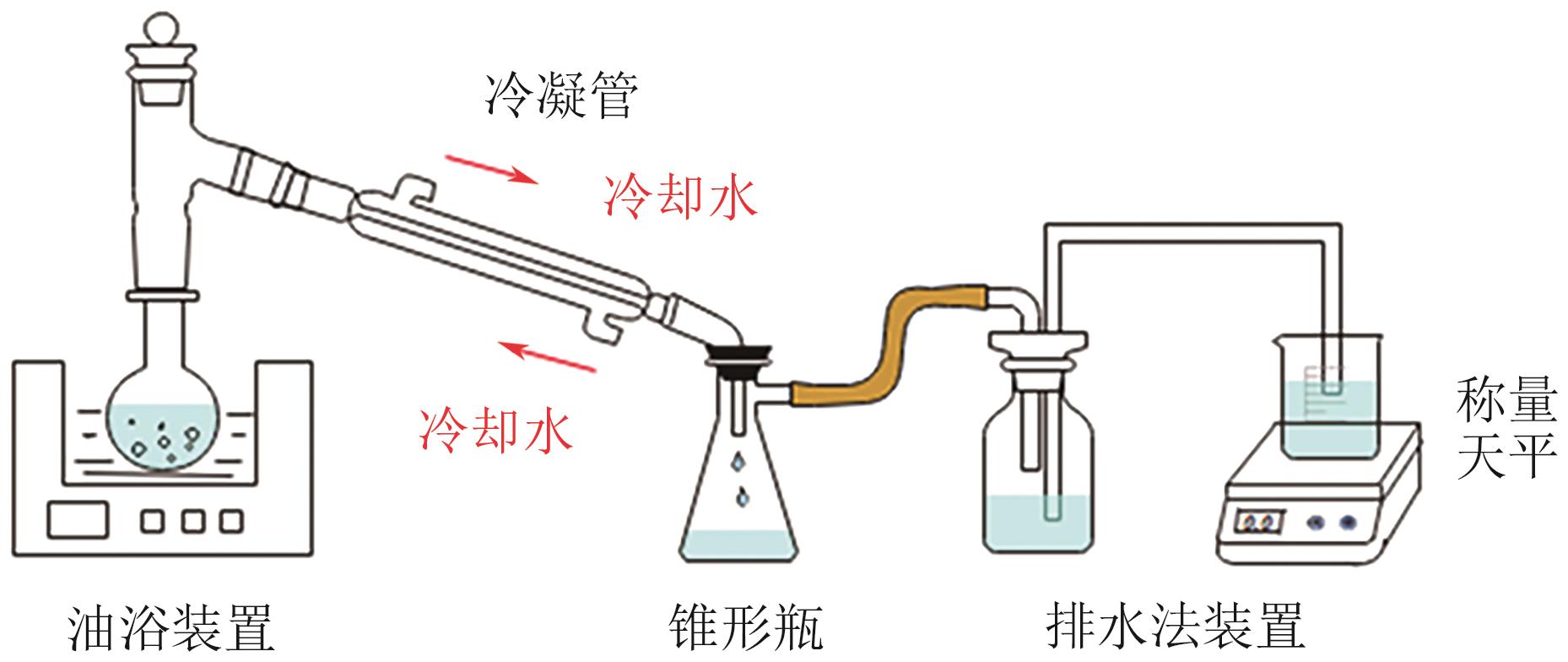
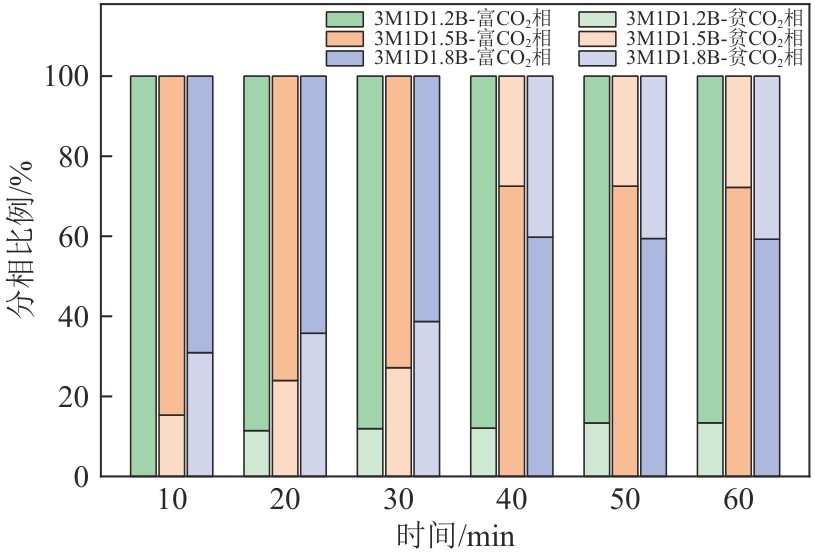
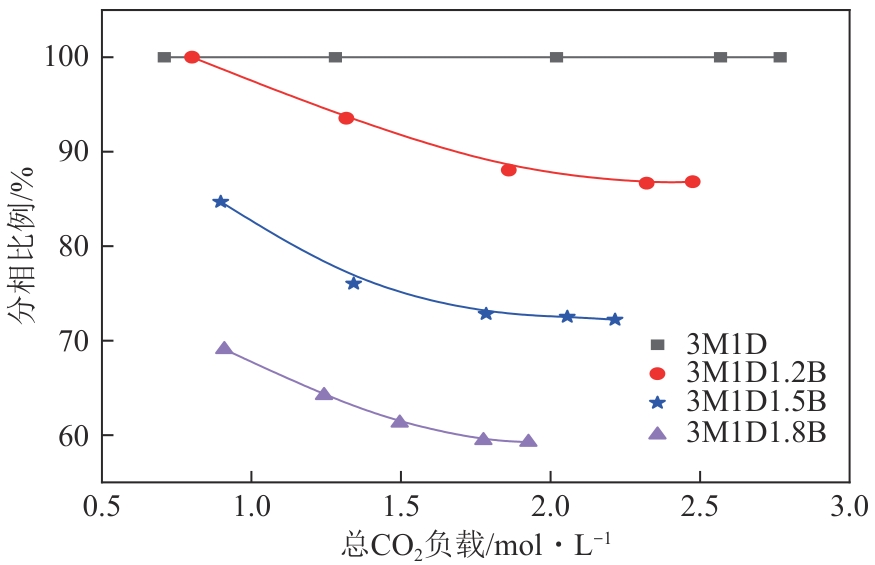
| [1] |
REICHSTEIN Markus, BAHN Michael, CIAIS Philippe, et al. Climate extremes and the carbon cycle[J]. Nature, 2013, 500(7462): 287-295.
|
| [2] |
ZHANG Xiaowen, HUANG Yufei, YANG Jian, et al. Amine-based CO2 capture aided by acid-basic bifunctional catalyst: Advancement of amine regeneration using metal modified MCM-41[J]. Chemical Engineering Journal, 2020, 383: 123077.
|
| [3] |
GOTO Kazuya, YOGO Katsunori, HIGASHII Takayuki. A review of efficiency penalty in a coal-fired power plant with post-combustion CO2 capture[J]. Applied Energy, 2013, 111: 710-720.
|
| [4] |
RAO Anand B, RUBIN Edward S. A technical, economic, and environmental assessment of amine-based CO2 capture technology for power plant greenhouse gas control[J]. Environmental Science & Technology, 2002, 36(20): 4467-4475.
|
| [5] |
Juan LYU, LIU Sen, LING Hao, et al. Development of a promising biphasic absorbent for postcombustion CO2 capture: Sulfolane + 2-(methylamino)ethanol + H2O[J]. Industrial & Engineering Chemistry Research, 2020, 59(32): 14496-14506.
|
| [6] |
ZHANG Shihan, SHEN Yao, WANG Lidong, et al. Phase change solvents for post-combustion CO2 capture: Principle, advances, and challenges[J]. Applied Energy, 2019, 239: 876-897.
|
| [7] |
BAI Liju, LU Shijian, ZHAO Qizheng, et al. Low-energy-consuming CO2 capture by liquid-liquid biphasic absorbents of EMEA/DEEA/PX[J]. Chemical Engineering Journal, 2022, 450: 138490.
|
| [8] |
ZHANG Shihan, SHEN Yao, SHAO Peijing, et al. Kinetics, thermodynamics, and mechanism of a novel biphasic solvent for CO2 capture from flue gas[J]. Environmental Science & Technology, 2018, 52(6): 3660-3668.
|
| [9] |
JIA Ruiqi, XU Yihang, ZHANG Jiaojiao, et al. A novel phase change absorbent with ionic liquid as promoter for low energy-consuming CO2 capture[J]. Separation and Purification Technology, 2023, 315: 123740.
|
| [10] |
LIAN Shaohan, SONG Chunfeng, LIU Qingling, et al. Recent advances in ionic liquids-based hybrid processes for CO2 capture and utilization[J]. Journal of Environmental Sciences, 2021, 99: 281-295.
|
| [11] |
LIU Fei, FANG Mengxiang, DONG Wenfeng, et al. Carbon dioxide absorption in aqueous alkanolamine blends for biphasic solvents screening and evaluation[J]. Applied Energy, 2019, 233: 468-477.
|
| [12] |
ZHOU Xiaobin, JING Guohua, Bihong LYU, et al. Low-viscosity and efficient regeneration of carbon dioxide capture using a biphasic solvent regulated by 2-amino-2-methyl-1-propanol[J]. Applied Energy, 2019, 235: 379-390.
|
| [13] |
WANG Lidong, LIU Shanshan, WANG Rujie, et al. Regulating phase separation behavior of a DEEA-TETA biphasic solvent using sulfolane for energy-saving CO2 capture[J]. Environmental Science & Technology, 2019, 53(21): 12873-12881.
|
| [14] |
LIU Fan, SHEN Yao, SHEN Li, et al. Sustainable ionic liquid organic solution with efficient recyclability and low regeneration energy consumption for CO2 capture[J]. Separation and Purification Technology, 2021, 275: 119123.
|
| [15] |
ZHOU Xiaobin, LI Xiaoling, WEI Jianwen, et al. Novel nonaqueous liquid-liquid biphasic solvent for energy-efficient carbon dioxide capture with low corrosivity[J]. Environmental Science & Technology, 2020, 54(24): 16138-16146.
|
| [16] |
JIANG Wufeng, LI Xiaoshan, GAO Ge, et al. Advances in applications of ionic liquids for phase change CO2 capture[J]. Chemical Engineering Journal, 2022, 445: 136767.
|
| [17] |
CHIU Li-Feng, LIU Hsiao-Fen, LI Menghui. Heat capacity of alkanolamines by differential scanning calorimetry[J]. Journal of Chemical & Engineering Data, 1999, 44(3): 631-636.
|
| [18] |
YANG Jie, YU Xinhai, AN Lin, et al. CO2 capture with the absorbent of a mixed ionic liquid and amine solution considering the effects of SO2 and O2 [J]. Applied Energy, 2017, 194: 9-18.
|
| [19] |
JIA Ruiqi, WU Qing, ZHANG Liangliang, et al. CO2 absorption mechanism and kinetic modeling of mixed amines with ionic liquid activation[J]. AIChE Journal, 2024, 70(9): e18493.
|
| [20] |
HAIDER Md Belal, HUSSAIN Zakir, KUMAR Rakesh. CO2 absorption and kinetic study in ionic liquid amine blends[J]. Journal of Molecular Liquids, 2016, 224: 1025-1031.
|
| [21] |
LEITES I L. Thermodynamics of CO2 solubility in mixtures monoethanolamine with organic solvents and water and commercial experience of energy saving gas purification technology[J]. Energy Conversion and Management, 1998, 39(16/17/18): 1665-1674.
|
| [22] |
MALINOWSKI Janusz J, DAUGULIS Andrew J. Salt effects in extraction of ethanol, 1-butanol and acetone from aqueous solutions[J]. AIChE Journal, 1994, 40(9): 1459-1465.
|
| [23] |
Mar OLAYA M, GARCÍA Angela N, MARCILLA Antonio. Liquid-liquid-solid equilibria for the quaternary system water + acetone + 1-butanol + sodium chloride at 25℃[J]. Journal of Chemical & Engineering Data, 1996, 41(4): 910-917.
|
 ), 贾瑞琪2, 张博1, 张娇娇2, 肖家旺1, 张亮亮2(
), 贾瑞琪2, 张博1, 张娇娇2, 肖家旺1, 张亮亮2( )
)
 ), JIA Ruiqi2, ZHANG Bo1, ZHANG Jiaojiao2, XIAO Jiawang1, ZHANG Liangliang2(
), JIA Ruiqi2, ZHANG Bo1, ZHANG Jiaojiao2, XIAO Jiawang1, ZHANG Liangliang2( )
)