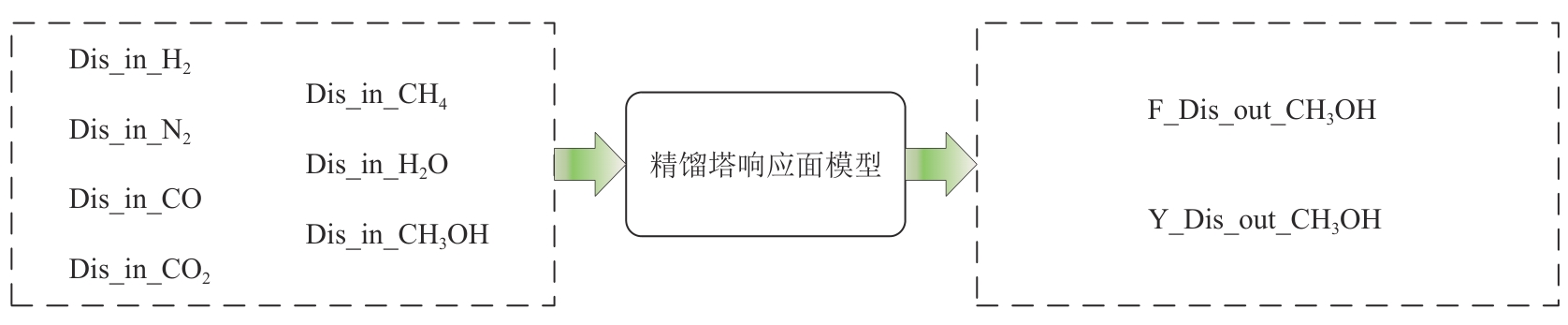
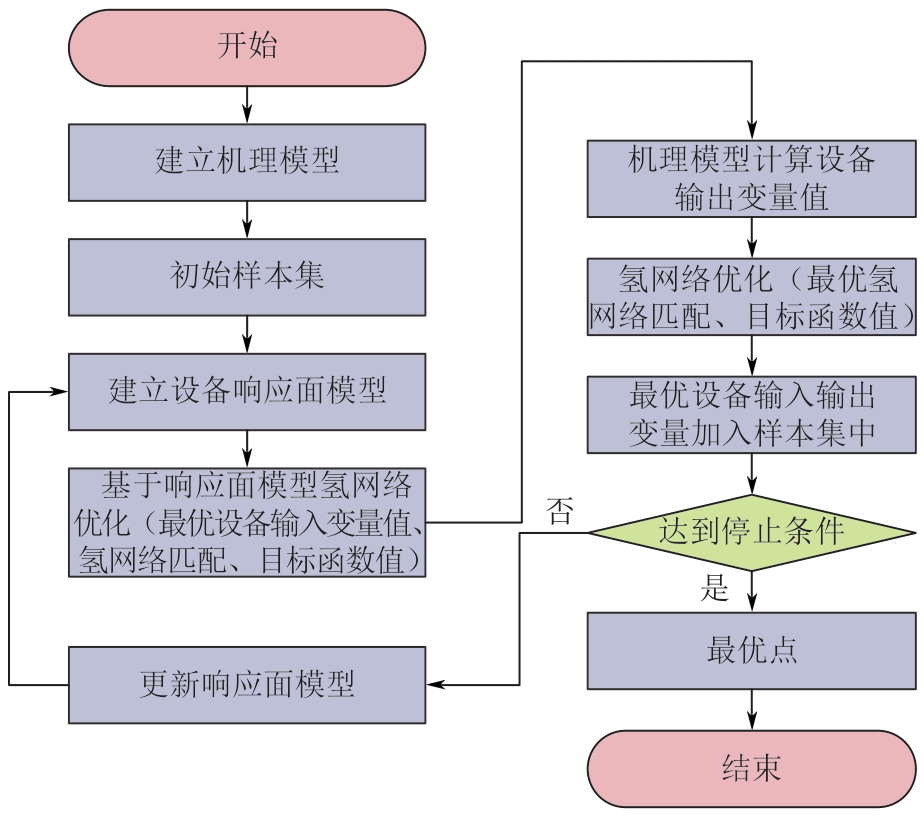
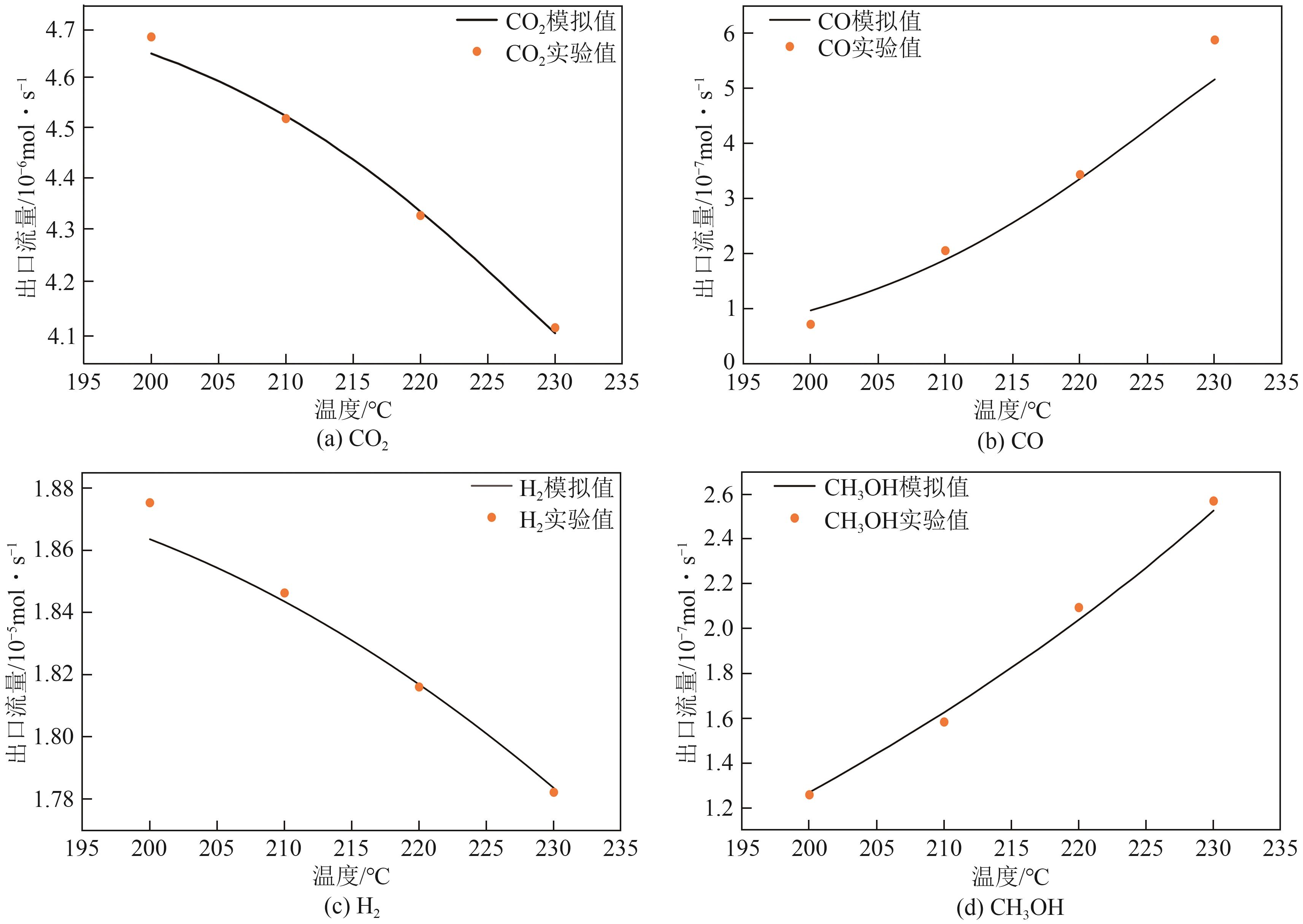
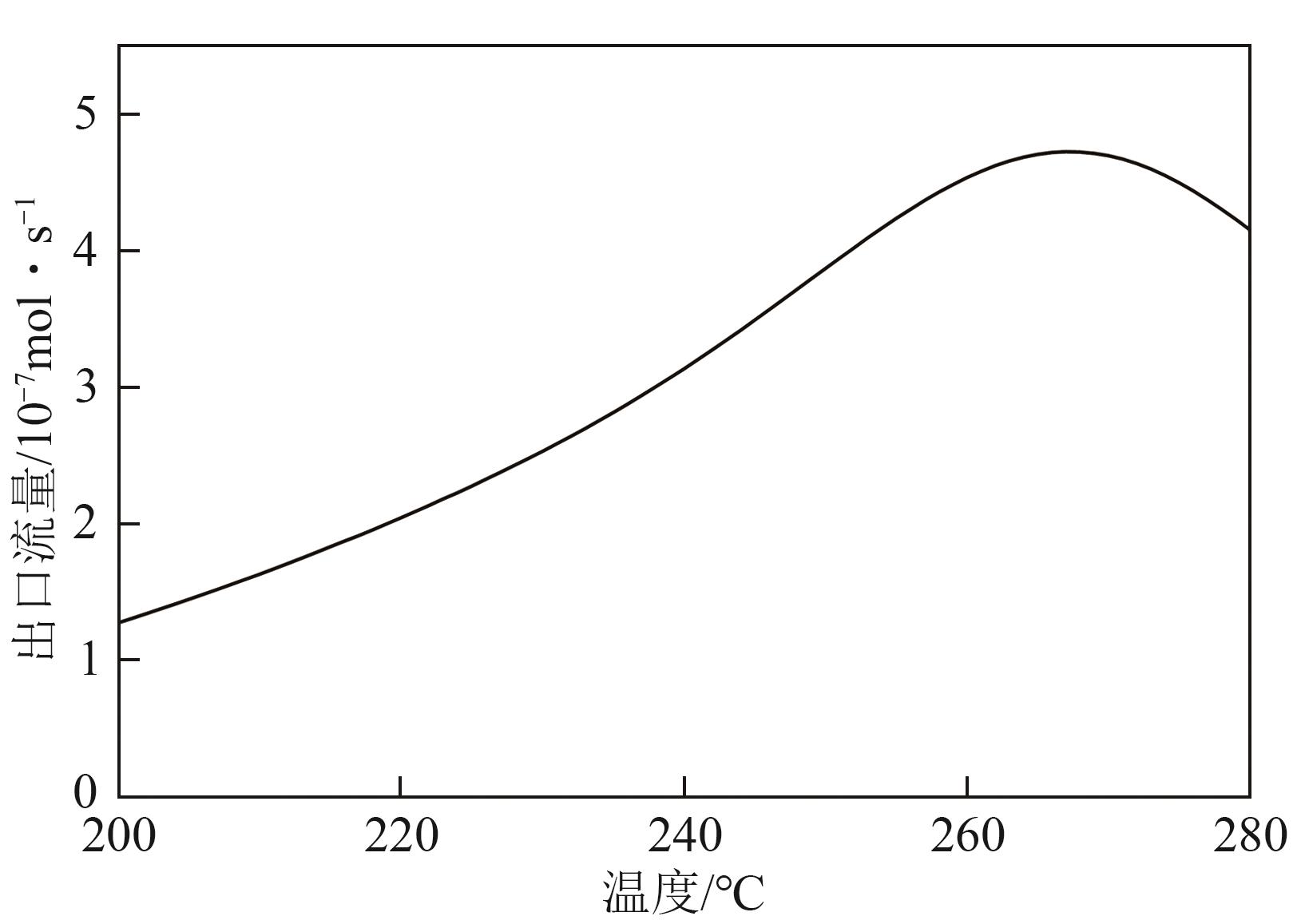
化工进展 ›› 2025, Vol. 44 ›› Issue (8): 4688-4700.DOI: 10.16085/j.issn.1000-6613.2024-1926
• 过程系统工程的模拟与仿真 • 上一篇
进化响应面驱动的CO2加氢制甲醇工艺与氢网络同步优化
黄灵军( ), 朱卿宇, 张宇, 孙维祺, 窦东阳(
), 朱卿宇, 张宇, 孙维祺, 窦东阳( ), 王启立(
), 王启立( )
)
- 中国矿业大学化工学院,江苏 徐州 221116
-
收稿日期:2024-11-22修回日期:2024-12-24出版日期:2025-08-25发布日期:2025-09-08 -
通讯作者:窦东阳,王启立 -
作者简介:黄灵军(1992—),男,博士,讲师,研究方向为过程系统工程。E-mail:hlj@cumt.edu.cn。 -
基金资助:中央高校基本科研业务费专项资金项目(XJ2023003201)
Simultaneous optimization of hydrogen network with CO₂ hydrogenation to methanol process based on evolutionary response surface method
HUANG Lingjun( ), ZHU Qingyu, ZHANG Yu, SUN Weiqi, DOU Dongyang(
), ZHU Qingyu, ZHANG Yu, SUN Weiqi, DOU Dongyang( ), WANG Qili(
), WANG Qili( )
)
- School of Chemical Engineering and Technology, China University of Mining and Technology, Xuzhou 221116, Jiangsu, China
-
Received:2024-11-22Revised:2024-12-24Online:2025-08-25Published:2025-09-08 -
Contact:DOU Dongyang, WANG Qili
摘要:
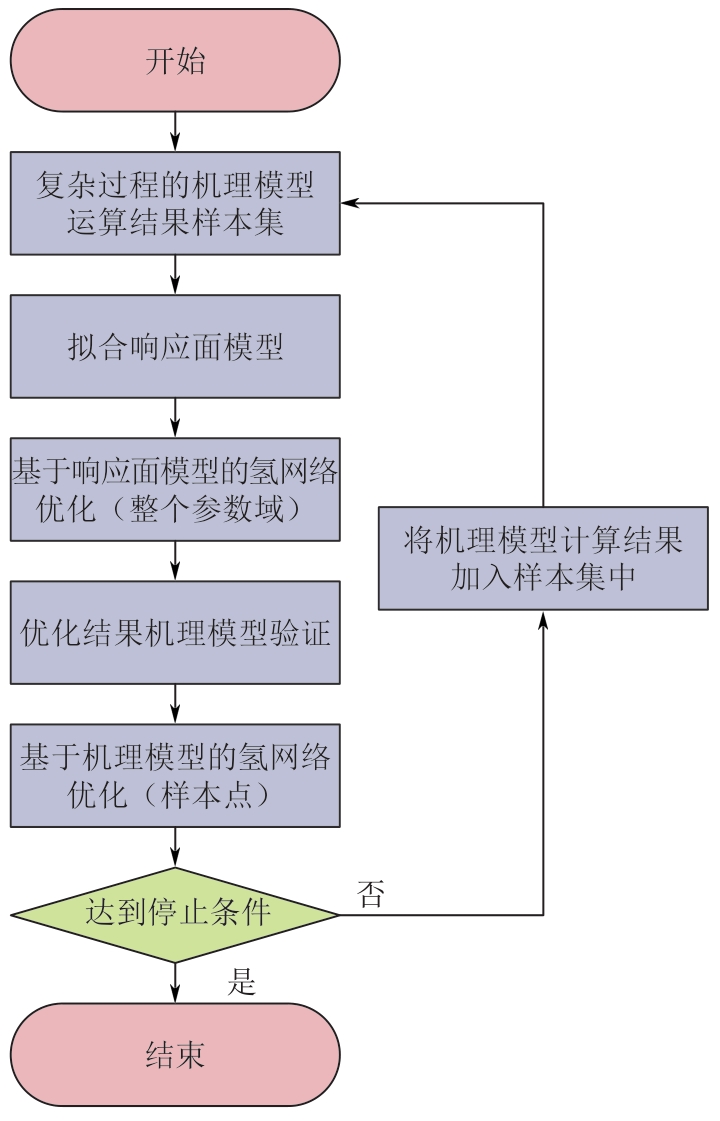
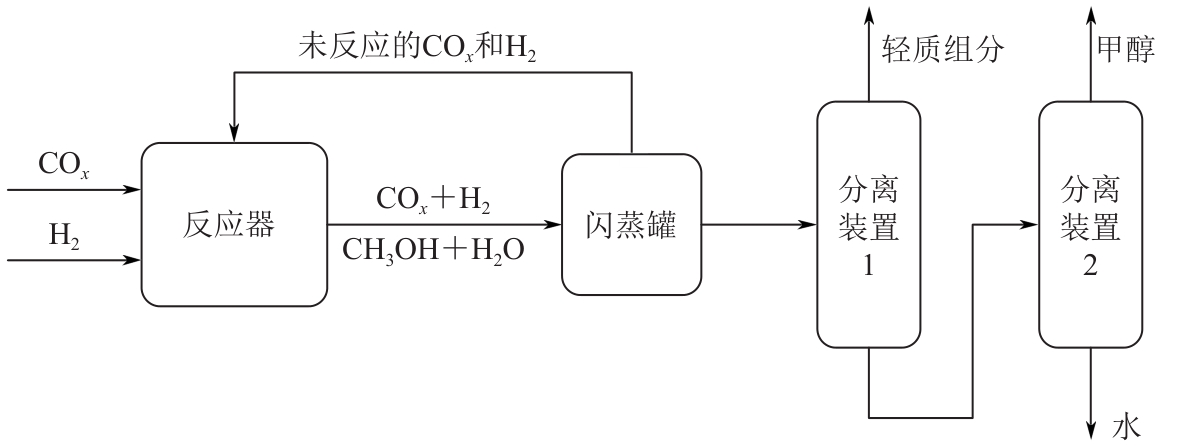
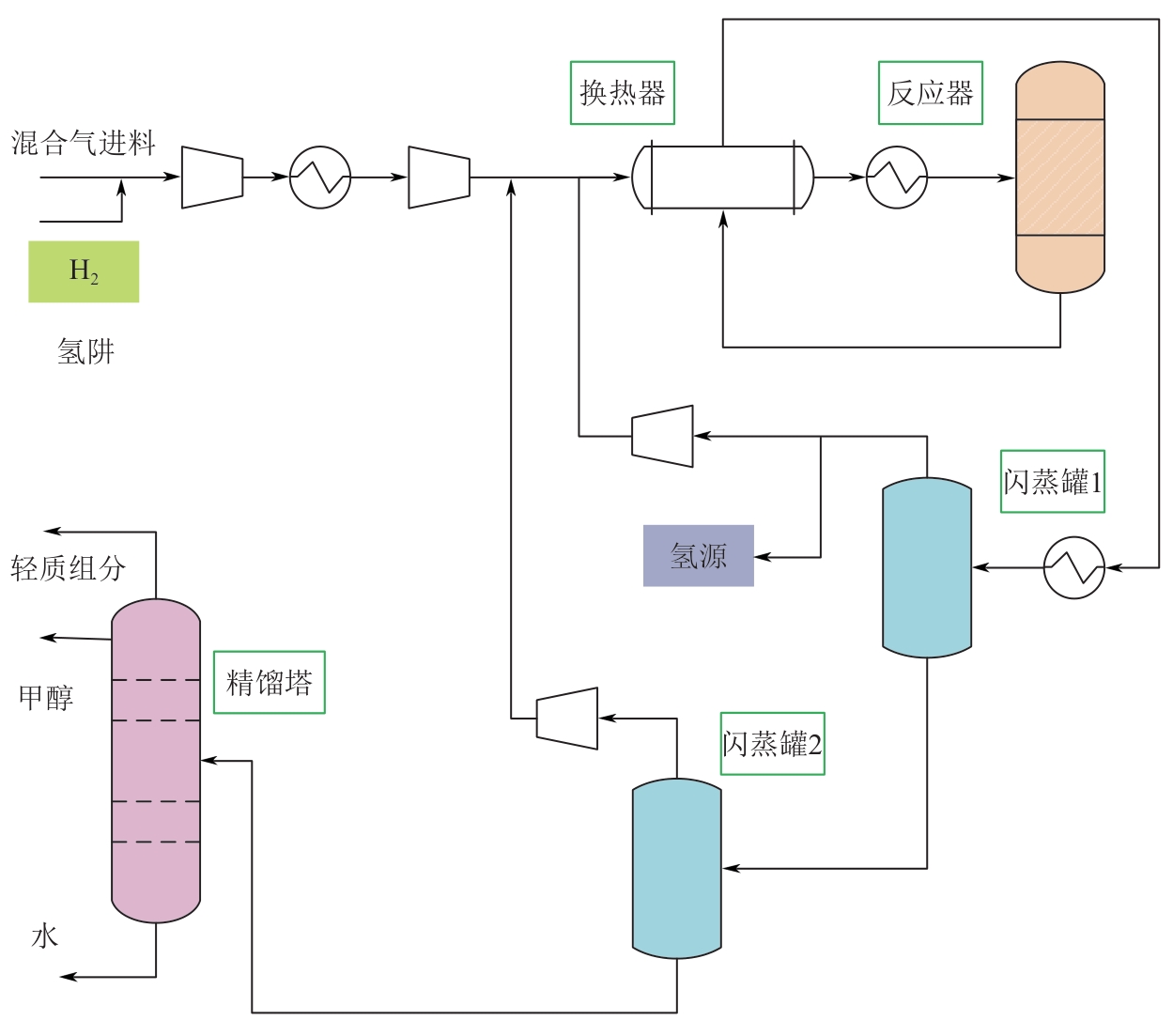
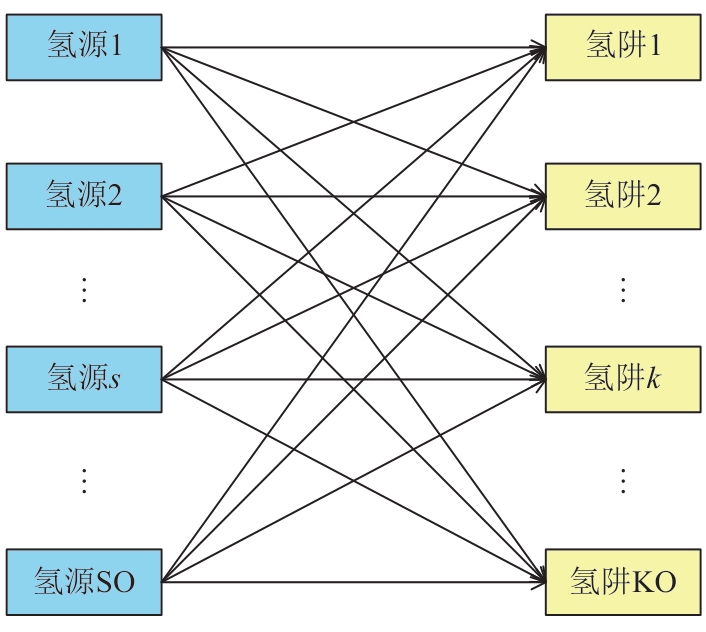
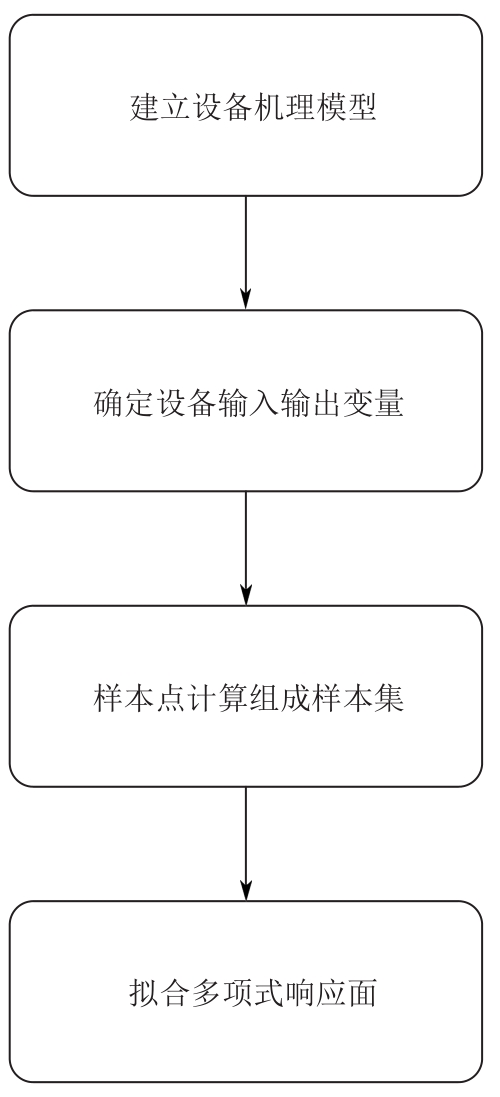
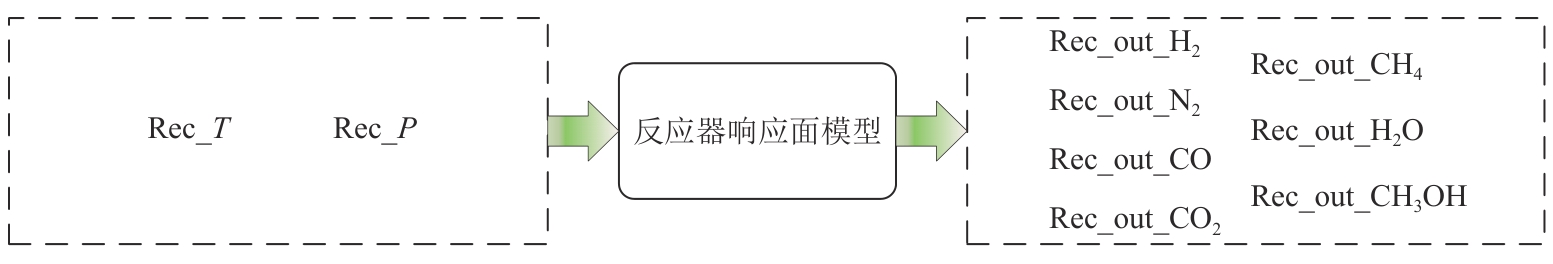
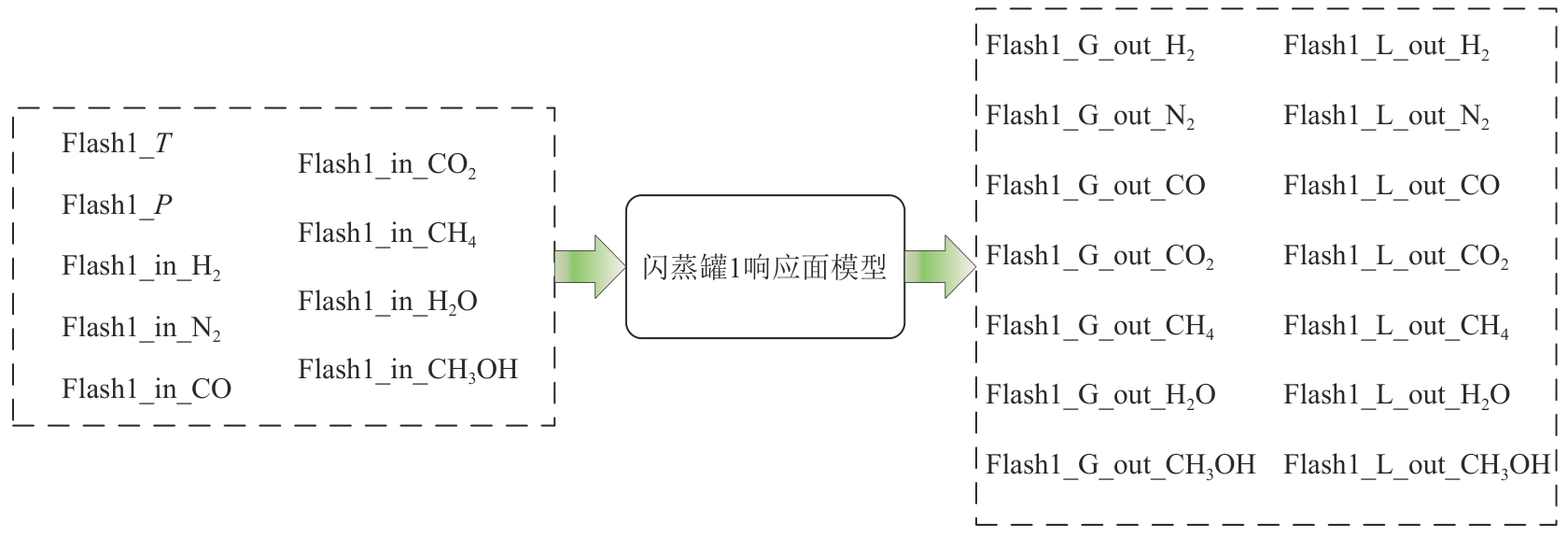
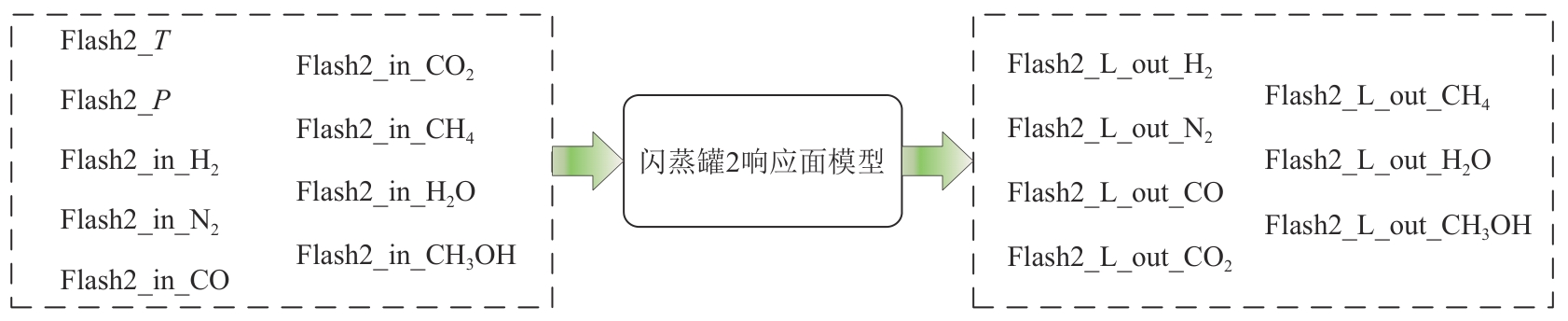
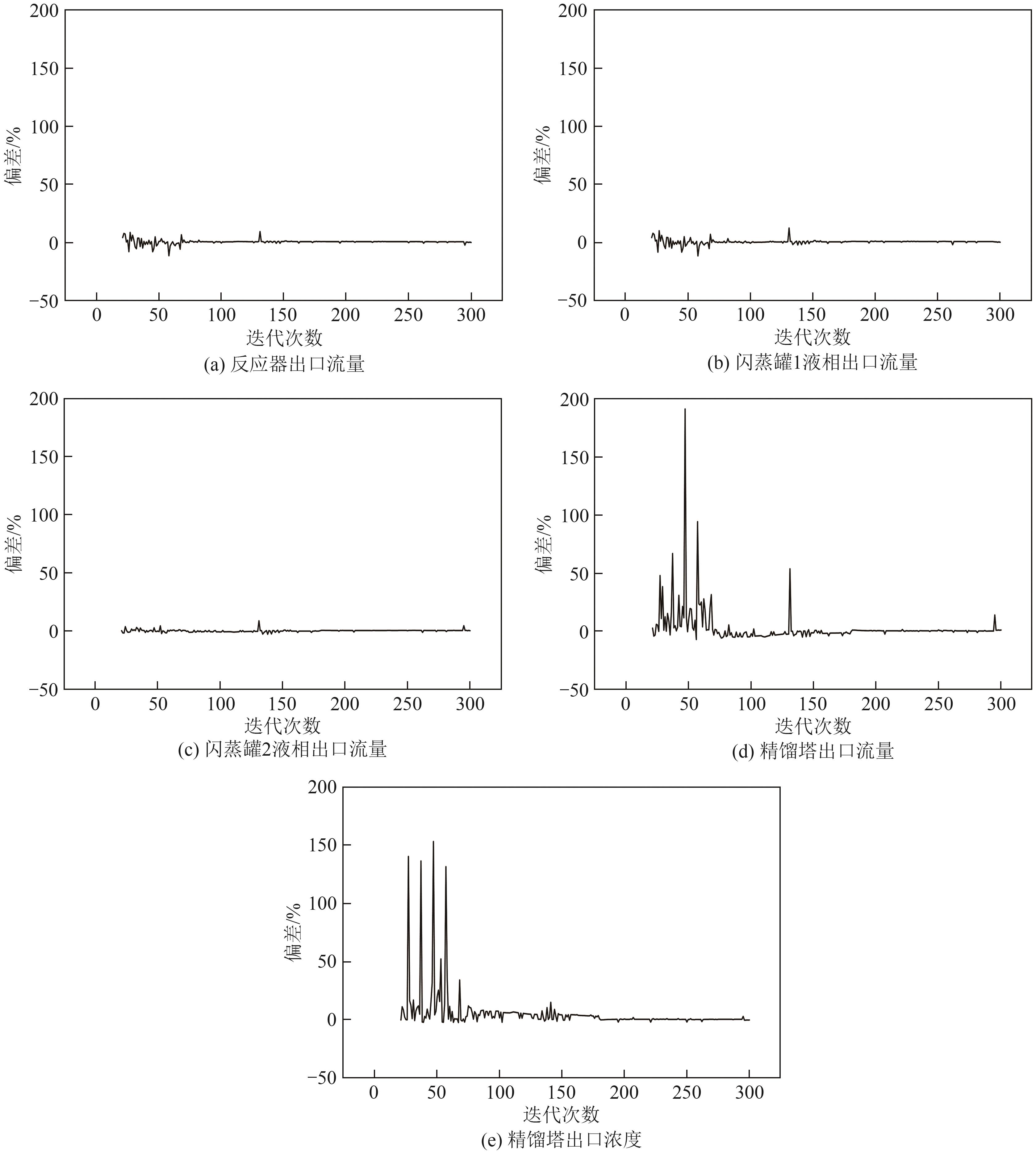
复杂工艺流程与氢网络的协同优化是炼厂氢网络集成中的难题。为此,本文提出了一种基于进化响应面的协同优化方法,同步优化甲醇工艺流程与氢网络。该方法构建了CO₂加氢制甲醇流程中反应器、闪蒸罐和精馏塔的机理模型,基于此得到的样本集建立相应的进化响应面模型,利用机理模型验证响应面模型的优化结果并进行修正,提出高效的优化流程框架。将此方法应用于某炼厂的氢网络集成优化,综合考虑了甲醇产量、设备制造成本和用氢成本等因素。结果表明,该方法能够在提升甲醇产量的同时优化炼厂的氢资源分配,降低设备制造与用氢成本,提升了炼厂七百多万元的年度经济效益。该方法求解高效,优化结果的准确性较传统方法显著提高,为炼厂氢网络与甲醇工艺流程的协同优化提供了有效的解决方案。
中图分类号:
引用本文
黄灵军, 朱卿宇, 张宇, 孙维祺, 窦东阳, 王启立. 进化响应面驱动的CO2加氢制甲醇工艺与氢网络同步优化[J]. 化工进展, 2025, 44(8): 4688-4700.
HUANG Lingjun, ZHU Qingyu, ZHANG Yu, SUN Weiqi, DOU Dongyang, WANG Qili. Simultaneous optimization of hydrogen network with CO₂ hydrogenation to methanol process based on evolutionary response surface method[J]. Chemical Industry and Engineering Progress, 2025, 44(8): 4688-4700.
| 反应 | M1的表达式 | M1值 | M2表达式 | M2值 |
|---|---|---|---|---|
| 式(1) | KCO | 2.16×10-5exp(46800/RT) | KCO/K1 | 9.11×107exp(-51638/RT) |
| 式(2) | 7.05×10-7exp(61700/RT) | 6.59×10-9exp(101384.87/RT) | ||
| 式(3) | 7.05×10-7exp(61700/RT) | 2.78×104exp(2946.87/RT) |
表1 推动力项常数表达式与计算结果
| 反应 | M1的表达式 | M1值 | M2表达式 | M2值 |
|---|---|---|---|---|
| 式(1) | KCO | 2.16×10-5exp(46800/RT) | KCO/K1 | 9.11×107exp(-51638/RT) |
| 式(2) | 7.05×10-7exp(61700/RT) | 6.59×10-9exp(101384.87/RT) | ||
| 式(3) | 7.05×10-7exp(61700/RT) | 2.78×104exp(2946.87/RT) |
| 反应 | ln M1 | ln M2 | |||
|---|---|---|---|---|---|
| A | B | A | B | ||
| 式(1) | -10.7428 | 5629.06 | 18.3272 | -6210.97 | |
| 式(2) | -14.1651 | 7421.22 | -18.8370 | 12194.48 | |
| 式(3) | -14.1651 | 7421.22 | 10.2330 | 354.45 | |
表2 用于Aspen Plus的推动力常数系数
| 反应 | ln M1 | ln M2 | |||
|---|---|---|---|---|---|
| A | B | A | B | ||
| 式(1) | -10.7428 | 5629.06 | 18.3272 | -6210.97 | |
| 式(2) | -14.1651 | 7421.22 | -18.8370 | 12194.48 | |
| 式(3) | -14.1651 | 7421.22 | 10.2330 | 354.45 | |
| 项 | 常数 | A | B | |
|---|---|---|---|---|
| 1 | 1 | 0 | 0 | p |
| 2 | -18.8717 | 10103.44 | ||
| 3 | KCO | -10.7428 | 5629.06 | |
| 4 | -29.6144 | 15732.50 | ||
| 5 | -14.165 | 7421.22 | ||
| 6 | -33.037 | 17524.66 |
表3 吸附项常数系数
| 项 | 常数 | A | B | |
|---|---|---|---|---|
| 1 | 1 | 0 | 0 | p |
| 2 | -18.8717 | 10103.44 | ||
| 3 | KCO | -10.7428 | 5629.06 | |
| 4 | -29.6144 | 15732.50 | ||
| 5 | -14.165 | 7421.22 | ||
| 6 | -33.037 | 17524.66 |
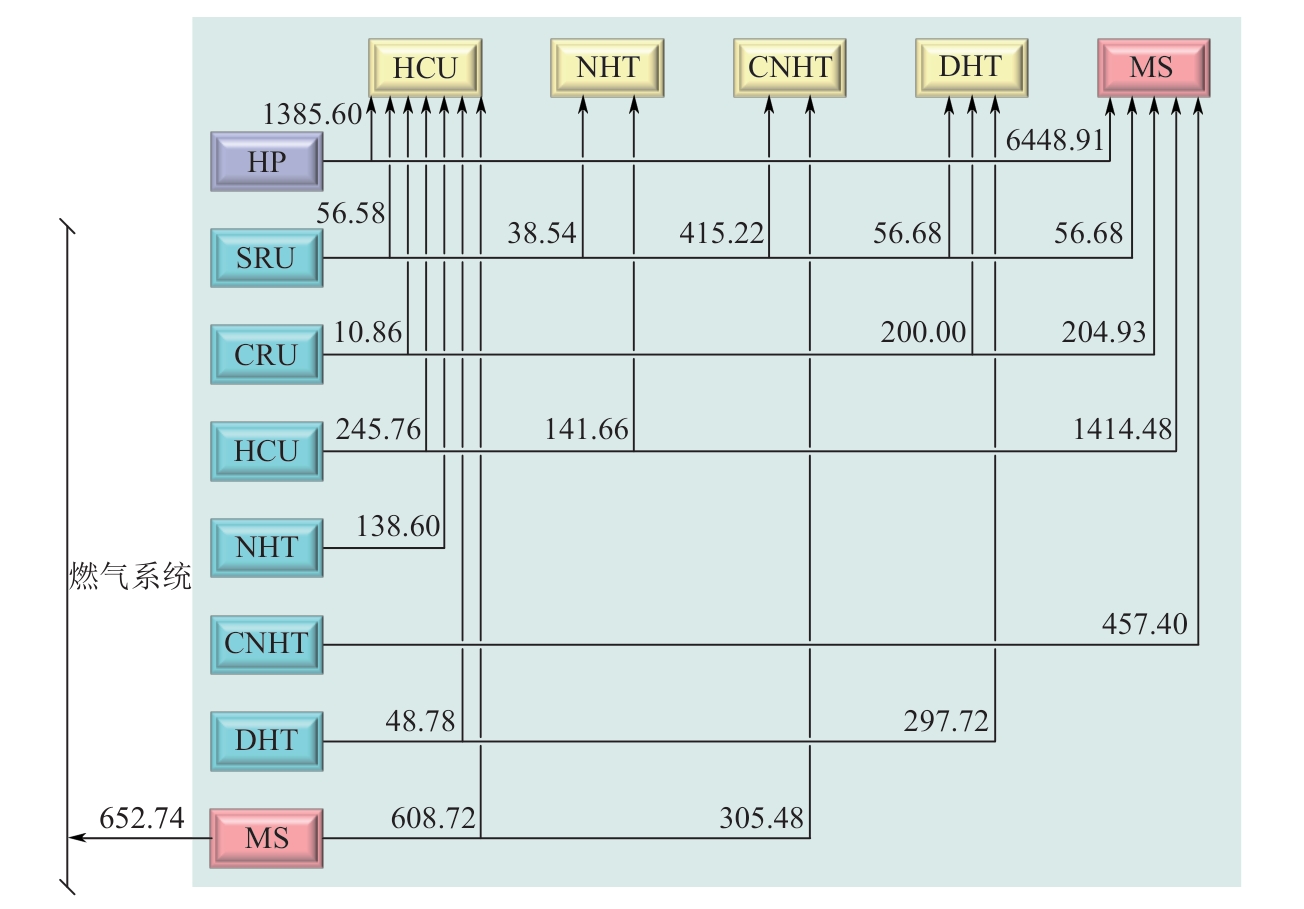
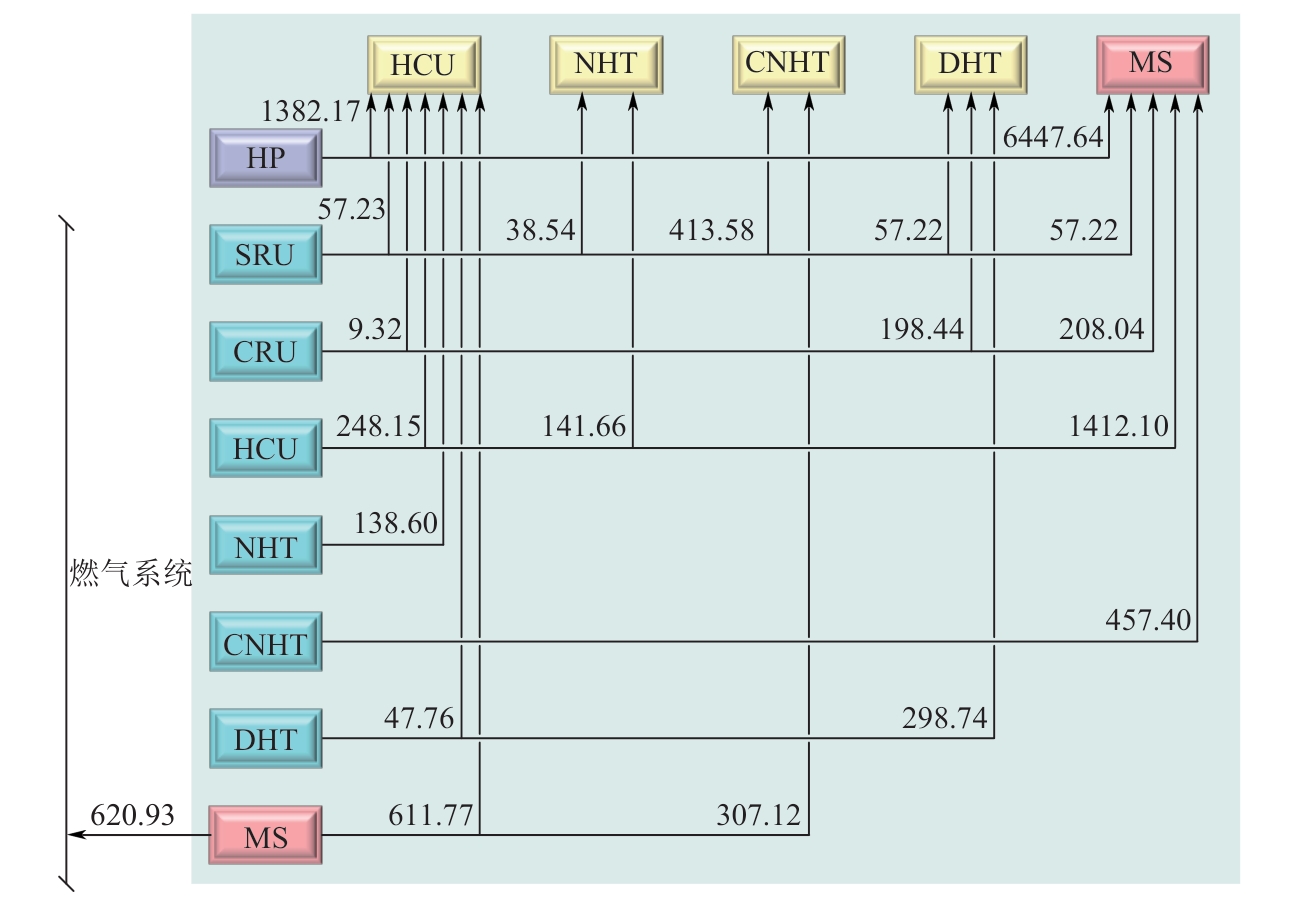
| 装置 | 氢源 | 装置 | 氢阱 | ||
|---|---|---|---|---|---|
| 摩尔分数 | 流量/kmol·h-1 | 摩尔分数 | 流量/kmol·h-1 | ||
| SRU | 0.9300 | 623.80 | HCU | 0.8061 | 2495.00 |
| CRU | 0.8000 | 415.80 | NHT | 0.7885 | 180.20 |
| HCU | 0.7500 | 1801.90 | DHT | 0.7757 | 554.40 |
| NHT | 0.7500 | 138.60 | CNHT | 0.7514 | 720.70 |
| DHT | 0.7300 | 346.50 | MS | 0.9000 | 8582.41 |
| CNHT | 0.7000 | 457.40 | |||
| MS | 0.6253 | 2232.74 | |||
| HP | 0.9500 | 7782.42 | |||
表4 氢源和氢阱参数
| 装置 | 氢源 | 装置 | 氢阱 | ||
|---|---|---|---|---|---|
| 摩尔分数 | 流量/kmol·h-1 | 摩尔分数 | 流量/kmol·h-1 | ||
| SRU | 0.9300 | 623.80 | HCU | 0.8061 | 2495.00 |
| CRU | 0.8000 | 415.80 | NHT | 0.7885 | 180.20 |
| HCU | 0.7500 | 1801.90 | DHT | 0.7757 | 554.40 |
| NHT | 0.7500 | 138.60 | CNHT | 0.7514 | 720.70 |
| DHT | 0.7300 | 346.50 | MS | 0.9000 | 8582.41 |
| CNHT | 0.7000 | 457.40 | |||
| MS | 0.6253 | 2232.74 | |||
| HP | 0.9500 | 7782.42 | |||
| 取值 | 反应器温度/℃ | 反应器压力/bar | 闪蒸罐1温度/℃ | 闪蒸罐1压力/bar | 闪蒸罐2温度/℃ | 闪蒸罐2压力/bar |
|---|---|---|---|---|---|---|
| 上限 | 280 | 120 | 45 | 119 | 45 | 3 |
| 下限 | 220 | 80 | 35 | 79 | 35 | 1 |
表5 决策变量取值上下限
| 取值 | 反应器温度/℃ | 反应器压力/bar | 闪蒸罐1温度/℃ | 闪蒸罐1压力/bar | 闪蒸罐2温度/℃ | 闪蒸罐2压力/bar |
|---|---|---|---|---|---|---|
| 上限 | 280 | 120 | 45 | 119 | 45 | 3 |
| 下限 | 220 | 80 | 35 | 79 | 35 | 1 |
| 实验次数 | 反应体积空速(GHSV)/m3·kg-1·h-1 | P/bar | T/℃ | 组分分压/bar | 转换率(实验)/% | 选择性(实验)/% | 转换率(模拟)/% | 选择性(模拟)/% | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H2 | CO2 | N2 | Ar | CO2 | H2 | CH3OH | CO | CO2 | H2 | CH3OH | CO | ||||||||
| 1 | 7800 | 50 | 200 | 34.9 | 8.9 | 6.2 | 0 | 4 .6 | 2.4 | 56 | 44 | 4.6 | 2.5 | 57 | 43 | ||||
| 2 | 7800 | 50 | 210 | 34.9 | 8.9 | 6.2 | 0 | 7.7 | 3.4 | 45 | 55 | 7.2 | 3.5 | 46 | 54 | ||||
| 3 | 7800 | 50 | 220 | 34.9 | 8.9 | 6.2 | 0 | 11.1 | 4.8 | 43 | 57 | 11.1 | 5.0 | 38 | 62 | ||||
| 4 | 7800 | 50 | 230 | 34.9 | 8.9 | 6.2 | 0 | 15.8 | 6.3 | 38 | 62 | 15.8 | 6.7 | 33 | 67 | ||||
| 5 | 7800 | 50 | 210 | 12.7 | 6.2 | 6.2 | 24.8 | 5.1 | 2.9 | 20 | 80 | 5.2 | 4.3 | 34 | 66 | ||||
| 6 | 7800 | 50 | 210 | 18.8 | 6.2 | 6.2 | 18.7 | 5.9 | 3.1 | 33 | 67 | 6.7 | 3.9 | 39 | 61 | ||||
| 7 | 7800 | 50 | 210 | 24.4 | 6.2 | 6.2 | 13.1 | 7.2 | 3.1 | 39 | 61 | 8.0 | 3.7 | 42 | 58 | ||||
| 8 | 7800 | 50 | 210 | 31.3 | 6.2 | 6.2 | 6.3 | 9.3 | 3.2 | 42 | 58 | 9.4 | 3.5 | 45 | 55 | ||||
| 9 | 7800 | 50 | 210 | 37.5 | 6.2 | 6.2 | 0 | 9.9 | 3.4 | 48 | 52 | 10.6 | 3.4 | 48 | 52 | ||||
| 10 | 7800 | 50 | 210 | 24.4 | 12 | 6.2 | 7.4 | 4.8 | 3.9 | 37 | 63 | 4.3 | 3.9 | 41 | 59 | ||||
| 11 | 7800 | 50 | 210 | 24.4 | 8.1 | 6.2 | 11.3 | 6.1 | 3.3 | 38 | 62 | 6.2 | 3.8 | 42 | 58 | ||||
| 12 | 7800 | 50 | 210 | 24.4 | 4.9 | 6.2 | 14.5 | 8.8 | 2.9 | 38 | 62 | 9.8 | 3.7 | 43 | 57 | ||||
| 13 | 7800 | 50 | 210 | 24.4 | 4.1 | 6.2 | 15.3 | 9.8 | 2.7 | 40 | 60 | 11.5 | 3.6 | 43 | 57 | ||||
| 14 | 7800 | 65 | 210 | 31.7 | 8.1 | 8.1 | 17.1 | 8.6 | 4.4 | 48 | 52 | 8.8 | 4.3 | 46 | 54 | ||||
| 15 | 7800 | 80 | 210 | 39 | 10 | 10 | 21 | 9.9 | 5.3 | 51 | 49 | 9.6 | 4.8 | 49 | 51 | ||||
| 16 | 11700 | 50 | 210 | 24.4 | 6.3 | 6.3 | 13.1 | 5.6 | 2.6 | 39 | 61 | 5.8 | 2.8 | 41 | 59 | ||||
| 17 | 23400 | 50 | 210 | 24.4 | 6.3 | 6.3 | 13.1 | 2.8 | 1.4 | 46 | 54 | 3.3 | 1.6 | 41 | 59 | ||||
表6 模拟结果与实验结果对比
| 实验次数 | 反应体积空速(GHSV)/m3·kg-1·h-1 | P/bar | T/℃ | 组分分压/bar | 转换率(实验)/% | 选择性(实验)/% | 转换率(模拟)/% | 选择性(模拟)/% | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H2 | CO2 | N2 | Ar | CO2 | H2 | CH3OH | CO | CO2 | H2 | CH3OH | CO | ||||||||
| 1 | 7800 | 50 | 200 | 34.9 | 8.9 | 6.2 | 0 | 4 .6 | 2.4 | 56 | 44 | 4.6 | 2.5 | 57 | 43 | ||||
| 2 | 7800 | 50 | 210 | 34.9 | 8.9 | 6.2 | 0 | 7.7 | 3.4 | 45 | 55 | 7.2 | 3.5 | 46 | 54 | ||||
| 3 | 7800 | 50 | 220 | 34.9 | 8.9 | 6.2 | 0 | 11.1 | 4.8 | 43 | 57 | 11.1 | 5.0 | 38 | 62 | ||||
| 4 | 7800 | 50 | 230 | 34.9 | 8.9 | 6.2 | 0 | 15.8 | 6.3 | 38 | 62 | 15.8 | 6.7 | 33 | 67 | ||||
| 5 | 7800 | 50 | 210 | 12.7 | 6.2 | 6.2 | 24.8 | 5.1 | 2.9 | 20 | 80 | 5.2 | 4.3 | 34 | 66 | ||||
| 6 | 7800 | 50 | 210 | 18.8 | 6.2 | 6.2 | 18.7 | 5.9 | 3.1 | 33 | 67 | 6.7 | 3.9 | 39 | 61 | ||||
| 7 | 7800 | 50 | 210 | 24.4 | 6.2 | 6.2 | 13.1 | 7.2 | 3.1 | 39 | 61 | 8.0 | 3.7 | 42 | 58 | ||||
| 8 | 7800 | 50 | 210 | 31.3 | 6.2 | 6.2 | 6.3 | 9.3 | 3.2 | 42 | 58 | 9.4 | 3.5 | 45 | 55 | ||||
| 9 | 7800 | 50 | 210 | 37.5 | 6.2 | 6.2 | 0 | 9.9 | 3.4 | 48 | 52 | 10.6 | 3.4 | 48 | 52 | ||||
| 10 | 7800 | 50 | 210 | 24.4 | 12 | 6.2 | 7.4 | 4.8 | 3.9 | 37 | 63 | 4.3 | 3.9 | 41 | 59 | ||||
| 11 | 7800 | 50 | 210 | 24.4 | 8.1 | 6.2 | 11.3 | 6.1 | 3.3 | 38 | 62 | 6.2 | 3.8 | 42 | 58 | ||||
| 12 | 7800 | 50 | 210 | 24.4 | 4.9 | 6.2 | 14.5 | 8.8 | 2.9 | 38 | 62 | 9.8 | 3.7 | 43 | 57 | ||||
| 13 | 7800 | 50 | 210 | 24.4 | 4.1 | 6.2 | 15.3 | 9.8 | 2.7 | 40 | 60 | 11.5 | 3.6 | 43 | 57 | ||||
| 14 | 7800 | 65 | 210 | 31.7 | 8.1 | 8.1 | 17.1 | 8.6 | 4.4 | 48 | 52 | 8.8 | 4.3 | 46 | 54 | ||||
| 15 | 7800 | 80 | 210 | 39 | 10 | 10 | 21 | 9.9 | 5.3 | 51 | 49 | 9.6 | 4.8 | 49 | 51 | ||||
| 16 | 11700 | 50 | 210 | 24.4 | 6.3 | 6.3 | 13.1 | 5.6 | 2.6 | 39 | 61 | 5.8 | 2.8 | 41 | 59 | ||||
| 17 | 23400 | 50 | 210 | 24.4 | 6.3 | 6.3 | 13.1 | 2.8 | 1.4 | 46 | 54 | 3.3 | 1.6 | 41 | 59 | ||||
| 项目 | 贝叶斯算法 | 进化响应面法 |
|---|---|---|
| 反应器温度/℃ | 247.19 | 237.9 |
| 反应器压力/bar | 112.42 | 120 |
| 闪蒸罐1温度/℃ | 35 | 35 |
| 闪蒸罐1压力/bar | 111.42 | 119 |
| 闪蒸罐2温度/℃ | 39.9 | 35 |
| 闪蒸罐2压力/bar | 1.76 | 1.4 |
| 氢气公用工程用量/kmol·h-1 | 7829.81 | 8034.51 |
| 甲醇产量/kmol·h-1 | 3291.56 | 3295.32 |
| 闪蒸罐1材料成本/CNY | 482114.57 | 494919.77 |
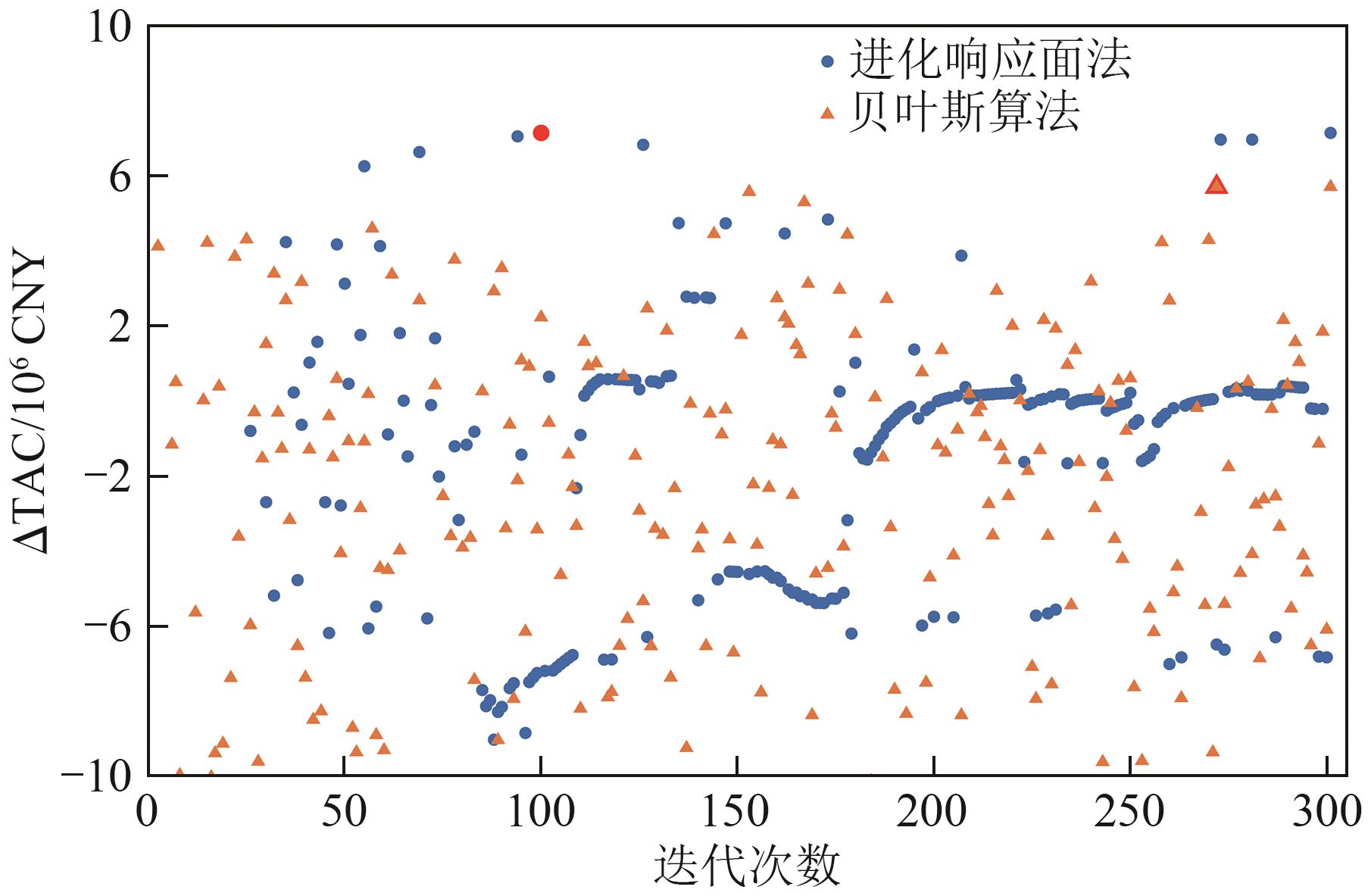
| 年度总效益增量/CNY | 5722022.60 | 7163708.71 |
| 最优点迭代次数 | 272 | 100 |
| 计算时间/s | 5333 | 4507 |
表7 贝叶斯算法与进化响应法结果对比
| 项目 | 贝叶斯算法 | 进化响应面法 |
|---|---|---|
| 反应器温度/℃ | 247.19 | 237.9 |
| 反应器压力/bar | 112.42 | 120 |
| 闪蒸罐1温度/℃ | 35 | 35 |
| 闪蒸罐1压力/bar | 111.42 | 119 |
| 闪蒸罐2温度/℃ | 39.9 | 35 |
| 闪蒸罐2压力/bar | 1.76 | 1.4 |
| 氢气公用工程用量/kmol·h-1 | 7829.81 | 8034.51 |
| 甲醇产量/kmol·h-1 | 3291.56 | 3295.32 |
| 闪蒸罐1材料成本/CNY | 482114.57 | 494919.77 |
| 年度总效益增量/CNY | 5722022.60 | 7163708.71 |
| 最优点迭代次数 | 272 | 100 |
| 计算时间/s | 5333 | 4507 |
| [1] | International Energy Agency. Global hydrogen review 2024[R/OL]. (2024-10-02)[2024-11-22].. |
| [2] | International Energy Agency. CO2 emissions in 2022[R/OL]. (2023-03-02)[2024-11-22].. |
| [3] | 杨学萍. 碳中和背景下现代煤化工技术路径探索[J].化工进展, 2022, 41(7): 3402-3412. |
| YANG Xueping. Exploration on technical path of modern coal chemical industry under the background of carbon neutralization[J]. Chemical Industry and Engineering Progress, 2022, 41(7): 3402-3412. | |
| [4] | 王江涛, 鹿晓斌. CO2促进“甲醇经济”与“氢经济”共同发展[J]. 现代化工, 2021, 41(7): 14-18, 25. |
| WANG Jiangtao, LU Xiaobin. Together development of “methanol economy” and “hydrogen economy” driven by CO2 utilization[J]. Modern Chemical Industry, 2021, 41(7): 14-18, 25. | |
| [5] | AN Xin, ZUO Yizan, ZHANG Qiang, et al. Methanol synthesis from CO2 hydrogenation with a Cu/Zn/Al/Zr fibrous catalyst[J]. Chinese Journal of Chemical Engineering, 2009, 17(1): 88-94. |
| [6] | PORTHA Jean-François, PARKHOMENKO Ksenia, KOBL Kilian, et al. Kinetics of methanol synthesis from carbon dioxide hydrogenation over copper-zinc oxide catalysts[J]. Industrial & Engineering Chemistry Research, 2017, 56(45): 13133-13145. |
| [7] | KISS Anton A, PRAGT J J, VOS H J, et al. Novel efficient process for methanol synthesis by CO2 hydrogenation[J]. Chemical Engineering Journal, 2016, 284: 260-269. |
| [8] | YANG Minbo, ZENG Siying, FENG Xiao, et al. Simulation-based modeling and optimization for refinery hydrogen network integration with light hydrocarbon recovery[J]. International Journal of Hydrogen Energy, 2022, 47(7): 4662-4673. |
| [9] | HUANG Lingjun, HONG Xiaodong, LIAO Zuwei, et al. Efficient hybrid strategy for simultaneous design of refinery hydrogen networks and pressure swing adsorption unit[J]. Journal of Cleaner Production, 2024, 466: 142858. |
| [10] | CONN Andrew R, LE DIGABEL Sébastien. Use of quadratic models with mesh-adaptive direct search for constrained black box optimization[J]. Optimization Methods and Software, 2013, 28(1): 139-158. |
| [11] | BOUKOUVALA Fani, IERAPETRITOU Marianthi G. Surrogate-based optimization of expensive flowsheet modeling for continuous pharmaceutical manufacturing[J]. Journal of Pharmaceutical Innovation, 2013, 8(2): 131-145. |
| [12] | REGIS Rommel G. Constrained optimization by radial basis function interpolation for high-dimensional expensive black-box problems with infeasible initial points[J]. Engineering Optimization, 2014, 46(2): 218-243. |
| [13] | FORRESTER Alexander, JONES Donald. Global optimization of deceptive functions with sparse sampling[C]//12th AIAA/ISSMO Multidisciplinary Analysis and Optimization Conference. Victoria, British Columbia, Canada: AIAA, 2008. |
| [14] | REGIS Rommel G, SHOEMAKER Christine A. A quasi-multistart framework for global optimization of expensive functions using response surface models[J]. Journal of Global Optimization, 2013, 56(4): 1719-1753. |
| [15] | 吴依凡, 夏志鹏, 吉旭, 等. 基于代理模型的炼厂氢网络与脱硫系统同步优化[J]. 华东理工大学学报(自然科学版), 2023, 49(2): 176-187. |
| WU Yifan, XIA Zhipeng, JI Xu, et al. Surrogate-assisted refinery hydrogen network optimization with hydrogen sulfide removal[J]. Journal of East China University of Science and Technology, 2023, 49(2): 176-187. | |
| [16] | MARTELLI Emanuele, AMALDI Edoardo, CONSONNI Stefano. Numerical optimization of heat recovery steam cycles: Mathematical model, two-stage algorithm and applications[J]. Computers & Chemical Engineering, 2011, 35(12): 2799-2823. |
| [17] | ABRAMSON Mark A, AUDET Charles. Convergence of mesh adaptive direct search to second-order stationary points[J]. SIAM Journal on Optimization, 2006, 17(2): 606-619. |
| [18] | GRAAF G H, SCHOLTENS H, STAMHUIS E J, et al. Intra-particle diffusion limitations in low-pressure methanol synthesis[J]. Chemical Engineering Science, 1990, 45(4): 773-783. |
| [19] | GRAAF G H, SIJTSEMA P J J M, STAMHUIS E J, et al. Chemical equilibria in methanol synthesis[J]. Chemical Engineering Science, 1986, 41(11): 2883-2890. |
| [20] | LUYBEN William L. Design and control of a methanol reactor/column process[J]. Industrial & Engineering Chemistry Research, 2010, 49(13): 6150-6163. |
| [21] | ALVES Joao J, TOWLER Gavin P. Analysis of refinery hydrogen distribution systems[J]. Industrial & Engineering Chemistry Research, 2002, 41(23): 5759-5769. |
| [1] | 吴博, 马琳萱, 张明峰, 曹丽娟, 周蕾, 王学重. 基于机器学习的超声衰减预测水滑石粒度分布[J]. 化工进展, 2025, 44(8): 4365-4374. |
| [2] | 赵用明, 卜亿峰, 王涛, 杜冰, 门卓武. 费托合成催化剂动态置换与稳态工艺的集成优化[J]. 化工进展, 2025, 44(8): 4536-4544. |
| [3] | 杨傲, 邓苇, 黎勇, 罗婧, 王梓霖, 张俊, 申威峰. 基于热力学拓扑理论的三塔变压精馏分离四氢呋喃/甲醇/乙醇多目标优化设计[J]. 化工进展, 2025, 44(8): 4582-4593. |
| [4] | 董丰莲, 李鹏, 魏志伟, 孙鑫, 徐赫锴, 何畅. 考虑原油采购选择的混炼加工优化[J]. 化工进展, 2025, 44(8): 4648-4656. |
| [5] | 杨嘉聪, 程光旭, 贾彤华, 姜召. 煤制甲醇与绿氢高效耦合新工艺模拟及技术经济分析[J]. 化工进展, 2025, 44(8): 4657-4668. |
| [6] | 贾子葶, 崔子元, 王彧斐. 规整化柔性厂区布局优化策略[J]. 化工进展, 2025, 44(8): 4669-4679. |
| [7] | 黄旭昆, 葛纪军, 徐盼, 毕荣山, 李国选. 聚芳酯多级逆流水洗过程的模拟与优化[J]. 化工进展, 2025, 44(8): 4680-4687. |
| [8] | 叶晓生, 袁婷, 贾鑫, 任庆霞. 多元复合纳米材料去除微囊藻毒素-LR研究进展[J]. 化工进展, 2025, 44(7): 4144-4157. |
| [9] | 于宁, 王秋月, 王志才, 高子怡, 柴永明, 董斌. 双位点协同调控增强钙钛矿氧化物的水氧化活性[J]. 化工进展, 2025, 44(7): 3976-3984. |
| [10] | 姚如伟, 宋乐音, 牛琴琴, 李聪明. Na-S双助剂修饰铁基催化剂催化CO2加氢制C2+醇[J]. 化工进展, 2025, 44(6): 3154-3162. |
| [11] | 李明, 周依, 南兰, 叶晓生. 自动优化连续合成研究进展[J]. 化工进展, 2025, 44(6): 3190-3198. |
| [12] | 周鹏辉, 曾琳, 代黎, 冯小波, 倪笛. 响应面法和熵权法对离心风机的多目标性能优化[J]. 化工进展, 2025, 44(6): 3271-3279. |
| [13] | 郑慧哲, 王浩泽, 蒋杰, 赵玲, 奚桢浩. 反应与传质耦合的PCTG共聚酯圆盘反应器建模与模拟[J]. 化工进展, 2025, 44(6): 3372-3381. |
| [14] | 单灵海, 段欢欢, 郑旭铭, 黄晓璜, 崔国民. 一种新的换热单元竞争强化策略优化换热网络[J]. 化工进展, 2025, 44(6): 3393-3404. |
| [15] | 李红伟, 许涵侨, 赵燕, 刘耀宗, 滕志君, 季东, 李贵贤. 铂基催化剂电催化甲醇氧化研究进展与展望[J]. 化工进展, 2025, 44(6): 3443-3456. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||