化工进展 ›› 2024, Vol. 43 ›› Issue (6): 3174-3186.DOI: 10.16085/j.issn.1000-6613.2023-0857
• 材料科学与技术 • 上一篇
单原子Ni、N共掺杂碳材料基催化剂电还原CO2制CO研究进展
- 安徽工业大学化学与化工学院,安徽省煤洁净转化与高值化利用重点实验室,安徽 马鞍山 243032
-
收稿日期:2023-05-25修回日期:2023-07-11出版日期:2024-06-15发布日期:2024-07-02 -
通讯作者:刘祥春,崔平 -
作者简介:蔚德磊(1987—),男,博士研究生,研究方向为CO2电化学还原。E-mail:yudelei@ahut.edu.cn。 -
基金资助:国家自然科学基金(22278001);煤清洁转化与高值化利用安徽省重点实验室开放课题(CHV21-03)
Recent advances in electroreduction of CO2 to CO using single atom Ni, N co-doped carbon-material based catalysts
YU Delei( ), HAN Kangshun, CHEN Yao, LIU Xiangchun(
), HAN Kangshun, CHEN Yao, LIU Xiangchun( ), CUI Ping(
), CUI Ping( )
)
- Anhui Province Key Laboratory of Coal Clean Conversion and High Valued Utilization, School of Chemistry and Chemical Engineering, Anhui University of Technology, Ma’anshan 243032, Anhui, China
-
Received:2023-05-25Revised:2023-07-11Online:2024-06-15Published:2024-07-02 -
Contact:LIU Xiangchun, CUI Ping
摘要:
单原子Ni、N共掺杂碳材料基催化剂(Ni-N-C)电催化CO2制CO,因其在较大的电流密度和较大的过电势下仍具有超高的CO选择性,引起了广泛关注。本文综述了Ni-N-C电催化CO2制CO研究进展,包括氮掺杂碳材料基催化剂和Ni-N-C电催化CO2制CO。总结了氮掺杂碳材料基催化剂中不同形态N的催化活性,目前为止,N在该类催化剂电化学还原CO2中的确切作用没有达成共识。此外,还总结了Ni-N-C的催化性能、催化机理及催化性能改性方法,探讨了与Ni配位的N原子和缺陷位对催化活性的影响规律,分析了各类碳载体的优缺点。Ni-N-C电化学还原CO2制CO研究已取得一定的进展,如电流密度可达工业级要求,但是其实现大规模工业化应用还需解决一些关键问题,如开发高活性和高稳定性Ni-N-C的定向制备技术;研发制备简易温和、成本低廉、具有抗腐蚀性、耐高温的先进碳载体材料。
中图分类号:
引用本文
蔚德磊, 韩康顺, 陈瑶, 刘祥春, 崔平. 单原子Ni、N共掺杂碳材料基催化剂电还原CO2制CO研究进展[J]. 化工进展, 2024, 43(6): 3174-3186.
YU Delei, HAN Kangshun, CHEN Yao, LIU Xiangchun, CUI Ping. Recent advances in electroreduction of CO2 to CO using single atom Ni, N co-doped carbon-material based catalysts[J]. Chemical Industry and Engineering Progress, 2024, 43(6): 3174-3186.
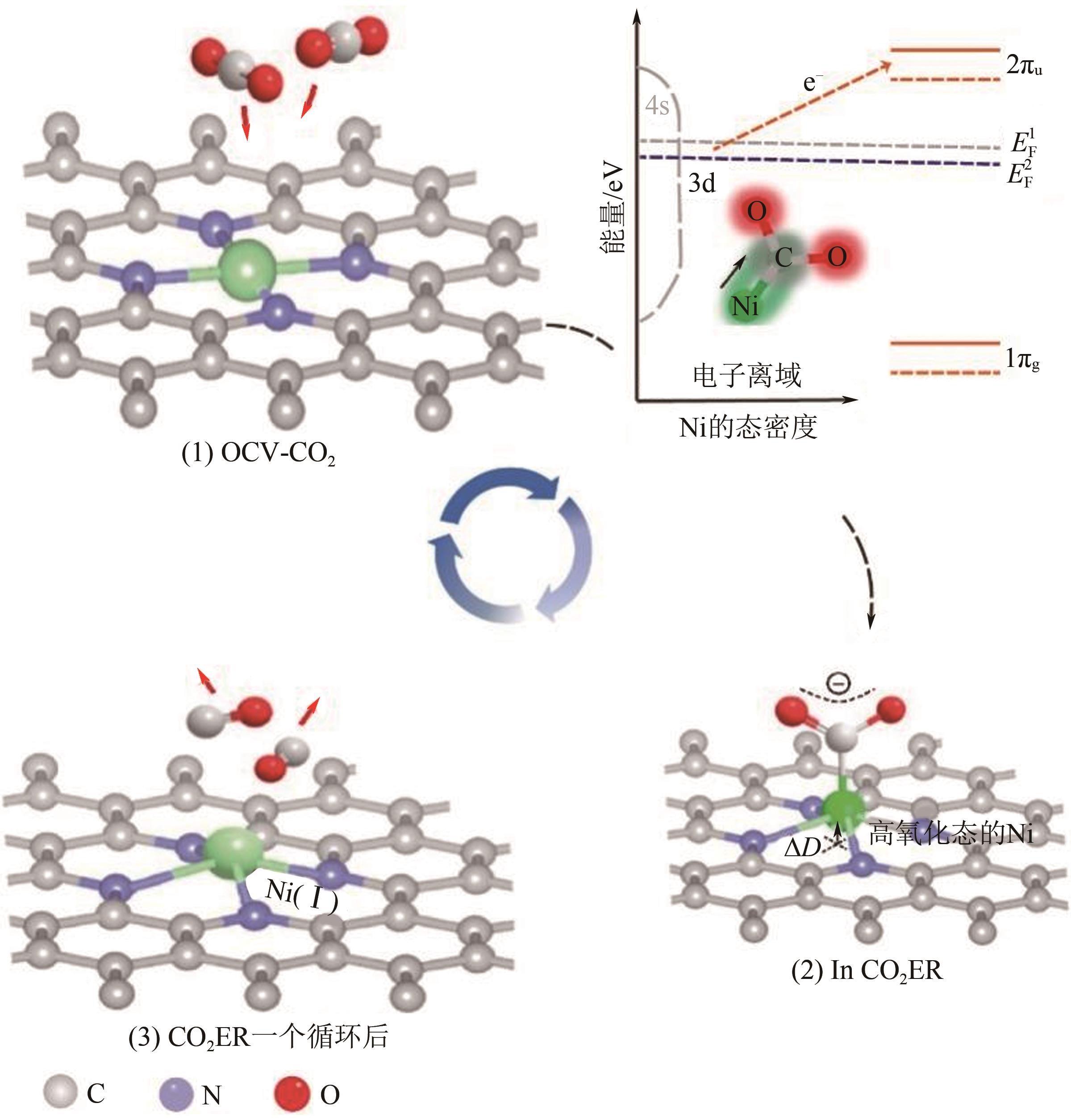
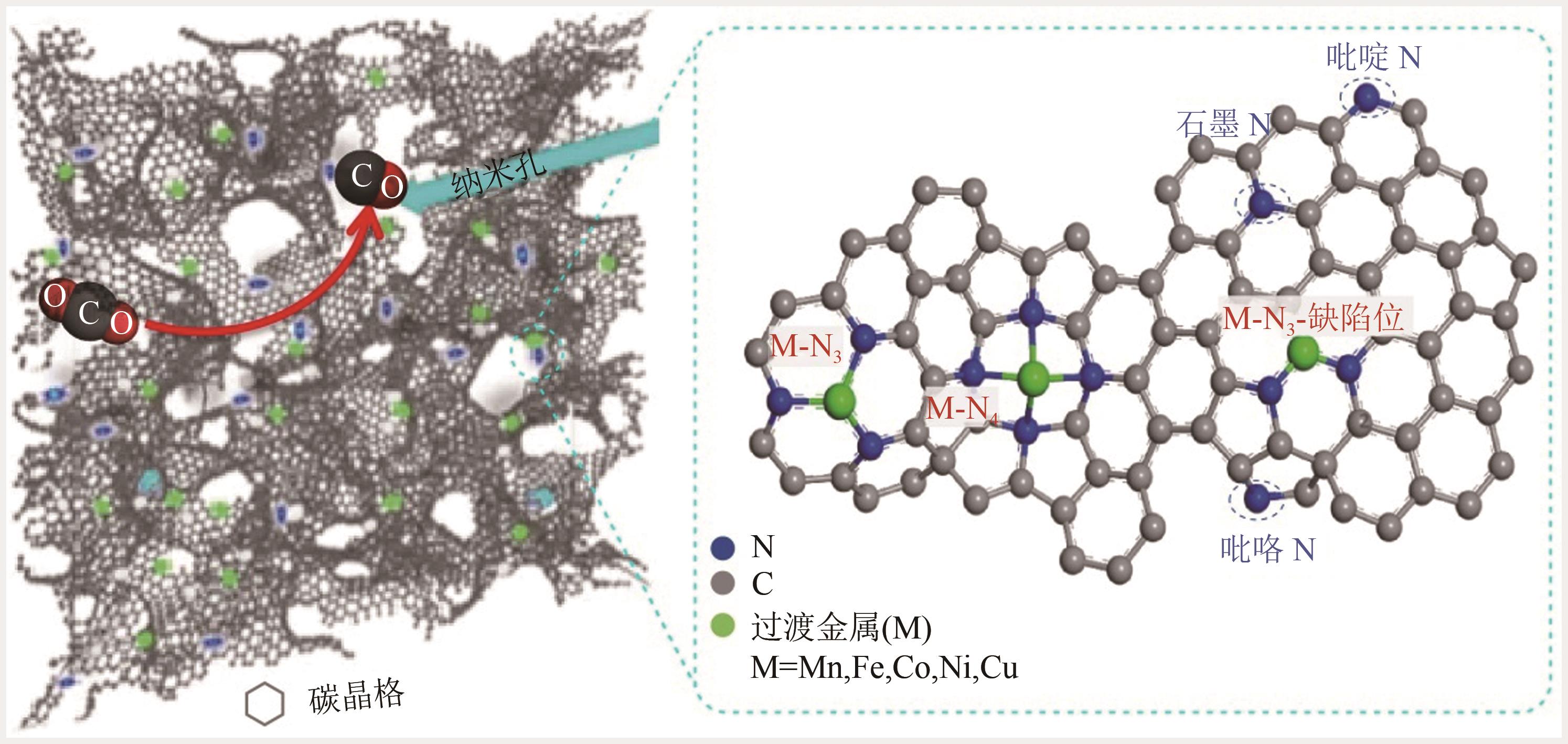
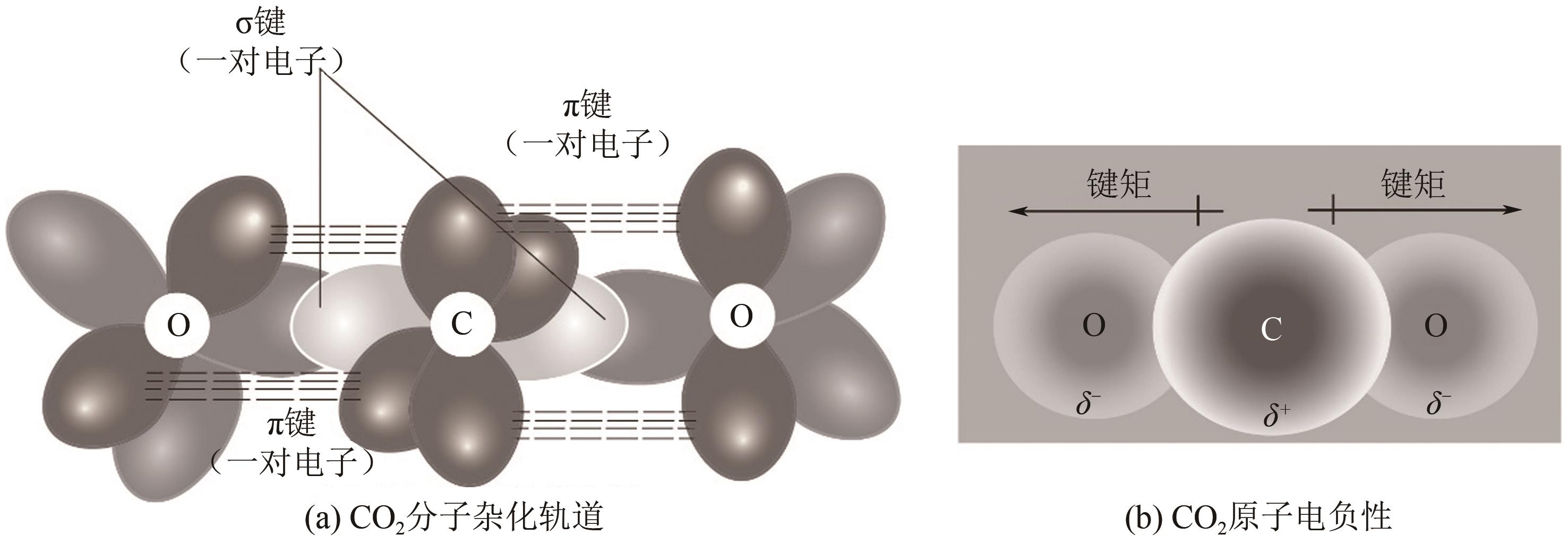
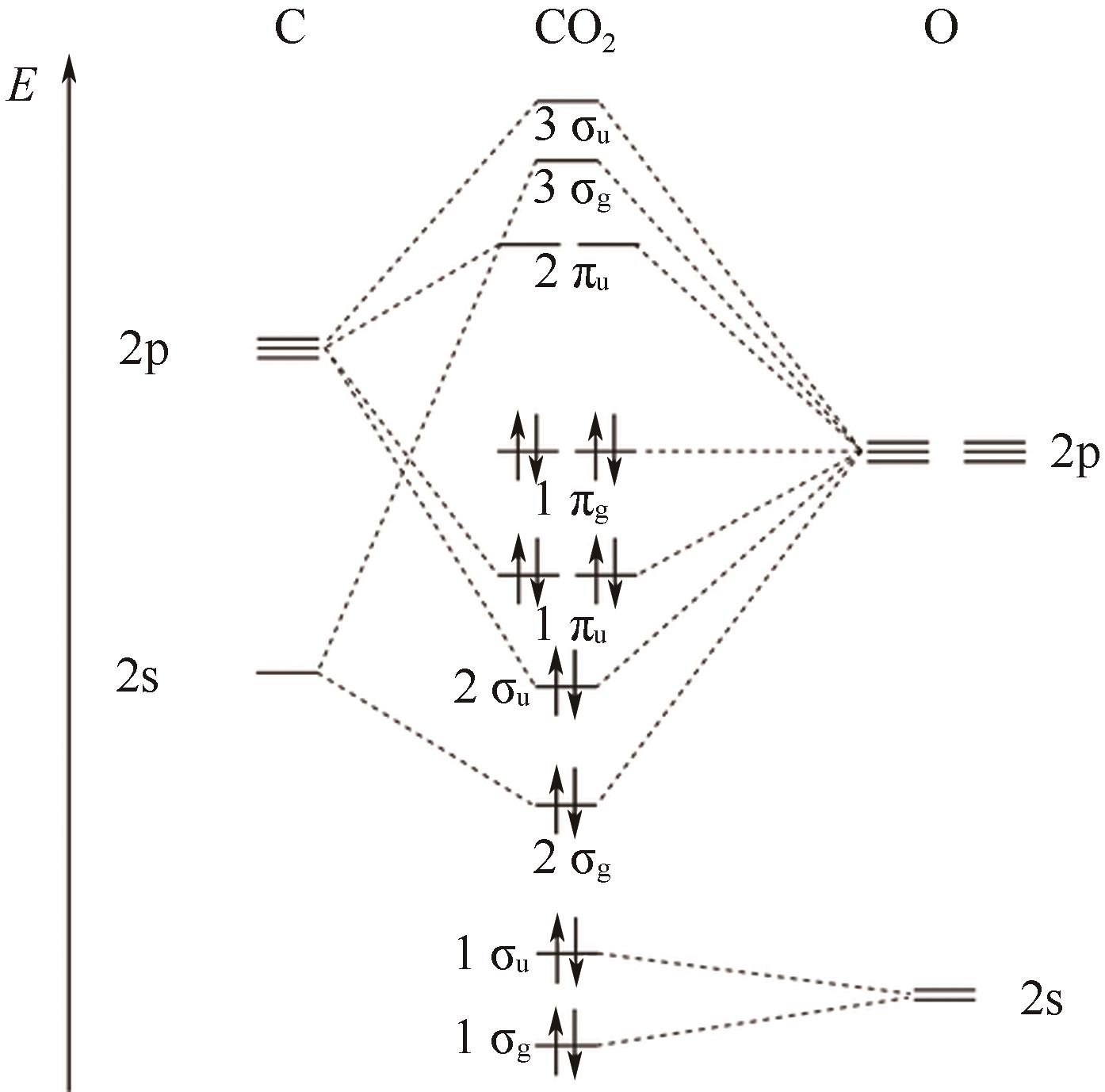
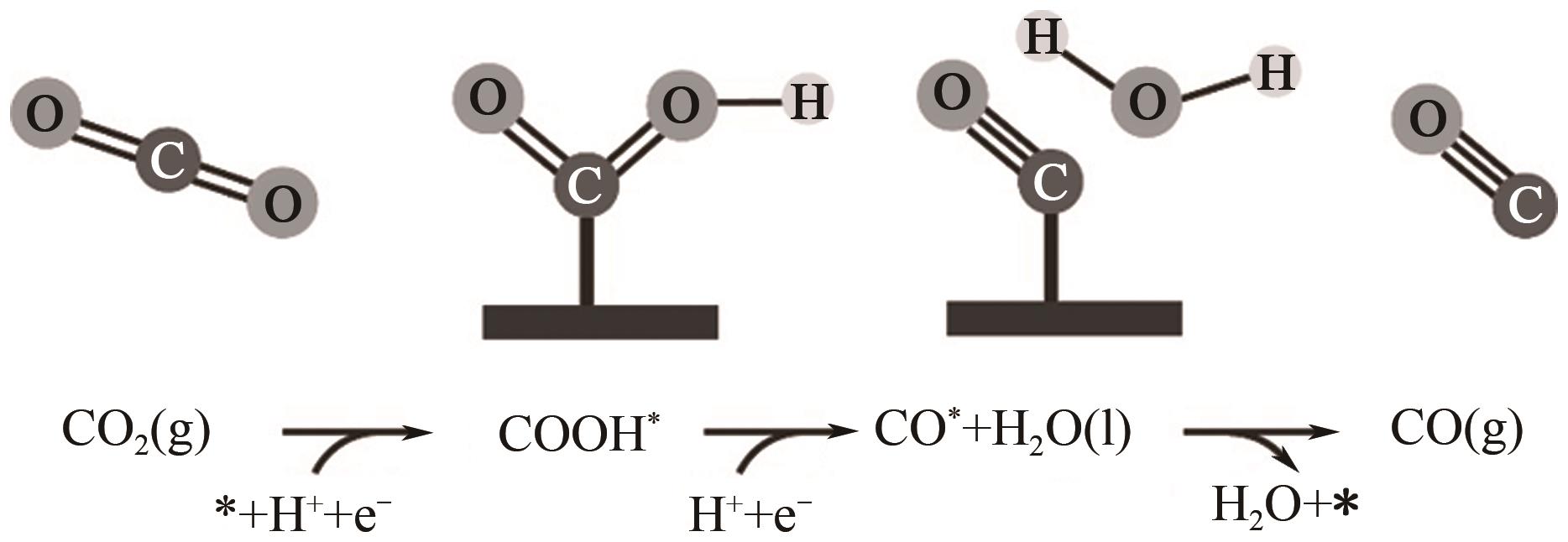
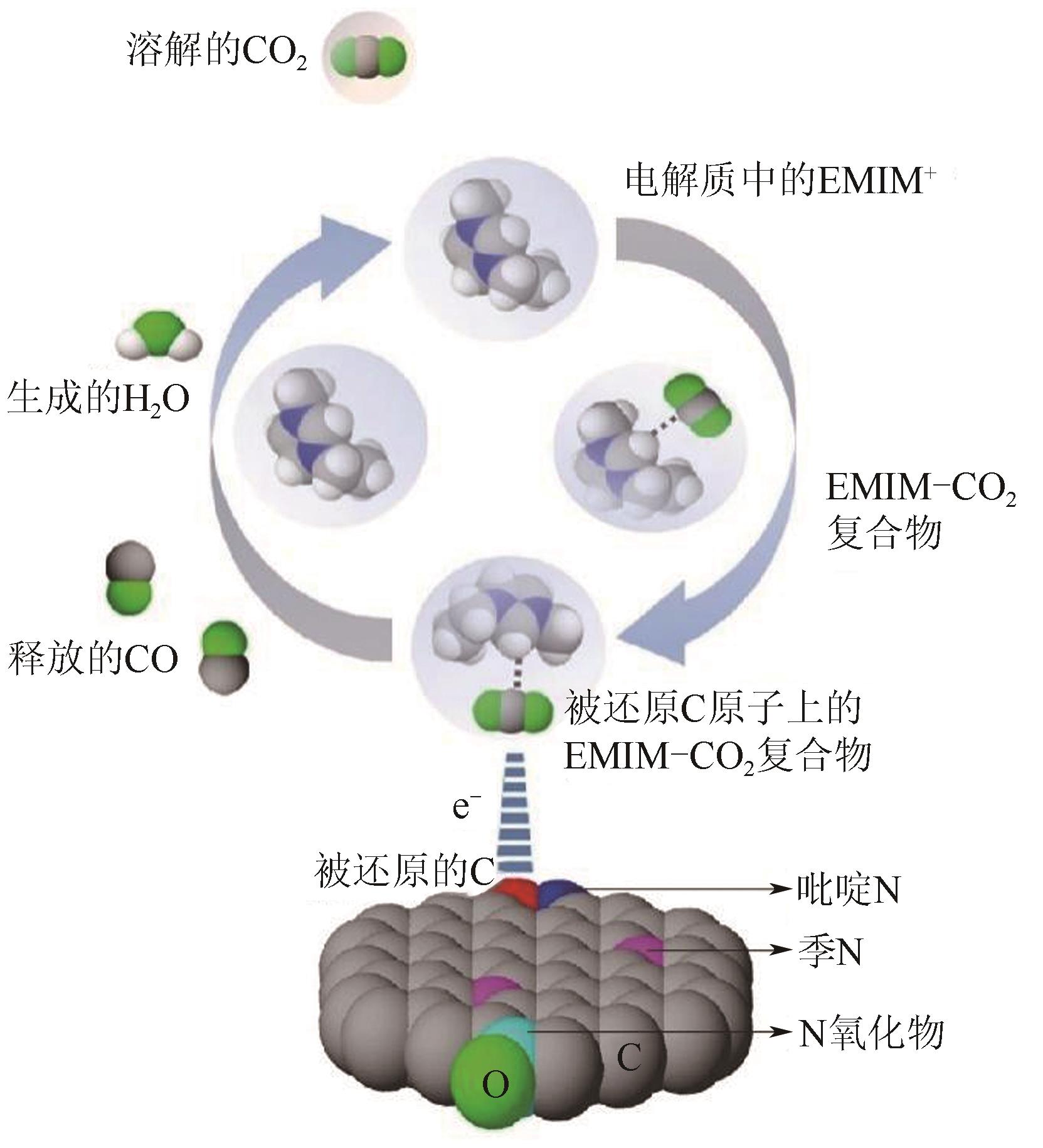
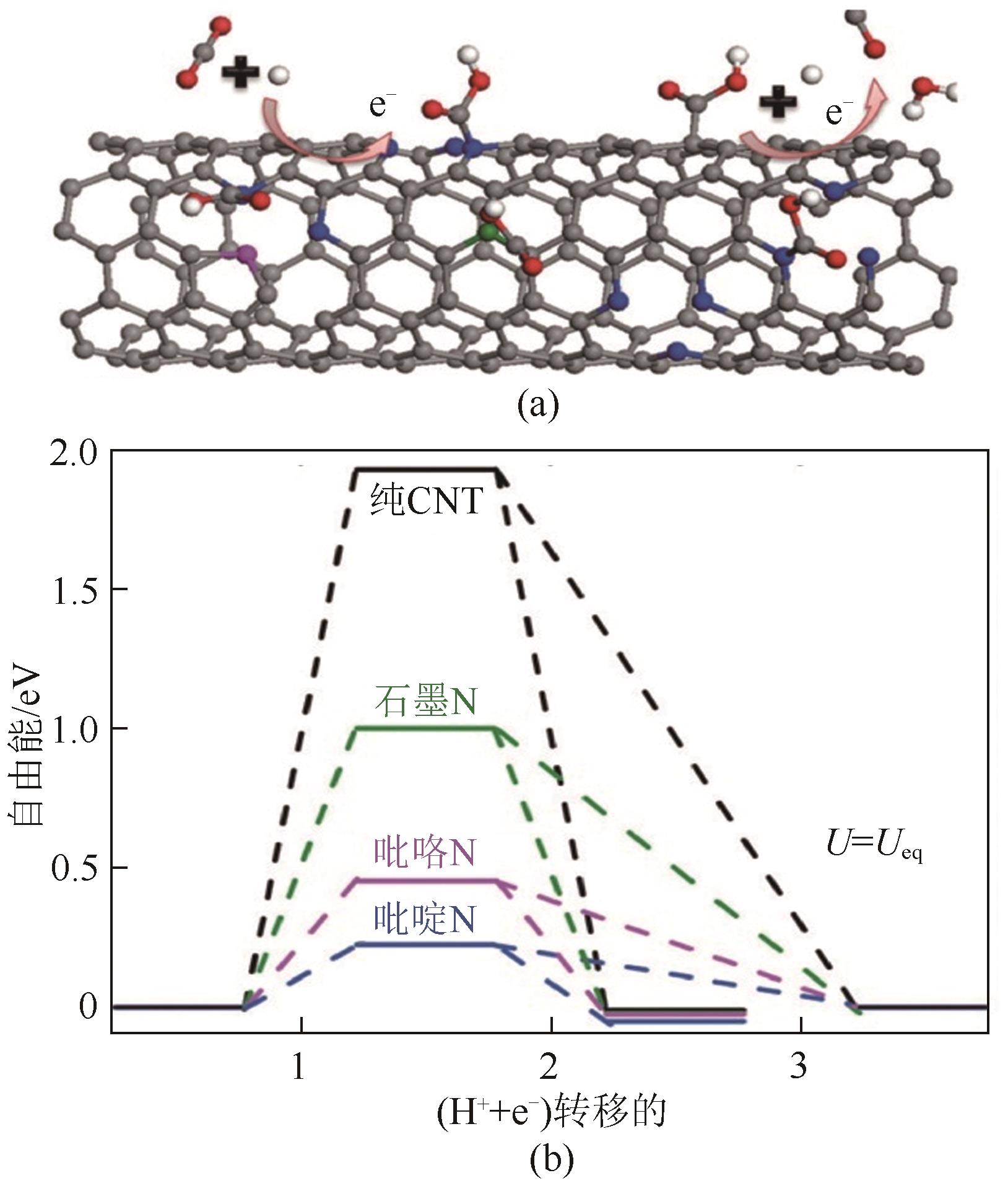
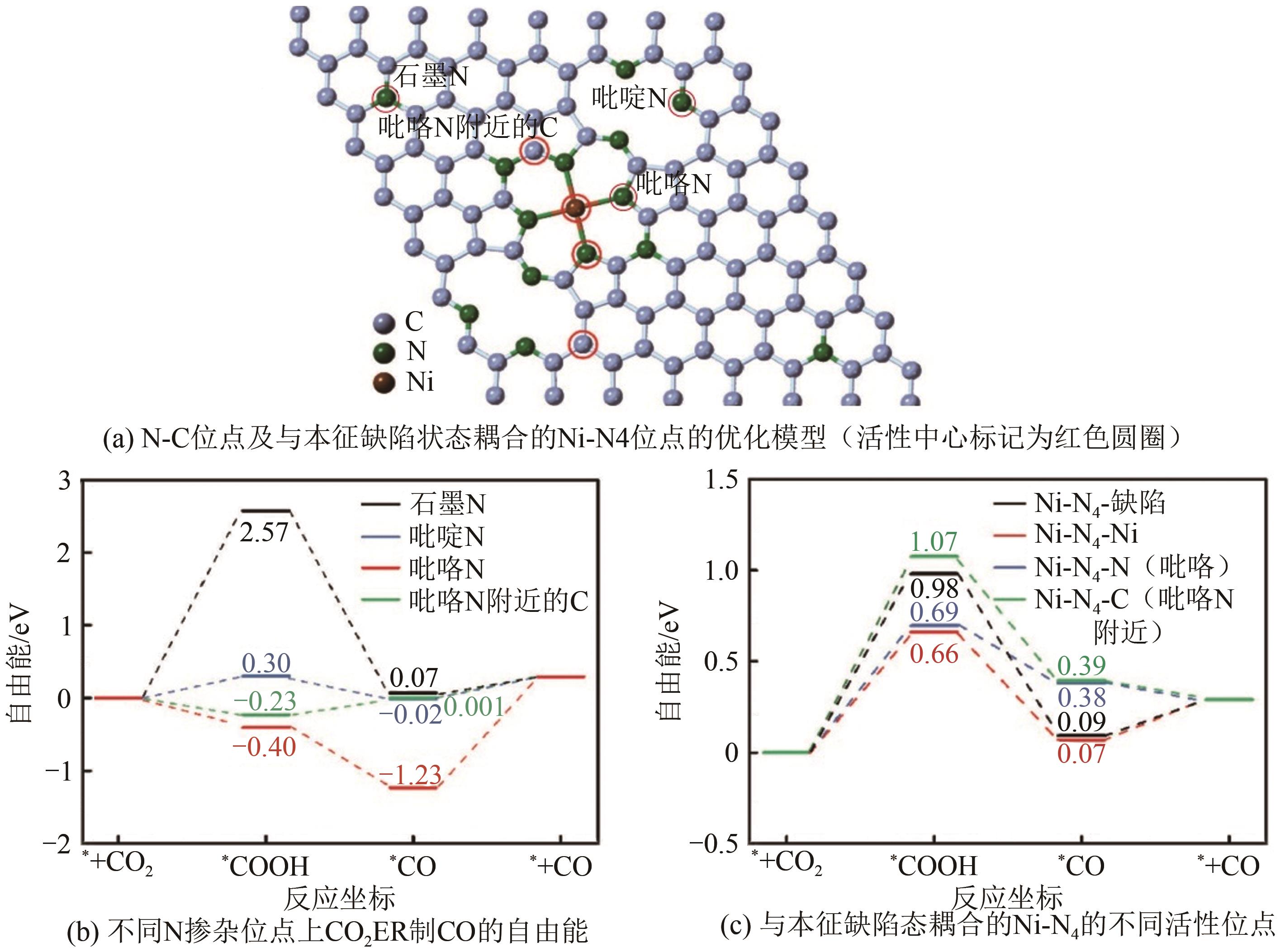
图10 CO2还原活性位点的结构演变[105](ΔD表示Ni原子向CO2电子转移产生的Ni原子离开平面的位移;右上角的示意图显示了Ni(Ⅰ)活性位点上CO2分子的活化过程;红色箭头表示从Ni(Ⅰ)到吸附的CO2的电子转移。EF1和EF2分别是形成Ni-CO2δ- 之前和之后的Ni-N-C的费米能级;1πg和2πu是CO2的分子轨道)
| 制备方法 | 碳材料载体或其前体 | 电化学性能 | 参考文献 | |
|---|---|---|---|---|
| 电解液 | 性能参数 | |||
| 900℃煅烧 | 改性石墨烯 | 0.1mol/L KHCO3 | FECO=90% @ -0.7~-0.9V(vs.RHE);TOF=2700h-1 @ -0.7V(vs.RHE); 稳定运行时长> 5h @ -0.65V(vs.RHE);塔菲尔斜率=126mV/dec | [ |
| 750℃煅烧 | 石墨烯纳米片 | 0.5mol/L KHCO3 | FECO=95%、电流密度=11mA/cm2 @ 620 mV;TOF = 6.8s-1 @ 0.57V; 稳定运行时长> 20h @ 12mA/cm2;塔菲尔斜率=110mV/dec | [ |
| 分子工程法引入甲氧基、1000℃煅烧 | 碳纳米管 | 1mol/L KHCO3 | FECO=99.5% @ 电流密度=10~300mA/cm2; TOF = 2.9s-1 @ -0.64V(vs.RHE);稳定运行时长> 40h @ 150mA/cm2 | [ |
| 微波诱导等离子体法引入S原子 | 氧化石墨烯 | 0.5mol/L KHCO3 | FECO=97%、电流密度=40mA/cm2@ -0.8~-0.9V(vs.RHE); TOF = 3965h-1 @ -0.79V(vs.RHE);稳定运行时长> 20h @ -0.8V | [ |
| 氰基修饰碳基底、670℃煅烧 | C3N4 | 0.5mol/L KHCO3 | FECO≥90% @ -0.58~-1.18V(vs.RHE);电流密度=46.8mA/cm2、 TOF = 22000h-1 @ -1.18V;稳定运行时长> 12h @ -0.88V(vs.RHE) | [ |
| 800~1000℃煅烧、模板法 | 多巴胺 | 0.5mol/L KHCO3 | FECO=96%、电流密度=35mA/cm2@ -1V(vs.RHE); 稳定运行时长>12h @ 2.4V | [ |
| 400~1000℃煅烧 | ZIF-8 | 0.5mol/L KHCO3 | FECO=92%、电流密度=92.1mA/cm2、TOF = 5500h-1 @ -0.9V(vs.RHE); 稳定运行时长> 70h @ -0.6V(vs.RHE) | [ |
| 1000℃煅烧 | ZIF-8 | 0.5mol/L KHCO3 | FECO=71.9%、电流密=10.48mA/cm2、TOF = 5273h-1 @ -0.89V; 稳定运行时长>60h @ -1.0V(vs.RHE);塔菲尔斜率=249mV/dec | [ |
| 静电纺丝法、750℃煅烧 | 石墨壳 | 0.1mol/L KHCO3 | FECO=93.2%、电流密度=4mA/cm2、稳定运行时长>20h @ -0.7V; 塔菲尔斜率=138.5mV/dec | [ |
| 拓扑化学转化法 | 葡萄糖、C3N4 | 0.5mol/L KHCO3 | FECO>90% @ -0.5~-0.9V;电流密度=28.6mA/cm2@ -0.81V; 塔菲尔斜率=103mV/dec | [ |
| 850℃煅烧 | 碳纳米管 | 0.5mol/L KHCO3 | FECO=97% @ -0.83V(vs.RHE);TOF =13860h-1、电流密度=17.5mA/cm2@ -0.94 V(vs.RHE);稳定运行时长>16h @ -0.79V(vs.RHE) | [ |
| 后离子交换法、900℃煅烧 | ZIF-8 | 0.5mol/L KHCO3 | FECO=95.6%、TOF=1425 h-1、电流密度=6.64mA/cm2、 稳定运行时长>10h @ -0.65V;塔菲尔斜率=99mV/dec | [ |
| 950℃煅烧 | 三(4-氨苯基)胺为原料制备酶 | 0.5mol/L KHCO3 | FECO=99.6%、电流密度=1.23A/cm2、TOF =69.7s-1 @ -2.4V(vs.RHE); 稳定运行时长>100h @ -2.2 V(vs.RHE) | [ |
| 静电纺丝法、1000℃煅烧 | 聚丙烯腈、聚苯乙烯 | 0.5mol/L KHCO3 | FECO=96.6% @ -0.8 V(vs.RHE);电流密度=15.5mA/cm2、 TOF=4606.5h-1 @ -1.0V;塔菲尔斜率=124.6mV/dec | [ |
| 600~800℃煅烧 | 苯代三聚氰胺、 氰尿酸 | 0.5mol/L KHCO3 | FECO>90%、电流密度=65mA/cm2、TOF=135000h-1 @ -0.9V(vs.RHE); 稳定运行时长>14h @ -0.7V(vs.RHE);塔菲尔斜率=124mV/dec | [ |
| 900℃煅烧、引入S原子 | 石墨烯 | 0.5mol/L KHCO3 | FECO=97%、电流密度=350A/gcatalyst、TOF = 14800h-1、 稳定运行时长>100h @ 0.61V;塔菲尔斜率=114mV/dec | [ |
| 600℃、1000℃煅烧 | 炭黑 | 0.5mol/L KHCO3 | FECO >95% @ -0.6~-0.84V(vs.RHE);电流密度=21mA/cm2 @ -0. 9V (vs.RHE);稳定运行时长>24h @ 0.55V;塔菲尔斜率=101mV/dec | [ |
| 静电纺丝法、900℃煅烧 | 多孔碳纤维薄膜 | 0.5mol/L KHCO3 | FECO=96%、电流密度=56.1mA/cm2 @ -0.7V(vs.RHE); 塔菲尔斜率=117mV/dec | [ |
表1 典型Ni-N-C的制备方法及其在H型电解池中催化性能总结
| 制备方法 | 碳材料载体或其前体 | 电化学性能 | 参考文献 | |
|---|---|---|---|---|
| 电解液 | 性能参数 | |||
| 900℃煅烧 | 改性石墨烯 | 0.1mol/L KHCO3 | FECO=90% @ -0.7~-0.9V(vs.RHE);TOF=2700h-1 @ -0.7V(vs.RHE); 稳定运行时长> 5h @ -0.65V(vs.RHE);塔菲尔斜率=126mV/dec | [ |
| 750℃煅烧 | 石墨烯纳米片 | 0.5mol/L KHCO3 | FECO=95%、电流密度=11mA/cm2 @ 620 mV;TOF = 6.8s-1 @ 0.57V; 稳定运行时长> 20h @ 12mA/cm2;塔菲尔斜率=110mV/dec | [ |
| 分子工程法引入甲氧基、1000℃煅烧 | 碳纳米管 | 1mol/L KHCO3 | FECO=99.5% @ 电流密度=10~300mA/cm2; TOF = 2.9s-1 @ -0.64V(vs.RHE);稳定运行时长> 40h @ 150mA/cm2 | [ |
| 微波诱导等离子体法引入S原子 | 氧化石墨烯 | 0.5mol/L KHCO3 | FECO=97%、电流密度=40mA/cm2@ -0.8~-0.9V(vs.RHE); TOF = 3965h-1 @ -0.79V(vs.RHE);稳定运行时长> 20h @ -0.8V | [ |
| 氰基修饰碳基底、670℃煅烧 | C3N4 | 0.5mol/L KHCO3 | FECO≥90% @ -0.58~-1.18V(vs.RHE);电流密度=46.8mA/cm2、 TOF = 22000h-1 @ -1.18V;稳定运行时长> 12h @ -0.88V(vs.RHE) | [ |
| 800~1000℃煅烧、模板法 | 多巴胺 | 0.5mol/L KHCO3 | FECO=96%、电流密度=35mA/cm2@ -1V(vs.RHE); 稳定运行时长>12h @ 2.4V | [ |
| 400~1000℃煅烧 | ZIF-8 | 0.5mol/L KHCO3 | FECO=92%、电流密度=92.1mA/cm2、TOF = 5500h-1 @ -0.9V(vs.RHE); 稳定运行时长> 70h @ -0.6V(vs.RHE) | [ |
| 1000℃煅烧 | ZIF-8 | 0.5mol/L KHCO3 | FECO=71.9%、电流密=10.48mA/cm2、TOF = 5273h-1 @ -0.89V; 稳定运行时长>60h @ -1.0V(vs.RHE);塔菲尔斜率=249mV/dec | [ |
| 静电纺丝法、750℃煅烧 | 石墨壳 | 0.1mol/L KHCO3 | FECO=93.2%、电流密度=4mA/cm2、稳定运行时长>20h @ -0.7V; 塔菲尔斜率=138.5mV/dec | [ |
| 拓扑化学转化法 | 葡萄糖、C3N4 | 0.5mol/L KHCO3 | FECO>90% @ -0.5~-0.9V;电流密度=28.6mA/cm2@ -0.81V; 塔菲尔斜率=103mV/dec | [ |
| 850℃煅烧 | 碳纳米管 | 0.5mol/L KHCO3 | FECO=97% @ -0.83V(vs.RHE);TOF =13860h-1、电流密度=17.5mA/cm2@ -0.94 V(vs.RHE);稳定运行时长>16h @ -0.79V(vs.RHE) | [ |
| 后离子交换法、900℃煅烧 | ZIF-8 | 0.5mol/L KHCO3 | FECO=95.6%、TOF=1425 h-1、电流密度=6.64mA/cm2、 稳定运行时长>10h @ -0.65V;塔菲尔斜率=99mV/dec | [ |
| 950℃煅烧 | 三(4-氨苯基)胺为原料制备酶 | 0.5mol/L KHCO3 | FECO=99.6%、电流密度=1.23A/cm2、TOF =69.7s-1 @ -2.4V(vs.RHE); 稳定运行时长>100h @ -2.2 V(vs.RHE) | [ |
| 静电纺丝法、1000℃煅烧 | 聚丙烯腈、聚苯乙烯 | 0.5mol/L KHCO3 | FECO=96.6% @ -0.8 V(vs.RHE);电流密度=15.5mA/cm2、 TOF=4606.5h-1 @ -1.0V;塔菲尔斜率=124.6mV/dec | [ |
| 600~800℃煅烧 | 苯代三聚氰胺、 氰尿酸 | 0.5mol/L KHCO3 | FECO>90%、电流密度=65mA/cm2、TOF=135000h-1 @ -0.9V(vs.RHE); 稳定运行时长>14h @ -0.7V(vs.RHE);塔菲尔斜率=124mV/dec | [ |
| 900℃煅烧、引入S原子 | 石墨烯 | 0.5mol/L KHCO3 | FECO=97%、电流密度=350A/gcatalyst、TOF = 14800h-1、 稳定运行时长>100h @ 0.61V;塔菲尔斜率=114mV/dec | [ |
| 600℃、1000℃煅烧 | 炭黑 | 0.5mol/L KHCO3 | FECO >95% @ -0.6~-0.84V(vs.RHE);电流密度=21mA/cm2 @ -0. 9V (vs.RHE);稳定运行时长>24h @ 0.55V;塔菲尔斜率=101mV/dec | [ |
| 静电纺丝法、900℃煅烧 | 多孔碳纤维薄膜 | 0.5mol/L KHCO3 | FECO=96%、电流密度=56.1mA/cm2 @ -0.7V(vs.RHE); 塔菲尔斜率=117mV/dec | [ |
| 50 | SUN Lu, YAN Chunjie, CHEN Ying, et al. Preparation of amorphous carbon nanotubes using attapulgite as template and furfuryl alcohol as carbon source[J]. Journal of Non-Crystalline Solids, 2012, 358(18/19): 2723-2726. |
| 51 | RODRÍGUEZ-MANZO Julio A, CRETU Ovidiu, BANHART Florian. Trapping of metal atoms in vacancies of carbon nanotubes and graphene[J]. ACS Nano, 2010, 4(6): 3422-3428. |
| 52 | NOVOSELOV K S, GEIM A K, MOROZOV S V, et al. Electric field effect in atomically thin carbon films[J]. Science, 2004, 306(5696): 666-669. |
| 53 | GONG Kuanping, DU Feng, XIA Zhenhai, et al. Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction[J]. Science, 2009, 323(5915): 760-764. |
| 54 | ZHENG Yao, JIAO Yan, CHEN Jun, et al. Nanoporous graphitic-C3N4@carbon metal-free electrocatalysts for highly efficient oxygen reduction[J]. Journal of the American Chemical Society, 2011, 133(50): 20116-20119. |
| 55 | LIU Ruili, WU Dongqing, FENG Xinliang, et al. Nitrogen-doped ordered mesoporous graphitic arrays with high electrocatalytic activity for oxygen reduction[J]. Angewandte Chemie International Edition, 2010, 49(14): 2565-2569. |
| 56 | LI Yanguang, ZHOU Wu, WANG Hailiang, et al. An oxygen reduction electrocatalyst based on carbon nanotube-graphene complexes[J]. Nature Nanotechnology, 2012, 7(6): 394-400. |
| 57 | YANG Shubin, ZHI Linjie, TANG Kun, et al. Efficient synthesis of heteroatom (N or S)-doped graphene based on ultrathin graphene oxide-porous silica sheets for oxygen reduction reactions[J]. Advanced Functional Materials, 2012, 22(17): 3634-3640. |
| 58 | SHARMA Pranav P, WU Jingjie, YADAV Ram Manohar, et al. Nitrogen-doped carbon nanotube arrays for high-efficiency electrochemical reduction of CO2: On the understanding of defects, defect density, and selectivity[J]. Angewandte Chemie International Edition, 2015, 54(46): 13701-13705. |
| 59 | VARELA Ana Sofia, SAHRAIE Nastaran Ranjbar, STEINBERG Julian, et al. Metal-doped nitrogenated carbon as an efficient catalyst for direct CO2 electroreduction to CO and hydrocarbons[J]. Angewandte Chemie International Edition, 2015, 54(37): 10758-10762. |
| 60 | ZHENG Tingting, ZHANG Menglu, WU Lianghuan, et al. Upcycling CO2 into energy-rich long-chain compounds via electrochemical and metabolic engineering[J]. Nature Catalysis, 2022, 5(5): 388-396. |
| 61 | ZHAO Di, YU Ke, SONG Pengyu, et al. Atomic-level engineering Fe1N2O2 interfacial structure derived from oxygen-abundant metal-organic frameworks to promote electrochemical CO2 reduction[J]. Energy & Environmental Science, 2022, 15(9): 3795-3804. |
| 62 | YE Yifan, CAI Fan, LI Haobo, et al. Surface functionalization of ZIF-8 with ammonium ferric citrate toward high exposure of Fe-N active sites for efficient oxygen and carbon dioxide electroreduction[J]. Nano Energy, 2017, 38: 281-289. |
| 63 | LIU Tianfu, Sajjad ALI, LIAN Zan, et al. CO2 electoreduction reaction on heteroatom-doped carbon cathode materials[J]. Journal of Materials Chemistry A, 2017, 5(41): 21596-21603. |
| 64 | VOIRY Damien, SHIN Hyeon Suk, Kian Ping LOH, et al. Low-dimensional catalysts for hydrogen evolution and CO2 reduction[J]. Nature Reviews Chemistry, 2018, 2: 105. |
| 65 | KUMAR Bijandra, ASADI Mohammad, PISASALE Davide, et al. Renewable and metal-free carbon nanofibre catalysts for carbon dioxide reduction[J]. Nature Communications, 2013, 4: 2819. |
| 66 | WEI Dacheng, LIU Yunqi, WANG Yu, et al. Synthesis of N-doped graphene by chemical vapor deposition and its electrical properties[J]. Nano Letters, 2009, 9(5): 1752-1758. |
| 67 | DENG Dehui, CHEN Xiaoqi, YU Liang, et al. A single iron site confined in a graphene matrix for the catalytic oxidation of benzene at room temperature[J]. Science Advances, 2015, 1(11): e1500462. |
| 68 | BOPPELLA Ramireddy, Muthu AUSTERIA P, KIM Yujin, et al. Pyrrolic N-stabilized monovalent Ni single-atom electrocatalyst for efficient CO2 reduction: Identifying the role of pyrrolic-N and synergistic electrocatalysis[J]. Advanced Functional Materials, 2022, 32(35): 2202351. |
| 69 | SMITH Rodney D L, PICKUP Peter G. Nitrogen-rich polymers for the electrocatalytic reduction of CO2 [J]. Electrochemistry Communications, 2010, 12(12): 1749-1751. |
| 70 | WANG Wei, SHANG Lu, CHANG Guojing, et al. Intrinsic carbon-defect-driven electrocatalytic reduction of carbon dioxide[J]. Advanced Materials, 2019, 31(19): 1808276. |
| 71 | WU Jingjie, MA Sichao, SUN Jing, et al. A metal-free electrocatalyst for carbon dioxide reduction to multi-carbon hydrocarbons and oxygenates[J]. Nature Communications, 2016, 7: 13869. |
| 72 | WU Jingjie, YADAV Ram Manohar, LIU Mingjie, et al. Achieving highly efficient, selective, and stable CO2 reduction on nitrogen-doped carbon nanotubes[J]. ACS Nano, 2015, 9(5): 5364-5371. |
| 73 | WU Jingjie, LIU Mingjie, SHARMA Pranav P, et al. Incorporation of nitrogen defects for efficient reduction of CO2 via two-electron pathway on three-dimensional graphene foam[J]. Nano Letters, 2016, 16(1): 466-470. |
| 74 | WANG Hong, JIA Jia, SONG Pengfei, et al. Efficient electrocatalytic reduction of CO2 by nitrogen-doped nanoporous carbon/carbon nanotube membranes: A step towards the electrochemical CO2 refinery[J]. Angewandte Chemie International Edition, 2017, 56(27): 7847-7852. |
| 1 | QIAO Jinli, LIU Yuyu, HONG Feng, et al. A review of catalysts for the electroreduction of carbon dioxide to produce low-carbon fuels[J]. Chemical Society Reviews, 2014, 43(2): 631-675. |
| 2 | LI Christina W, CISTON Jim, KANAN Matthew W. Electroreduction of carbon monoxide to liquid fuel on oxide-derived nanocrystalline copper[J]. Nature, 2014, 508(7497): 504-507. |
| 3 | WU Yueshen, JIANG Zhan, LU Xu, et al. Domino electroreduction of CO2 to methanol on a molecular catalyst[J]. Nature, 2019, 575(7784): 639-642. |
| 4 | 姚青, 俞志勇, 黄小青. 单原子催化剂的合成及其能源电催化应用的研究进展[J]. 高等学校化学学报, 2022, 43(9): 149-167. |
| YAO Qing, YU Zhiyong, HUANG Xiaoqing. Progress in synthesis and energy-related electrocatalysis of single-atom catalysts [J]. Chemical Journal of Chinese Universities, 2022, 43(9): 149-167. | |
| 5 | CYRILLE Costentin, MARC Robert, Savéant JEAN-MICHEL. Catalysis of the electrochemical reduction of carbon dioxide[J]. Chemical Society Reviews, 2013, 42(6): 2423-2436. |
| 6 | IZUMI Yasuo. Recent advances in the photocatalytic conversion of carbon dioxide to fuels with water and/or hydrogen using solar energy and beyond[J]. Coordination Chemistry Reviews, 2013, 257(1): 171-186. |
| 7 | LIU Min, PANG Yuanjie, ZHANG Bo, et al. Enhanced electrocatalytic CO2 reduction via field-induced reagent concentration[J]. Nature, 2016, 537(7620): 382-386. |
| 8 | 王磊, 蒋勇, 钟达忠, 等. 碳化的MOF用于电催化还原二氧化碳制备乙烯和乙醇[J]. 化工学报, 2022, 73(8): 3576-3585. |
| WANG Lei, JIANG Yong, ZHONG Dazhong, et al. Carbonized metal-organic framework for carbon dioxide reduction to ethylene and ethanol[J]. CIESC Journal, 2022, 73(8): 3576-3585. | |
| 9 | DUAN Xiaochuan, XU Jiantie, WEI Zengxi, et al. Metal-free carbon materials for CO2 electrochemical reduction[J]. Advanced Materials, 2017, 29(41): 1701784. |
| 10 | AGARWAL Arun S, ZHAI Yumei, Hill Davion, et al. The electrochemical reduction of carbon dioxide to formate/formic acid: Engineering and economic feasibility[J]. ChemSusChem, 2011, 4(9): 1301-1310. |
| 11 | JHONG Huei-Ru, MA Sichao, KENIS Paul. Electrochemical conversion of CO2 to useful chemicals: Current status, remaining challenges, and future opportunities[J]. Current Opinion in Chemical Engineering, 2013, 2(2): 191-199. |
| 75 | WANG Hongxia, CHEN Yabin, HOU Xiaoli, et al. Nitrogen-doped graphenes as efficient electrocatalysts for the selective reduction of carbon dioxide to formate in aqueous solution[J]. Green Chemistry, 2016, 18(11): 3250-3256. |
| 76 | LI Wanlu, SEREDYCH Mykola, Enrique RODRÍGUEZ-CASTELLÓN, et al. Metal-free nanoporous carbon as a catalyst for electrochemical reduction of CO2 to CO and CH4 [J]. ChemSusChem, 2016, 9(6): 606-616. |
| 77 | XU Junyuan, KAN Yuhe, HUANG Rui, et al. Revealing the origin of activity in nitrogen-doped nanocarbons towards electrocatalytic reduction of carbon dioxide[J]. ChemSusChem, 2016, 9(10): 1085-1089. |
| 78 | CHAI Guoliang, GUO Zhengxiao. Highly effective sites and selectivity of nitrogen-doped graphene/CNT catalysts for CO2 electrochemical reduction[J]. Chemical Science, 2016, 7(2): 1268-1275. |
| 79 | ZHENG Tingting, JIANG Kun, WANG Haotian. Recent advances in electrochemical CO2-to-CO conversion on heterogeneous catalysts[J]. Advanced Materials, 2018, 30(48): 1802066. |
| 80 | ZENG Qingting, YANG Guangxing, CHEN Jianhao, et al. Effects of nitrogen and oxygen on electrochemical reduction of CO2 in nitrogen-doped carbon black[J]. Carbon, 2023, 202: 1-11. |
| 81 | LIU Weiqi, QI Jiawei, BAI Peiyao, et al. Utilizing spatial confinement effect of N atoms in micropores of coal-based metal-free material for efficiently electrochemical reduction of carbon dioxide[J]. Applied Catalysis B: Environmental, 2020, 272: 118974. |
| 82 | LI Chen, WANG Yuwei, XIAO Nan, et al. Nitrogen-doped porous carbon from coal for high efficiency CO2 electrocatalytic reduction[J]. Carbon, 2019, 151: 46-52. |
| 83 | TORNOW Claire E, THORSON Michael R, MA Sichao, et al. Nitrogen-based catalysts for the electrochemical reduction of CO2 to CO[J]. Journal of the American Chemical Society, 2012, 134(48): 19520-19523. |
| 84 | COSTENTIN Cyrille, DROUET Samuel, ROBERT Marc, et al. A local proton source enhances CO2 electroreduction to CO by a molecular Fe catalyst[J]. Science, 2012, 338(6103): 90-94. |
| 85 | BHUGUN Iqbal, LEXA Doris, Jean-Michel SAVÉANT. Catalysis of the electrochemical reduction of carbon dioxide by iron(0) porphyrins: Synergystic effect of weak Brönsted acids[J]. Journal of the American Chemical Society, 1996, 118(7): 1769-1776. |
| 86 | FROEHLICH Jesse D, KUBIAK Clifford P. The homogeneous reduction of CO2 by [Ni(cyclam)]+: Increased catalytic rates with the addition of a CO scavenger[J]. Journal of the American Chemical Society, 2015, 137(10): 3565-3573. |
| 87 | FROEHLICH Jesse D, KUBIAK Clifford P. Homogeneous CO2 reduction by Ni(cyclam) at a glassy carbon electrode[J]. Inorganic Chemistry, 2012, 51(7): 3932-3934. |
| 12 | FINN Colin, SCHNITTGER Sorcha, YELLOWLEES Lesley J, et al. Molecular approaches to the electrochemical reduction of carbon dioxide[J]. Chemical Communications, 2012, 48(10): 1392-1399. |
| 13 | LU Qi, ROSEN Jonathan, ZHOU Yang, et al. A selective and efficient electrocatalyst for carbon dioxide reduction[J]. Nature Communications, 2014, 5: 3242. |
| 14 | MISTRY Hemma, VARELA Ana Sofia, BONIFACIO Cecile S, et al. Highly selective plasma-activated copper catalysts for carbon dioxide reduction to ethylene[J]. Nature Communications, 2016, 7: 12123. |
| 15 | 于丰收, 湛佳宇, 张鲁华. p区金属基电催化还原二氧化碳制甲酸催化剂研究进展[J]. 化学进展, 2022, 34(4): 983-991. |
| YU Fengshou, ZHAN Jiayu, ZHANG Luhua. The progress on electrochemical CO2-to-formate conversion by p-block metal based catalysts[J]. Progress in Chemistry, 2022, 34(4): 983-991. | |
| 16 | LEE Seunghwa, KIM Dahee, LEE Jaeyoung. Electrocatalytic production of C3-C4 compounds by conversion of CO2 on a chloride-induced Bi-phasic Cu2O-Cu catalyst[J]. Angewandte Chemie (International Ed in English), 2015, 54(49): 14701-14705. |
| 17 | NGUYEN Dang Le Tri, KIM Younghye, HWANG Yun Jeong, et al. Progress in development of electrocatalyst for CO2 conversion to selective CO production[J]. Carbon Energy, 2020, 2(1): 72-98. |
| 18 | JU Wen, BAGGER Alexander, HAO Guangping, et al. Understanding activity and selectivity of metal-nitrogen-doped carbon catalysts for electrochemical reduction of CO2 [J]. Nature Communications, 2017, 8: 944. |
| 19 | BLIGAARD T, NØRSKOV J K, DAHL S, et al. The Brønsted-Evans-Polanyi relation and the volcano curve in heterogeneous catalysis[J]. Journal of Catalysis, 2004, 224(1): 206-217. |
| 20 | LI Jingkun, Paulina PRŠLJA, SHINAGAWA Tatsuya, et al. Volcano trend in electrocatalytic CO2 reduction activity over atomically dispersed metal sites on nitrogen-doped carbon[J]. ACS Catalysis, 2019, 9(11): 10426-10439. |
| 21 | HANSEN Heine A, VARLEY Joel B, PETERSON Andrew A, et al. Understanding trends in the electrocatalytic activity of metals and enzymes for CO2 reduction to CO[J]. The Journal of Physical Chemistry Letters, 2013, 4(3): 388-392. |
| 22 | 张超. 单原子催化剂电催化还原二氧化碳研究进展[J]. 应用化学, 2022, 39(6): 871-887. |
| ZHANG Chao. Research prospect of single atom catalysts towards electrocatalytic reduction of carbon dioxide[J]. Chinese Journal of Applied Chemistry, 2022, 39(6): 871-887. | |
| 88 | KELLY Craig A, MULAZZANI Quinto G, VENTURI Margherita, et al. The thermodynamics and kinetics of CO2 and H+ binding to Ni(cyclam)+ in aqueous solution[J]. Journal of the American Chemical Society, 1995, 117(17): 4911-4919. |
| 89 | SONG Jinshuai, KLEIN Eric L, Neese Frank, et al. The mechanism of homogeneous CO2 reduction by Ni(cyclam): Product selectivity, concerted proton-electron transfer and C—O bond cleavage[J]. Inorganic Chemistry, 2014, 53(14): 7500-7507. |
| 90 | SU Panpan, IWASE Kazuyuki, NAKANISHI Shuji, et al. Nickel-nitrogen-modified graphene: An efficient electrocatalyst for the reduction of carbon dioxide to carbon monoxide[J]. Small, 2016, 12(44): 6083-6089. |
| 91 | JIANG Kun, SIAHROSTAMI Samira, ZHENG Tingting, et al. Isolated Ni single atoms in graphene nanosheets for high-performance CO2 reduction[J]. Energy & Environmental Science, 2018, 11(4): 893-903. |
| 92 | ZHANG Xiao, WANG Yang, GU Meng, et al. Molecular engineering of dispersed nickel phthalocyanines on carbon nanotubes for selective CO2 reduction[J]. Nature Energy, 2020, 5(9): 684-692. |
| 93 | JIA Chen, TAN Xin, ZHAO Yong, et al. Sulfur-dopant-promoted electroreduction of CO2 over coordinatively unsaturated Ni-N2 moieties[J]. Angewandte Chemie International Edition, 2021, 60(43): 23342-23348. |
| 94 | DAIYAN Rahman, ZHU Xiaofeng, TONG Zizheng, et al. Transforming active sites in nickel-nitrogen-carbon catalysts for efficient electrochemical CO2 reduction to CO[J]. Nano Energy, 2020, 78: 105213. |
| 95 | LI Yi, ADLI Nadia Mohd, SHAN Weitao, et al. Atomically dispersed single Ni site catalysts for high-efficiency CO2 electroreduction at industrial-level current densities[J]. Energy & Environmental Science, 2022, 15(5): 2108-2119. |
| 96 | QIU H J, Ito Yoshikazu, CONG Weitao, et al. Nanoporous graphene with single-atom nickel dopants: An efficient and stable catalyst for electrochemical hydrogen production[J]. Angewandte Chemie International Edition, 2015, 54(47): 14031-14035. |
| 97 | FAN Lili, LIU Pengfei, YAN Xuecheng, et al. Atomically isolated nickel species anchored on graphitized carbon for efficient hydrogen evolution electrocatalysis[J]. Nature Communications, 2016, 7: 10667. |
| 98 | ZHAO Changming, DAI Xinyao, YAO Tao, et al. Ionic exchange of metal-organic frameworks to access single nickel sites for efficient electroreduction of CO2 [J]. Journal of the American Chemical Society, 2017, 139(24): 8078-8081. |
| 99 | Young Jin SA, JUNG Hyejin, SHIN Dongyup, et al. Thermal transformation of molecular Ni2+-N4 sites for enhanced CO2 electroreduction activity[J]. ACS Catalysis, 2020, 10(19): 10920-10931. |
| 100 | ZHANG Yan, JIAO Long, YANG Weijie, et al. Rational fabrication of low-coordinate single-atom Ni electrocatalysts by MOFs for highly selective CO2 reduction[J]. Angewandte Chemie International Edition, 2021, 60(14): 7607-7611. |
| 23 | ZHANG Wenjun, HU Yi, MA Lianbo, et al. Progress and perspective of electrocatalytic CO2 Reduction for renewable carbonaceous fuels and chemicals[J]. Advanced Science, 2018, 5(1): 1700275. |
| 24 | GAO Shan, LIN Yue, JIAO Xingchen, et al. Partially oxidized atomic cobalt layers for carbon dioxide electroreduction to liquid fuel[J]. Nature, 2016, 529(7584): 68-71. |
| 25 | FRANCKE Robert, SCHILLE Benjamin, ROEMELT Michael. Homogeneously catalyzed electroreduction of carbon dioxide: Methods, mechanisms, and catalysts[J]. Chemical Reviews, 2018, 118(9): 4631-4701. |
| 26 | KUHL Kendra P, HATSUKADE Toru, CAVE Etosha R, et al. Electrocatalytic conversion of carbon dioxide to methane and methanol on transition metal surfaces[J]. Journal of the American Chemical Society, 2014, 136(40): 14107-14113. |
| 27 | SHI Chuan, HANSEN Heine A, LAUSCHE Adam C, et al. Trends in electrochemical CO2 reduction activity for open and close-packed metal surfaces[J]. Physical Chemistry Chemical Physics, 2014, 16(10): 4720-4727. |
| 28 | GONG Ming, ZHOU Wu, TSAI Mon-Che, et al. Nanoscale nickel oxide/nickel heterostructures for active hydrogen evolution electrocatalysis[J]. Nature Communications, 2014, 5: 4695. |
| 29 | HORI Yoshio, WAKEBE Hidetoshi, TSUKAMOTO Toshio, et al. Electrocatalytic process of CO selectivity in electrochemical reduction of CO2 at metal electrodes in aqueous media[J]. Electrochimica Acta, 1994, 39(11/12): 1833-1839. |
| 30 | FISHER Barbara J, EISENBERG Richard. Electrocatalytic reduction of carbon dioxide by using macrocycles of nickel and cobalt[J]. Journal of the American Chemical Society, 1980, 102(24): 7361-7363. |
| 31 | BENSON Eric E, KUBIAK Clifford P, SATHRUM Aaron J, et al. Electrocatalytic and homogeneous approaches to conversion of CO2 to liquid fuels[J]. Chemical Society Reviews, 2009, 38(1): 89-99. |
| 32 | JIANG Kun, SIAHROSTAMI Samira, AKEY Austin J, et al. Transition-metal single atoms in a graphene shell as active centers for highly efficient artificial photosynthesis[J]. Chem, 2017, 3(6): 950-960. |
| 33 | 秦永吉, 罗俊. 单原子催化剂在CO2转化中的应用[J]. 高等学校化学学报, 2022, 43(9): 13-28. |
| QIN Yongji, LUO Jun. Applications of single-atom catalysts in CO2 conversion [J]. Chemical Journal of Chinese Universities, 2022, 43(9): 13-28. | |
| 34 | LARRAZÁBAL Gastón O, MARTÍN Antonio J, Javier PÉREZ-RAMÍREZ. Building blocks for high performance in electrocatalytic CO2 reduction: Materials, optimization strategies, and device engineering[J]. The Journal of Physical Chemistry Letters, 2017, 8(16): 3933-3944. |
| 35 | YOO Jong Suk, CHRISTENSEN Rune, VEGGE Tejs, et al. Theoretical insight into the trends that guide the electrochemical reduction of carbon dioxide to formic acid[J]. ChemSusChem, 2016, 9(4): 358-363. |
| 101 | HUANG Jiarun, QIU Xiaofeng, ZHAO Zhenhua, et al. Single-product faradaic efficiency for electrocatalytic of CO2 to CO at current density larger than 1.2A/cm2 in neutral aqueous solution by a single-atom nanozyme[J]. Angewandte Chemie International Edition, 2022, 61(44): e202210985. |
| 102 | CAO Xueying, ZHAO Lanling, WULAN Bari, et al. Atomic bridging structure of nickel-nitrogen-carbon for highly efficient electrocatalytic reduction of CO2 [J]. Angewandte Chemie International Edition, 2022, 134(6): e202113918. |
| 103 | RONG Xin, WANG Hongjuan, LU Xiuli, et al. Controlled synthesis of a vacancy-defect single-atom catalyst for boosting CO2 electroreduction[J]. Angewandte Chemie International Edition, 2020, 59(5): 1961-1965. |
| 104 | YAN Chengcheng, LI Haobo, YE Yifan, et al. Coordinatively unsaturated nickel-nitrogen sites towards selective and high-rate CO2 electroreduction[J]. Energy & Environmental Science, 2018, 11(5): 1204-1210. |
| 105 | YANG Hongbin, HUNG Sungfu, LIU Song, et al. Atomically dispersed Ni(Ⅰ) as the active site for electrochemical CO2 reduction[J]. Nature Energy, 2018, 3(2): 140-147. |
| 106 | MENGES Fabian S, CRAIG Stephanie M, Niklas TÖTSCH, et al. Capture of CO2 by a cationic nickel(Ⅰ) complex in the gas phase and characterization of the bound, activated CO2 molecule by cryogenic ion vibrational predissociation spectroscopy[J]. Angewandte Chemie International Edition, 2016, 55(4): 1282-1285. |
| 107 | FUJITA Etsuko, FURENLID Lars R, RENNER Mark W. Direct XANES evidence for charge transfer in CO-CO2 complexes[J]. Journal of the American Chemical Society, 1997, 119(19): 4549-4550. |
| 108 | DING X, ROGATIS L DE, VESSELLI E, et al. Interaction of carbon dioxide with Ni(110): A combined experimental and theoretical study[J]. Physical Review B, 2007, 76(19): 195425. |
| 109 | FREUND H J, ROBERTS M W. Surface chemistry of carbon dioxide[J]. Surface Science Reports, 1996, 25(8): 225-273. |
| 110 | ZHENG Tingting, JIANG Kun, Na TA, et al. Large-scale and highly selective CO2 electrocatalytic reduction on nickel single-atom catalyst[J]. Joule, 2019, 3(1): 265-278. |
| 111 | YANG Hengpan, LIN Qing, ZHANG Chao, et al. Carbon dioxide electroreduction on single-atom nickel decorated carbon membranes with industry compatible current densities[J]. Nature Communications, 2020, 11: 593. |
| 112 | MUKHERJEE Shreya, YANG Xiaoxuan, SHAN Weitao, et al. Atomically dispersed single Ni site catalysts for nitrogen reduction toward electrochemical ammonia synthesis using N2 and H2O[J]. Small Methods, 2020, 4(6): 1900821. |
| 113 | JIAO Long, YANG Weijie, WAN Gang, et al. Single-atom electrocatalysts from multivariate metal-organic frameworks for highly selective reduction of CO2 at low pressures[J]. Angewandte Chemie International Edition, 2020, 59(46): 20589-20595. |
| 36 | BURDYNY Thomas, SMITH Wilson A. CO2 reduction on gas-diffusion electrodes and why catalytic performance must be assessed at commercially-relevant conditions[J]. Energy & Environmental Science, 2019, 12(5): 1442-1453. |
| 37 | VARELA Ana Sofia, JU Wen, BAGGER Alexander, et al. Electrochemical reduction of CO2 on metal-nitrogen-doped carbon catalysts[J]. ACS Catalysis, 2019, 9(8): 7270-7284. |
| 38 | KOSHY David M, CHEN Shucheng, LEE Dong Un, et al. Understanding the origin of highly selective CO2 electroreduction to CO on Ni, N-doped carbon catalysts[J]. Angewandte Chemie International Edition, 2020, 59(10): 4043-4050. |
| 39 | 唐甜蜜, 王振旅, 管景奇. 调控单位点M-N-C电催化剂的电子结构提升二氧化碳还原性能[J]. 物理化学学报, 2023, 39(4): 2208033. |
| TANG Tianmi, WANG Zhenlyu, GUAN Jingqi. Electronic structure regulation of single-site M-N-C electrocatalysts for carbon dioxide reduction[J]. Acta Physico-Chimica Sinica, 2023, 39(4): 2208033. | |
| 40 | 董灵玉, 葛睿, 原亚飞, 等. 多孔炭基二氧化碳电催化材料研究进展[J]. 化工学报, 2020, 71(6): 2492-2509. |
| DONG Lingyu, GE Rui, YUAN Yafei, et al. Recent advances in porous carbon-based carbon dioxide electrocatalytic materials[J]. CIESC Journal, 2020, 71(6): 2492-2509. | |
| 41 | LI Qing, CAO Ruiguo, CHO Jaephil, et al. Nanostructured carbon-based cathode catalysts for nonaqueous lithium-oxygen batteries[J]. Physical Chemistry Chemical Physics, 2014, 16(27): 13568-13582. |
| 42 | WANG Qiyou, LIU Kang, HU Kangman, et al. Attenuating metal-substrate conjugation in atomically dispersed nickel catalysts for electroreduction of CO2 to CO[J]. Nature Communications, 2022, 13: 6082. |
| 43 | YANG Xiaofeng, WANG Aiqin, QIAO Botao, et al. Single-atom catalysts: A new frontier in heterogeneous catalysis[J]. Accounts of Chemical Research, 2013, 46(8): 1740-1748. |
| 44 | LI Xiaogang, BI Wentuan, CHEN Minglong, et al. Exclusive Ni-N4 sites realize near-unity CO selectivity for electrochemical CO2 reduction[J]. Journal of the American Chemical Society, 2017, 139(42): 14889-14892. |
| 45 | 曾奇. 铁-氮共掺杂碳基材料的结构设计及其电催化二氧化碳还原性能的研究[D]. 杭州: 浙江大学, 2021. |
| ZENG Qi. Structural design of iron-nitrogen co-doped carbon based materials and its application for carbon dioxide electroreduction [D]. Hangzhou: Zhejiang University, 2021. | |
| 46 | SUN Zhenyu, MA Tao, TAO Hengcong, et al. Fundamentals and challenges of electrochemical CO2 reduction using two-dimensional materials[J]. Chem, 2017, 3(4): 560-587. |
| 47 | LIU Song, YANG Hongbin, HUNG Sung-Fu, et al. Elucidating the electrocatalytic CO2 reduction reaction over a model single-atom nickel catalyst[J]. Angewandte Chemie International Edition, 2020, 59(2): 798-803. |
| 48 | 张育新, 王灿, 舒文祥. 二氧化碳的还原及其利用研究进展[J]. 化工进展, 2023, 42(2): 944-956. |
| ZHANG Yuxin, WANG Can, SHU Wenxiang. Review on the research progress of carbon dioxide reduction and utilization [J]. Chemical Industry and Engineering Progress, 2023, 42(2): 944-956. | |
| 49 | PETERSON Andrew A, Frank ABILD-PEDERSEN, STUDT Felix, et al. How copper catalyzes the electroreduction of carbon dioxide into hydrocarbon fuels[J]. Energy & Environmental Science, 2010, 3(9): 1311-1315. |
| [1] | 陈富强, 仲兆平, 戚仁志. 铜基催化剂电还原二氧化碳为甲酸研究进展[J]. 化工进展, 2024, 43(6): 3051-3060. |
| [2] | 葛全倩, 徐迈, 梁铣, 王凤武. MOFs材料在光电催化领域应用的研究进展[J]. 化工进展, 2023, 42(9): 4692-4705. |
| [3] | 符淑瑢, 王丽娜, 王东伟, 刘蕊, 张晓慧, 马占伟. 析氧助催化剂增强光阳极光电催化分解水性能研究进展[J]. 化工进展, 2023, 42(5): 2353-2370. |
| [4] | 张会霞, 周立山, 张程蕾, 钱光磊, 谢陈鑫, 朱令之. Bi2S3/TiO2纳米锥光阳极的制备及其光电催化降解土霉素[J]. 化工进展, 2023, 42(10): 5548-5557. |
| [5] | 王亚楠, 孟秀霞, 张维民, 杨乃涛. 表/界面调控策略在氢/氧反应电极中的研究进展[J]. 化工进展, 2022, 41(5): 2416-2428. |
| [6] | 薛李静, 费星, 刘江淋, 吴林军, 仇中杰, 许权洲, 钟晓文, 林绪亮, 秦延林. 木质素基碳材料催化剂的制备及应用研究进展[J]. 化工进展, 2022, 41(5): 2441-2450. |
| [7] | 华亚妮, 冯少广, 党欣悦, 郝文斌, 张保文, 高展. CO2电催化还原产合成气研究进展[J]. 化工进展, 2022, 41(3): 1224-1240. |
| [8] | 郑元波, 张前, 石坚, 李佳霖, 梅苏宁, 余秦伟, 杨建明, 吕剑. 电催化还原CO2生成多种产物催化剂研究进展[J]. 化工进展, 2022, 41(3): 1209-1223. |
| [9] | 唐金琼, 孔勇, 沈晓冬. 碳化物衍生碳的制备及其应用研究进展[J]. 化工进展, 2022, 41(2): 791-802. |
| [10] | 翟重渊, 赵丹荻, 何亚鹏, 黄惠, 陈步明, 郭忠诚. 掺硼金刚石阳极电催化降解新兴抗生素类污染物研究进展[J]. 化工进展, 2022, 41(12): 6615-6626. |
| [11] | 杨妍, 刘国涛, 余庆慧, 李晓娟, 张颖. 多孔炭材料改性纳米零价铁的研究进展[J]. 化工进展, 2021, 40(S2): 198-202. |
| [12] | 葛睿, 胡旭, 董灵玉, 李丹, 郝广平. 电化学耦合阴极二氧化碳还原与阳极氧化合成[J]. 化工进展, 2021, 40(9): 5132-5144. |
| [13] | 余素云, 梁乐程, 崔志明. 酸性环境中甲醇电氧化催化剂的研究进展[J]. 化工进展, 2021, 40(9): 4962-4974. |
| [14] | 王艺蒙, 刘建军, 左胜利, 李抗. MoS2光电催化剂活性位点的优化和效能研究进展[J]. 化工进展, 2021, 40(7): 3747-3759. |
| [15] | 范文龙, 李林哲, 薛志伟, 孟秀霞, 张津津, 于方永, 杨乃涛. 电催化合成氨研究进展[J]. 化工进展, 2021, 40(6): 3005-3019. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||