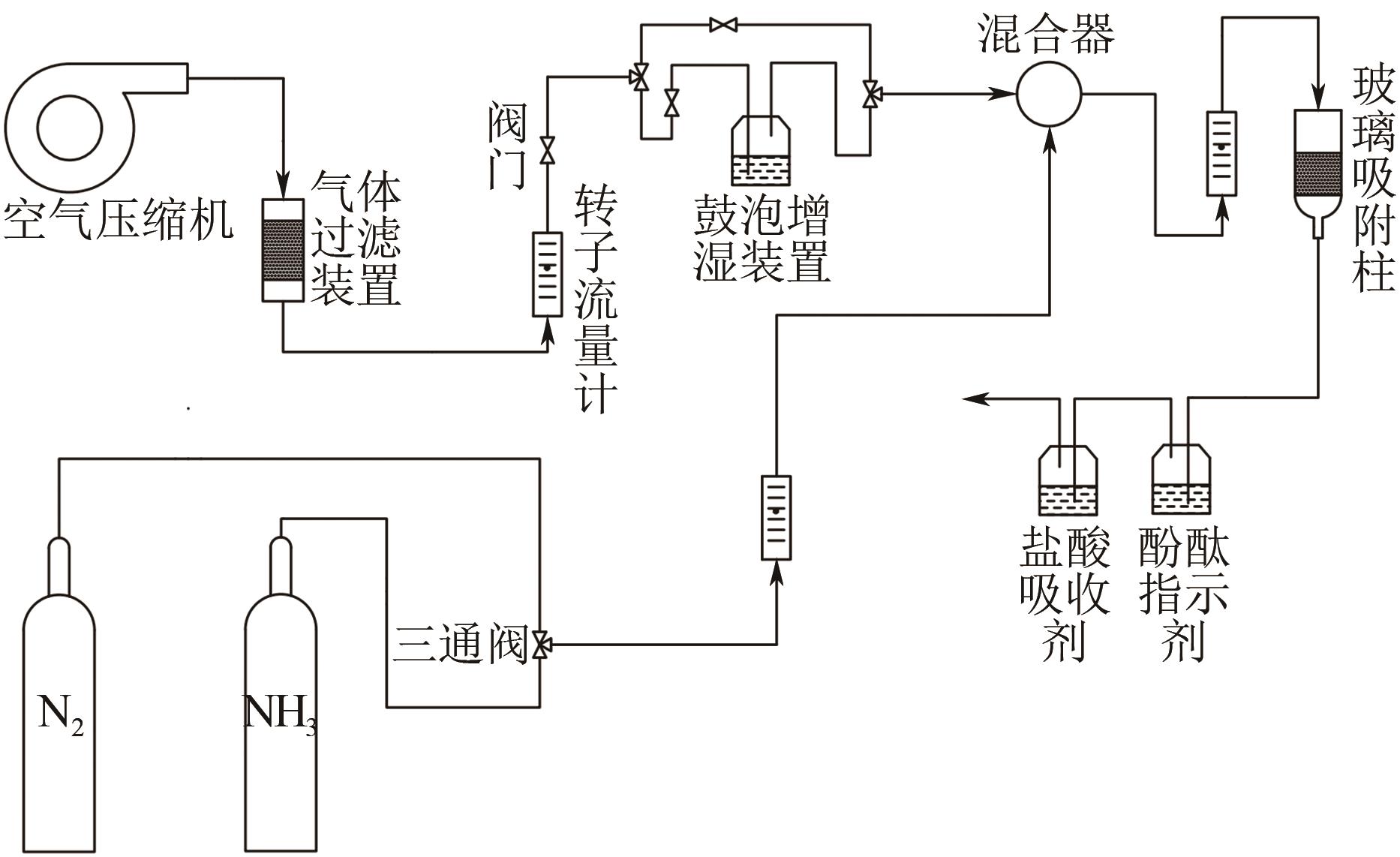
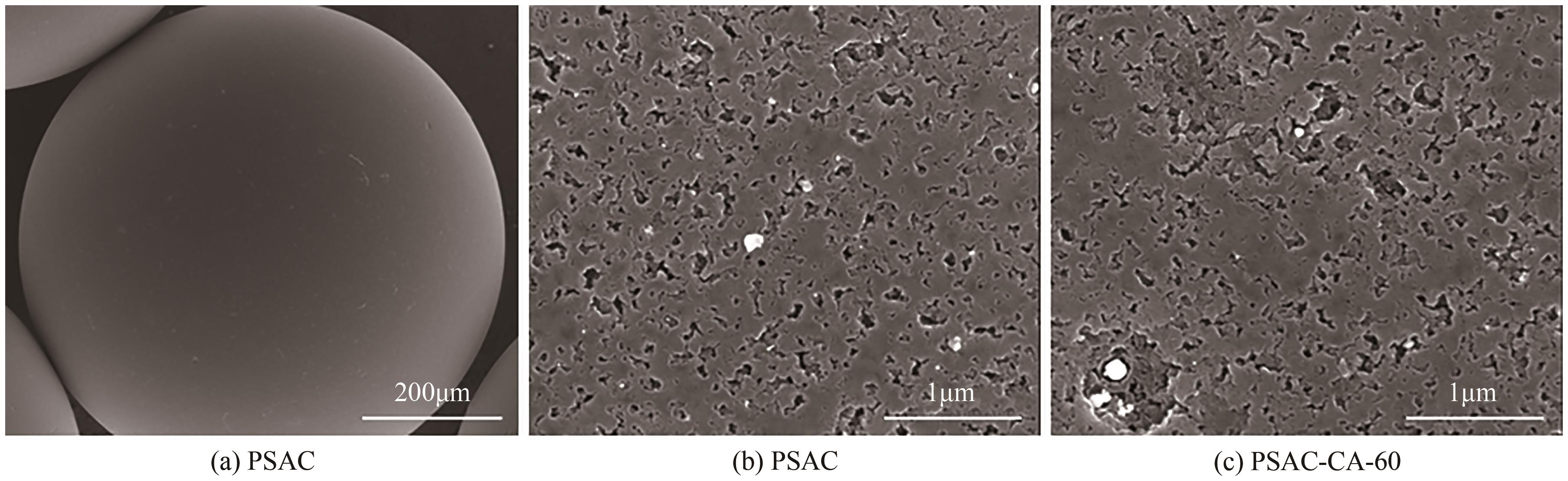
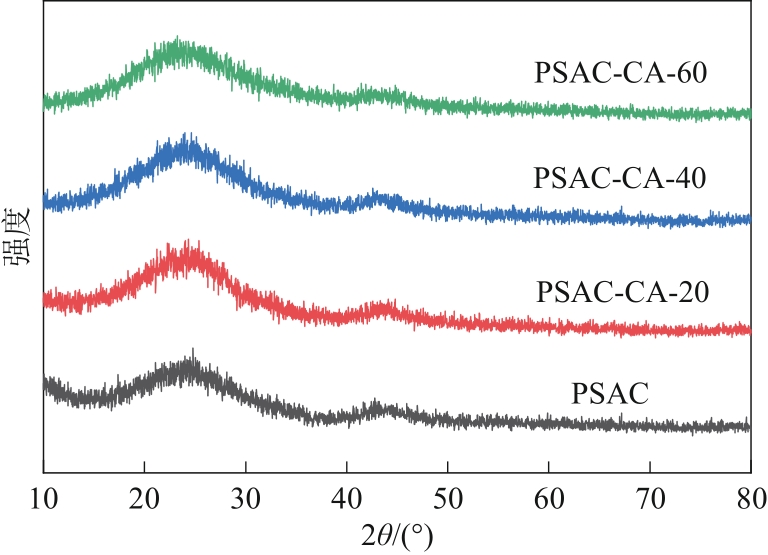
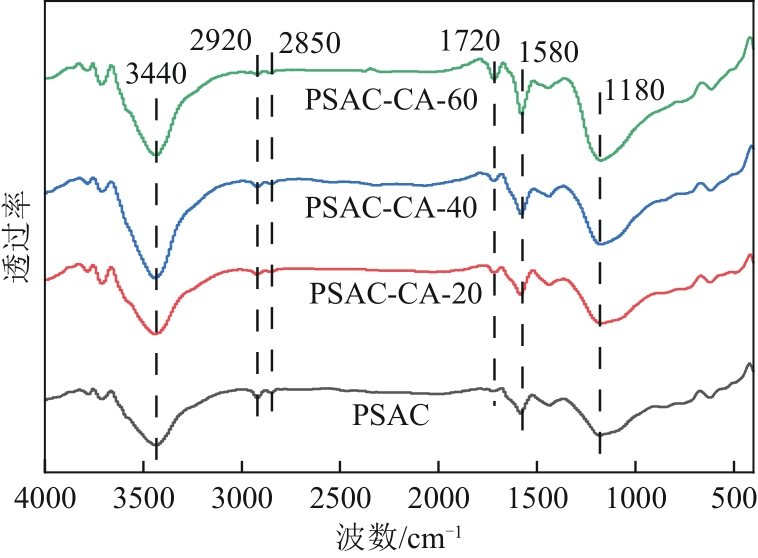
| 1 |
Huyen Thanh VO, KIM Jiyull, KIM Na Yeon, et al. Effect of pore texture property of mesoporous alumina on adsorption performance of ammonia gas[J]. Journal of Industrial and Engineering Chemistry, 2020, 91: 129-138.
|
| 2 |
HU Tingting, LIU Fang, DOU Shuai, et al. Selective adsorption of trace gaseous ammonia from air by a sulfonic acid-modified silica xerogel: Preparation, characterization and performance[J]. Chemical Engineering Journal, 2022, 443: 136357.
|
| 3 |
MAITLO Hubdar ALI, MAITLO Ghulamullah, SONG Xiangru, et al. A figure of merits-based performance comparison of various advanced functional nanomaterials for adsorptive removal of gaseous ammonia[J]. Science of the Total Environment, 2022, 822: 153428.
|
| 4 |
BEHERA Sailesh N, SHARMA Mukesh, ANEJA Viney P, et al. Ammonia in the atmosphere: A review on emission sources, atmospheric chemistry and deposition on terrestrial bodies[J]. Environmental Science and Pollution Research, 2013, 20(11): 8092-8131.
|
| 5 |
陈沛坤, 张袁斌, 崔希利, 等. 氨气深度脱除材料与技术研究进展[J]. 化工进展, 2021, 40(7): 3957-3975.
|
|
CHEN Peikun, ZHANG Yuanbin, CUI Xili, et al. Progress in materials and technologies for deep removal of ammonia gas[J]. Chemical Industry and Engineering Progress, 2021, 40(7): 3957-3975.
|
| 6 |
WANG Xiaohong, CHENG Hairong, YE Guangzheng, et al. Key factors and primary modification methods of activated carbon and their application in adsorption of carbon-based gases: A review[J]. Chemosphere, 2022, 287: 131995.
|
| 7 |
金春江, 王鲁元, 陈惠敏, 等. 一步快速活化法制备生物质活性炭及其对乙酸乙酯的吸附再生[J]. 化工进展, 2021, 40(S1): 446-455.
|
|
JIN Chunjiang, WANG Luyuan, CHEN Huimin, et al. Preparation of biomass activated carbon by one step rapid activation and its adsorption regeneration for ethyl acetate[J]. Chemical Industry and Engineering Progress, 2021, 40(S1): 446-455.
|
| 8 |
樊相汝, 羊依金, 郭旭晶, 等. 软锰矿-含油污泥基活性炭对亚甲基蓝的吸附特性[J]. 化工进展, 2022, 41(12): 6664-6671.
|
|
FAN Xiangru, YANG Yijin, GUO Xujing, et al. Study on the adsorption characteristics of methylene blue by soft manganese ore-oil sludge-based activated carbon[J]. Chemical Industry and Engineering Progress, 2022, 41(12): 6664-6671.
|
| 9 |
BANDOSZ Teresa J, PETIT Camille. On the reactive adsorption of ammonia on activated carbons modified by impregnation with inorganic compounds[J]. Journal of Colloid and Interface Science, 2009, 338(2): 329-345.
|
| 10 |
WANG Jitong, JIANG Wuyou, ZHANG Zixiao, et al. Mesoporous carbon beads impregnated with transition metal chlorides for regenerative removal of ammonia in the atmosphere[J]. Industrial & Engineering Chemistry Research, 2017, 56(12): 3283-3290.
|
| 11 |
郭军军, 金彦任, 崔洪, 等. 空气氧化对沥青基球形活性炭的改性研究[J]. 炭素技术, 2021, 40(5): 63-67.
|
|
GUO Junjun, JIN Yanren, CUI Hong, et al. Effect of air modification on pitch-based spherical activated carbon[J]. Carbon Techniques, 2021, 40(5): 63-67.
|
| 12 |
谢菲, 王艳莉, 詹亮, 等. 沥青基球形活性炭对CO2的吸脱附行为研究[J]. 无机材料学报, 2011, 26(2): 149-154.
|
|
XIE Fei, WANG Yanli, ZHAN Liang, et al. Adsorption/desorption performance of CO2 on pitch-based spherical activated carbons[J]. Journal of Inorganic Materials, 2011, 26(2): 149-154.
|
| 13 |
RASHID Umma Salma, BEZBARUAH Achintya N. Citric acid modified granular activated carbon for enhanced defluoridation[J]. Chemosphere, 2020, 252: 126639.
|
| 14 |
CHEN J Paul, WU Shunnian, CHONG Kai-Hau. Surface modification of a granular activated carbon by citric acid for enhancement of copper adsorption[J]. Carbon, 2003, 41(10): 1979-1986.
|
| 15 |
傅成诚, 梅凡民, 周亮, 等. 改性活性炭对氨气吸附性能的研究[J]. 纺织高校基础科学学报, 2009, 22(3): 386-389.
|
|
FU Chengcheng, MEI Fanmin, ZHOU Liang, et al. Adsorption properties of modified activated carbon onto amonia[J]. Basic Sciences Journal of Textile Universities, 2009, 22(3): 386-389.
|
| 16 |
LI Qi, ZHU Youyu, ZHAO Pinyi, et al. Commercial activated carbon as a novel precursor of the amorphous carbon for high-performance sodium-ion batteries anode[J]. Carbon, 2018, 129: 85-94.
|
| 17 |
AKPOTU Samson O, MOODLEY Brenda. Synthesis and characterization of citric acid grafted MCM-41 and its adsorption of cationic dyes[J]. Journal of Environmental Chemical Engineering, 2016, 4(4): 4503-4513.
|
| 18 |
WANG Xiangyu, WANG Anqi, MA Jun. Visible-light-driven photocatalytic removal of antibiotics by newly designed C3N4@MnFe2O4-graphene nanocomposites[J]. Journal of Hazardous Materials, 2017, 336: 81-92.
|
| 19 |
CHEN Bo, ZHU Zhiliang, MA Jie, et al. Surfactant assisted Ce-Fe mixed oxide decorated multiwalled carbon nanotubes and their arsenic adsorption performance[J]. Journal of Materials Chemistry A, 2013, 1(37): 11355-11367.
|
| 20 |
MADY Amr Hussein, BAYNOSA Marjorie Lara, TUMA Dirk, et al. Facile microwave-assisted green synthesis of Ag-ZnFe2O4@rGO nanocomposites for efficient removal of organic dyes under UV- and visible-light irradiation[J]. Applied Catalysis B: Environmental, 2017, 203: 416-427.
|
| 21 |
BEHERA Arjun, MANSINGH Sriram, Kundan Kumar DAS, et al. Synergistic ZnFe2O4-carbon allotropes nanocomposite photocatalyst for norfloxacin degradation and Cr (Ⅵ) reduction[J]. Journal of Colloid and Interface Science, 2019, 544: 96-111.
|
| 22 |
THOMMES Matthias, KANEKO Katsumi, NEIMARK Alexander V, et al. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report)[J]. Pure and Applied Chemistry, 2015, 87(9/10): 1051-1069.
|
| 23 |
SEREDYCH Mykola, Denisa HULICOVA-JURCAKOVA, LU Gaoqing, et al. Surface functional groups of carbons and the effects of their chemical character, density and accessibility to ions on electrochemical performance[J]. Carbon, 2008, 46(11): 1475-1488.
|
| 24 |
HUANG Chen-Chia, LI Hongsong, CHEN Chien-Hung. Effect of surface acidic oxides of activated carbon on adsorption of ammonia[J]. Journal of Hazardous Materials, 2008, 159(2/3): 523-527.
|
| 25 |
ZHANG Yufang, XIAO Jianfei, ZHANG Tian C, et al. Synthesis of CuSiO3-loaded P-doped porous biochar derived from phytic acid-activated lemon peel for enhanced adsorption of NH3 [J]. Separation and Purification Technology, 2022, 283: 120179.
|
| 26 |
Maraisa GONÇALVES, Laura SÁNCHEZ-GARCÍA, DE OLIVEIRA JARDIM Erika, et al. Ammonia removal using activated carbons: Effect of the surface chemistry in dry and moist conditions[J]. Environmental Science & Technology, 2011, 45(24): 10605-10610.
|
 ), LIANG Xiaoyi(
), LIANG Xiaoyi( )
)