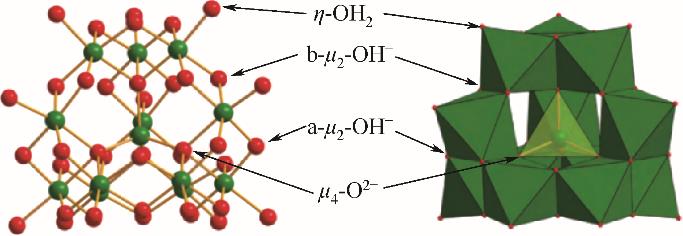
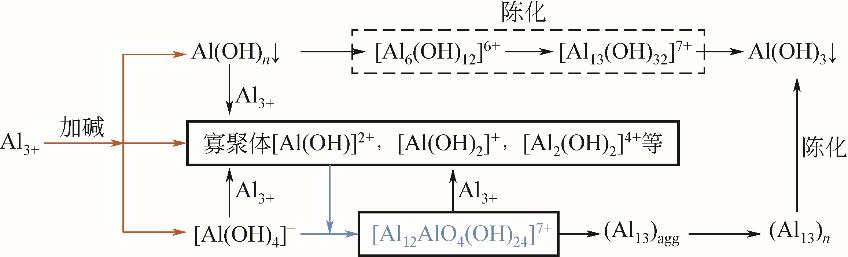
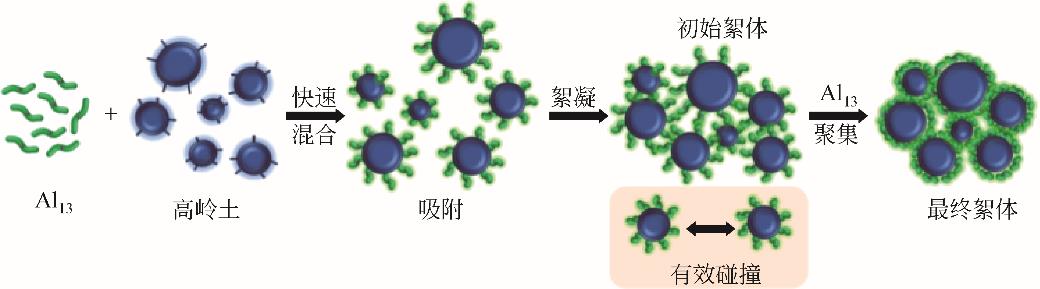
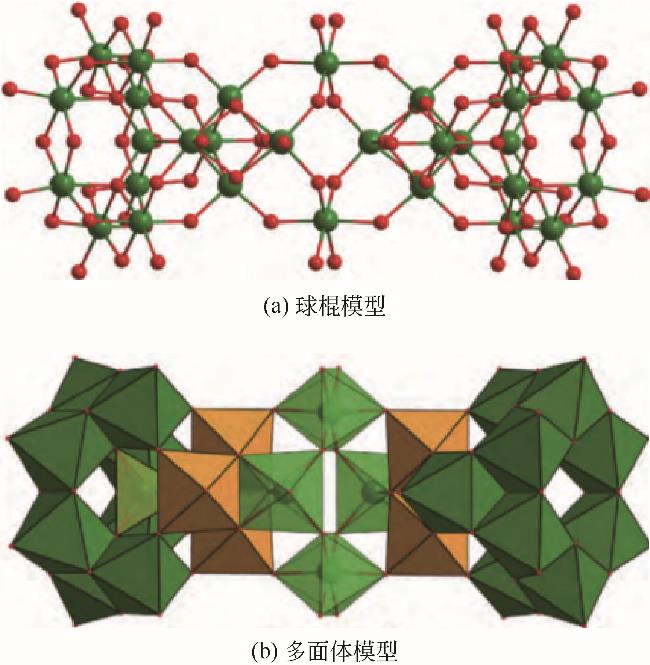
化工进展 ›› 2023, Vol. 42 ›› Issue (S1): 479-488.DOI: 10.16085/j.issn.1000-6613.2023-1056
水处理工艺中铝盐水解物的毒性、形态及控制研究进展
- 兰州交通大学环境与市政工程学院,甘肃 兰州 730070
-
收稿日期:2023-06-27修回日期:2023-10-26出版日期:2023-10-25发布日期:2023-11-30 -
通讯作者:毛玉红 -
作者简介:王敏(1999—),女,硕士研究生,研究方向为给水处理理论与技术。E-mail:wangminjob@126.com。 -
基金资助:国家自然科学基金(51968038)
Progress on the toxicity, morphology and control of aluminum salt hydrolysates in water treatment process
WANG Min( ), MAO Yuhong(
), MAO Yuhong( ), CHEN Chao, BAI Dan
), CHEN Chao, BAI Dan
- Environmental and Municipal Engineering, Lanzhou Jiaotong University, Lanzhou 730070, Gansu, China
-
Received:2023-06-27Revised:2023-10-26Online:2023-10-25Published:2023-11-30 -
Contact:MAO Yuhong
摘要:
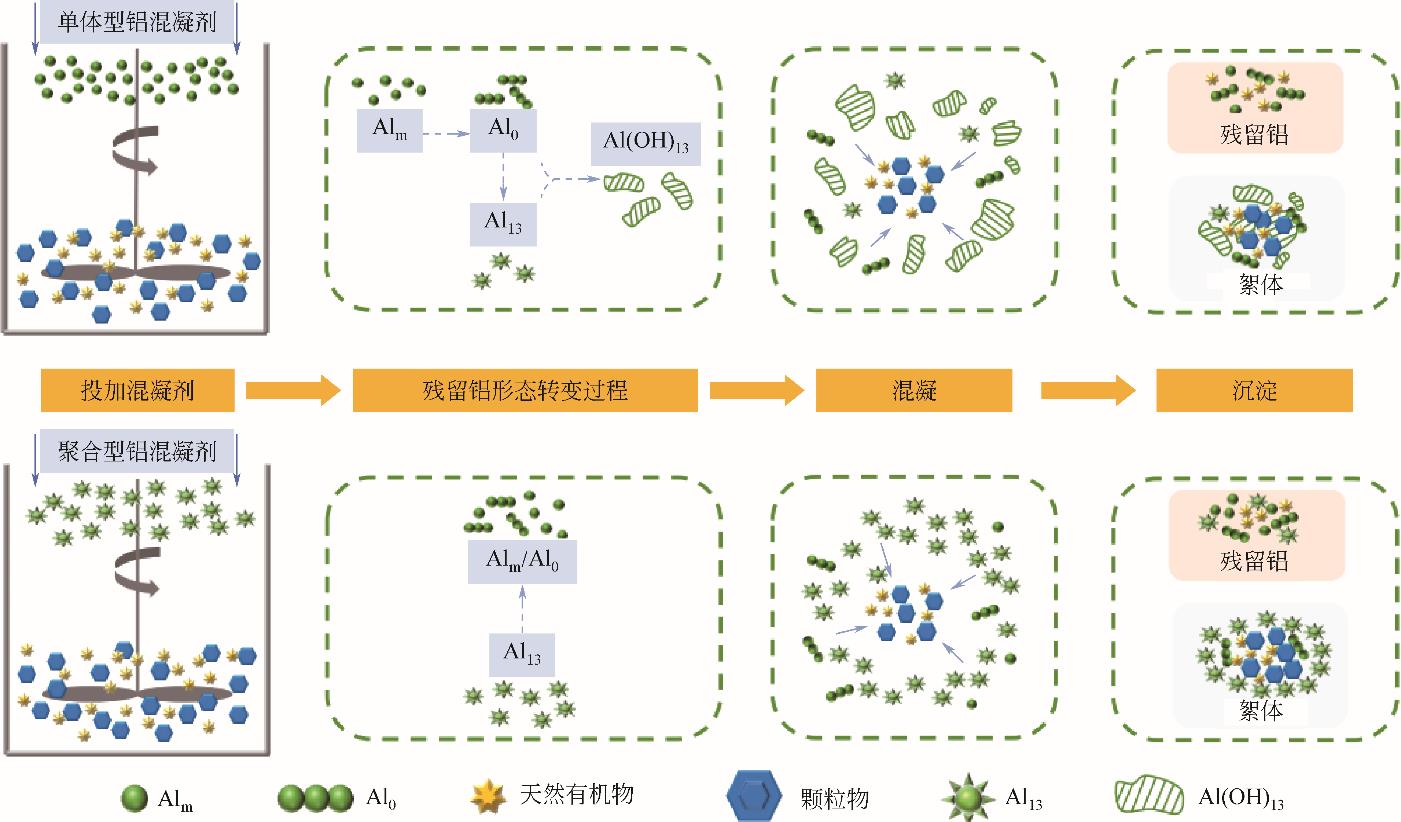
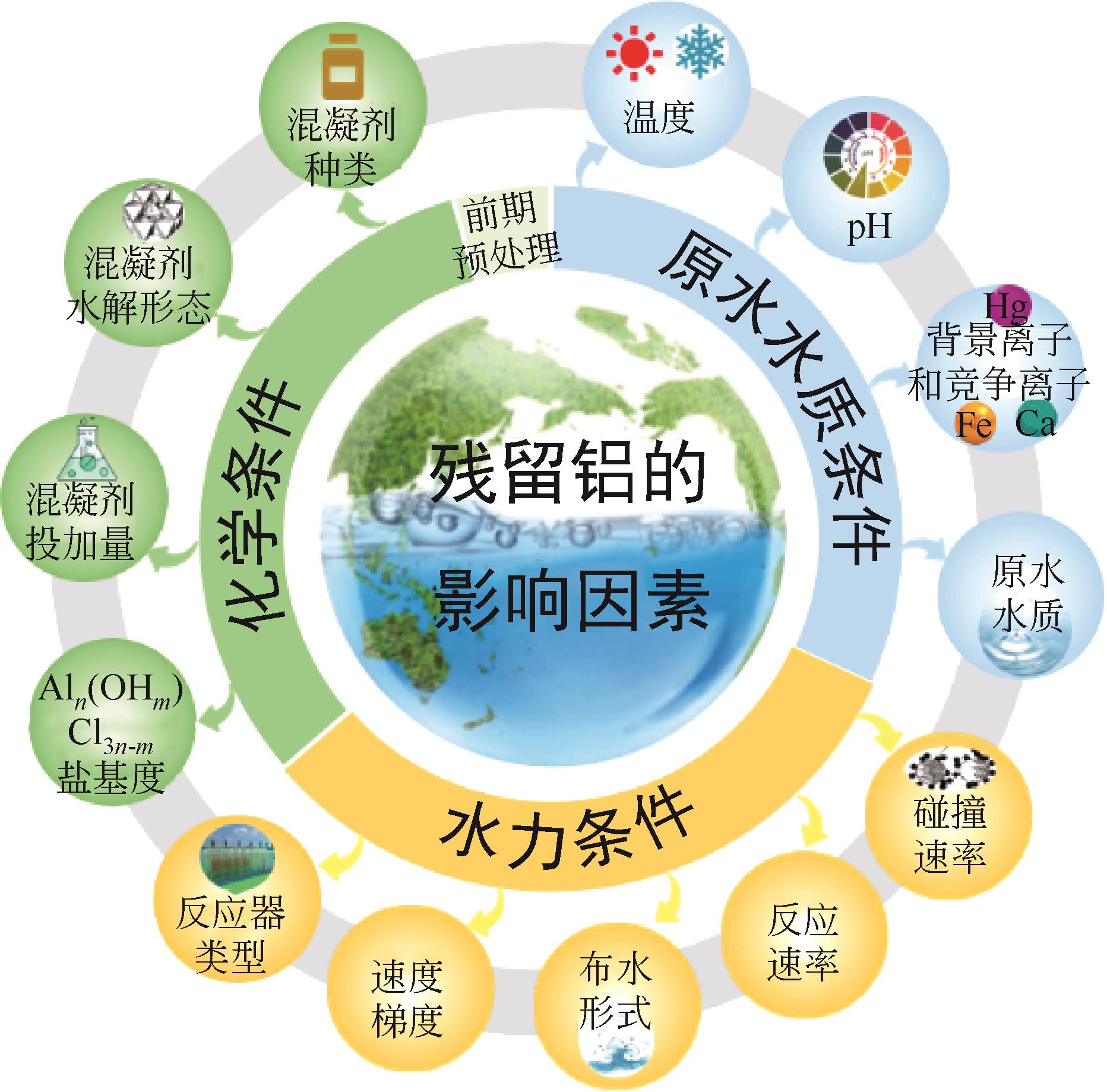
铝盐混凝剂因其形成絮体大而密实,具有较好的除浊脱色性能,在混凝领域广泛应用,但却存在处理水铝残留问题。本文为更好地促进铝盐混凝剂在水处理领域的应用,详细介绍了铝盐水解物对人体的毒性效应、对输配水管网系统和饮用水深度处理工艺的影响。总结了残留铝生成机理和形态分析方法,阐述了优势形态Al13和Al30的水解过程以及其发挥优势混凝作用的原因。分析了原水水质条件、化学条件、水力条件和前期预处理对残留铝含量的影响。最后,点明了未来控制残留铝的策略和技术。指出未来应结合人工智能复配合成纳米级新型混凝剂,关注混凝过程中水力条件对残留铝的影响,并开发能够精准测量分析水中各类铝形态的方法,不断创新强化净水工艺,进一步完善残留铝控制措施,保障出水水质安全。
中图分类号:
引用本文
王敏, 毛玉红, 陈超, 白丹. 水处理工艺中铝盐水解物的毒性、形态及控制研究进展[J]. 化工进展, 2023, 42(S1): 479-488.
WANG Min, MAO Yuhong, CHEN Chao, BAI Dan. Progress on the toxicity, morphology and control of aluminum salt hydrolysates in water treatment process[J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 479-488.
| 国家或机构 | 残留铝限制标准/mg·L-1 |
|---|---|
| 世界卫生组织 | 0.2 |
| 欧盟 | 0.2 |
| 美国 | 0.05~0.20 |
| 日本 | 0.2 |
| 中国 | 0.2 |
表1 国内外生活饮用水残留铝浓度限值标准
| 国家或机构 | 残留铝限制标准/mg·L-1 |
|---|---|
| 世界卫生组织 | 0.2 |
| 欧盟 | 0.2 |
| 美国 | 0.05~0.20 |
| 日本 | 0.2 |
| 中国 | 0.2 |
| 分类 | 原理 | 优缺点 | 适用情况 | 备注 | 文献 |
|---|---|---|---|---|---|
| Ferron逐时络合比色法 | 根据 Al3+及其不稳定配合物与光谱试剂反应动力学的差异来分析铝的形态 | 不用分离,不破坏溶液平衡;干扰严重,灵敏度不高 | 高浓度条件下的铝 | 检测限0.05 mg/L; 线性范围0.1~1.5mg/L | [ |
| 铬天青S分光光度法 | 操作简单,不需要昂贵仪器;酸度难以控制,不稳定 | 生活饮用水和水源水中的铝 | 检测限0.008mg/L; 回收率94%~106% | [ | |
| 8-羟基喹啉-5-磺酸分光光度法 | 灵敏度高,对铝形态干扰小 | 快速反应铝的测定 | 检测限0.01mg/L; 回收率92.5%~101% | [ | |
| 离子交换色谱法 | 利用某一特定的色谱系统进行溶液中铝各组分的分析 | 分离柱可多次测定水样; 无法直接得到无机单核铝的含量,误差大且操作烦琐 | 同时分析低浓度的多种离子 | 检测限0.02mg/L; 回收率94%~102% | [ |
| 高效液相色谱法 | 对铝具有独特选择性 | 饮用水中铝的测定 | 检测限0.015mg/L; 线性范围0.03~0.3mg/L | [ | |
| 流动注射分析法 | 重现性好,能准确控制反应时间,可与多种检测仪联用 | 现配样品的处理 | 检测限0.002mg/L | [ | |
| 邻苯二酚紫-示波计时电位法 | 通过调节pH和选择合适的配体两条途径来分析铝形态 | 设备简单、步骤简便、灵敏度高、无需分离;加热或通氧会破坏水样中各种铝形态间的原始平衡,干扰准确性 | 天然水体中各种 铝形态的分析 | 检测限0.003mg/L; 相对标准偏差6.5% | [ |
| 钙镁试剂-示波计时电位法 | 天然水体中各组分单核铝的测定 | 检测限0.015mg/L; 回收率95%~107% | [ | ||
| 27Al-NMR核磁共振法 | 铝的不同物种的27Al信号化学位移和线宽不同,可进行形态分析 | 快速、非破坏性、高灵敏度 及可定量 | 环境生物中铝含量分析 | — | [ |
表2 常见铝浓度分析方法
| 分类 | 原理 | 优缺点 | 适用情况 | 备注 | 文献 |
|---|---|---|---|---|---|
| Ferron逐时络合比色法 | 根据 Al3+及其不稳定配合物与光谱试剂反应动力学的差异来分析铝的形态 | 不用分离,不破坏溶液平衡;干扰严重,灵敏度不高 | 高浓度条件下的铝 | 检测限0.05 mg/L; 线性范围0.1~1.5mg/L | [ |
| 铬天青S分光光度法 | 操作简单,不需要昂贵仪器;酸度难以控制,不稳定 | 生活饮用水和水源水中的铝 | 检测限0.008mg/L; 回收率94%~106% | [ | |
| 8-羟基喹啉-5-磺酸分光光度法 | 灵敏度高,对铝形态干扰小 | 快速反应铝的测定 | 检测限0.01mg/L; 回收率92.5%~101% | [ | |
| 离子交换色谱法 | 利用某一特定的色谱系统进行溶液中铝各组分的分析 | 分离柱可多次测定水样; 无法直接得到无机单核铝的含量,误差大且操作烦琐 | 同时分析低浓度的多种离子 | 检测限0.02mg/L; 回收率94%~102% | [ |
| 高效液相色谱法 | 对铝具有独特选择性 | 饮用水中铝的测定 | 检测限0.015mg/L; 线性范围0.03~0.3mg/L | [ | |
| 流动注射分析法 | 重现性好,能准确控制反应时间,可与多种检测仪联用 | 现配样品的处理 | 检测限0.002mg/L | [ | |
| 邻苯二酚紫-示波计时电位法 | 通过调节pH和选择合适的配体两条途径来分析铝形态 | 设备简单、步骤简便、灵敏度高、无需分离;加热或通氧会破坏水样中各种铝形态间的原始平衡,干扰准确性 | 天然水体中各种 铝形态的分析 | 检测限0.003mg/L; 相对标准偏差6.5% | [ |
| 钙镁试剂-示波计时电位法 | 天然水体中各组分单核铝的测定 | 检测限0.015mg/L; 回收率95%~107% | [ | ||
| 27Al-NMR核磁共振法 | 铝的不同物种的27Al信号化学位移和线宽不同,可进行形态分析 | 快速、非破坏性、高灵敏度 及可定量 | 环境生物中铝含量分析 | — | [ |
| 1 | CHEN Kaiyue, LIU Yuting, HUNG Jui-Ting, et al. Synergism of Fe and Al salts for the coagulation of dissolved organic matter: Structural developments of Fe/Al-organic matter associations[J]. Chemosphere, 2023, 316: 137737. |
| 2 | Izabela KRUPIŃSKA. Aluminium drinking water treatment residuals and their toxic impact on human health[J]. Molecules, 2020, 25(3): 641. |
| 3 | 国家市场监督管理总局, 国家标准化管理委员会. 生活饮用水卫生标准: [S]. 北京: 中国标准出版社, 2022. |
| Ministry of Health, PRC, Standardization Administration of China, Standardization Administration of the People's Republic of China. Standards for drinking water quality: [S]. Beijing: Standards Press of China, 2022. | |
| 4 | MARTINEZ Caroline Silveira, ALTERMAN Caroline D C, VERA Gema, et al. Egg white hydrolysate as a functional food ingredient to prevent cognitive dysfunction in rats following long-term exposure to aluminum[J]. Scientific Reports, 2019, 9: 1868. |
| 5 | WANG Fei, KANG Pan, LI Zhaoyang, et al. Role of MLL in the modification of H3K4me3 in aluminium-induced cognitive dysfunction[J]. Chemosphere, 2019, 232: 121-129. |
| 6 | LU Xiaoting, XU Shimeng, ZHANG Yunwei, et al. Longitudinal study of the effects of occupational aluminium exposure on workers’ cognition[J]. Chemosphere, 2021, 271: 129569. |
| 7 | ALSAEED R, ALAJI B, EBRAHIM M. Predicting turbidity and aluminum in drinking water treatment plants using hybrid network (GA-ANN) and GEP[J]. Drinking Water Engineering & Science Discussions, 2021. DOI:10.5194/dwes-2021-8 . |
| 8 | LI Huan, XUE Xingli, LI Zhaoyang, et al. Aluminium-induced synaptic plasticity injury via the PHF8-H3K9me2-BDNF signalling pathway[J]. Chemosphere, 2020, 244: 125445. |
| 9 | CHEN Shengyi, WENG Minghung, Li Zihying, et al. Protective effects of camellia and olive oils against cognitive impairment via gut microbiota-brain communication in rats[J]. Food & Function, 2022, 13(13): 7168-7180. |
| 10 | Deiweson SOUZA-MONTEIRO, NUNES Paula Beatriz, FERREIRA Railson, et al. Aluminum-induced toxicity in salivary glands of mice after long-term exposure: Insights into the redox state and morphological analyses[J]. Biological Trace Element Research, 2020, 198(2): 575-582. |
| 11 | ESQUERRE Nicolas, BASSO Lilian, DUBUQUOY Caroline, et al. Aluminum ingestion promotes colorectal hypersensitivity in rodents[J]. Cellular and Molecular Gastroenterology and Hepatology, 2019, 7(1): 185-196. |
| 12 | NIE Yingying, YANG Jingming, ZHOU Longjian, et al. Marine fungal metabolite butyrolactone Ⅰ prevents cognitive deficits by relieving inflammation and intestinal microbiota imbalance on aluminum trichloride-injured zebrafish[J]. Journal of Neuroinflammation, 2022, 19(1): 39. |
| 13 | IGWENAGU Ephraim, IGBOKWE Ikechukwu Onyebuchi, EGBE-NWIYI Tobias Nnia. Fasting hyperglycaemia, glucose intolerance and pancreatic islet necrosis in albino rats associated with subchronic oral aluminium chloride exposure[J]. Comparative Clinical Pathology, 2020, 29(1): 75-81. |
| 14 | WEI Xi, WEI Hua, YANG Dawei, et al. Effect of aluminum exposure on glucose metabolism and its mechanism in rats[J]. Biological Trace Element Research, 2018, 186(2): 450-456. |
| 15 | ARAFAT Esraa A, EL-SAYED Doaa S, HUSSEIN Hussein K, et al. Entomotherapeutic role of Periplaneta americana extract in alleviating aluminum oxide nanoparticles-induced testicular oxidative impairment in migratory locusts (Locusta migratoria) as an ecotoxicological model[J]. Antioxidants, 2023, 12(3): 653. |
| 16 | BADAWOUD Mohammed H, Gamal ABDEL-AZIZ, EL-FARK M M, et al. The effect of aluminum exposure on maternal health and fetal growth in rats[J]. Cureus, 2022, 14(11): e31775. |
| 17 | YOKEL Robert A. Aluminum reproductive toxicity: A summary and interpretation of scientific reports[J]. Critical Reviews in Toxicology, 2020, 50(7): 551-593. |
| 18 | Soon-Thiam KHU, XIN Changchun, WANG Tianzhi, et al. Effects of hydraulic conditions on biofilm detached in drinking water distribution system[J]. Journal of Water Process Engineering, 2023, 53: 103882. |
| 19 | KURAJICA L, UJEVIĆ BOŠNJAK M, KINSELA A S, et al. Effects of changing supply water quality on drinking water distribution networks: Changes in NOM optical properties, disinfection byproduct formation, and Mn deposition and release[J]. Science of the Total Environment, 2021, 762: 144159. |
| 20 | 李礼, 赵蓓, 柴文, 等. 城市供水管网中铝形态特征分析[J]. 中国给水排水, 2022, 38(5): 9-13. |
| LI Li, ZHAO Bei, CHAI Wen, et al. Analysis on morphological characteristics of aluminum in urban drinking water distribution system[J]. China Water & Wastewater, 2022, 38(5): 9-13. | |
| 21 | 聂煜东, 李金, 张贤明. 水处理过程中膜污染问题及其预处理技术研究进展[J]. 化工进展, 2021, 40(4): 2278-2289. |
| NIE Yudong, LI Jin, ZHANG Xianming. Research progress on membrane fouling and its pretreatment technology in water treatment[J]. Chemical Industry and Engineering Progress, 2021, 40(4): 2278-2289. | |
| 22 | YUAN Ziyi, LI Yunfei, LI Tianyu, et al. Identifying key residual aluminum species responsible for aggravation of nanofiltration membrane fouling in drinking water treatment[J]. Journal of Membrane Science, 2022, 659: 120833. |
| 23 | LIU Danyang, CABRERA Johny, ZHONG Lijuan, et al. Using loose nanofiltration membrane for lake water treatment: A pilot study[J]. Frontiers of Environmental Science & Engineering, 2021, 15(4): 69. |
| 24 | LIU Hongyuan, LIU Haoran, XIE Yawei. Fate and fractionation of aluminum in a full-scale Al-based drinking water treatment plant[J]. Journal of Water Supply: Research and Technology-Aqua, 2020, 69(5): 469-477. |
| 25 | MEGHZILI Bachir, SOUAD Brakchi, ABDELKRIM Azzouz. Risk of residual aluminum in treated waters with aluminum sulphate[J]. Advances in Research, 2016, 6(5): 1-8. |
| 26 | DUAN Shuxuan, XU Hui, XIAO Feng, et al. Effects of Al species on coagulation efficiency, residual Al and floc properties in surface water treatment[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2014, 459: 14-21. |
| 27 | LIU Libing, YANG Qinxue, WANG Pin, et al. Efficient purification of Al30 by organic complexation method[J]. Journal of Environmental Sciences, 2019, 80: 240-247. |
| 28 | LIU Libing, LU Sen, AN Guangyu, et al. Historical development of Al30 highlighting the unique characteristics and application in water treatment: A review[J]. Coordination Chemistry Reviews, 2022, 473: 214807. |
| 29 | 王东升, 安广宇, 刘丽冰, 等. Al13的分子学及其在环境工程中的应用[J]. 环境工程学报, 2018, 12(6): 1565-1584. |
| WANG Dongsheng, AN Guangyu, LIU Libing, et al. Molecules of Al13 and its application in environmental engineering[J]. Chinese Journal of Environmental Engineering, 2018, 12(6): 1565-1584. | |
| 30 | WANG Pin, JIAO Ruyuan, LIU Libing, et al. Optimized coagulation pathway of Al13: Effect of in situ aggregation of Al13 [J]. Chemosphere, 2019, 230: 76-83. |
| 31 | ALLOUCHE Lionel, TAULELLE Francis. Conversion of Al13 Keggin ε into Al30: A reaction controlled by aluminum monomers[J]. Inorganic Chemistry Communications, 2003, 6(9): 1167-1170. |
| 32 | CARDONA Yaneth, KORILI Sophia A, GIL Antonio. Understanding the formation of Al13 and Al30 polycations to the development of microporous materials based on Al13- and Al30-PILC montmorillonites: A review[J]. Applied Clay Science, 2021, 203: 105996. |
| 33 | LIU Libing, LU Sen, DEMISSIE Hailu, et al. Formation of Al30 aggregates and its correlation to the coagulation effect[J]. Chemosphere, 2021, 278: 130493. |
| 34 | YUAN Ziyi, LI Tianyu, ZHANG Jianfeng, et al. Fluorescence-based method for fast quantification of active aluminums in natural and treated water[J]. Journal of Hazardous Materials, 2022, 433: 128815. |
| 35 | WANG Pin, DING Shunke, AN Guangyu, et al. Removal of disinfection by-product precursors by Al-based coagulants: A comparative study on coagulation performance[J]. Journal of Hazardous Materials, 2021, 420: 126558. |
| 36 | WANG Xi, XU Hui, WANG Dongsheng. Mechanism of fluoride removal by AlCl3 and Al13: The role of aluminum speciation[J]. Journal of Hazardous Materials, 2020, 398: 122987. |
| 37 | FENG Li, LUAN Zhaokun, TANG Hongxiao. Species analysis methods for hydrolysis polymerization of aluminum[J]. Journal of Environmental Sciences, 1998(1): 33-38. |
| 38 | MESQUITA Raquel B R, RANGEL António O S S. Development of sequential injection methodologies for the spectrophotometric direct and kinetic determination of aluminium in natural and waste waters[J]. Journal of the Brazilian Chemical Society, 2008, 19(6): 1171-1179. |
| 39 | MEMON M, BHANGER M I. Micellar liquid chromatographic determination of aluminum as its complex with 8-hydroxyquinoline-5-sulfonic acid[J]. Acta Chromatographica, 2004, 14(14): 172-179. |
| 40 | LETTERMAN Raymond D, DRISCOLL Charles T. Survey of residual aluminum in filtered water[J]. Journal AWWA, 1988, 80(4): 154-158. |
| 41 | SATO Makoto, MATSUDA Jun, MURAYAMA Harunobu, et al. Analytical chemistry for environmental and human health. Determination of aluminium in biological samples and drinking water by kinetic-differentiation mode HPLC with fluorescent detection[J]. Bunseki Kagaku, 2000, 49(6): 429-435. |
| 42 | CASSELLA Ricardo J, MAGALHÃES Otto I B, COUTO Marcos Tadeu, et al. Synthesis and application of a functionalized resin for flow injection/F AAS copper determination in waters[J]. Talanta, 2005, 67(1): 121-128. |
| 43 | Jamaluddin AHMED M, HOSSAN Jamal. Spectrophotometric determination of aluminium by morin[J]. Talanta, 1995, 42(8): 1135-1142. |
| 44 | 干宁, 雷建平, 李伟, 等. 钙镁试剂-示波计时电位法快速测定天然水中不同形态铝[J]. 环境化学, 2002, 21(4): 397-403. |
| GAN Ning, LEI Jianping, LI Wei, et al. Determination of aluminum fractions in natural waters by a.c.oscillopolarography using calmagite[J]. Environmental Chemistry, 2002, 21(4): 397-403. | |
| 45 | 王文东, 杨宏伟, 蒋晶, 等. 天然水体中铝形态分析方法研究进展[J]. 净水技术, 2009, 28(6): 8-12, 29. |
| WANG Wendong, YANG Hongwei, JIANG Jing, et al. Study progress on analysis method of aluminum species in natural water[J]. Water Purification Technology, 2009, 28(6): 8-12, 29. | |
| 46 | XU Hui, ZHANG Dawei, XU Zhizhen, et al. Study on the effects of organic matter characteristics on the residual aluminum and flocs in coagulation processes[J]. Journal of Environmental Sciences, 2018, 63: 307-317. |
| 47 | WAN Biao, ELZINGA Evert J, HUANG Rixiang, et al. Molecular mechanism of linear polyphosphate adsorption on iron and aluminum oxides[J]. The Journal of Physical Chemistry C, 2020, 124(52): 28448-28457. |
| 48 | WANG Wendong, LI Hua, WANG Xiaochang, et al. Spatial variations of aluminum species in drinking water supplies in Xi'an studied applying geographic information system[J]. Journal of Environmental Sciences, 2010, 22(4): 519-525. |
| 49 | VAN BENSCHOTEN John E, EDZWALD James K. Measuring aluminum during water treatment: Methodology and application[J]. Journal AWWA, 1990, 82(5): 71-78. |
| 50 | 王文东, 杨宏伟, 蒋晶, 等. 水温和pH对饮用水中铝形态分布的影响[J]. 环境科学, 2009, 30(8): 2259-2262. |
| WANG Wendong, YANG Hongwei, JIANG Jing, et al. Effects of temperature and pH on the distribution of aluminum species in drinking water[J]. Environmental Science, 2009, 30(8): 2259-2262. | |
| 51 | MA Min, GU Junnong, LI Yuxian, et al. Residual aluminum control for source water with high risk of overproof coagulant residue: A novel application of principal component analysis[J]. Journal of Environmental Chemical Engineering, 2017, 5(3): 2605-2610. |
| 52 | 贺晓娟, 袁本松, 王砚, 等. 微山湖水质对控制残余铝的影响及生产运行分析[J]. 中国给水排水, 2020, 36(3): 45-48. |
| HE Xiaojuan, YUAN Bensong, WANG Yan, et al. Influence of water quality of Weishanhu Lake on residual aluminum and production operation[J]. China Water & Wastewater, 2020, 36(3): 45-48. | |
| 53 | 甘振东, 李鑫玮, 张健, 等. 原水pH和出厂水残余铝偏高调控技术研究与应用[J]. 给水排水, 2023, 59(1): 47-52. |
| GAN Zhendong, LI Xinwei, ZHANG Jian, et al. Research and application of regulation technology for high pH of raw water and residual aluminum[J]. Water & Wastewater Engineering, 2023, 59(1): 47-52. | |
| 54 | ZHOU Yuxuan, YAN Mingquan, LIU Ruiping, et al. Investigating the effect of hardness cations on coagulation: The aspect of neutralisation through A l ( Ⅲ ) -dissolved organic matter (DOM) binding[J]. Water Research, 2017, 115: 22-28. |
| 55 | KAJJUMBA George William, FISCHER Daniel, RISSO Le Anna, et al. Application of cerium and lanthanum coagulants in wastewater treatment—A comparative assessment to magnesium, aluminum, and iron coagulants[J]. Chemical Engineering Journal, 2021, 426: 131268. |
| 56 | JIANG Zhenzhen, ZHU Junren. Preparation, characterization and coagulation performance of a composite coagulant: Polymeric aluminum ferric sulfate[J]. IOP Conference Series: Earth and Environmental Science, 2021, 647(1): 012059. |
| 57 | EDZWALD James K. Aluminum in drinking water: Occurrence, effects, and control[J]. American Water Works Association, 2020, 112(5): 34-41. |
| 58 | 吴福雨, 李能能, 樊小东, 等. 混凝剂中的铝形态对黄河水源水中出水余铝的影响[J]. 环境工程学报, 2022, 16(12): 3926-3934. |
| WU Fuyu, LI Nengneng, FAN Xiaodong, et al. Effects of aluminum form in coagulant on residual aluminum in effluent of the source water of Yellow River[J]. Chinese Journal of Environmental Engineering, 2022, 16(12): 3926-3934. | |
| 59 | Dong-Min SON, KANG Lim-Seok. Evaluation of coagulation-UF process considering residual aluminuim concentration as seawater desalination pretreatment[J]. Journal of Korean Society of Environmental Engineers, 2013, 35(7): 495-502. |
| 60 | 赵晓非, 张晓阳, 杨腾飞, 等. 碱化度对聚合氯化铝混凝除硅的影响[J]. 化工进展, 2015, 34(12): 4375-4378, 4397. |
| ZHAO Xiaofei, ZHANG Xiaoyang, YANG Tengfei, et al. Influence of basicity of polyaluminum chloride on removal of silicon[J]. Chemical Industry and Engineering Progress, 2015, 34(12): 4375-4378, 4397. | |
| 61 | 李润生, 金立新, 李凯, 等. 高盐基度PAC降低饮用水中残留铝的研究[J]. 中国给水排水, 2013, 29(7): 52-55. |
| LI Runsheng, JIN Lixin, LI Kai, et al. High basicity polyaluminium chloride for reduction of residual aluminium in drinking water[J]. China Water & Wastewater, 2013, 29(7): 52-55. | |
| 62 | 毛玉红, 常青, 曾立云. 混凝过程中絮体形貌的PIV成像观测与表征[J]. 中国环境科学, 2014, 34(4): 951-957. |
| MAO Yuhong, CHANG Qing, ZENG Liyun. Characterization and measurement of the flocs during the coagulation process by PIV[J]. China Environmental Science, 2014, 34(4): 951-957. | |
| 63 | 刘海龙, 王东升, 王敏, 等. 强化混凝对水力条件的要求[J]. 中国给水排水, 2006, 22(5): 1-4. |
| LIU Hailong, WANG Dongsheng, WANG Min, et al. Requirement for hydrodynamic conditions in enhanced coagulation[J]. China Water & Wastewater, 2006, 22(5): 1-4. | |
| 64 | LI Mengzhuo, CHENG Jixia, ZOU Fang, et al. Effects of pre-oxidation on residual dissolved aluminum in coagulated water: A pilot-scale study[J]. Water research, 2021, 190: 116682. |
| 65 | YANG Zhonglian, GAO Baoyu, YUE Qinyan, et al. Effect of pH on the coagulation performance of Al-based coagulants and residual aluminum speciation during the treatment of humic acid-kaolin synthetic water[J]. Journal of Hazardous Materials, 2010, 178(1/2/3): 596-603. |
| 66 | 李勐卓, 程继夏, 顾军农, 等. 铁-铝盐混凝剂混合投加工艺控制溶解性残余铝的机理[J]. 环境工程学报, 2021, 15(2): 580-587. |
| LI Mengzhuo, CHENG Jixia, GU Junnong, et al. Mechanism of controlling dissolved residual aluminum in simultaneous addition of iron and aluminum salt coagulants[J]. Chinese Journal of Environmental Engineering, 2021, 15(2): 580-587. | |
| 67 | 曲江东, 徐慧, 徐建坤, 等. 原水性质对新型含Ca2+复合混凝剂混凝过程的影响[J]. 环境科学, 2019, 40(1): 263-272. |
| QU Jiangdong, XU Hui, XU Jiankun, et al. Influence of the coagulation mechanism on the coagulation performances using new composite coagulants: Role of the raw water characteristics[J]. Environmental Science, 2019, 40(1): 263-272. | |
| 68 | MISHRA Mansi, CHAUHAN M S. Biosorption as a novel approach for removing aluminium from water treatment plant residual—A review[J]. Water Quality Management, 2018, 79: 93-99. |
| 69 | YU Huibo, ZHANG Xianqiu, HAN Xue, et al. Nanofiltration membrane fouling and control caused by residual aluminum in feed water[J]. Water, Air, & Soil Pollution, 2022, 233(1): 1. DOI: 10.1007/S11270-021-05470-z . |
| 70 | WANG Wendong, LI Hua, DING Zhenzhen, et al. Effects of advanced oxidation pretreatment on residual aluminum control in high humic acid water purification[J]. Journal of Environmental Sciences, 2011, 23(7): 1079-1085. |
| [1] | 赵晓非, 张晓阳, 杨腾飞, 范蕾, 王顺武, 于庆龙. 碱化度对聚合氯化铝混凝除硅的影响[J]. 化工进展, 2015, 34(12): 4375-4378,4397. |
| [2] | 李 军1,王纯正2,马占华1,孙兰义1. 隔壁塔用于苯、甲苯、二甲苯分离的控制[J]. 化工进展, 2013, 32(04): 757-762. |
| [3] | 杨兰琴1,冯雷雨1,2,陈银广1. 中国水环境中全氟化合物的污染水平及控制策略[J]. 化工进展, 2012, 31(10): 2304-2312. |
| [4] | 韦朝海,何勤聪,帅 伟,任 源,成国飞,潘维龙. 精细化工废水的污染特性分析及其控制策略 [J]. 化工进展, 2009, 28(11): 2047-. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||