化工进展 ›› 2023, Vol. 42 ›› Issue (S1): 299-309.DOI: 10.16085/j.issn.1000-6613.2023-1169
用于SO2去极化电解制氢的铂基催化剂
- 清华大学核能与新能源技术研究院,北京 100084
-
收稿日期:2023-07-10修回日期:2023-09-12出版日期:2023-10-25发布日期:2023-11-30 -
通讯作者:张平 -
作者简介:谢璐垚(2000—),女,硕士研究生,研究方向为新型电解制氢技术。E-mail:xiely22@mails.tsinghua.edu.cn。 -
基金资助:国家科技重大专项(ZX06901)
Platinum-based catalysts for SO2 depolarized electrolysis
XIE Luyao( ), CHEN Songzhe, WANG Laijun, ZHANG Ping(
), CHEN Songzhe, WANG Laijun, ZHANG Ping( )
)
- Institute of Nuclear and New Energy Technology, Tsinghua University, Beijing 100084, China
-
Received:2023-07-10Revised:2023-09-12Online:2023-10-25Published:2023-11-30 -
Contact:ZHANG Ping
摘要:
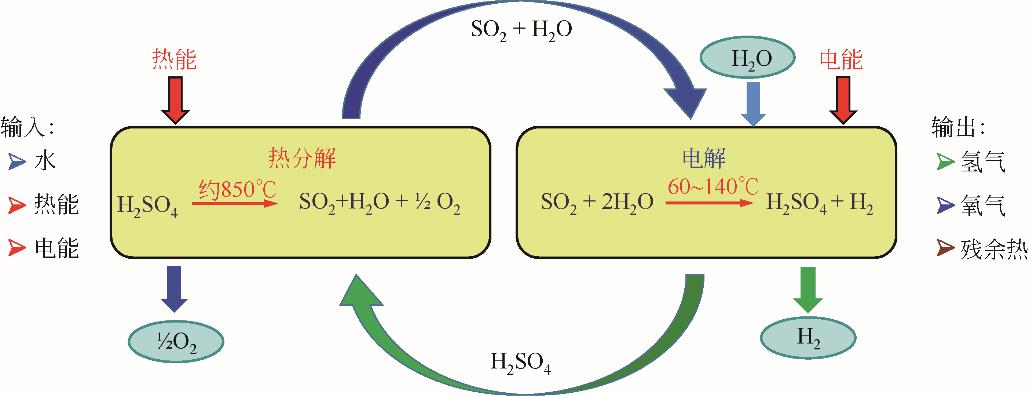
综述了铂基SO2去极化电解(SDE)阳极催化剂的研究进展。SDE阳极反应条件苛刻,铂基催化剂因具备良好的导电性、抗腐蚀性,并能够有效抵抗H2S等硫物质的毒化,成为SDE阳极催化剂的首选。通过引入Al、Cr、Ni等非贵金属元素,可有效提高铂基催化剂性能并减少Pt的用量。在载体方面,综述和讨论了活性炭、石墨、炭黑、石墨烯以及SiC/TiC等对铂基催化剂性能的影响,此外分析了催化剂制备工艺对催化剂结构参数和性能的影响。尽管已经取得了很多研究成果,但当前对铂基SDE阳极催化剂的长期稳定性、多金属催化剂各金属元素间的相互作用等方面的研究尚较少,进一步优化催化剂设计、加强载体筛选及其改性,开发新的制备工艺,提高Pt利用率及催化剂的活性和稳定性,是未来相关研究的关键所在。
中图分类号:
引用本文
谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309.
XIE Luyao, CHEN Songzhe, WANG Laijun, ZHANG Ping. Platinum-based catalysts for SO2 depolarized electrolysis[J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 299-309.
| 路径1(HSO3*中间体) | 路径2(SO3*中间体) |
|---|---|
表1 SO2在水相中两种可能的氧化反应路径[23]
| 路径1(HSO3*中间体) | 路径2(SO3*中间体) |
|---|---|
| 周期 | 元素 | 催化活性研究方法 | 密度泛函理论分析结果[ | 实验测试结果 |
|---|---|---|---|---|
| 第五周期 | Nb | 密度泛函理论分析 | 0.6~1.2V电压范围内反应活性较高 | — |
| Mo | 密度泛函理论分析 | 模拟的归一化SO2氧化速率满 | — | |
| Ru | 密度泛函理论分析 实验测试 | 模拟的归一化SO2氧化速率较慢 | 25℃、50% H2SO4(饱和SO2)条件下催化活性较好[ | |
| 50℃、2mol/L H2SO4(10-3mol/L SO2)条件下催化活性较差[ | ||||
| 第五周期 | Rh | 密度泛函理论分析 实验测试 | 模拟的归一化SO2氧化速率较慢 | 25℃、50% H2SO4(饱和SO2)条件下几乎没有催化活性[ |
| 50℃、2mol/L H2SO4(10-3mol/L SO2)条件下催化活性最高,但活性随电解过程衰减严重[ | ||||
| Pd | 密度泛函理论分析 实验测试 | 模拟的归一化SO2氧化速率较快 | 25℃、50% H2SO4(饱和SO2)条件下催化活性最好[ | |
| Ag | 密度泛函理论分析 | 模拟的归一化SO2氧化速率较慢 | — | |
| 第六周期 | Ta | 密度泛函理论分析 | 0.6~1.2V电压范围内反应活性较高 | — |
| W | 密度泛函理论分析 | 模拟的归一化SO2氧化速率慢 | — | |
| Re | 实验测试 | — | 25℃、50% H2SO4(饱和SO2)条件下几乎没有催化活性[ | |
| Os | 密度泛函理论分析 | 模拟的归一化SO2氧化速率较慢 | — | |
| Ir | 密度泛函理论分析 实验测试 | 模拟的归一化SO2氧化速率较慢 | 25℃、50% H2SO4(饱和SO2)条件下几乎没有催化活性[ | |
| 50℃、2mol/L H2SO4(10-3mol/L SO2)条件下催化活性较差[ | ||||
| Pt | 密度泛函理论分析 实验测试 | 反应活性中等,模拟的归一化SO2氧化速率快 | 25℃、50% H2SO4(饱和SO2)条件下催化活性较好[ | |
| 50℃、2mol/L H2SO4(10-3mol/L SO2)条件下催化性能较好(最优选择) | ||||
| Au | 密度泛函理论分析 实验测试 | 模拟的归一化SO2氧化速率快 | 25℃、50% H2SO4(饱和SO2)条件下催化活性较好[ | |
| 50℃、2mol/L H2SO4(10-3mol/L SO2)条件下催化活性较好[ |
表2 五、六周期过渡金属元素催化SO2氧化研究结果[19,23,33]
| 周期 | 元素 | 催化活性研究方法 | 密度泛函理论分析结果[ | 实验测试结果 |
|---|---|---|---|---|
| 第五周期 | Nb | 密度泛函理论分析 | 0.6~1.2V电压范围内反应活性较高 | — |
| Mo | 密度泛函理论分析 | 模拟的归一化SO2氧化速率满 | — | |
| Ru | 密度泛函理论分析 实验测试 | 模拟的归一化SO2氧化速率较慢 | 25℃、50% H2SO4(饱和SO2)条件下催化活性较好[ | |
| 50℃、2mol/L H2SO4(10-3mol/L SO2)条件下催化活性较差[ | ||||
| 第五周期 | Rh | 密度泛函理论分析 实验测试 | 模拟的归一化SO2氧化速率较慢 | 25℃、50% H2SO4(饱和SO2)条件下几乎没有催化活性[ |
| 50℃、2mol/L H2SO4(10-3mol/L SO2)条件下催化活性最高,但活性随电解过程衰减严重[ | ||||
| Pd | 密度泛函理论分析 实验测试 | 模拟的归一化SO2氧化速率较快 | 25℃、50% H2SO4(饱和SO2)条件下催化活性最好[ | |
| Ag | 密度泛函理论分析 | 模拟的归一化SO2氧化速率较慢 | — | |
| 第六周期 | Ta | 密度泛函理论分析 | 0.6~1.2V电压范围内反应活性较高 | — |
| W | 密度泛函理论分析 | 模拟的归一化SO2氧化速率慢 | — | |
| Re | 实验测试 | — | 25℃、50% H2SO4(饱和SO2)条件下几乎没有催化活性[ | |
| Os | 密度泛函理论分析 | 模拟的归一化SO2氧化速率较慢 | — | |
| Ir | 密度泛函理论分析 实验测试 | 模拟的归一化SO2氧化速率较慢 | 25℃、50% H2SO4(饱和SO2)条件下几乎没有催化活性[ | |
| 50℃、2mol/L H2SO4(10-3mol/L SO2)条件下催化活性较差[ | ||||
| Pt | 密度泛函理论分析 实验测试 | 反应活性中等,模拟的归一化SO2氧化速率快 | 25℃、50% H2SO4(饱和SO2)条件下催化活性较好[ | |
| 50℃、2mol/L H2SO4(10-3mol/L SO2)条件下催化性能较好(最优选择) | ||||
| Au | 密度泛函理论分析 实验测试 | 模拟的归一化SO2氧化速率快 | 25℃、50% H2SO4(饱和SO2)条件下催化活性较好[ | |
| 50℃、2mol/L H2SO4(10-3mol/L SO2)条件下催化活性较好[ |
| 引入过渡金属元素 | 测试体系 | 测试条件 | 催化性能是否提升(与Pt/C相比) | 备注 |
|---|---|---|---|---|
| Ir | 两电极体系 (“三明治”型MEA) | 25℃,30% H2SO4 (饱和SO2)[ | 电压1.6~1.8V时电流密度较大 | 结论不同原因可能是双金属比例、测试体系以及碳载体不同造成的 |
80℃,56% H2SO4 (饱和SO2)[ | 电压0.7~1.2V时归一化电流密度大 | |||
| 三电极体系 | 25℃,30% H2SO4 (饱和SO2)[ | — | ||
| Ru | 三电极体系 | 25℃,30% H2SO4 (饱和SO2)[ | — | 结论不同原因可能是催化剂构型不同造成的 |
20℃,56% H2SO4 (饱和SO2)[ | 电压0.5~1.3V时归一化电流密度大 | |||
两电极体系 (“三明治”型MEA) | 80℃,56% H2SO4 (饱和SO2)[ | 电压0.7~1.2V时归一化电流密度大 | ||
| Rh | 两电极体系 (“三明治”型MEA) | 25℃,30% H2SO4 (饱和SO2)[ | — | 结论不同原因可能是双金属比例、测试环境不同造成的 |
80℃,56% H2SO4 (饱和SO2)[ | 归一化电流密度提高 | |||
| Al | 两电极体系 (“三明治”型MEA) | 室温、3mol/L H2SO4 (含0.9mol/L SO2)[ | 催化活性提高 | |
| Cr | 两电极体系 (“三明治”型MEA) | 25℃,30% H2SO4 (饱和SO2)[ | ECSA略有增加;开路电压小;电压1.4~1.8V时电流密度大 | Cr的引入或改变Pt的电子结构,增加Pt原子d电子层空轨道数,产生了电子效应和几何效应 |
| 三电极体系 | ||||
| Co | 三电极体系 | 25℃,30% H2SO4 (饱和SO2)[ | ECSA略有增加 | |
| Fe | 三电极体系 | 25℃,30% H2SO4 (饱和SO2)[ | ECSA略有增加 | |
| Pd | 两电极体系 (“三明治”型MEA) | 25℃,30% H2SO4 (饱和SO2)[ | — | 结论不同原因可能是双金属比例、测试体系、测试环境、载体不同造成的 |
80℃,56% H2SO4 (饱和SO2)[ | 归一化电流升高;稳定性增加 | |||
| 三电极体系 | 25℃,1mol/L H2SO4, 100mmol/L SO2[ | SO2氧化电解的平均起始电压小,稳定性增加 | ||
| Cu | 两电极体系 (“三明治”型MEA) | 80℃,56% H2SO4 (饱和SO2)[ | — | |
| Ni | 三电极体系 | 25℃,56% H2SO4 (饱和SO2)[ | 电压0.6~1.0V时归一化电流密度大 | |
| Co、Cr | 三电极体系 | 25℃,30% H2SO4 (饱和SO2)[ | — | |
| Pd、Al | 三电极体系 | 60℃,1mol/L H2SO4+1mol/L Na2SO3[ | 归一化电流密度大 |
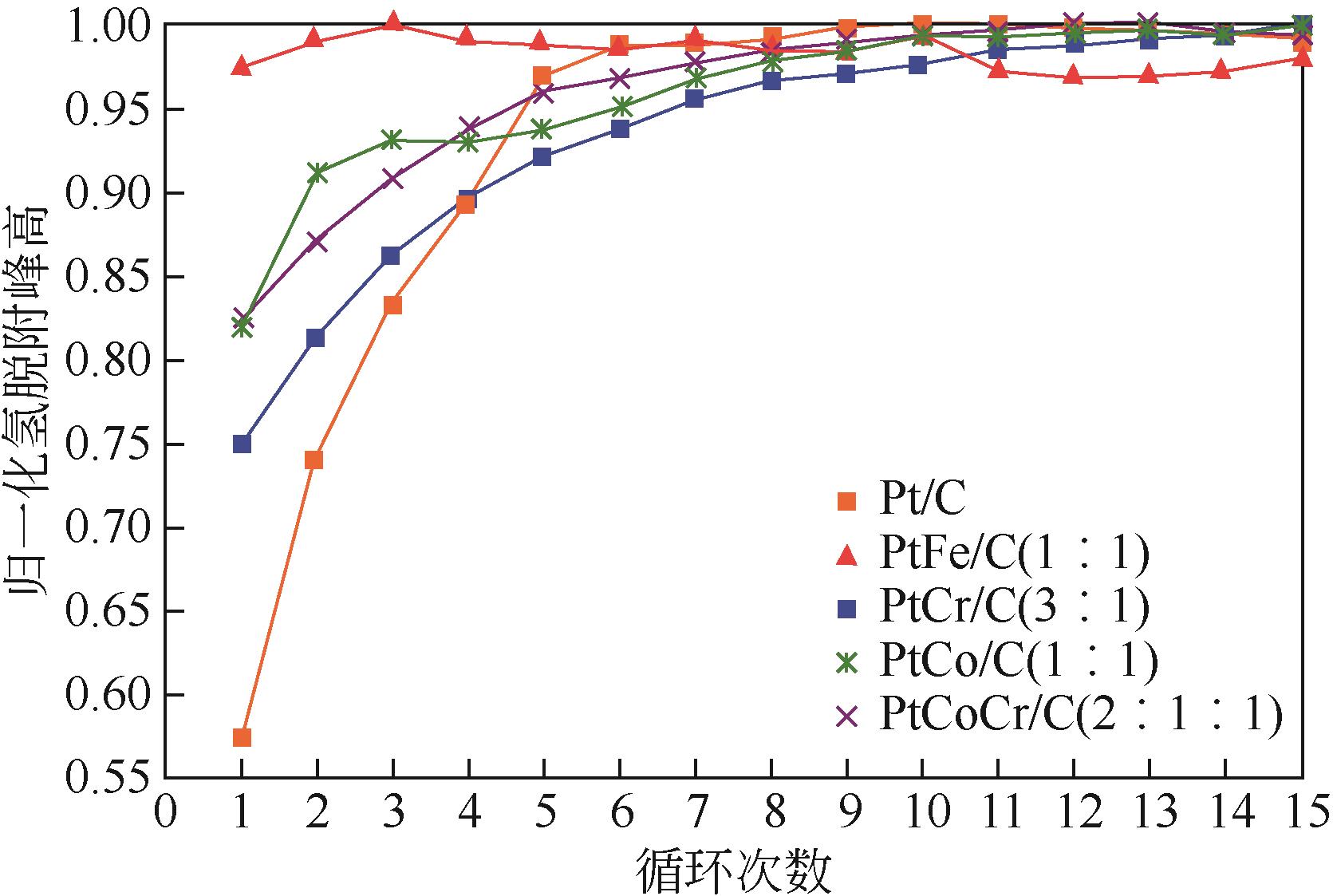
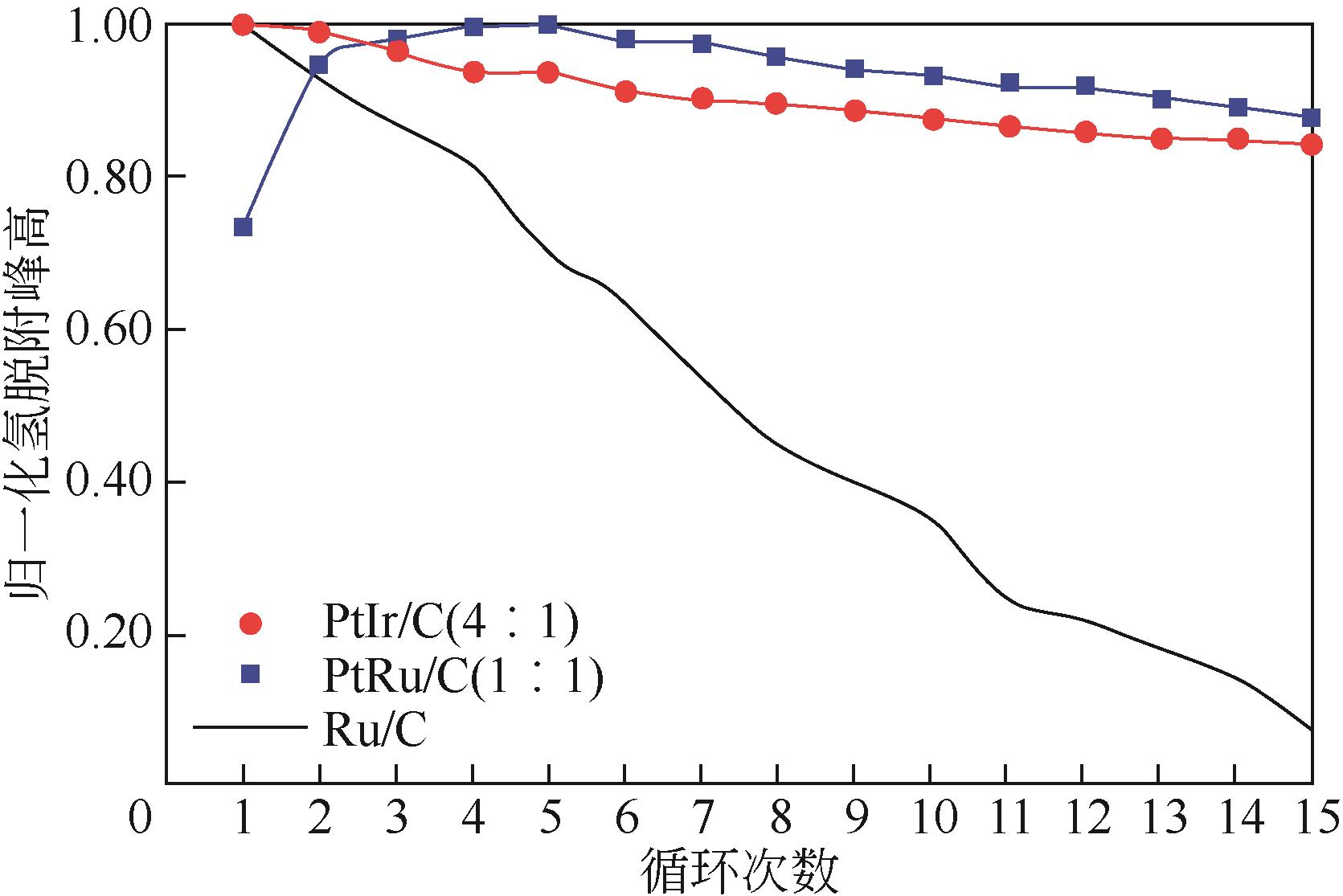
表3 双金属/多金属铂基催化剂研究结果汇总
| 引入过渡金属元素 | 测试体系 | 测试条件 | 催化性能是否提升(与Pt/C相比) | 备注 |
|---|---|---|---|---|
| Ir | 两电极体系 (“三明治”型MEA) | 25℃,30% H2SO4 (饱和SO2)[ | 电压1.6~1.8V时电流密度较大 | 结论不同原因可能是双金属比例、测试体系以及碳载体不同造成的 |
80℃,56% H2SO4 (饱和SO2)[ | 电压0.7~1.2V时归一化电流密度大 | |||
| 三电极体系 | 25℃,30% H2SO4 (饱和SO2)[ | — | ||
| Ru | 三电极体系 | 25℃,30% H2SO4 (饱和SO2)[ | — | 结论不同原因可能是催化剂构型不同造成的 |
20℃,56% H2SO4 (饱和SO2)[ | 电压0.5~1.3V时归一化电流密度大 | |||
两电极体系 (“三明治”型MEA) | 80℃,56% H2SO4 (饱和SO2)[ | 电压0.7~1.2V时归一化电流密度大 | ||
| Rh | 两电极体系 (“三明治”型MEA) | 25℃,30% H2SO4 (饱和SO2)[ | — | 结论不同原因可能是双金属比例、测试环境不同造成的 |
80℃,56% H2SO4 (饱和SO2)[ | 归一化电流密度提高 | |||
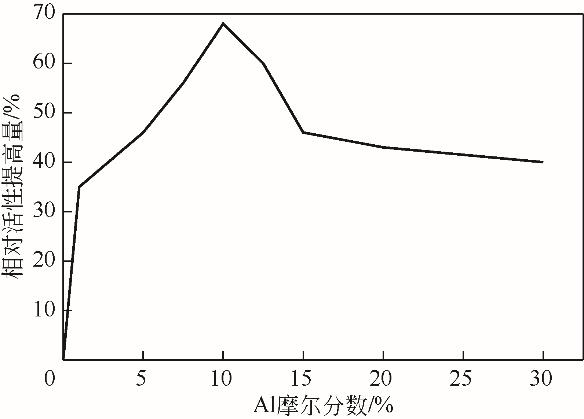
| Al | 两电极体系 (“三明治”型MEA) | 室温、3mol/L H2SO4 (含0.9mol/L SO2)[ | 催化活性提高 | |
| Cr | 两电极体系 (“三明治”型MEA) | 25℃,30% H2SO4 (饱和SO2)[ | ECSA略有增加;开路电压小;电压1.4~1.8V时电流密度大 | Cr的引入或改变Pt的电子结构,增加Pt原子d电子层空轨道数,产生了电子效应和几何效应 |
| 三电极体系 | ||||
| Co | 三电极体系 | 25℃,30% H2SO4 (饱和SO2)[ | ECSA略有增加 | |
| Fe | 三电极体系 | 25℃,30% H2SO4 (饱和SO2)[ | ECSA略有增加 | |
| Pd | 两电极体系 (“三明治”型MEA) | 25℃,30% H2SO4 (饱和SO2)[ | — | 结论不同原因可能是双金属比例、测试体系、测试环境、载体不同造成的 |
80℃,56% H2SO4 (饱和SO2)[ | 归一化电流升高;稳定性增加 | |||
| 三电极体系 | 25℃,1mol/L H2SO4, 100mmol/L SO2[ | SO2氧化电解的平均起始电压小,稳定性增加 | ||
| Cu | 两电极体系 (“三明治”型MEA) | 80℃,56% H2SO4 (饱和SO2)[ | — | |
| Ni | 三电极体系 | 25℃,56% H2SO4 (饱和SO2)[ | 电压0.6~1.0V时归一化电流密度大 | |
| Co、Cr | 三电极体系 | 25℃,30% H2SO4 (饱和SO2)[ | — | |
| Pd、Al | 三电极体系 | 60℃,1mol/L H2SO4+1mol/L Na2SO3[ | 归一化电流密度大 |
| 1 | 董馨浍. 中国氢能产业现状与未来[EB/OL]. [2022-11-28]. . |
| DONG Xinhui. Status and euture of China's hydrogen energy industry [EB/OL]. [2022-11-28]. . | |
| 2 | 曾朵红, 阮巧燕. 氢能源行业深度报告: 绿氢,第四次能源革命的载体[EB/OL]. [2023-03-08]. . |
| ZENG Duohong, RUAN Qiaoyan. Hydrogen energy industry in-depth report: Green Hydrogen, the carrier of the fourth energy revolution[EB/OL]. [2023-03-08]. . | |
| 3 | FUNK J E, REINSTROM R M. Energy requirements in production of hydrogen from water[J]. Industrial & Engineering Chemistry Process Design and Development, 1966, 5(3): 336-342. |
| 4 | 陈晶澈, 张彦威, 周俊虎. 两步式热化学循环分解水制氢研究进展[J]. 能源工程, 2016(2): 21-27. |
| CHEN Jingche, ZHANG Yanwei, ZHOU Junhu. Research progress of two-step thermochemical cycle of water splitting[J]. Energy Engineering, 2016(2): 21-27. | |
| 5 | JOMARD F, FERAUD J P, CAIRE J P. Numerical modeling for preliminary design of the hydrogen production electrolyzer in the Westinghouse hybrid cycle[J]. International Journal of Hydrogen Energy, 2008, 33(4): 1142-1152. |
| 6 | BRECHER L E, WU C K. Electrolytic decomposition of water: US3888750[P]. 1975-06-10. |
| 7 | BRECHER L E, SPEWOCK S, WARDE C J. The Westinghouse sulfur cycle for the thermochemical decomposition of water[J]. International Journal of Hydrogen Energy, 1977, 2(1): 7-15. |
| 8 | 王荣荣. 二氧化硫去极化反应催化剂的制备与性能研究[D]. 北京: 北京化工大学, 2021. |
| WANG Rongrong. Study on preparation and performance of catalyst for sulfur dioxide depolarization reaction [D]. Beijing: Beijing University of Chemical Technology, 2021. | |
| 9 | 张平, 于波, 陈靖, 等. 热化学循环分解水制氢研究进展[J]. 化学进展, 2005, 17(4): 643-650. |
| ZHANG Ping, YU Bo, CHEN Jing, et al. Study on the Hydrogen Production by Thermochemical Water Splitting[J]. Progress in Chemistry, 2005, 17(4): 643-650. | |
| 10 | GORENSEK M B, STASER J A, STANFORD T G, et al. A thermodynamic analysis of the SO2/H2SO4 system in SO2-depolarized electrolysis[J]. International Journal of Hydrogen Energy, 2009, 34(15): 6089-6095. |
| 11 | ARMSTRONG D A, HUIE R E, KOPPENOL W H, et al. Standard electrode potentials involving radicals in aqueous solution: Inorganic radicals (IUPAC Technical Report)[J]. Pure and Applied Chemistry, De Gruyter, 2015, 87(11/12): 1139-1150. |
| 12 | STEIMKE J L, STEEPER T J. Characterization testing of H2O-SO2 electrolyzer at ambient pressure[R]. United States: N. p., 2005:1-56. |
| 13 | STASER J, RAMASAMY R P, SIVASUBRAMANIAN P, et al. Effect of water on the electrochemical oxidation of gas-phase SO2 in a PEM electrolyzer for H2 production[J]. Electrochemical and Solid-State Letters, IOP Publishing, 2007, 10(11): E17. |
| 14 | STASER J A, GORENSEK M B, WEIDNER J W. Quantifying individual potential contributions of the hybrid sulfur electrolyzer[J]. Journal of the Electrochemical Society, 2010, 157(6): B952. |
| 15 | DING Xifeng, CHEN Songzhe, WANG Laijun, et al. Study on electrochemical impedance spectroscopy and cell voltage composition of a PEM SO2-depolarized electrolyzer using graphite felt as diffusion layer[J]. Electrochimica Acta, 2022, 426: 140837. |
| 16 | COLÓN-MERCADO H R, GORENSEK M B, FUJIMOTO C H, et al. High-performance SO2-depolarized electrolysis cell using advanced polymer electrolyte membranes[J]. International Journal of Hydrogen Energy, 2022, 47(1): 57-68. |
| 17 | GORENSEK M B, SUMMERS W A. Hybrid sulfur flowsheets using PEM electrolysis and a bayonet decomposition reactor[J]. International Journal of Hydrogen Energy, 2009, 34(9): 4097-4114. |
| 18 | STASER J A, GORENSEK M B, WEIDNER J W. Quantifying individual potential contributions of the hybrid sulfur electrolyzer[J]. Journal of the Electrochemical Society, 2010, 157(6): B952. |
| 19 | APPLEBY A J, PINCHON B. Electrochemical aspects of the H2SO4-SO2 thermoelectrochemical cycle for hydrogen production[J]. International Journal of Hydrogen Energy, 1980, 5(3): 253-267. |
| 20 | LU P W T, GARCIA E R, AMMON R L. Recent developments in the technology of sulphur dioxide depolarized electrolysis[J]. Journal of Applied Electrochemistry, 1981, 11(3): 347-355. |
| 21 | O’BRIEN J A, HINKLEY J T, DONNE S W, et al. The electrochemical oxidation of aqueous sulfur dioxide: A critical review of work with respect to the hybrid sulfur cycle[J]. Electrochimica Acta, 2010, 55(3): 573-591. |
| 22 | LU P W T, AMMON R L. Sulfur dioxide depolarized electrolysis for hydrogen production: Development status[J]. International Journal of Hydrogen Energy, 1982, 7(7): 563-575. |
| 23 | KRIEK R J, ROSSMEISL J, SIAHROSTAMI S, et al. H2 production through electro-oxidation of SO2: Identifying the fundamental limitations[J]. Physical Chemistry Chemical Physics, The Royal Society of Chemistry, 2014, 16(20): 9572-9579. |
| 24 | NOYES A A, STEINOUR H H. The potential of inert electrodes in solutions of sulfurous acid and its behavior as an oxidizing and reducing agent[J]. Journal of the American Chemical Society, 1929, 51(5): 1409-1428. |
| 25 | BARBIER J, LAMY-PITARA E, MARECOT P, et al. Role of sulfur in catalytic hydrogenation reactions[M]. Advances in Catalysis. Amsterdam: Elsevier, 1990: 279-318. |
| 26 | KRÜGER A J, KRIEG H M, BESSARABOV D. Effect of H2S on SO2-depolarised water electrolysis[J]. International Journal of Hydrogen Energy, 2015, 40(13): 4442-4450. |
| 27 | MARSHALL A, BØRRESEN B, HAGEN G, et al. Electrochemical characterisation of Ir x Sn1- x O2 powders as oxygen evolution electrocatalysts[J]. Electrochimica Acta, 2006, 51(15): 3161-3167. |
| 28 | MARSHALL A, BØRRESEN B, HAGEN G, et al. Hydrogen production by advanced proton exchange membrane (PEM) water electrolysers—Reduced energy consumption by improved electrocatalysis[J]. Energy, 2007, 32(4): 431-436. |
| 29 | 卢雯婷, 陈敬超, 冯晶, 等. 贵金属催化剂的应用研究进展[J]. 稀有金属材料与工程, 2012, 41(1): 184-188. |
| LU Wenting, CHEN Jingchao, FENG Jing, et al. Research progress of noble metal catalyst application[J]. Rare Metal Materials and Engineering, 2012, 41(1): 184-188. | |
| 30 | 黄荣光. 贵金属催化剂的制备[J]. 贵金属, 1982, 4(1): 51-59. |
| HUANG Rongguang. Preparation of precious metal catalysts[J]. Precious Metals, 1982, 4(1): 51-59. | |
| 31 | 熊峻, 陈吉祥, 张继炎. 氯代硝基苯催化加氢合成氯代苯胺的催化剂研究进展[J]. 化学试剂, 2006, 28(6): 331-335. |
| XIONG Jun, CHEN Jixiang, ZHANG Jiyan. Recent development of catalysts for hydrogenation of chloronitrobenzene to chloroaniline[J]. Chemical Reagents, 2006,28 (6): 331-335. | |
| 32 | 张萍俊. 质子交换膜水电解池的性能优化及动态响应的研究[D]. 大连: 大连交通大学, 2019. |
| ZHANG Pingjun. Study on performance optimization and dynamic response of proton exchange membrane water electrolyic cell[D]. Dalian: Dalian Jiaotong University, 2019. | |
| 33 | LU P W T, AMMON R L. An investigation of electrode materials for the anodic oxidation of sulfur dioxide in concentrated sulfuric acid[J]. Journal of the Electrochemical Society, 1980, 127(12): 2610-2616. |
| 34 | LEE S K, KIM C H, CHO W C, et al. The effect of Pt loading amount on SO2 oxidation reaction in an SO2-depolarized electrolyzer used in the hybrid sulfur (HyS) process[J]. International Journal of Hydrogen Energy, 2009, 34(11): 4701-4707. |
| 35 | COLÓN-MERCADO H, ELVINGTON M, HOBBS D. Close-out report for HyS electrolyzer component development work at savannah river national laboratory[J]. SRNL-STI-2010-00019, Savannah River Site (SRS), Aiken, SC (United States), 2010: 1-21. |
| 36 | XUE Lulu, ZHANG Ping, CHEN Songzhe, et al. Pt-based bimetallic catalysts for SO2-depolarized electrolysis reaction in the hybrid sulfur process[J]. International Journal of Hydrogen Energy, 2014, 39(26): 14196-14203. |
| 37 | LEE J, LANGER S H. Electrochemical sulphur dioxide oxidation with platinum-aluminum electrocatalysts[J]. Journal of Applied Electrochemistry, 1995, 25(4): 353-357. |
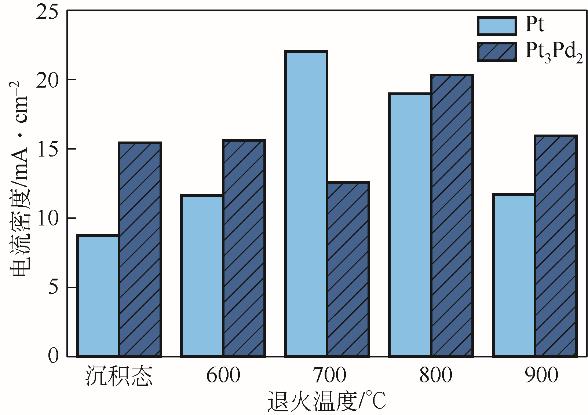
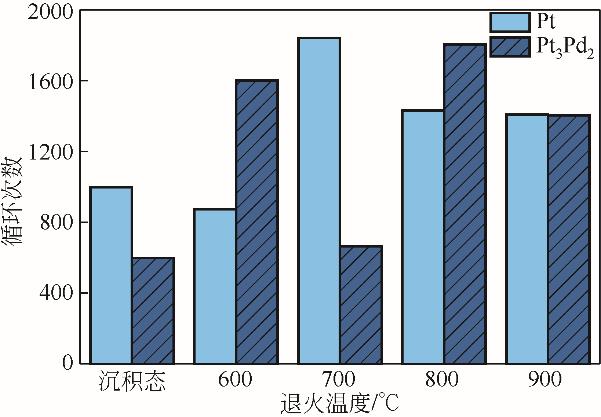
| 38 | FALCH A, LATES V, KRIEK R J. Combinatorial plasma sputtering of Pt x Pd y thin film electrocatalysts for aqueous SO2 electro-oxidation[J]. Electrocatalysis, 2015, 6(3): 322–330. |
| 39 | FALCH A, LATES V A, KOTZÉ H S, et al. The effect of rapid thermal annealing on sputtered Pt and Pt3Pd2 thin film electrocatalysts for aqueous SO2 electro-oxidation[J]. Electrocatalysis, 2016, 7(1): 33-41. |
| 40 | FALCH A, BADETS V A, LABRUGÈRE C, et al. Co-sputtered Pt x Pd y Al z thin film electrocatalysts for the production of hydrogen via SO2(aq) electro-oxidation[J]. Electrocatalysis, 2016, 7(5): 376-390. |
| 41 | HUANG Biyi, HE Yong, WANG Zhihua, et al. Ru@Pt/C core-shell catalyst for SO2 electrocatalytic oxidation in electrochemical Bunsen reaction[J]. Electrochimica Acta, 2020, 331: 135315. |
| 42 | ZHANG Shuhan, HUANG Biyi, HE Yong, et al. Demetallized Pt x Ni y /C catalyst for SO2 electrochemical oxidation in the SI/HyS hydrogen production cycles[J]. International Journal of Hydrogen Energy, 2021, 46(17): 10161-10171. |
| 43 | TANG Libin, LI Xueming, JI Rongbin, et al. Bottom-up synthesis of large-scale graphene oxide nanosheets[J]. Journal of Materials Chemistry, 2012, 22(12): 5676-5683. |
| 44 | PEI Songfeng, ZHAO Jinping, DU Jinhong, et al. Direct reduction of graphene oxide films into highly conductive and flexible graphene films by hydrohalic acids[J]. Carbon, 2010, 48(15): 4466-4474. |
| 45 | PEI Songfeng, CHENG Huiming. The reduction of graphene oxide[J]. Carbon, 2012, 50(9): 3210-3228. |
| 46 | HUANG Biyi, HE Yong, WANG Zhihua, et al. High-performance Pt catalyst with graphene/carbon black as a hybrid support for SO2 electrocatalytic oxidation[J]. Langmuir, American Chemical Society, 2020, 36(1): 20-27. |
| 47 | BAYRAKÇEKEN Y A, DAŞ E. Chemically synthesized reduced graphene oxide-carbon black based hybrid catalysts for PEM fuel cells[J]. International Journal of Hydrogen Energy, 2018, 43(40): 18691-18701. |
| 48 | LOBATO Justo, ZAMORA Hector, PLAZA Jorge, et al. Enhancement of high temperature PEMFC stability using catalysts based on Pt supported on SiC based materials[J]. Applied Catalysis B: Environmental, 2016, 198: 516-524. |
| 49 | ZAMORA H, PLAZA J, VELHAC P, et al. SiCTiC as catalyst support for HT-PEMFCs. Influence of Ti content[J]. Applied Catalysis B: Environmental, 2017, 207: 244-254. |
| 50 | LOBATO J, DÍAZ-ABAD S, PELÁEZ M C, et al. Synthesis and characterization of Pt on novel catalyst supports for the H2 production in the Westinghouse cycle[J]. International Journal of Hydrogen Energy, 2020, 45(47): 25672-25680. |
| 51 | DUAN Sibin, DU Zhe, FAN Hongsheng, et al. Nanostructure optimization of platinum-based nanomaterials for catalytic applications[J]. Nanomaterials, 2018, 8(11): 949. |
| 52 | LOUKRAKPAM Rameshwori, LUO Jin, HE Tinget al. Nanoengineered PtCo and PtNi catalysts for oxygen reduction reaction: An assessment of the structural and electrocatalytic properties[J]. The Journal of Physical Chemistry C, 2011, 115(5): 1682-1694. |
| 53 | 梁健. 多元醇还原法制备Pt/RGONRs及其氧还原反应性能[D]. 大连: 大连理工大学, 2018. |
| LIANG Jian. Preparation of Pt/RGONRs by polyol reduction method and its oxygen reduction reaction performance[D]. Dalian: Dalian University of Technology, 2018. | |
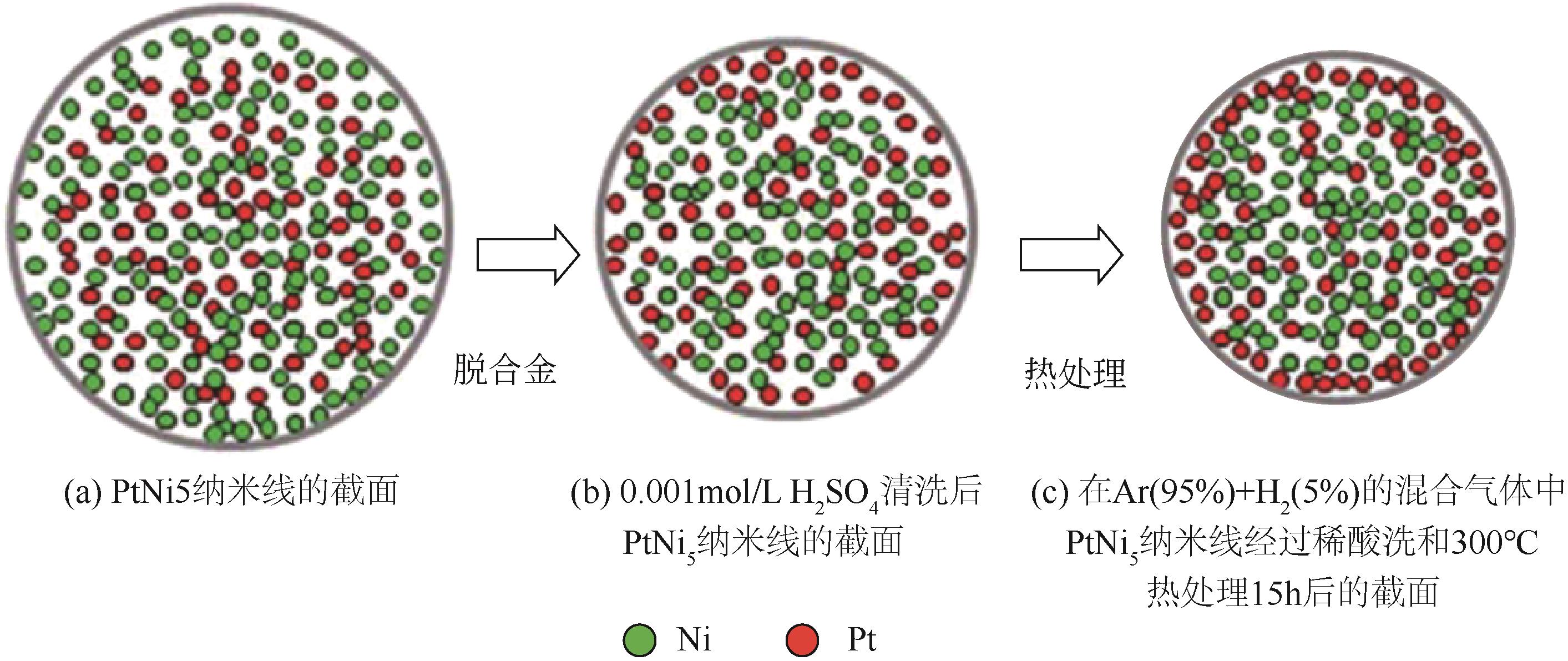
| 54 | SHUI J, ZHANG J, LI J C. Making Pt-shell Pt30Ni70 nanowires by mild dealloying and heat treatments with little Ni loss[J]. Journal of Materials Chemistry, 2011, 21(17): 6225-6229. |
| [1] | 张亚娟, 徐惠, 胡贝, 史星伟. 化学镀法制备NiCoP/rGO/NF高效电解水析氢催化剂[J]. 化工进展, 2023, 42(8): 4275-4282. |
| [2] | 王蕴青, 杨国锐, 延卫. 过渡金属磷化物的改性方法及其在电化学析氢中的应用[J]. 化工进展, 2023, 42(7): 3532-3549. |
| [3] | 陈怡欣, 甄摇摇, 陈瑞浩, 吴继伟, 潘丽美, 姚翀, 罗杰, 卢春山, 丰枫, 王清涛, 张群峰, 李小年. 铂基纳米催化剂的制备及在加氢领域的进展[J]. 化工进展, 2023, 42(6): 2904-2915. |
| [4] | 符淑瑢, 王丽娜, 王东伟, 刘蕊, 张晓慧, 马占伟. 析氧助催化剂增强光阳极光电催化分解水性能研究进展[J]. 化工进展, 2023, 42(5): 2353-2370. |
| [5] | 肖周荣, 李国柱, 王涖, 张香文, 谷建民, 王德松. 液体碳氢燃料蒸汽重整制氢催化剂研究进展[J]. 化工进展, 2022, 41(S1): 97-107. |
| [6] | 胡兵, 徐立军, 何山, 苏昕, 汪继伟. 碳达峰与碳中和目标下PEM电解水制氢研究进展[J]. 化工进展, 2022, 41(9): 4595-4604. |
| [7] | 闫鹏, 程易. 用于分布式制氢的甲烷蒸汽重整膜反应器的数值模拟[J]. 化工进展, 2022, 41(7): 3446-3454. |
| [8] | 陶礼, 杨启容, 李昭莹, 亓昊, 王力伟, 马欣如. 基于分子动力学模拟的轮胎橡胶催化热解制氢机理[J]. 化工进展, 2022, 41(6): 3010-3021. |
| [9] | 张轩, 樊昕晔, 吴振宇, 郑丽君. 氢能供应链成本分析及建议[J]. 化工进展, 2022, 41(5): 2364-2371. |
| [10] | 方书起, 王毓谦, 李攀, 陈志勇, 陈玮, 白净, 常春. 生物油催化重整制氢研究进展[J]. 化工进展, 2022, 41(3): 1330-1339. |
| [11] | 王集杰, 韩哲, 陈思宇, 汤驰洲, 沙峰, 唐珊, 姚婷婷, 李灿. 太阳燃料甲醇合成[J]. 化工进展, 2022, 41(3): 1309-1317. |
| [12] | 徐明, 邵明飞, 刘清雅, 段雪. 电解水制氢耦合碳酸盐还原展望[J]. 化工进展, 2022, 41(3): 1121-1124. |
| [13] | 冯翔, 杨朝合, CHEN De. 加快生物质废弃物吸附增强制可再生氢气[J]. 化工进展, 2022, 41(3): 1107-1110. |
| [14] | 万磊, 徐子昂, 王培灿, 许琴, 王保国. 电解水制氢的耐碱离子膜研究进展[J]. 化工进展, 2022, 41(3): 1556-1568. |
| [15] | 陈健, 姬存民, 卜令兵. 碳中和背景下工业副产气制氢技术研究与应用[J]. 化工进展, 2022, 41(3): 1479-1486. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||