化工进展 ›› 2023, Vol. 42 ›› Issue (10): 5531-5537.DOI: 10.16085/j.issn.1000-6613.2022-2157
改性煤矸石基沸石对水中腐殖酸的吸附性能
- 北京工业大学城市建设学部,北京 100124
-
收稿日期:2022-11-21修回日期:2023-01-28出版日期:2023-10-15发布日期:2023-11-11 -
通讯作者:赵白航 -
作者简介:朱义浩(1998—),男,硕士研究生,研究方向为煤矸石资源化利用。E-mail: zhuyihao18@163.com。 -
基金资助:煤炭开采水资源保护与利用国家重点实验室开放基金课题(46004018202001)
Humic acid adsorption removal by modified coal gangue-based zeolite
ZHU Yihao( ), ZHAO Baihang(
), ZHAO Baihang( ), WANG Chun, ZHANG Yuqing, YANG Haishan
), WANG Chun, ZHANG Yuqing, YANG Haishan
- Department of Urban Construction, Beijing University of Technology, Beijing 100124, China
-
Received:2022-11-21Revised:2023-01-28Online:2023-10-15Published:2023-11-11 -
Contact:ZHAO Baihang
摘要:
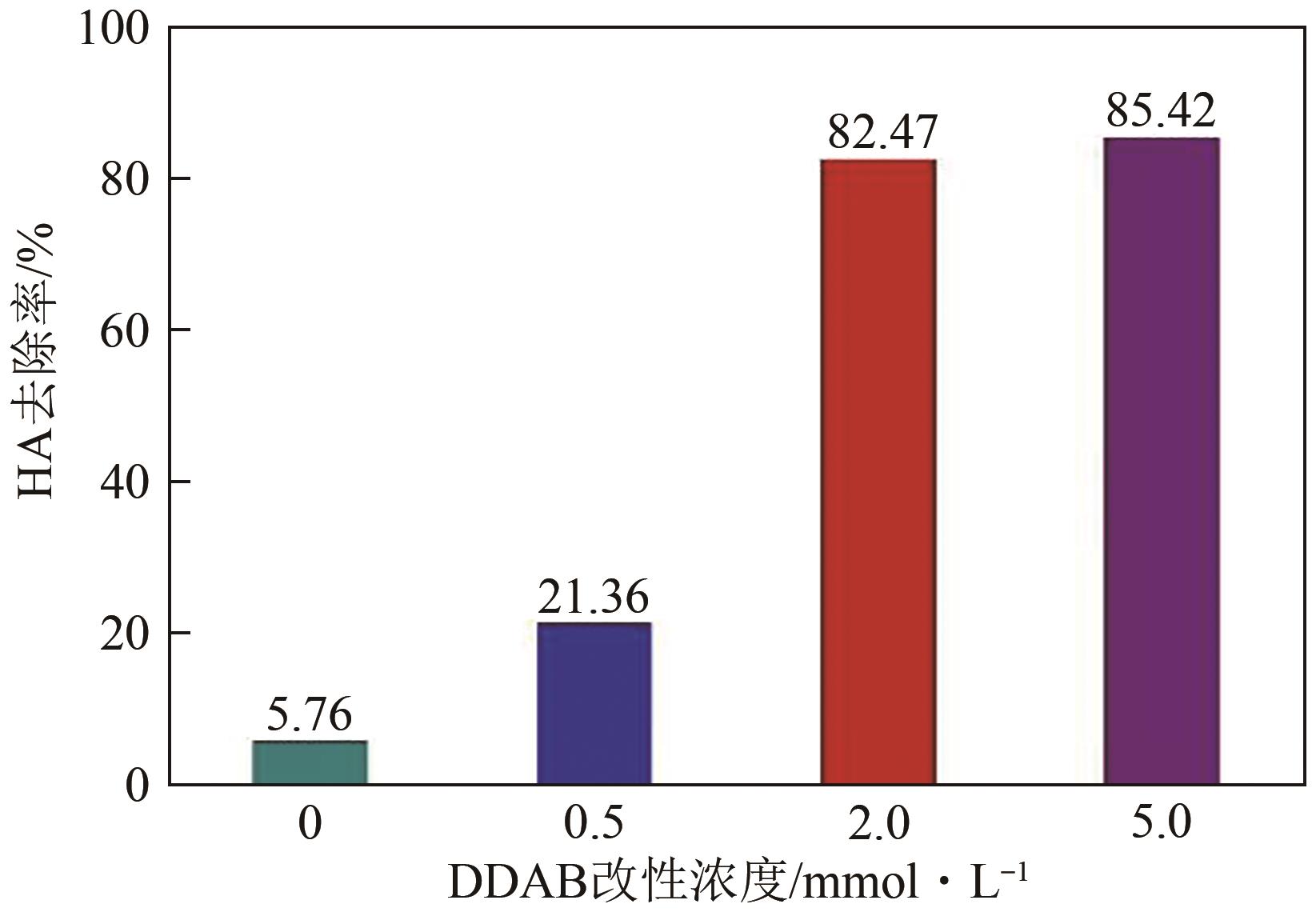
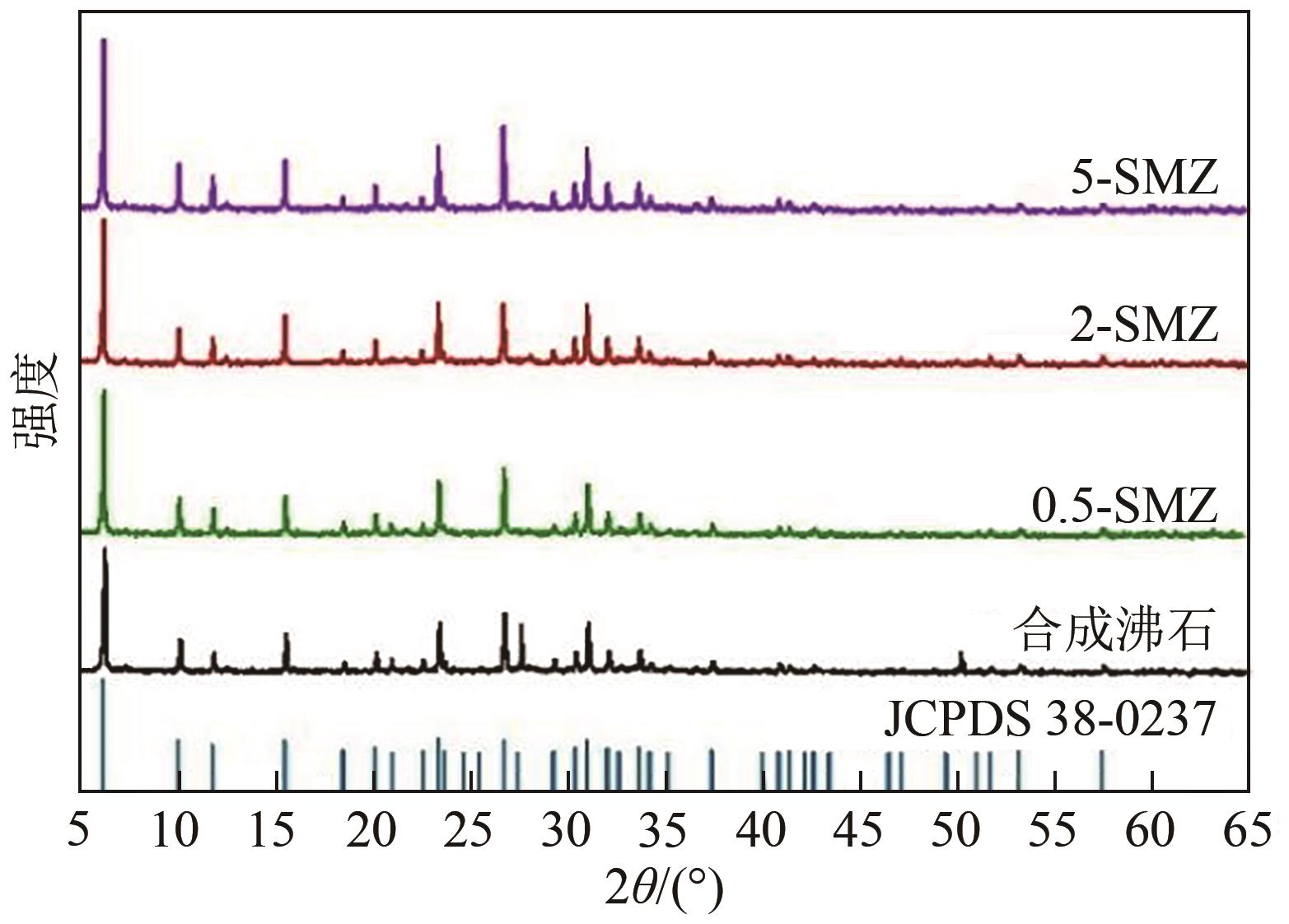
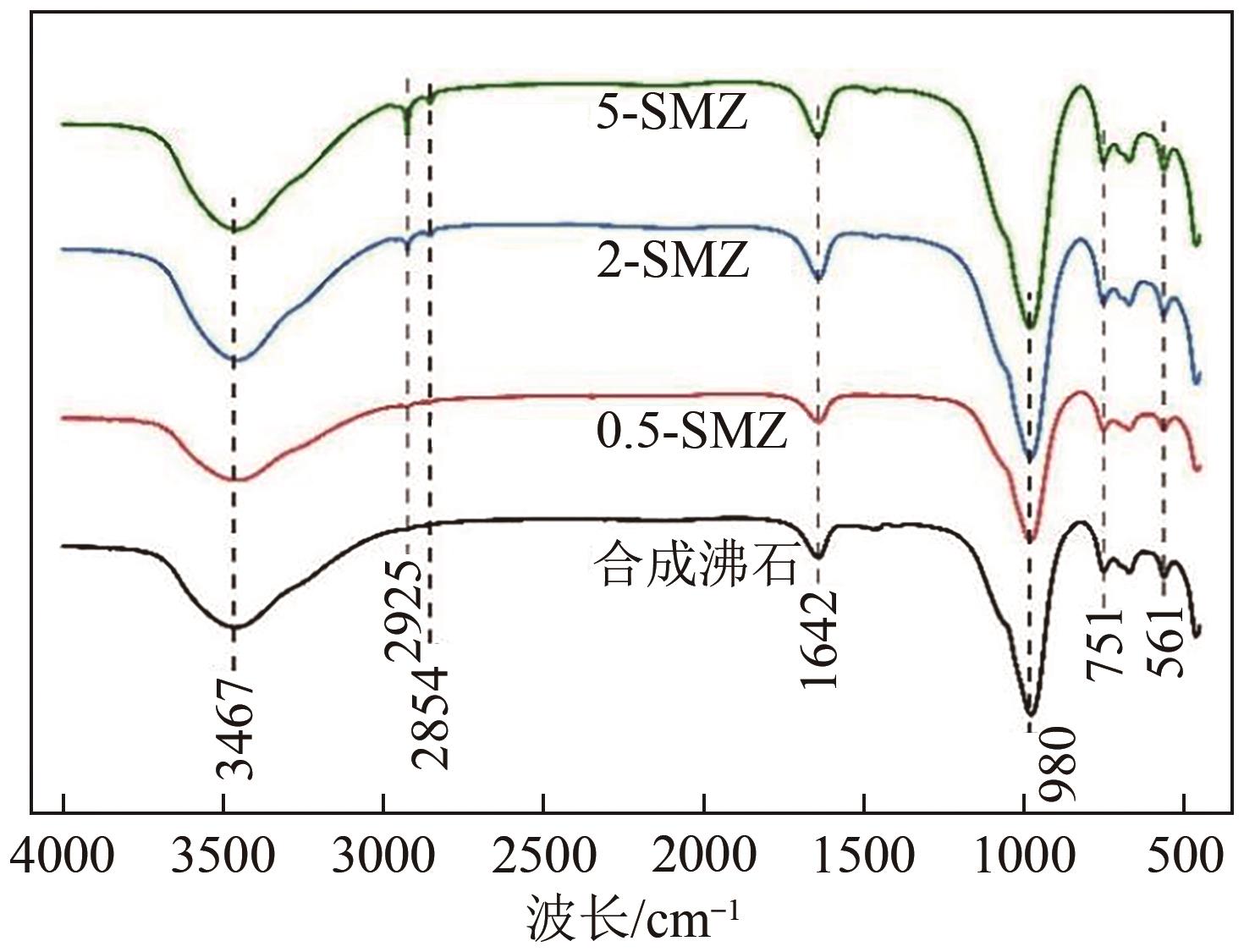
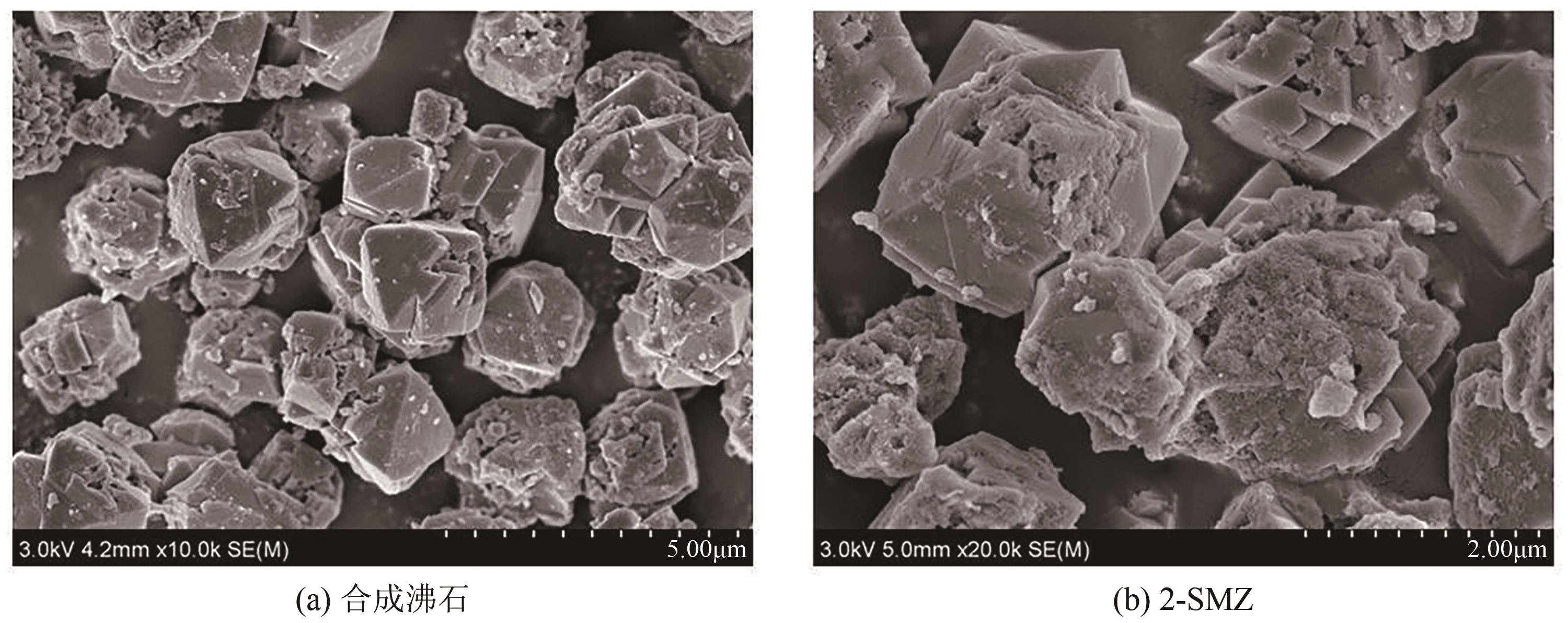
以煤矸石为原料,采用NaOH碱熔水热合成法制备NaX型沸石,并采用阳离子表面活性剂双十二烷基二甲基溴化铵(DDAB)对其进行改性。采用比表面积测试法、X射线衍射仪、扫描电子显微镜和傅里叶变换红外光谱等表征手段对改性沸石(surfactant modified zeolite,SMZ)进行表征分析。选用CCD响应曲面法构建以腐殖酸(humic acid,HA)浓度、初始pH和改性沸石投加量为影响因素,HA去除率为响应值的预测模型,确定最佳吸附条件。结果表明,SMZ表面变得粗糙,DDAB成功负载到沸石外表面。各影响因素对吸附效果的影响顺序依次为:pH>HA浓度>吸附剂投加量。最佳吸附条件下的HA浓度为10mg/L、pH为4.55、改性沸石投加量为5.5g/L,此时对应的HA去除率为88.71%。本研究表明,煤矸石基改性沸石对HA的吸附性能明显提升,对去除水中HA具有潜在的应用价值。
中图分类号:
引用本文
朱义浩, 赵白航, 王淳, 张雨晴, 杨海山. 改性煤矸石基沸石对水中腐殖酸的吸附性能[J]. 化工进展, 2023, 42(10): 5531-5537.
ZHU Yihao, ZHAO Baihang, WANG Chun, ZHANG Yuqing, YANG Haishan. Humic acid adsorption removal by modified coal gangue-based zeolite[J]. Chemical Industry and Engineering Progress, 2023, 42(10): 5531-5537.
| 因素 | 编码 | 各因素水平编码值 | ||||
|---|---|---|---|---|---|---|
| -α | -1 | 0 | 1 | α | ||
| pH | X1 | 0.78 | 3 | 6.25 | 9.5 | 11.72 |
| 腐殖酸浓度/mg·L-1 | X2 | 1.48 | 10 | 22.5 | 35 | 43.52 |
| 改性沸石投加量/g·L-1 | X3 | 0.14 | 1.5 | 3.5 | 5.5 | 6.86 |
表1 响应曲面法设计因素与水平编码值
| 因素 | 编码 | 各因素水平编码值 | ||||
|---|---|---|---|---|---|---|
| -α | -1 | 0 | 1 | α | ||
| pH | X1 | 0.78 | 3 | 6.25 | 9.5 | 11.72 |
| 腐殖酸浓度/mg·L-1 | X2 | 1.48 | 10 | 22.5 | 35 | 43.52 |
| 改性沸石投加量/g·L-1 | X3 | 0.14 | 1.5 | 3.5 | 5.5 | 6.86 |
| 样品 | 比表面积/m2·g-1 | 孔容/cm3·g-1 | 平均孔径/nm |
|---|---|---|---|
| 合成沸石 | 370.63 | 0.24 | 3.78 |
| 2-SMZ | 241.63 | 0.17 | 4.87 |
表2 合成沸石、2-SMZ的孔结构参数
| 样品 | 比表面积/m2·g-1 | 孔容/cm3·g-1 | 平均孔径/nm |
|---|---|---|---|
| 合成沸石 | 370.63 | 0.24 | 3.78 |
| 2-SMZ | 241.63 | 0.17 | 4.87 |
| 编号 | X1 | X2 | X3 | 去除率/% | |
|---|---|---|---|---|---|
| 实际值 | 预测值 | ||||
| 1 | 6.25 | 22.5 | 3.5 | 74.2 | 72.57 |
| 2 | 9.5 | 35.0 | 1.5 | 30.4 | 30.21 |
| 3 | 6.25 | 22.5 | 0.14 | 45.3 | 46.1 |
| 4 | 11.72 | 22.5 | 3.5 | 69.5 | 27.39 |
| 5 | 6.25 | 22.5 | 3.5 | 69.5 | 72.57 |
| 6 | 3.0 | 10.0 | 1.5 | 80.2 | 77.03 |
| 7 | 6.25 | 22.5 | 6.86 | 81.8 | 80.83 |
| 8 | 6.25 | 43.52 | 3.5 | 58.7 | 62.03 |
| 9 | 6.25 | 22.5 | 3.5 | 75.8 | 72.57 |
| 10 | 6.25 | 22.5 | 3.5 | 73.5 | 72.57 |
| 11 | 0.78 | 22.5 | 3.5 | 58.8 | 60.67 |
| 12 | 6.25 | 22.5 | 3.5 | 73.8 | 72.57 |
| 13 | 3.0 | 35.0 | 1.5 | 62.7 | 59.41 |
| 14 | 9.5 | 35.0 | 5.5 | 56.6 | 56.90 |
| 15 | 3.0 | 10.0 | 5.5 | 89.3 | 86.63 |
| 16 | 6.25 | 22.5 | 3.5 | 69.3 | 72.57 |
| 17 | 3.0 | 35.0 | 5.5 | 74.3 | 72.56 |
| 18 | 6.25 | 1.48 | 3.5 | 87.4 | 88.13 |
| 19 | 9.5 | 10.0 | 5.5 | 63.9 | 67.32 |
| 20 | 9.5 | 10.0 | 1.5 | 48.3 | 47.17 |
表3 试验设计及响应值
| 编号 | X1 | X2 | X3 | 去除率/% | |
|---|---|---|---|---|---|
| 实际值 | 预测值 | ||||
| 1 | 6.25 | 22.5 | 3.5 | 74.2 | 72.57 |
| 2 | 9.5 | 35.0 | 1.5 | 30.4 | 30.21 |
| 3 | 6.25 | 22.5 | 0.14 | 45.3 | 46.1 |
| 4 | 11.72 | 22.5 | 3.5 | 69.5 | 27.39 |
| 5 | 6.25 | 22.5 | 3.5 | 69.5 | 72.57 |
| 6 | 3.0 | 10.0 | 1.5 | 80.2 | 77.03 |
| 7 | 6.25 | 22.5 | 6.86 | 81.8 | 80.83 |
| 8 | 6.25 | 43.52 | 3.5 | 58.7 | 62.03 |
| 9 | 6.25 | 22.5 | 3.5 | 75.8 | 72.57 |
| 10 | 6.25 | 22.5 | 3.5 | 73.5 | 72.57 |
| 11 | 0.78 | 22.5 | 3.5 | 58.8 | 60.67 |
| 12 | 6.25 | 22.5 | 3.5 | 73.8 | 72.57 |
| 13 | 3.0 | 35.0 | 1.5 | 62.7 | 59.41 |
| 14 | 9.5 | 35.0 | 5.5 | 56.6 | 56.90 |
| 15 | 3.0 | 10.0 | 5.5 | 89.3 | 86.63 |
| 16 | 6.25 | 22.5 | 3.5 | 69.3 | 72.57 |
| 17 | 3.0 | 35.0 | 5.5 | 74.3 | 72.56 |
| 18 | 6.25 | 1.48 | 3.5 | 87.4 | 88.13 |
| 19 | 9.5 | 10.0 | 5.5 | 63.9 | 67.32 |
| 20 | 9.5 | 10.0 | 1.5 | 48.3 | 47.17 |
| 参数 | 平方和 | 自由度 | 均方值 | F值 | p值 |
|---|---|---|---|---|---|
| 合计 | 5311.3 | 19 | |||
| 模型 | 5110.11 | 9 | 567.79 | 28.22 | <0.0001 |
| X1 | 1768.26 | 1 | 1768.26 | 87.89 | <0.0001 |
| X2 | 822.23 | 1 | 822.23 | 40.87 | <0.0001 |
| X3 | 1123.80 | 1 | 1123.80 | 55.86 | <0.0001 |
| X1X2 | 6.66 | 1 | 6.66 | 0.33 | 0.5777 |
| X1X3 | 55.65 | 1 | 55.65 | 2.77 | 0.1273 |
| X2X3 | 21.45 | 1 | 21.45 | 1.07 | 0.3261 |
| X12 | 1221.39 | 1 | 1221.39 | 60.71 | <0.0001 |
| X22 | 11.36 | 1 | 11.36 | 0.56 | 0.4697 |
| X32 | 87.99 | 1 | 87.99 | 4.37 | 0.0630 |
| 残差 | 201.19 | 10 | 20.12 | ||
| 失拟度 | 165.69 | 5 | 33.14 | 4.67 | 0.0581 |
| 误差项 | 35.51 | 5 | 7.10 |
表4 方差分析
| 参数 | 平方和 | 自由度 | 均方值 | F值 | p值 |
|---|---|---|---|---|---|
| 合计 | 5311.3 | 19 | |||
| 模型 | 5110.11 | 9 | 567.79 | 28.22 | <0.0001 |
| X1 | 1768.26 | 1 | 1768.26 | 87.89 | <0.0001 |
| X2 | 822.23 | 1 | 822.23 | 40.87 | <0.0001 |
| X3 | 1123.80 | 1 | 1123.80 | 55.86 | <0.0001 |
| X1X2 | 6.66 | 1 | 6.66 | 0.33 | 0.5777 |
| X1X3 | 55.65 | 1 | 55.65 | 2.77 | 0.1273 |
| X2X3 | 21.45 | 1 | 21.45 | 1.07 | 0.3261 |
| X12 | 1221.39 | 1 | 1221.39 | 60.71 | <0.0001 |
| X22 | 11.36 | 1 | 11.36 | 0.56 | 0.4697 |
| X32 | 87.99 | 1 | 87.99 | 4.37 | 0.0630 |
| 残差 | 201.19 | 10 | 20.12 | ||
| 失拟度 | 165.69 | 5 | 33.14 | 4.67 | 0.0581 |
| 误差项 | 35.51 | 5 | 7.10 |
| 吸附剂 | 去除率/% | 参考文献 |
|---|---|---|
| 纳米MgO | 92 | [ |
| 磁性树脂 | 82 | [ |
| HDTMA改性粉煤灰基沸石 | 73 | [ |
| 壳聚糖改性沸石 | 66.2 | [ |
| TiO2修饰沸石 | 80 | [ |
| DDAB改性沸石 | 84.62 | 本研究 |
表5 不同吸附材料去除腐殖酸的效果对比
| 吸附剂 | 去除率/% | 参考文献 |
|---|---|---|
| 纳米MgO | 92 | [ |
| 磁性树脂 | 82 | [ |
| HDTMA改性粉煤灰基沸石 | 73 | [ |
| 壳聚糖改性沸石 | 66.2 | [ |
| TiO2修饰沸石 | 80 | [ |
| DDAB改性沸石 | 84.62 | 本研究 |
| 11 | JIN Yuxuan, LI Li, LIU Ze, et al. Synthesis and characterization of low-cost zeolite NaA from coal gangue by hydrothermal method[J]. Advanced Powder Technology, 2021, 32(3): 791-801. |
| 12 | Vesna KRSTIĆ. Role of zeolite adsorbent in water treatment[M]//Handbook of Nanomaterials for Wastewater Treatment. Amsterdam: Elsevier, 2021: 417-481. |
| 13 | Bo LYU, DONG Bobing, ZHANG Chuanxiang, et al. Effective adsorption of methylene blue from aqueous solution by coal gangue-based zeolite granules in a fluidized bed: Fluidization characteristics and continuous adsorption[J]. Powder Technology, 2022, 408: 117764. |
| 14 | LI Hui, LI Mingjun, ZHENG Feng, et al. Efficient removal of water pollutants by hierarchical porous zeolite-activated carbon prepared from coal gangue and bamboo[J]. Journal of Cleaner Production, 2021, 325: 129322. |
| 15 | Yingwei LYU, MA Baozhong, LIU Yubo, et al. Adsorption behavior and mechanism of mixed heavy metal ions by zeolite adsorbent prepared from lithium leach residue[J]. Microporous and Mesoporous Materials, 2022, 329: 111553. |
| 16 | 王琳琳, 张智明, 丁阿强, 等. 沸石材料的改性及其对水体污染物的吸附性能[J]. 化工进展, 2018, 37(6): 2269-2281. |
| WANG Linlin, ZHANG Zhiming, DING Aqiang, et al. Modification of zeolite materials and their adsorption properties for the pollutants in aqueous solution[J]. Chemical Industry and Engineering Progress, 2018, 37(6): 2269-2281. | |
| 17 | ZHANG Hongling, XIA Mingzhu, WANG Fengyun, et al. Adsorption properties and mechanism of montmorillonite modified by two Gemini surfactants with different chain lengths for three benzotriazole emerging contaminants: Experimental and theoretical study[J]. Applied Clay Science, 2021, 207: 106086. |
| 18 | XIE Qiang, XIE Jie, WANG Zhe, et al. Adsorption of organic pollutants by surfactant modified zeolite as controlled by surfactant chain length[J]. Microporous and Mesoporous Materials, 2013, 179: 144-150. |
| 19 | HEDAYATI Monireh S, LI Loretta Y. Removal of polycyclic aromatic hydrocarbons from aqueous media using modified clinoptilolite[J]. Journal of Environmental Management, 2020, 273: 111113. |
| 20 | 方巧, 林建伟, 詹艳慧, 等. 溴化十六烷基吡啶改性沸石对水中甲基橙的吸附[J]. 环境工程学报, 2014, 8(6): 2211-2217. |
| FANG Qiao, LIN Jianwei, ZHAN Yanhui, et al. Adsorption of methyl orange from aqueous solution on cetylpyridinium bromide(CPB)-modified zeolite[J]. Chinese Journal of Environmental Engineering, 2014, 8(6): 2211-2217. | |
| 21 | Şakir YLMAZ. Facile synthesis of surfactant-modified layered double hydroxide magnetic hybrid composite and its application for bisphenol A adsorption: Statistical optimization of operational variables[J]. Surfaces and Interfaces, 2022, 32: 102171. |
| 22 | SPIRIDONOV A M, SOKOLOVA M D, FEDOSEEVA V I, et al. Adsorption complexes ‘zeolite-cationic surfactant’: Properties and surface activity in a polymer composite material based on ultra-high-molecular-weight polyethylene[J]. Materials Today Chemistry, 2021, 20: 100441. |
| 23 | MURUKUTTI Mahima Kumar, JENA Hrudananda. Synthesis of nano-crystalline zeolite-A and zeolite-X from Indian coal fly ash, its characterization and performance evaluation for the removal of Cs+ and Sr2+ from simulated nuclear waste[J]. Journal of Hazardous Materials, 2022, 423: 127085. |
| 24 | 彭莎. 改性沸石吸附水中典型污染物的性能与机理研究[D]. 武汉: 武汉大学, 2016. |
| PENG Sha. Study on performance and mechanism of modified zeolite for adsorption of typical pollutants in water[D]. Wuhan: Wuhan University, 2016. | |
| 25 | 晏才雅. 表面活性剂改性沸石和生物炭对土壤中As和Cd的稳定修复[D]. 长沙: 湖南大学, 2021. |
| YAN Caiya. Stable remediation of As and Cd in soil by surfactant modified zeolite and biochar[D]. Changsha: Hunan University, 2021. | |
| 26 | 张译心. 表面活性剂改性沸石吸附雌激素的机理研究[D]. 长春: 吉林大学, 2018. |
| ZHANG Yixin. Study on the mechanism of estrogen adsorption by zeolite modified by surfactant[D]. Changchun: Jilin University, 2018. | |
| 27 | HAILU Solomon Legese, NAIR Balachandran Unni, Mesfin REDI-ABSHIRO, et al. Preparation and characterization of cationic surfactant modified zeolite adsorbent material for adsorption of organic and inorganic industrial pollutants[J]. Journal of Environmental Chemical Engineering, 2017, 5(4): 3319-3329. |
| 28 | 何敏祯. HDTMA改性沸石对三氯生的吸附行为与机理研究[D]. 广州: 华南理工大学, 2012. |
| HE Minzhen. Study on adsorption behavior and mechanism of triclosan by HDTMA modified zeolite[D]. Guangzhou: South China University of Technology, 2012. | |
| 29 | LI Chunjie, DONG Yang, WU Deyi, et al. Surfactant modified zeolite as adsorbent for removal of humic acid from water[J]. Applied Clay Science, 2011, 52(4): 353-357. |
| 1 | 高凌峰. 煤矸石组分特征及资源化利用现状分析[J]. 江西煤炭科技, 2022(4): 233-235, 238. |
| GAO Lingfeng. Analysis of composition characteristics and resource utilization of coal gangue[J]. Jiangxi Coal Science & Technology, 2022(4): 233-235, 238. | |
| 2 | 张伟龙, 刘刚. 煤矸石资源化利用技术研究新进展[J]. 陕西煤炭, 2022, 41(5): 149-152. |
| ZHANG Weilong, LIU Gang. New progress in research on resource utilization technology of coal gangue[J]. Shaanxi Coal, 2022, 41(5): 149-152. | |
| 3 | 常纪文, 杜根杰, 杜建磊, 等. 我国煤矸石综合利用的现状、问题与建议[J]. 中国环保产业, 2022(8): 13-17. |
| CHANG Jiwen, DU Genjie, DU Jianlei, et al. Current situation of the comprehensive utilization of coal gangue in China and the related problems and recommendations[J]. China Environmental Protection Industry, 2022(8): 13-17. | |
| 4 | WU Yuguo, YU Xiaoyang, HU Shengyong, et al. Experimental study of the effects of stacking modes on the spontaneous combustion of coal gangue[J]. Process Safety and Environmental Protection, 2019, 123: 39-47. |
| 5 | Qikai LYU, DONG Xinfa, ZHU Zhiwen, et al. Environment-oriented low-cost porous mullite ceramic membrane supports fabricated from coal gangue and bauxite[J]. Journal of Hazardous Materials, 2014, 273: 136-145. |
| 6 | ZHU Xiaobo, GONG Wenhui, LI Wang, et al. Reclamation of waste coal gangue activated by Stenotrophomonas maltophilia for mine soil improvement: Solubilizing behavior of bacteria on nutrient elements[J]. Journal of Environmental Management, 2022, 320: 115865. |
| 7 | PENG Hong, LI Yang, JIA Xianglong. Experimental study on thermoelectric generation Device based on accumulated temperature waste heat of coal gangue[J]. Energy Reports, 2022, 8: 210-219. |
| 8 | LI Xiang, PAN Mengbo, TAO Mengya, et al. Preparation of high closed porosity foamed ceramics from coal gangue waste for thermal insulation applications[J]. Ceramics International, 2022, 48(24): 37055-37063. |
| 9 | ZHOU Jianmin, ZHENG Feng, LI Hui, et al. Optimization of post-treatment variables to produce hierarchical porous zeolites from coal gangue to enhance adsorption performance[J]. Chemical Engineering Journal, 2020, 381: 122698. |
| 10 | QUAN Cui, CHU Hua, ZHOU Yingying, et al. Amine-modified silica zeolite from coal gangue for CO2 capture[J]. Fuel, 2022, 322: 124184. |
| 30 | NODEHI Reza, SHAYESTEH Hadi, KELISHAMI Ahmad Rahbar. Enhanced adsorption of Congo red using cationic surfactant functionalized zeolite particles[J]. Microchemical Journal, 2020, 153: 104281. |
| 31 | TOHDEE Kanogwan, KAEWSICHAN Lupong. Potential of BCDMACl modified bentonite in simultaneous adsorption of heavy metal Ni (Ⅱ) and humic acid[J]. Journal of Environmental Chemical Engineering, 2018, 6(4): 5616-5624. |
| 32 | LIN Jianwei, ZHAN Yanhui. Adsorption of humic acid from aqueous solution onto unmodified and surfactant-modified chitosan/zeolite composites[J]. Chemical Engineering Journal, 2012, 200: 202-213. |
| 33 | KASRAEE Mahboobeh, DEHGHANI Mohammad Hadi, MAHVI Amir Hossein, et al. Adsorptive removal of humic substances using cationic surfactant-modified nano pumice from water environment: Optimization, isotherm, kinetic and thermodynamic studies[J]. Chemosphere, 2022, 307: 135983. |
| 34 | ZHAN Yanhui, LIN Jianwei, LI Jia. Preparation and characterization of surfactant-modified hydroxyapatite/zeolite composite and its adsorption behavior toward humic acid and copper (Ⅱ)[J]. Environmental Science and Pollution Research, 2013, 20(4): 2512-2526. |
| 35 | ZHOU Juanjuan, XIA Yan, GONG Yanyan, et al. Efficient natural organic matter removal from water using nano-MgO coupled with microfiltration membrane separation[J]. Science of the Total Environment, 2020, 711: 135120. |
| 36 | 黄昕, 许金明, 钱怡冉, 等. 粒径对磁性树脂去除腐殖酸性能的影响[J]. 供水技术, 2020,14(5):1-4. |
| HUANG Xin, XU Jinming, QIAN Yiran, et al. Effect of particle size of magnetic resins on its removal effect of humic acid[J]. Water Technology, 2020, 14(5): 1-4. | |
| 37 | XIE Jie, LI Chunjie, CHI Lina, et al. Chitosan modified zeolite as a versatile adsorbent for the removal of different pollutants from water[J]. Fuel, 2013, 103: 480-485. |
| 38 | LIU Sanly, May LIM, AMAL Rose. TiO2-coated natural zeolite: Rapid humic acid adsorption and effective photocatalytic regeneration[J]. Chemical Engineering Science, 2014, 105: 46-52. |
| [1] | 李梦圆, 郭凡, 李群生. 聚乙烯醇生产中回收工段第三、第四精馏塔的模拟与优化[J]. 化工进展, 2023, 42(S1): 113-123. |
| [2] | 张瑞杰, 刘志林, 王俊文, 张玮, 韩德求, 李婷, 邹雄. 水冷式复叠制冷系统的在线动态模拟与优化[J]. 化工进展, 2023, 42(S1): 124-132. |
| [3] | 王福安. 300kt/a环氧丙烷工艺反应器降耗减排分析[J]. 化工进展, 2023, 42(S1): 213-218. |
| [4] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [5] | 陈匡胤, 李蕊兰, 童杨, 沈建华. 质子交换膜燃料电池气体扩散层结构与设计研究进展[J]. 化工进展, 2023, 42(S1): 246-259. |
| [6] | 王家庆, 宋广伟, 李强, 郭帅成, DAI Qingli. 橡胶混凝土界面改性方法及性能提升路径[J]. 化工进展, 2023, 42(S1): 328-343. |
| [7] | 崔守成, 徐洪波, 彭楠. 两种MOFs材料用于O2/He吸附分离的模拟分析[J]. 化工进展, 2023, 42(S1): 382-390. |
| [8] | 王正坤, 黎四芳. 双子表面活性剂癸炔二醇的绿色合成[J]. 化工进展, 2023, 42(S1): 400-410. |
| [9] | 陈崇明, 陈秋, 宫云茜, 车凯, 郁金星, 孙楠楠. 分子筛基CO2吸附剂研究进展[J]. 化工进展, 2023, 42(S1): 411-419. |
| [10] | 许春树, 姚庆达, 梁永贤, 周华龙. 共价有机框架材料功能化策略及其对Hg(Ⅱ)和Cr(Ⅵ)的吸附性能研究进展[J]. 化工进展, 2023, 42(S1): 461-478. |
| [11] | 顾永正, 张永生. HBr改性飞灰对Hg0的动态吸附及动力学模型[J]. 化工进展, 2023, 42(S1): 498-509. |
| [12] | 孙玉玉, 蔡鑫磊, 汤吉海, 黄晶晶, 黄益平, 刘杰. 反应精馏合成甲基丙烯酸甲酯工艺优化及节能[J]. 化工进展, 2023, 42(S1): 56-63. |
| [13] | 郭强, 赵文凯, 肖永厚. 增强流体扰动强化变压吸附甲硫醚/氮气分离的数值模拟[J]. 化工进展, 2023, 42(S1): 64-72. |
| [14] | 李春利, 韩晓光, 刘加朋, 王亚涛, 王晨希, 王洪海, 彭胜. 填料塔液体分布器的研究进展[J]. 化工进展, 2023, 42(9): 4479-4495. |
| [15] | 刘炫麟, 王驿凯, 戴苏洲, 殷勇高. 热泵中氨基甲酸铵分解反应特性及反应器结构优化[J]. 化工进展, 2023, 42(9): 4522-4530. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||