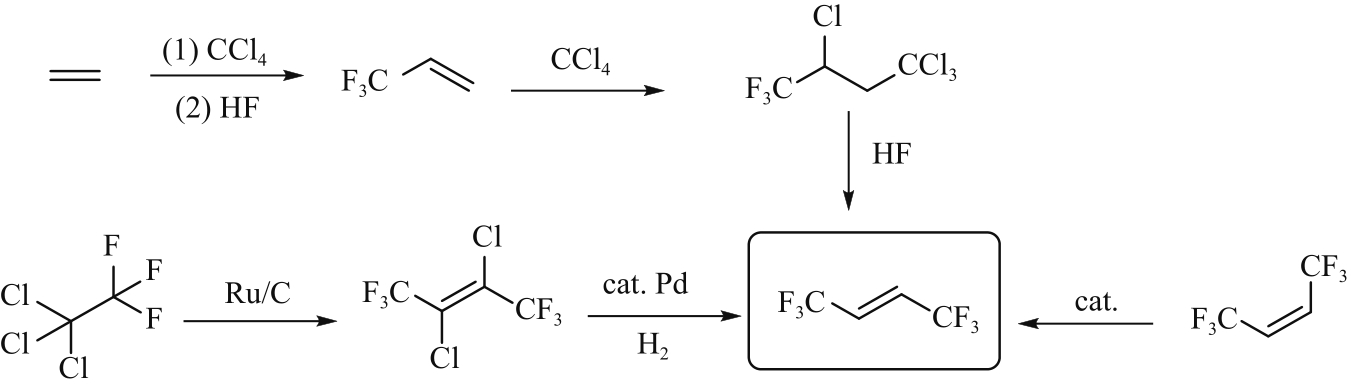
化工进展 ›› 2023, Vol. 42 ›› Issue (8): 4093-4107.DOI: 10.16085/j.issn.1000-6613.2023-0724
六氟化硫替代气体的研究现状及未来发展趋势
杨志强1( ), 曾纪珺1(
), 曾纪珺1( ), 马义丁1, 尉涛1, 赵波1, 刘英哲1, 张伟1, 吕剑1(
), 马义丁1, 尉涛1, 赵波1, 刘英哲1, 张伟1, 吕剑1( ), 李兴文2, 张博雅2, 唐念3, 李丽3, 孙东伟3
), 李兴文2, 张博雅2, 唐念3, 李丽3, 孙东伟3
- 1.西安近代化学研究所氟氮化工资源高效开发与利用国家重点实验室,陕西 西安 710065
2.西安交通大学电力 设备电气绝缘国家重点实验室,陕西 西安 710049
3.广东电网有限责任公司电力科学研究院,广东 广州 510699
-
收稿日期:2023-05-04修回日期:2023-08-10出版日期:2023-08-15发布日期:2023-09-19 -
通讯作者:吕剑 -
作者简介:杨志强(1984—),男,博士,研究员,主要研究领域为含氟专用化学品的热物性及其应用基础。E-mail: zqyangs@stu.xjtu.edu.cn
曾纪珺(1984—),男,博士研究生,研究员,主要研究领域为环保氟代烃的催化反应合成及工程化技术。E-mail: huajun_8484@163.com。 -
基金资助:国家自然科学基金(52277162);中国南方电网有限责任公司重点研发项目(GDKJXM20220361)
Research status and future trend of sulfur hexafluoride alternatives
YANG Zhiqiang1( ), ZENG Jijun1(
), ZENG Jijun1( ), MA Yiding1, YU Tao1, ZHAO Bo1, LIU Yingzhe1, ZHANG Wei1, LYU Jian1(
), MA Yiding1, YU Tao1, ZHAO Bo1, LIU Yingzhe1, ZHANG Wei1, LYU Jian1( ), LI Xingwen2, ZHANG Boya2, TANG Nian3, LI Li3, SUN Dongwei3
), LI Xingwen2, ZHANG Boya2, TANG Nian3, LI Li3, SUN Dongwei3
- 1.State Key Laboratory of Fluorine & Nitrogen Chemicals, Xi’an Modern Chemistry Research Institute, Xi’an 710065, Shaanxi,China
2.State Key Laboratory of Electrical Insulation and Power Equipment, Xi’an Jiaotong University, Xi’an 710049, Shaanxi, China
3.Electric Power Research Institute of Guangdong Power Grid Co. , Ltd. , Guangzhou 510080, Guangdong, China
-
Received:2023-05-04Revised:2023-08-10Online:2023-08-15Published:2023-09-19 -
Contact:LYU Jian
摘要:
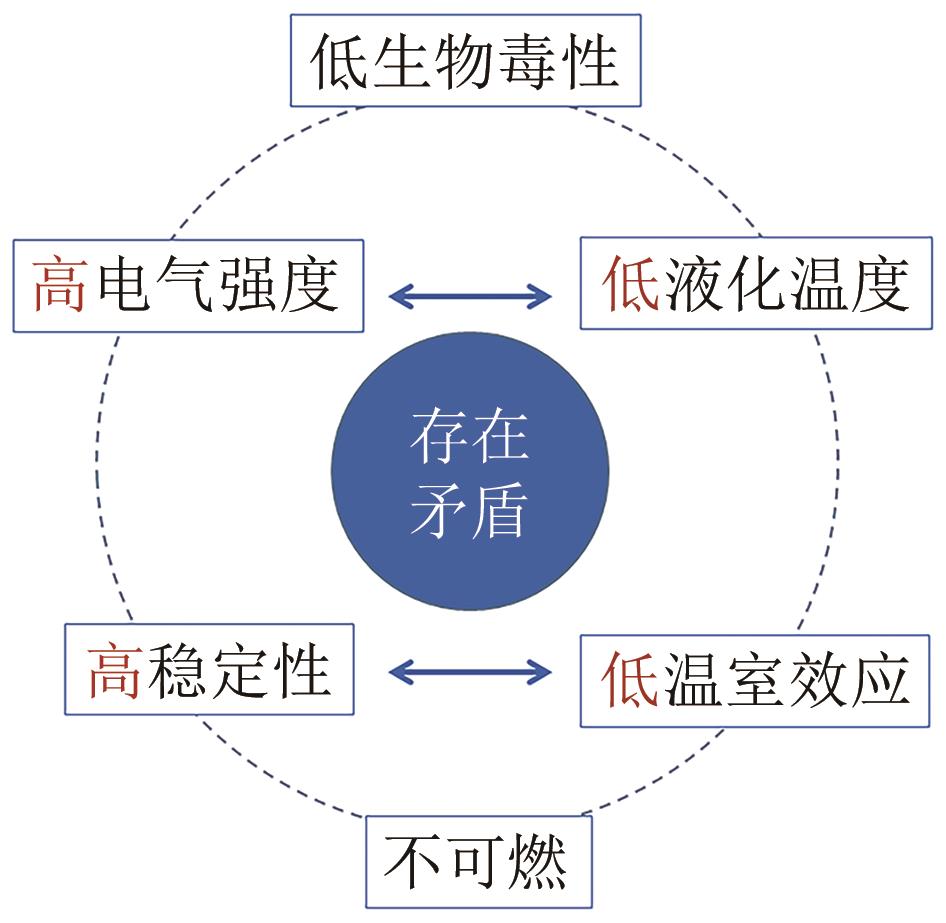
六氟化硫(SF6)是电力行业应用最为广泛的绝缘和灭弧介质。然而,SF6具有极高的温室效应潜值(GWP = 23900),其引起的环境问题逐渐成为制约我国电网绿色发展的重要因素。本文首先回顾了SF6替代气体试错法和计算机辅助法的筛选历程,综述了全氟酮、全氟异丁腈、三氟硫氮、氢氟烯烃等候选物在绝缘性能和制备方法两方面的研究成果。随后聚焦SF6替代气体筛选中的核心问题,分别从物理定义、基团贡献、定量构效关系和机器学习的角度,回顾了绝缘气体各性能预测方法的研究进展,指出同时兼顾计算效率、精确性和泛用性的预测方法是未来的发展趋势。基于目前的研究现状,提出了高通量分子设计和共沸绝缘气体两种研发思路,以期为我国绝缘环保气体的研发提供有益的借鉴。
中图分类号:
引用本文
杨志强, 曾纪珺, 马义丁, 尉涛, 赵波, 刘英哲, 张伟, 吕剑, 李兴文, 张博雅, 唐念, 李丽, 孙东伟. 六氟化硫替代气体的研究现状及未来发展趋势[J]. 化工进展, 2023, 42(8): 4093-4107.
YANG Zhiqiang, ZENG Jijun, MA Yiding, YU Tao, ZHAO Bo, LIU Yingzhe, ZHANG Wei, LYU Jian, LI Xingwen, ZHANG Boya, TANG Nian, LI Li, SUN Dongwei. Research status and future trend of sulfur hexafluoride alternatives[J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4093-4107.
| 绝缘气体 | 化学式 | 相对绝缘强度 | 标准大气压下的沸点/℃ | GWP |
|---|---|---|---|---|
| 六氟化硫 | SF6 | 1 | -64 | 23900 |
| 全氟戊酮 | C5F10O | 约2 | 26.9 | 1 |
| 全氟已酮 | C6F12O | >2 | 49 | 1 |
| 全氟异丁腈 | C4F7N | 约2.2 | -4.7 | 2210 |
| 三氟硫氮 | NSF3 | 1.35 | -27.1 | 916 |
| 四氟丙烯(E-HFO1234ze) | C3H2F4 | 0.82 | -18.9 | 4 |
| 六氟丁二烯(E-HFO1336mzz) | C4H2F4 | 1.6 | 7.5 | 10 |
| 二氧化碳 | CO2 | 约0.3 | -78.5 | 1 |
| 氮气 | N2 | -196 | 0 | |
| 空气 | — | -194 | 0 | |
| 氧气 | O2 | -182 | 0 |
表1 常见绝缘气体的基本性质
| 绝缘气体 | 化学式 | 相对绝缘强度 | 标准大气压下的沸点/℃ | GWP |
|---|---|---|---|---|
| 六氟化硫 | SF6 | 1 | -64 | 23900 |
| 全氟戊酮 | C5F10O | 约2 | 26.9 | 1 |
| 全氟已酮 | C6F12O | >2 | 49 | 1 |
| 全氟异丁腈 | C4F7N | 约2.2 | -4.7 | 2210 |
| 三氟硫氮 | NSF3 | 1.35 | -27.1 | 916 |
| 四氟丙烯(E-HFO1234ze) | C3H2F4 | 0.82 | -18.9 | 4 |
| 六氟丁二烯(E-HFO1336mzz) | C4H2F4 | 1.6 | 7.5 | 10 |
| 二氧化碳 | CO2 | 约0.3 | -78.5 | 1 |
| 氮气 | N2 | -196 | 0 | |
| 空气 | — | -194 | 0 | |
| 氧气 | O2 | -182 | 0 |
| 编号 | 年份 | 模型 | 参量 | 效果 | 文献 |
|---|---|---|---|---|---|
| 1 | 1987 | 基团类型和数目 | 未考虑各基团所处的化学环境不同。对中等大小的分子描述较好(测试集中的标准偏差为17.9K),但对于较小或较大分子的预测误差较大 | [ | |
| 2 | 1994 | 模型1的改进版 | 基团类型和数目 | 根据基团所处的化学环境对其贡献进行了更细致的规定。考虑了基团间的协同作用。数据得到了明显扩充,达到4426种分子,计算标准偏差为24.6K | [ |
| 3 | 2004 | 分子的饱和度、芳香性、氢键等 | 训练集为2812种分子,对其测试集中199种分子预测的标准偏差为6.37K。基团拆分繁复,且在基团贡献中要同时考虑该基团α位和β位上的原子或基团,容易在计算过程中造成冲突 | [ | |
| 4 | 2016 | T1反映了分子中的基团组成情况对沸点的影响;T2反映了分子量对沸点的影响 | 模型包含122种基团和2036种有机分子沸点,较好地反映了含有36个以下碳原子、分子量小于555的有机分子沸点,平均偏差4.35K。但是方法非常复杂,通过复杂的查表计算才能获得多个模型系数,且该方法的基团并非以最小单位拆分,造成基团拆分方法不唯一,实际操作中难以达成最优效果 | [ |
表2 基团贡献法预测气体沸点的模型
| 编号 | 年份 | 模型 | 参量 | 效果 | 文献 |
|---|---|---|---|---|---|
| 1 | 1987 | 基团类型和数目 | 未考虑各基团所处的化学环境不同。对中等大小的分子描述较好(测试集中的标准偏差为17.9K),但对于较小或较大分子的预测误差较大 | [ | |
| 2 | 1994 | 模型1的改进版 | 基团类型和数目 | 根据基团所处的化学环境对其贡献进行了更细致的规定。考虑了基团间的协同作用。数据得到了明显扩充,达到4426种分子,计算标准偏差为24.6K | [ |
| 3 | 2004 | 分子的饱和度、芳香性、氢键等 | 训练集为2812种分子,对其测试集中199种分子预测的标准偏差为6.37K。基团拆分繁复,且在基团贡献中要同时考虑该基团α位和β位上的原子或基团,容易在计算过程中造成冲突 | [ | |
| 4 | 2016 | T1反映了分子中的基团组成情况对沸点的影响;T2反映了分子量对沸点的影响 | 模型包含122种基团和2036种有机分子沸点,较好地反映了含有36个以下碳原子、分子量小于555的有机分子沸点,平均偏差4.35K。但是方法非常复杂,通过复杂的查表计算才能获得多个模型系数,且该方法的基团并非以最小单位拆分,造成基团拆分方法不唯一,实际操作中难以达成最优效果 | [ |
| 编号 | 年份 | 模型 | 参量 | 效果 | 文献 |
|---|---|---|---|---|---|
| 1 | 1982 | 电离能εi 和极化率α | 采用最小二乘法线性拟合了41种气体分子的绝缘强度与电离能、极化率之间的关系,模型相关系数R2=0.828,个别气体计算偏差较大,偏差最大达到了0.73 | [ | |
| 2 | 2004 | 积分光吸收强度IOA | 采用DFT方法在BLYP/DNP水平下计算43种分子IOA参数,模型的相关系数R仅为0.85(R2=0.7259),误差较大,且存在IOA计算量大、难计算的弊端 | [ | |
| 3 | 2013 | 绝热电离能 | 采用DFT方法在BP86/def-TZVP和BP86/def2-QZVPP水平下计算了67种分子的描述符,建立了极性和非极性气体的两个构效关系模型,极性气体的模型相关系数R=0.84(R2=0.71),非极性气体的模型相关系数R=0.96(R2=0.71)。两个模型建模过程比较复杂,且样本量多的极性气体相关系数比样本量少的非极性分子相关系数更低 | [ | |
| 4 | 2016 | 极化率与电子亲合能 | 采用DFT方法在M06-2X/6-311+G(3df)水平下计算了24种分子的描述符,获得了相关系数R仅为0.78(R2=0.602)的线性模型 | [ | |
| 5 | 2016 | 垂直电子亲和能Ev,等效截面积S | 采用DFT方法计算了几种典型有机气体的描述符,模型样本量少,对外部气体的预测能力弱 | [ | |
| 6 | 2017 | 相互作用性质函数(GIPF):As, | 采用DFT方法在M06-2X/6-31++G(d,p)水平下计算了43种分子的描述符,最优模型相关系数R2达到0.985 | [ | |
| 7 | 2018 | 分子质量xmw,电负性指数xEn,电子数xne,偶极矩xμ,极化率xa | 采用DFT方法在B3LYP/6-311G++(d,p)水平下计算了104种气体分子的描述符,模型相关系数R=0.948 | [ | |
| 8 | 2018 | 表面积As,表面静电势分布情况 | 采用DFT方法在M06-2X/6-31G+(d)水平下计算得到43种分子的描述符,模型相关系数R=0.993,平均绝对偏差MAD=0.0609,均方根误差σ=0.0802,最大偏差δmax=0.28 | [ | |
| 9 | 2019 | 第一电离能、第一电子亲和能、偶极矩和极化率等 | 用DFT方法在B3LYP/6-311G++(d,p)水平下计算了37种分子的描述符,相关系数R2=0.895 | [ | |
| 10 | 2021 | 分子表面积As,正静电势面积 | 采用DFT方法在M06-2X/6-31G++(d,p)水平下计算得到65种分子的描述符,模型的相关系数0.844 | [ |
表3 QSPR预测绝缘强度的模型
| 编号 | 年份 | 模型 | 参量 | 效果 | 文献 |
|---|---|---|---|---|---|
| 1 | 1982 | 电离能εi 和极化率α | 采用最小二乘法线性拟合了41种气体分子的绝缘强度与电离能、极化率之间的关系,模型相关系数R2=0.828,个别气体计算偏差较大,偏差最大达到了0.73 | [ | |
| 2 | 2004 | 积分光吸收强度IOA | 采用DFT方法在BLYP/DNP水平下计算43种分子IOA参数,模型的相关系数R仅为0.85(R2=0.7259),误差较大,且存在IOA计算量大、难计算的弊端 | [ | |
| 3 | 2013 | 绝热电离能 | 采用DFT方法在BP86/def-TZVP和BP86/def2-QZVPP水平下计算了67种分子的描述符,建立了极性和非极性气体的两个构效关系模型,极性气体的模型相关系数R=0.84(R2=0.71),非极性气体的模型相关系数R=0.96(R2=0.71)。两个模型建模过程比较复杂,且样本量多的极性气体相关系数比样本量少的非极性分子相关系数更低 | [ | |
| 4 | 2016 | 极化率与电子亲合能 | 采用DFT方法在M06-2X/6-311+G(3df)水平下计算了24种分子的描述符,获得了相关系数R仅为0.78(R2=0.602)的线性模型 | [ | |
| 5 | 2016 | 垂直电子亲和能Ev,等效截面积S | 采用DFT方法计算了几种典型有机气体的描述符,模型样本量少,对外部气体的预测能力弱 | [ | |
| 6 | 2017 | 相互作用性质函数(GIPF):As, | 采用DFT方法在M06-2X/6-31++G(d,p)水平下计算了43种分子的描述符,最优模型相关系数R2达到0.985 | [ | |
| 7 | 2018 | 分子质量xmw,电负性指数xEn,电子数xne,偶极矩xμ,极化率xa | 采用DFT方法在B3LYP/6-311G++(d,p)水平下计算了104种气体分子的描述符,模型相关系数R=0.948 | [ | |
| 8 | 2018 | 表面积As,表面静电势分布情况 | 采用DFT方法在M06-2X/6-31G+(d)水平下计算得到43种分子的描述符,模型相关系数R=0.993,平均绝对偏差MAD=0.0609,均方根误差σ=0.0802,最大偏差δmax=0.28 | [ | |
| 9 | 2019 | 第一电离能、第一电子亲和能、偶极矩和极化率等 | 用DFT方法在B3LYP/6-311G++(d,p)水平下计算了37种分子的描述符,相关系数R2=0.895 | [ | |
| 10 | 2021 | 分子表面积As,正静电势面积 | 采用DFT方法在M06-2X/6-31G++(d,p)水平下计算得到65种分子的描述符,模型的相关系数0.844 | [ |
| 编号 | 年份 | 模型 | 参量 | 效果 | 文献 |
|---|---|---|---|---|---|
| 1 | 1982 | 电离能εi 和极化率α | 训练集依托于实验值,拟合度不高,只能用作定性、半定量分析 | [ | |
| 2 | 1993 | 分子范德华表面积As,分子表面的静电势分布情况 | 使用量子化学方法对C、H、O、N、Cl、Br类化合物的分子表面静电势进行计算。由99种化合物组成的测试集的平均误差为36.5K | [ | |
| 3 | 2013 | 模型按照极性、非极性和全集合 | 垂直电子亲和能、极化率、分子偶极矩、分子量、电子数等 | 对67种绝缘气体组成的数据集拟合得到一系列构效关系模型。对于非极性分子,模型拟合度R2最高达到了0.92。但对于极性分子,模型拟合度较低,对全集合分子的拟合度更低,不足0.5,难以达到实用要求 | [ |
| 4 | 2013 | 垂直电子亲和能、极化率、分子偶极矩、分子量、电子数等 | 对含3~5个碳原子的羰基化合物沸点进行拟合,结果表明对这一分子集合,沸点预测的标准偏差为28K,但是模型中各个变量的阶数都很低,难以理解其物理意义,且不能排除过拟合可能 | [ | |
| 5 | 2017 | 分子范德华表面积、分子表面静电势分布、正负电荷分离程度和通用相互作用函数 | 模型2的改进版。54种气体沸点的拟合度R2=0.985,与实验数据的相对偏差σ=0.08。应用此模型对其之前研究中涉及的气体分子进行预测并比较发现相关系数低于0.9,标准偏差最大达到0.27,显示出该模型高度依赖数据集的选择,泛用能力较低 | [ | |
| 6 | 2017 | 分子范德华表面积、分子表面正负电势分布的平均偏差、化学硬度 | 模型5修正版,以代替了描述分子表面正负电势面积乘积的电荷平衡度,同时引入化学硬度参数用以描述分子失电子的难易程度。修正后的模型在其测试集内的拟合度R2=0.985 | [ |
表4 QSPR预测气体沸点的模型
| 编号 | 年份 | 模型 | 参量 | 效果 | 文献 |
|---|---|---|---|---|---|
| 1 | 1982 | 电离能εi 和极化率α | 训练集依托于实验值,拟合度不高,只能用作定性、半定量分析 | [ | |
| 2 | 1993 | 分子范德华表面积As,分子表面的静电势分布情况 | 使用量子化学方法对C、H、O、N、Cl、Br类化合物的分子表面静电势进行计算。由99种化合物组成的测试集的平均误差为36.5K | [ | |
| 3 | 2013 | 模型按照极性、非极性和全集合 | 垂直电子亲和能、极化率、分子偶极矩、分子量、电子数等 | 对67种绝缘气体组成的数据集拟合得到一系列构效关系模型。对于非极性分子,模型拟合度R2最高达到了0.92。但对于极性分子,模型拟合度较低,对全集合分子的拟合度更低,不足0.5,难以达到实用要求 | [ |
| 4 | 2013 | 垂直电子亲和能、极化率、分子偶极矩、分子量、电子数等 | 对含3~5个碳原子的羰基化合物沸点进行拟合,结果表明对这一分子集合,沸点预测的标准偏差为28K,但是模型中各个变量的阶数都很低,难以理解其物理意义,且不能排除过拟合可能 | [ | |
| 5 | 2017 | 分子范德华表面积、分子表面静电势分布、正负电荷分离程度和通用相互作用函数 | 模型2的改进版。54种气体沸点的拟合度R2=0.985,与实验数据的相对偏差σ=0.08。应用此模型对其之前研究中涉及的气体分子进行预测并比较发现相关系数低于0.9,标准偏差最大达到0.27,显示出该模型高度依赖数据集的选择,泛用能力较低 | [ | |
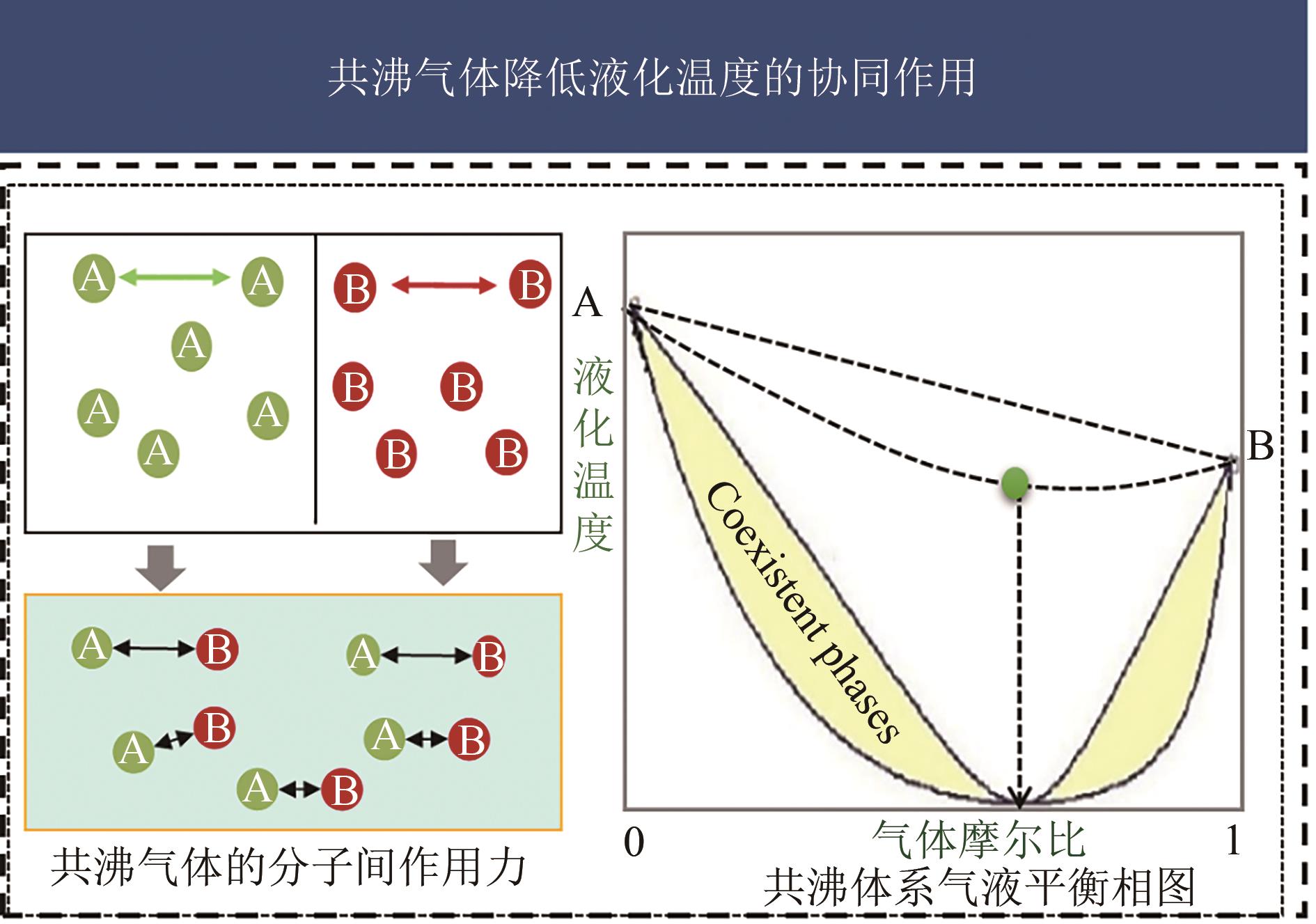
| 6 | 2017 | 分子范德华表面积、分子表面正负电势分布的平均偏差、化学硬度 | 模型5修正版,以代替了描述分子表面正负电势面积乘积的电荷平衡度,同时引入化学硬度参数用以描述分子失电子的难易程度。修正后的模型在其测试集内的拟合度R2=0.985 | [ |
| 序号 | 分子结构 | 绝缘强度 | 沸点/K |
|---|---|---|---|
| 1 |  | 0.87 | 209 |
| 2 |  | 1.09 | 250 |
| 3 |  | 1.17 | 273 |
表5 具有应用潜力的亚胺化合物
| 序号 | 分子结构 | 绝缘强度 | 沸点/K |
|---|---|---|---|
| 1 |  | 0.87 | 209 |
| 2 |  | 1.09 | 250 |
| 3 |  | 1.17 | 273 |
| 1 | 范建斌, 李鹏, 李金忠, 等. ±800kV特高压直流GIL关键技术研究[J]. 中国电机工程学报, 2008, 28(13): 1-7. |
| FAN Jianbin, LI Peng, LI Jinzhong, et al. Study on key technology of ±800kV UHVDC GIL[J]. Proceedings of the CSEE, 2008, 28(13): 1-7. | |
| 2 | 李兴文, 赵虎. SF6替代气体的研究进展综述[J]. 高电压技术, 2016, 42(6): 1695-1701. |
| LI Xingwen, ZHAO Hu. Review of research progress in SF6 substitute gases[J]. High Voltage Engineering, 2016, 42(6): 1695-1701. | |
| 3 | OWENS John, XIAO Ang, BONK Jason, et al. Recent development of two alternative gases to SF6 for high voltage electrical power applications[J]. Energies, 2021, 14(16): 5051. |
| 4 | SOVACOOL Benjamin K, GRIFFITHS Steve, KIM Jinsoo, et al. Climate change and industrial F-gases: A critical and systematic review of developments, sociotechnical systems and policy options for reducing synthetic greenhouse gas emissions[J]. Renewable and Sustainable Energy Reviews, 2021, 141: 110759. |
| 5 | RABIE Mohamed, FRANCK Christian M. Computational screening of new high voltage insulation gases with low global warming potential[J]. IEEE Transactions on Dielectrics and Electrical Insulation, 2015, 22(1): 296-302. |
| 6 | YU Xiaojuan, HOU Hua, WANG Baoshan. Prediction on dielectric strength and boiling point of gaseous molecules for replacement of SF6 [J]. Journal of Computational Chemistry, 2017, 38(10): 721-729. |
| 7 | XIAO Song, ZHANG Xiaoxing, TANG Ju, et al. A review on SF6 substitute gases and research status of CF3I gases[J]. Energy Reports, 2018, 4: 486-496. |
| 8 | Andreas HÖSL, PACHIN Juriy, Eda EGÜZ, et al. Positive synergy of SF6 and HFO1234ze(E)[J]. IEEE Transactions on Dielectrics and Electrical Insulation, 2020, 27(1): 322-324. |
| 9 | CHRISTOPHOROU L G, SAUERS I, JAMES D R, et al. Recent advances in gaseous dielectrics at oak ridge national laboratory[J]. IEEE Transactions on Electrical Insulation, 1984, EI-19(6): 550-566. |
| 10 | WANG Chunlin, COOPER Bridgette, WU Yi, et al. Why SF6 eats electrons: Identifying high electrical strength molecules from their electron collision properties[J]. Journal of Physics B: Atomic, Molecular and Optical Physics, 2021, 54(2): 025202. |
| 11 | BIASIUTTI G. Homogeneous field breakdown strength characteristics of some dielectric gases[M]//Gaseous Dielectrics Ⅲ. Amsterdam: Elsevier, 2013: 174-182. |
| 12 | VIJH Ashok K. Electric strenght and molecular properties of gaseous dielectrics[J]. IEEE Transactions on Electrical Insulation, 1977, EI-12(4): 313-315. |
| 13 | KAZAKOV Andrei, MCLINDEN Mark O, FRENKEL Michael. Computational design of new refrigerant fluids based on environmental, safety, and thermodynamic characteristics[J]. Industrial & Engineering Chemistry Research, 2012: 120917100332001. |
| 14 | BLOWERS Paul, HOLLINGSHEAD Kyle. Estimations of global warming potentials from computational chemistry calculations for CH2F2 and other fluorinated methyl species verified by comparison to experiment[J]. The Journal of Physical Chemistry A, 2009, 113(20): 5942-5950. |
| 15 | OYARO Nathan, SELLEVÅG Stig R, NIELSEN Claus J. Atmospheric chemistry of hydrofluoroethers: reaction of a series of hydrofluoroethers with OH radicals and Cl atoms, atmospheric lifetimes, and global warming potentials[J]. The Journal of Physical Chemistry A, 2005, 109(2): 337-346. |
| 16 | MCLINDEN Mark O, Steven BROWN J, BRIGNOLI Riccardo, et al. Limited options for low-global-warming-potential refrigerants[J]. Nature Communications, 2017, 8: 14476. |
| 17 | WOOTTON R E, KEGELMAN M R, BAUER A W, et al. Gases superior to SF6 for insulation and interruption[R]. Westinghouse Research and Development Center, 1982. |
| 18 | DEVINS J C. Replacement gases for SF6 [J]. IEEE Transactions on Electrical Insulation, 1980, EI-15(2): 81-86. |
| 19 | ZHANG Chaohai, SHI Huixuan, CHENG Lin, et al. First principles based computational scheme for designing new SF6 replacements[J]. IEEE Transactions on Dielectrics and Electrical Insulation, 2016, 23(5): 2572-2578. |
| 20 | 王宝山, 余小娟, 侯华, 等. 六氟化硫绝缘替代气体的构效关系与分子设计技术现状及发展[J]. 电工技术学报, 2020, 35(1): 21-33. |
| WANG Baoshan, YU Xiaojuan, HOU Hua, et al. Review on the developments of structure-activity relationship and molecular design of the replacement dielectric gases for SF6 [J]. Transactions of China Electrotechnical Society, 2020, 35(1): 21-33. | |
| 21 | YU Xiaojuan, HOU Hua, WANG Baoshan. A priori theoretical model for discovery of environmentally sustainable perfluorinated compounds[J]. The Journal of Physical Chemistry A, 2018, 122(13): 3462-3469. |
| 22 | TU Youping, CHEN Geng, WANG Cong, et al. Feasibility of C3F7CN/CO2 gas mixtures in high-voltage DC GIL: A review on recent advances[J]. High Voltage, 2020, 5(4): 377-386. |
| 23 | MANTILLA J D, GARIBOLDI N, GROB S, et al. Investigation of the insulation performance of a new gas mixture with extremely low GWP[C]//2014 IEEE Electrical Insulation Conference (EIC). June 8-11, 2014, Philadelphia, PA, USA. IEEE, 2014: 469-473. |
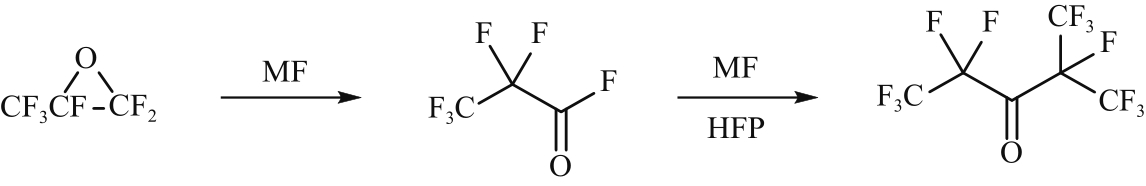
| 24 | SMITH R D, FAWCETT F S, COFFMAN D D. The chemistry of carbonyl fluoride. II. Synthesis of perfluoroisopropyl ketones[J]. Journal of the American Chemical Society, 1962, 84(22): 4285-4288. |
| 25 | WLASSICS Ivan, TORTELLI Vito, CARELLA Serena, et al. Perfluoro allyl fluorosulfate (FAFS): A versatile building block for new fluoroallylic compounds[J]. Molecules, 2011, 16(8): 6512-6540. |
| 26 | FENICHEV I M, BERENBLIT V V, BISPEN T A, et al. Catalytic synthesis of certain perfluorinated ketones and study of their structure by 19F NMR spectroscopy[J]. Russian Journal of Applied Chemistry, 2013, 86(8): 1243-1251. |
| 27 | RIVERS Paul E, MINDAY Richard M, BEHR Fred E, et al. Use of fluorinated ketones in fire extinguishing compositions: US6478979[P]. 2002-11-12. |
| 28 | BORNENGO G, CARLINI F M, PONTEVIVO M, et al. Process for the preparation of 2-perfluoropropoxyperfluoro-propionyl fluoride: US 4729856[P]. 1988-05-08. |
| 29 | NECHMI H E, BEROUAL A, GIRODET A, et al. Fluoronitriles/CO2 gas mixture as promising substitute to SF6 for insulation in high voltage applications[J]. IEEE Transactions on Dielectrics and Electrical Insulation, 2016, 23(5): 2587-2593. |
| 30 | KIEFFEL Yannick, IRWIN Todd, PONCHON Philippe, et al. Green gas to replace SF6 in electrical grids[J]. IEEE Power and Energy Magazine, 2016, 14(2): 32-39. |
| 31 | FAWCETT F S, TULLOCK C W, COFFMAN D D. The chemistry of carbonyl fluoride. I. The fluorination of organic compounds[J]. Journal of the American Chemical Society, 1962, 84(22): 4275-4285. |
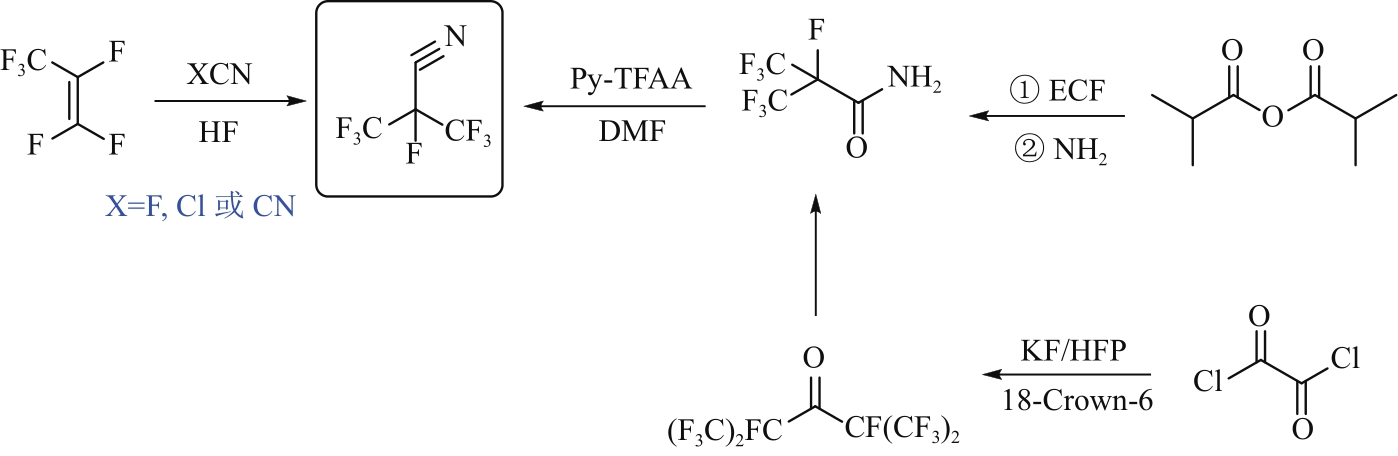
| 32 | GAO Zhanyang, WANG Min, WANG Shiyao, et al. Novel and efficient synthesis of insulating gas-heptafluoroisobutyronitrile from hexafluoropropylene[J]. Royal Society Open Science, 2019, 6(3): 181751. |
| 33 | OXENRIDER B, BEYLEVELD W, WOOLF C, et al. Process for preparing fluoroperhaloalkyl nitriles: US3752840[P]. 1973-08-14. |
| 34 | COSTELLO Michael G, FLYNN Richard M, BULINSKI Michael J. Fluorinated nitriles as dielectric gases: US10573426[P]. 2020-02-25. |
| 35 | LI Yi, ZHANG Xiaoxing, ZHANG Ji, et al. Assessment on the toxicity and application risk of C4F7N: A new SF6 alternative gas[J]. Journal of Hazardous Materials, 2019, 368: 653-660. |
| 36 | EIBECK Richard E. Dielectric gaseous mixture of thiazyltrifluoride and SF6 : US3390091[P]. 1968-06-25. |
| 37 | GLEMSER Oskar, Rüdiger MEWS. Chemistry of thiazyl fluoride (NSF) and thiazyl trifluoride (NSF3): A quarter century of sulfur-nitrogen-fluorine chemistry[J]. Angewandte Chemie International Edition in English, 1980, 19(11): 883-899. |
| 38 | DENG Jiayan, PENG Min, GAO Zhanyang, et al. Synthesis and dielectric properties of the eco-friendly insulating gas thiazyl trifluoride[J]. RSC Advances, 2020, 10(5): 2740-2746. |
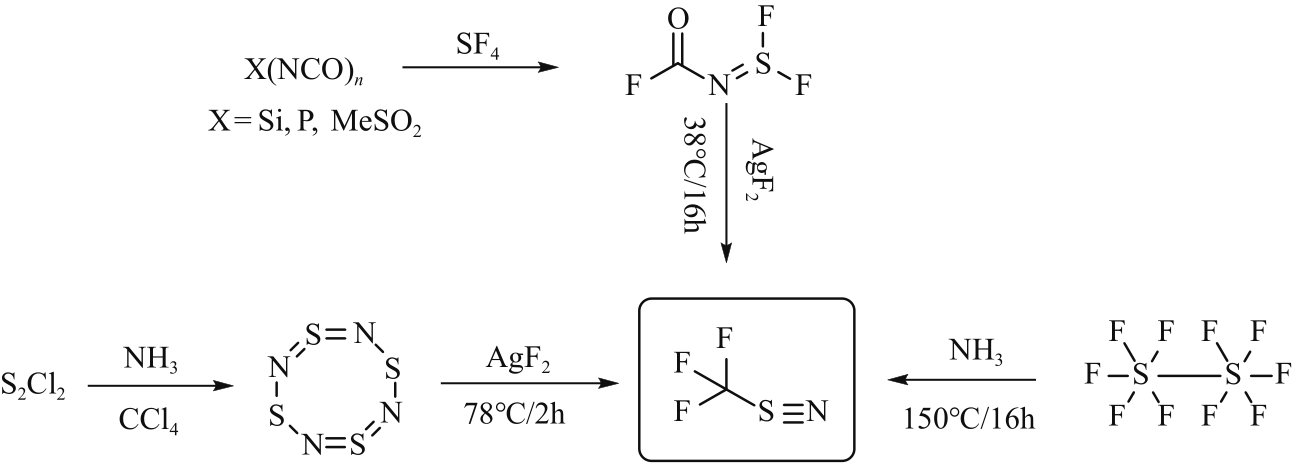
| 39 | CLIFFORD Alan F, KOBAYASHI Calvin S. The preparation and properties of N-fluoroformyliminosulfur difluoride, SF2=NCOF[J]. Inorganic Chemistry, 1965, 4(4): 571-574. |
| 40 | PREVE Christophe, PICCOZ Daniel, MALADEN Romain. Application of HFO1234ZEE in MV switchgear AS SF6 alternative gas[J]. CIRED-Open Access Proceedings Journal, 2017, 2017(1): 42-45. |
| 41 | PACHIN Juriy, Andreas HÖSL, FRANCK Christian M. Measurements of the electron swarm parameters of R1225ye(Z) (C3HF5) and its mixtures with N2 and CO2 [J]. Journal of Physics D: Applied Physics, 2019, 52(23): 235204. |
| 42 | CHACHEREAU A, RABIE M, FRANCK C M. Electron swarm parameters of the hydrofluoroolefine HFO1234ze[J]. Plasma Sources Science and Technology, 2016, 25(4): 045005. |
| 43 | EGÜZ E A, PACHIN J, FRANCK C M. Discussion on the mechanism leading to positive synergism in SF6 mixtures with HFO1234ze(E)[J]. Journal of Physics D: Applied Physics, 2022, 55(31): 315203. |
| 44 | JIA Zhaohua, MAO Wei, BAI Yanbo, et al. Hollownano-MgF2 supported catalysts: Highly active and stable in gas-phase dehydrofluorination of 1,1,1,3,3-pentafluoropropane[J]. Applied Catalysis B: Environmental, 2018, 238: 599-608. |
| 45 | MAO Wei, BAI Yanbo, JIA Zhaohua, et al. Highly efficient gas-phase dehydrofluorination of 1,1,1,3,3-pentafluoropropane to 1,3,3,3-tetrafluoropropene over mesoporous nano-aluminum fluoride prepared from a polyol mediated sol-gel process[J]. Applied Catalysis A: General, 2018, 564: 147-156. |
| 46 | LIU Jie, WANG Feng, ZHONG Lipeng, et al. Theoretical study of the decomposition mechanism of a novel eco-friendly insulation medium HFO-1336mzz(E) considering the effect of trace humidity[J]. Journal of Physics D: Applied Physics, 2022, 55(4): 045201. |
| 47 | 唐念, 熊嘉宇, 周永言, 等. 环保气体HFO-1336mzz(E)及其混合气体的绝缘性能研究[J]. 电工技术学报, 2021, 36(13): 2871-2879. |
| TANG Nian, XIONG Jiayu, ZHOU Yongyan, et al. Insulation performance of environmental-friendly gas HFO-1336mzz(E) and its mixtures[J]. Transactions of China Electrotechnical Society, 2021, 36(13): 2871-2879. | |
| 48 | TANG N, XIONG J, WANG K, et al. Insulation performance of environmental-friendly gas HFO-1336mzz(E) and its mixtures[J]. Diangong Jishu Xuebao/Transactions of China Electrotechnical Society, 2021, 36(13): 2871-2879. |
| 49 | 王凯, 周然, 张博雅, 等. 环境友好型HFO-1336mzz(E)混合气体在负荷开关中的灭弧性能仿真研究[J]. 高电压技术, 2023, 49(3): 1038-1045. |
| WANG Kai, ZHOU Ran, ZHANG Boya, et al. Simulation study on the arc extinguishing performance of eco-friendly HFO-1336mzz(E) mixture gas in a load switch[J]. High Voltage Engineering, 2023, 49(3): 1038-1045. | |
| 50 | JOHNSON R W, MOELLER W C, VAN DE PUY M. Process for combining chlorine-containing molecules to synthesize fluorine containing products: WO 9505353[P]. 1994-08-10. |
| 51 | NAPPA M J, PENG S, PETROV V A. Catalytic isomerization of Z-1,1,1,4,4,4-hexafluoro-2-butene: EP 3331846[P]. 2016-08-04. |
| 52 | TUNG Hsueh Sung, WANG Haiyou. Process for the manufacture of hexafluoro-2-butene: US8426655[P]. 2013-04-23. |
| 53 | 韩升, 曾纪珺, 赵波, 等. 催化氟化合成反式-1,1,1,4,4,4-六氟-2-丁烯的工艺研究[J]. 应用化工, 2020, 49(9): 2257-2260. |
| HAN Sheng, ZENG Jijun, ZHAO Bo, et al. Synthesis of trans-1,1,1,4,4,4-hexafluoro-2-butene by catalytic fluorination[J]. Applied Chemical Industry, 2020, 49(9): 2257-2260. | |
| 54 | ZHANG Boya, WANG Kai, YAO Yuyang, et al. Insulation characteristics of HFO-1336mzz(E) and its mixtures as eco-friendly alternatives to SF6 for medium-voltage switchgears[J]. IEEE Transactions on Dielectrics and Electrical Insulation, 2023, 30(2): 536-545. |
| 55 | GROVEN Steven D, DESGRANGES C, DELHOMMELLE J. Prediction of the boiling and critical points of polycyclic aromatic hydrocarbons via Wang-Landau simulations and machine learning[J]. Fluid Phase Equilibria, 2019, 484: 225-231. |
| 56 | SAHA Avijit, GRENGA Temistocle, DESHMUKH Abhishek Y, et al. Numerical modeling of single droplet flash boiling behavior of e-fuels considering internal and external vaporization[J]. Fuel, 2022, 308: 121934. |
| 57 | HIGHWOOD E J, SHINE K P. Radiative forcing and global warming potentials of 11 halogenated compounds[J]. Journal of Quantitative Spectroscopy and Radiative Transfer, 2000, 66(2): 169-183. |
| 58 | LASHOF Daniel A, AHUJA Dilip R. Relative contributions of greenhouse gas emissions to global warming[J]. Nature, 1990, 344(6266): 529-531. |
| 59 | WUEBBLES Donald J. Weighing functions for ozone depletion and greenhouse gas effects on climate[J]. Annual Review of Energy and the Environment, 1995, 20: 45-70. |
| 60 | DEMORE W B. Experimental and estimated rate constants for the reactions of hydroxyl radicals with several halocarbons[J]. The Journal of Physical Chemistry, 1996, 100(14): 5813-5820. |
| 61 | NAIK Vaishali, JAIN Atul K, PATTEN Kenneth O, et al. Consistent sets of atmospheric lifetimes and radiative forcings on climate for CFC replacements: HCFCs and HFCs[J]. Journal of Geophysical Research: Atmospheres, 2000, 105(D5): 6903-6914. |
| 62 | EYRING Henry. The activated complex and the absolute rate of chemical reactions[J]. Chemical Reviews, 1935, 17(1): 65-77. |
| 63 | WIGNER E. On the quantum correction for thermodynamic equilibrium[J]. Physical Review, 1932, 40(5): 749-759. |
| 64 | Lucas Bao Junwei, TRUHLAR Donald G. Variational transition state theory: Theoretical framework and recent developments[J]. Chemical Society Reviews, 2017, 46(24): 7548-7596. |
| 65 | VIEGAS Luís P. Simplified protocol for the calculation of multiconformer transition state theory rate constants applied to tropospheric OH-initiated oxidation reactions[J]. The Journal of Physical Chemistry A, 2021, 125(21): 4499-4512. |
| 66 | ALECU I M, ZHENG Jingjing, ZHAO Yan, et al. Computational thermochemistry: Scale factor databases and scale factors for vibrational frequencies obtained from electronic model chemistries[J]. Journal of Chemical Theory and Computation, 2010, 6(9): 2872-2887. |
| 67 | PINNOCK Simon, HURLEY Michael D, SHINE Keith P, et al. Radiative forcing of climate by hydrochlorofluorocarbons and hydrofluorocarbons[J]. Journal of Geophysical Research: Atmospheres, 1995, 100(D11): 23227-23238. |
| 68 | PAPASAVVA Stella, TAI Stephanie, ILLINGER Karl H, et al. Infrared radiative forcing of CFC substitutes and their atmospheric reaction products[J]. Journal of Geophysical Research: Atmospheres, 1997, 102(D12): 13643-13650. |
| 69 | URATA Shingo, TAKADA Akira, UCHIMARU Tadafumi, et al. Rate constants estimation for the reaction of hydrofluorocarbons and hydrofluoroethers with OH radicals[J]. Chemical Physics Letters, 2003, 368(1/2): 215-223. |
| 70 | CHANDRA Asit K, UCHIMARU Tadafumi, URATA Shingo, et al. Estimation of rate constants for hydrogen atom abstraction by OH radicals using the C? H bond dissociation enthalpies: Haloalkanes and haloethers[J]. International Journal of Chemical Kinetics, 2002, 35(3): 130-138. |
| 71 | KLAMT A. Estimation of gas-phase hydroxyl radical rate constants of organic compounds from molecular orbital calculations[J]. Chemosphere, 1993, 26(7): 1273-1289. |
| 72 | KLAMT Andreas. Estimation of gas-phase hydroxyl radical rate constants of oxygenated compounds based on molecular orbital calculations[J]. Chemosphere, 1996, 32(4): 717-726. |
| 73 | 侯华, 王宝山. 六氟化硫替代气体绝缘强度的官能团加和理论方法[J]. 高等学校化学学报, 2021, 42(12): 3709-3715. |
| HOU Hua, WANG Baoshan. Group additivity theoretical model for the prediction of dielectric strengths of the alternative gases to SF6 [J]. Chemical Journal of Chinese Universities, 2021, 42(12): 3709-3715. | |
| 74 | KWOK Eric S C, ATKINSON Roger. Estimation of hydroxyl radical reaction rate constants for gas-phase organic compounds using a structure-reactivity relationship: An update[J]. Atmospheric Environment, 1995, 29(14): 1685-1695. |
| 75 | ATKINSON Roger. Kinetics and mechanisms of the gas-phase reactions of the hydroxyl radical with organic compounds under atmospheric conditions[J]. Chemical Reviews, 1986, 86(1): 69-201. |
| 76 | ATKINSON Roger. Estimation of gas-phase hydroxyl radical rate constants for organic chemicals[J]. Environmental Toxicology and Chemistry, 1988, 7(6): 435-442. |
| 77 | MINAKATA Daisuke, LI Ke, WESTERHOFF Paul, et al. Development of a group contribution method to predict aqueous phase hydroxyl radical (HO·) reaction rate constants[J]. Environmental Science & Technology, 2009, 43(16): 6220-6227. |
| 78 | Ralph KÜHNE, EBERT Ralf-Uwe, Gerrit SCHÜÜRMANN. Chemical domain of QSAR models from atom-centered fragments[J]. Journal of Chemical Information and Modeling, 2009, 49(12): 2660-2669. |
| 79 | Ralph KÜHNE, EBERT Ralf-Uwe, Gerrit SCHÜÜRMANN. Estimation of compartmental half-lives of organic compounds-structural similarity versus EPI-suite[J]. QSAR & Combinatorial Science, 2007, 26(4): 542-549. |
| 80 | WANG Yanan, CHEN Jingwen, LI Xuehua, et al. Predicting rate constants of hydroxyl radical reactions with organic pollutants: Algorithm, validation, applicability domain, and mechanistic interpretation[J]. Atmospheric Environment, 2009, 43(5): 1131-1135. |
| 81 | LI Chao, YANG Xianhai, LI Xuehua, et al. Development of a model for predicting hydroxyl radical reaction rate constants of organic chemicals at different temperatures[J]. Chemosphere, 2014, 95: 613-618. |
| 82 | JOBACK K G, REID R C. Estimation of pure-component properties from group-contributions[J]. Chemical Engineering Communications, 1987, 57(1/2/3/4/5/6): 233-243. |
| 83 | STEIN S E, BROWN R L. Estimation of normal boiling points from group contributions[J]. Journal of Chemical Information and Computer Sciences, 1994, 34(3): 581-587. |
| 84 | YASH Nannoolal, Rarey JÜRGEN, RAMJUGERNATH D, et al. Estimation of pure component properties: Part 1. Estimation of the normal boiling point of non-electrolyte organic compounds via group contributions and group interactions[J]. Fluid Phase Equilibria, 2004, 226: 45-63. |
| 85 | GHASEMITABAR Habib, MOVAGHARNEJAD Kamyar. Estimation of the normal boiling point of organic compounds via a new group contribution method[J]. Fluid Phase Equilibria, 2016, 411: 13-23. |
| 86 | BRAND K P. Dielectric strength, boiling point and toxicity of gases-different aspects of the same basic molecular properties[J]. IEEE Transactions on Electrical Insulation, 1982, EI-17(5): 451-456. |
| 87 | MEURICE N, SANDRE E, ASLANIDES A, et al. Simple theoretical estimation of the dielectric strength of gases[J]. IEEE Transactions on Dielectrics and Electrical Insulation, 2004, 11(6): 946-948. |
| 88 | RABIE Mohamed, DAHL Dominik A, DONALD Steven M A, et al. Predictors for gases of high electrical strength[J]. IEEE Transactions on Dielectrics and Electrical Insulation, 2013, 20(3): 856-863. |
| 89 | JIAO Juntao, XIAO Dengming, ZHAO Xiaoling, et al. Analysis of the molecules structure and vertical electron affinity of organic gas impact on electric strength[J]. Plasma Science and Technology, 2016, 18(5): 554-559. |
| 90 | 梁艺丹. 基于密度泛函理论的SF6替代气体电气性能评价方法研究[D]. 长沙: 湖南大学, 2018. |
| LIANG Yidan. Research on evaluation method of electrical properties of SF6 substitution gas based on density functional theory[D]. Changsha: Hunan University, 2018. | |
| 91 | 侯华, 余小娟, 周文俊, 等. 绝缘气体介电强度的构效关系[J]. 高等学校化学学报, 2018, 39(11): 2477-2484. |
| HOU Hua, YU Xiaojuan, ZHOU Wenjun, et al. Theoretical investigations on the structure-activity relationship to the dielectric strength of the insulation gases[J]. Chemical Journal of Chinese Universities, 2018, 39(11): 2477-2484. | |
| 92 | 陈庆国, 邱睿, 林林, 等. 基于密度泛函理论的SF6潜在替代气体筛选[J]. 高电压技术, 2019, 45(4):1026-1033. |
| CHEN Qingguo, QIU Rui, LIN Lin, et al. Selection of potential substitutes for SF6 based on density functional theory[J]. High Voltage Engineering, 2019, 45(4): 1026-1033. | |
| 93 | MURRAY Jane S, LANE Pat, BRINCK Tore, et al. Relationships of critical constants and boiling points to computed molecular surface properties[J]. The Journal of Physical Chemistry, 1993, 97(37): 9369-9373. |
| 94 | RABIE M, FRANCK C. Predicting the electric strength of proposed SF6 replacement gases by means of density functional theory[C]//18th International Symposium on High Voltage Engineering, Seoul, Korea, 2013: 1381-1386. |
| 95 | 邱馨仪. SF6替代气体绝缘强度构效关系及分子设计方法[D]. 北京:华北电力大学, 2021. |
| QIU Xinyi. Structure-property relationship to the dielectric strength and molecular design method of SF6 alternative gas [D]. Beijing: North China Electric Power University, 2021. | |
| 96 | SUN H, LIANG L, WANG C, et al. Prediction of the Electrical Strength and Boiling Temperature of the Substitutes for Greenhouse Gas SF6 Using Neural Network and Random Forest[J]. IEEE Access, 2020:124204-124216. |
| 97 | LIU Bo, KARIMI NOURODDIN Maryam. Application of artificial intelligent approach to predict the normal boiling point of refrigerants[J]. International Journal of Chemical Engineering, 2023, 2023: 1-9. |
| 98 | TINGLE Benjamin I, TANG Khanh G, CASTANON Mar, et al. ZINC-22─A free multi-billion-scale database of tangible compounds for ligand discovery[J]. Journal of Chemical Information and Modeling, 2023, 63(4): 1166-1176. |
| 99 | REYMOND Jean-Louis. The chemical space project[J]. Accounts of Chemical Research, 2015, 48(3): 722-730. |
| 100 | WEN Linyuan, YU Tao, LAI Weipeng, et al. Accelerating molecular design of cage energetic materials with zero oxygen balance through large-scale database search[J]. The Journal of Physical Chemistry Letters, 2021, 12(47): 11591-11597. |
| 101 | WEN Linyuan, WANG Bozhou, YU Tao, et al. Accelerating the search of CHONF-containing highly energetic materials by combinatorial library design and high-throughput screening[J]. Fuel, 2022, 310: 122241. |
| 102 | LAI Weipeng, LIAN Peng, LIU Yingzhe, et al. Design and theoretical study of 15 novel high energy density compounds[J]. Journal of Molecular Modeling, 2014, 20(11): 2479. |
| 103 | LAI Weipeng, YU Tao, LIU Yingzhe, et al. Study on the computer-aided design of high energetic compounds based on the 1,2,3,4-tetrazine-1,3-dioxide frame[J]. Journal of Molecular Modeling, 2017, 23(12): 340. |
| 104 | WEN Linyuan, YU Tao, LAI Weipeng, et al. Intra-ring bridging: A strategy for molecular design of highly energetic nitramines[J]. Chinese Journal of Chemistry, 2021, 39(10): 2857-2864. |
| 105 | WEN Linyuan, YU Tao, LAI Weipeng, et al. Transferring the available fused cyclic scaffolds for high—Throughput combinatorial design of highly energetic materials via database mining[J]. Fuel, 2022, 324: 124591. |
| 106 | SEKIYA Akira, DESMARTEAU Darryl D. Novel N-fluoroamines via the chlorofluorination of compounds with carbon-nitrogen triple bonds[J]. Journal of the American Chemical Society, 1979, 101(25): 7640-7641. |
| 107 | SARWAR Ghulam, KIRCHMEIER Robert L, SHREEVE Jeanne M. Secondary (polyfluoroalkyl) chloroamines: Precursors to fluoroazaalkenes[J]. Inorganic Chemistry, 1990, 29(3): 571-572. |
| 108 | SARWAR Ghulam, KIRCHMEIER Robert L, SHREEVE Jean’ne M. Insertion of tetrafluoroethylene and trifluorochloroethylene into nitrogen-chlorine bonds. A new route to perfluoroazaalkenes[J]. Inorganic Chemistry, 1989, 28(11): 2187-2189. |
| 109 | BAUKNIGHT Charles W, DESMARTEAU Darryl D. Reactions of n-bromodifluoromethanimine[J]. The Journal of Organic Chemistry, 1988, 53(19): 4443-4447. |
| 110 | SEKIYA Akira, DESMARTEAU Darryl D. Reaction of N-chloro-N-fluoroperhaloalkylamines with mercury. facile synthesis of N-fluoro imines and N-fluoro amines[J]. The Journal of Organic Chemistry, 1981, 46(7): 1277-1280. |
| 111 | FRANCK Christian M, CHACHEREAU Alise, PACHIN Juriy. SF6-free gas-insulated switchgear: Current status and future trends[J]. IEEE Electrical Insulation Magazine, 2021, 37(1): 7-16. |
| 112 | RABIE Mohamed, FRANCK Christian M. Comparison of gases for electrical insulation: Fundamental concepts[J]. IEEE Transactions on Dielectrics and Electrical Insulation, 2018, 25(2): 649-656. |
| 113 | ZHAO Yanxing, LI Zhibin, ZHANG Xiaojun, et al. Azeotropic refrigerants and its application in vapor compression refrigeration cycle[J]. International Journal of Refrigeration, 2019, 108: 1-13. |
| 114 | 杨志强, 吕剑, 曾纪珺, 等. 一种寻找SF6替代气体寻找方法: CN202211583364.8[P]. 2022-11-18. |
| YANG Zhiqiang, LU Jian, ZENG Jijun, et al. A search method for finding alternative gases for SF6 : CN202211583364.8[P]. 2022-11-18. | |
| 115 | 杨志强, 吕剑, 唐晓博,等. 一种用于替代六氟化硫的绝缘混合气体及其应用. CN 202211585171.4[P].2022-11-18. |
| YANG Zhiqiang, LU Jian, TANG Xiaobo, et al. An insulating gas mixture for replacing sulfur hexafluoride and its application: CN 202211585171.4[P]. 2022-11-18. | |
| 116 | JALILIAN M R. Spectra and structure of binary azeotropes[J]. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2008, 69(3): 812-815. |
| 117 | WAKISAKA Akihiro, MATSUURA Kazuo, URANAGA Makoto, et al. Azeotropy of alcohol-water mixtures from the viewpoint of cluster-level structures[J]. Journal of Molecular Liquids, 2011, 160(2): 103-108. |
| 118 | KEASLER Samuel J, CHARAN Sophia M, WICK Collin D, et al. Transferable potentials for phase equilibria-united atom description of five- and six-membered cyclic alkanes and ethers[J]. The Journal of Physical Chemistry B, 2012, 116(36): 11234-11246. |
| 119 | QI Junjie, ZENG Fanlai, JIA Hanbing, et al. Research progress on the formation mechanism of azeotrope and its separation process in microwave field[J]. Journal of Chemical Technology & Biotechnology, 2022, 97(5): 1045-1063. |
| 120 | FONSECA José M S, DOHRN Ralf, PEPER Stephanie. High-pressure fluid-phase equilibria: Experimental methods and systems investigated (2005—2008)[J]. Fluid Phase Equilibria, 2011, 300(1/2): 1-69. |
| 121 | DOHRN Ralf, PEPER Stephanie, FONSECA José M S. High-pressure fluid-phase equilibria: Experimental methods and systems investigated (2000—2004)[J]. Fluid Phase Equilibria, 2010, 288(1/2): 1-54. |
| 122 | CONSTANTINESCU Dana, Jürgen GMEHLING. Further development of modified UNIFAC (dortmund): Revision and extension 6[J]. Journal of Chemical & Engineering Data, 2016, 61(8): 2738-2748. |
| 123 | Jürgen GMEHLING, WITTIG Roland, Jürgen LOHMANN, et al. A modified UNIFAC (dortmund) model. 4. Revision and extension[J]. Industrial & Engineering Chemistry Research, 2002, 41(6): 1678-1688. |
| 124 | JAUBERT Jean-Noël, PRIVAT Romain. Relationship between the binary interaction parameters (kij ) of the Peng-Robinson and those of the Soave-Redlich-Kwong equations of state: Application to the definition of the PR2SRK model[J]. Fluid Phase Equilibria, 2010, 295(1): 26-37. |
| 125 | NGUYEN Van T, TAN S Johnathan, DO D D, et al. Application of kinetic Monte Carlo method to the vapour-liquid equilibria of associating fluids and their mixtures[J]. Molecular Simulation, 2016, 42(8): 642-654. |
| [1] | 张杰, 王放放, 夏忠林, 赵光金, 马双忱. “双碳”目标下SF6排放现状、减排手段分析及未来展望[J]. 化工进展, 2023, 42(S1): 447-460. |
| [2] | 祖立武, 毕莹, 赵缤慧, 李纪东, 杨晴, 丛姗姗. 苯并𫫇嗪树脂研究进展[J]. 化工进展, 2022, 41(8): 4224-4240. |
| [3] | 唐坤, 刘奇磊, 张磊, 刘琳琳, 都健, 孟庆伟. 基于高阶基团贡献法与COSMO-SAC模型的溶剂设计方法[J]. 化工进展, 2021, 40(S2): 48-55. |
| [4] | 张新民,冯恩娟,徐正华,严 生. 聚羧酸类减水剂的分子设计与结构性能关系 [J]. 化工进展, 2008, 27(6): 913-. |
| [5] | 张学岗,张军保,宋 静,宋海华. 基于MGASA的计算机辅助分子设计 [J]. 化工进展, 2008, 27(12): 2019-. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||