化工进展 ›› 2023, Vol. 42 ›› Issue (7): 3875-3883.DOI: 10.16085/j.issn.1000-6613.2022-1631
CeO2/TiO2吸附剂煤气脱汞产物的浸出规律
陆洋( ), 周劲松(
), 周劲松( ), 周启昕, 王瑭, 刘壮, 李博昊, 周灵涛
), 周启昕, 王瑭, 刘壮, 李博昊, 周灵涛
- 浙江大学能源清洁利用国家重点实验室,浙江 杭州 310027
-
收稿日期:2022-09-05修回日期:2022-12-13出版日期:2023-07-15发布日期:2023-08-14 -
通讯作者:周劲松 -
作者简介:陆洋(1998—),男,硕士研究生,研究方向为煤气脱汞。E-mail:22027011@zju.edu.cn。 -
基金资助:国家自然科学基金(52176136)
Leaching mechanism of Hg-absorption products on CeO2/TiO2 sorbentsin syngas
LU Yang( ), ZHOU Jinsong(
), ZHOU Jinsong( ), ZHOU Qixin, WANG Tang, LIU Zhuang, LI Bohao, ZHOU Lingtao
), ZHOU Qixin, WANG Tang, LIU Zhuang, LI Bohao, ZHOU Lingtao
- State Key Laboratory of Clean Energy Utilization,Zhejiang University, Hangzhou 310027, Zhejiang, China
-
Received:2022-09-05Revised:2022-12-13Online:2023-07-15Published:2023-08-14 -
Contact:ZHOU Jinsong
摘要:
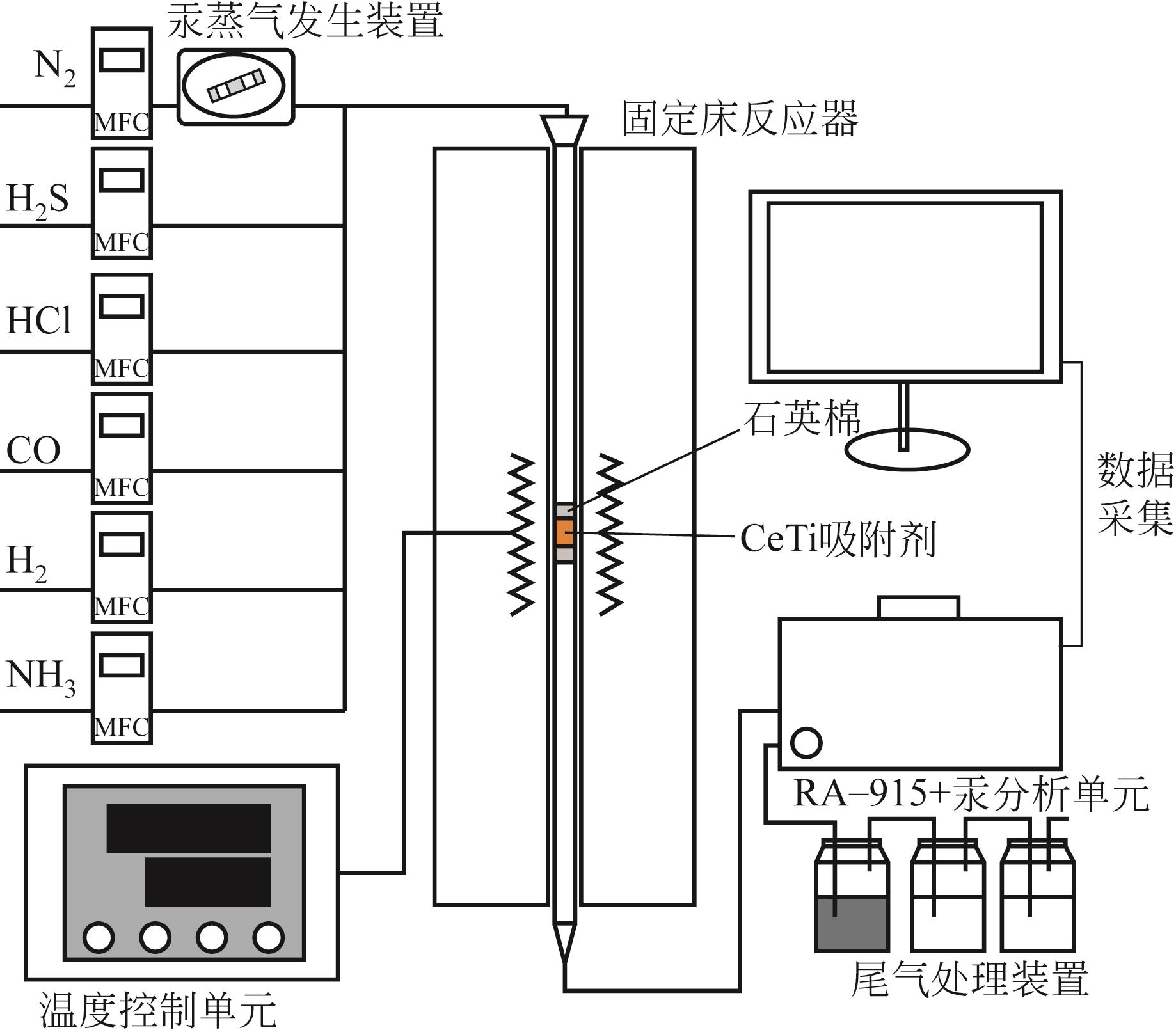
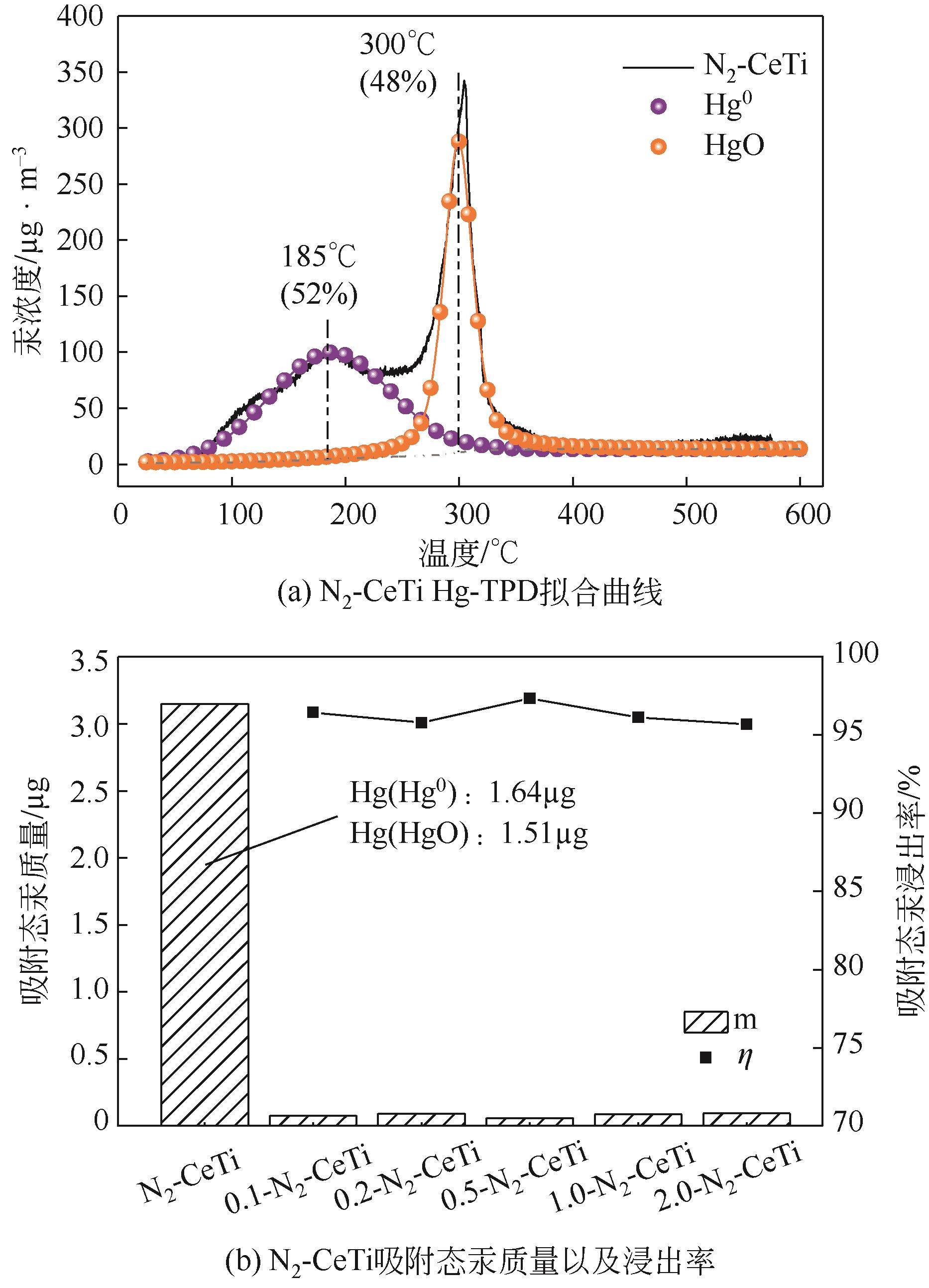
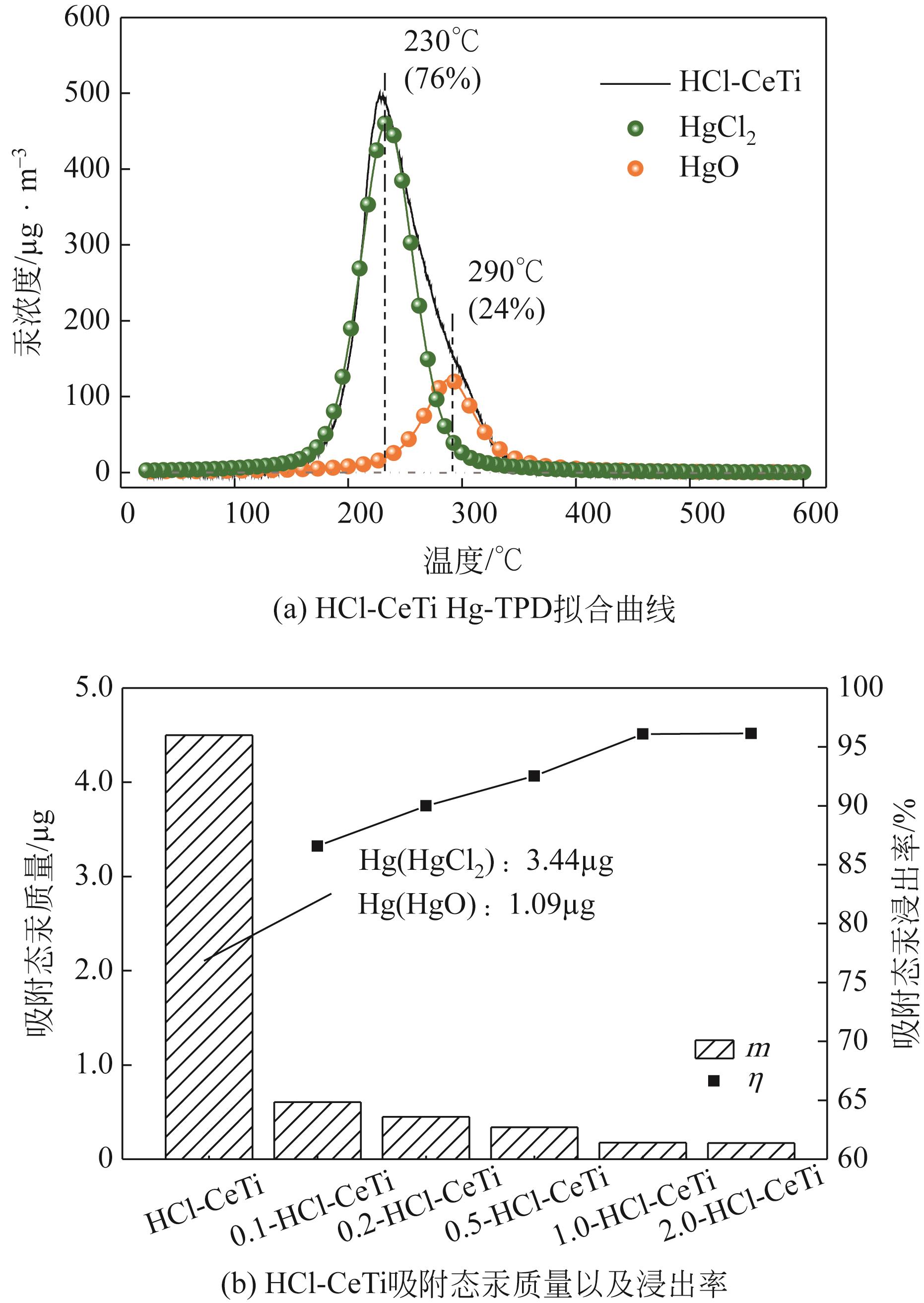
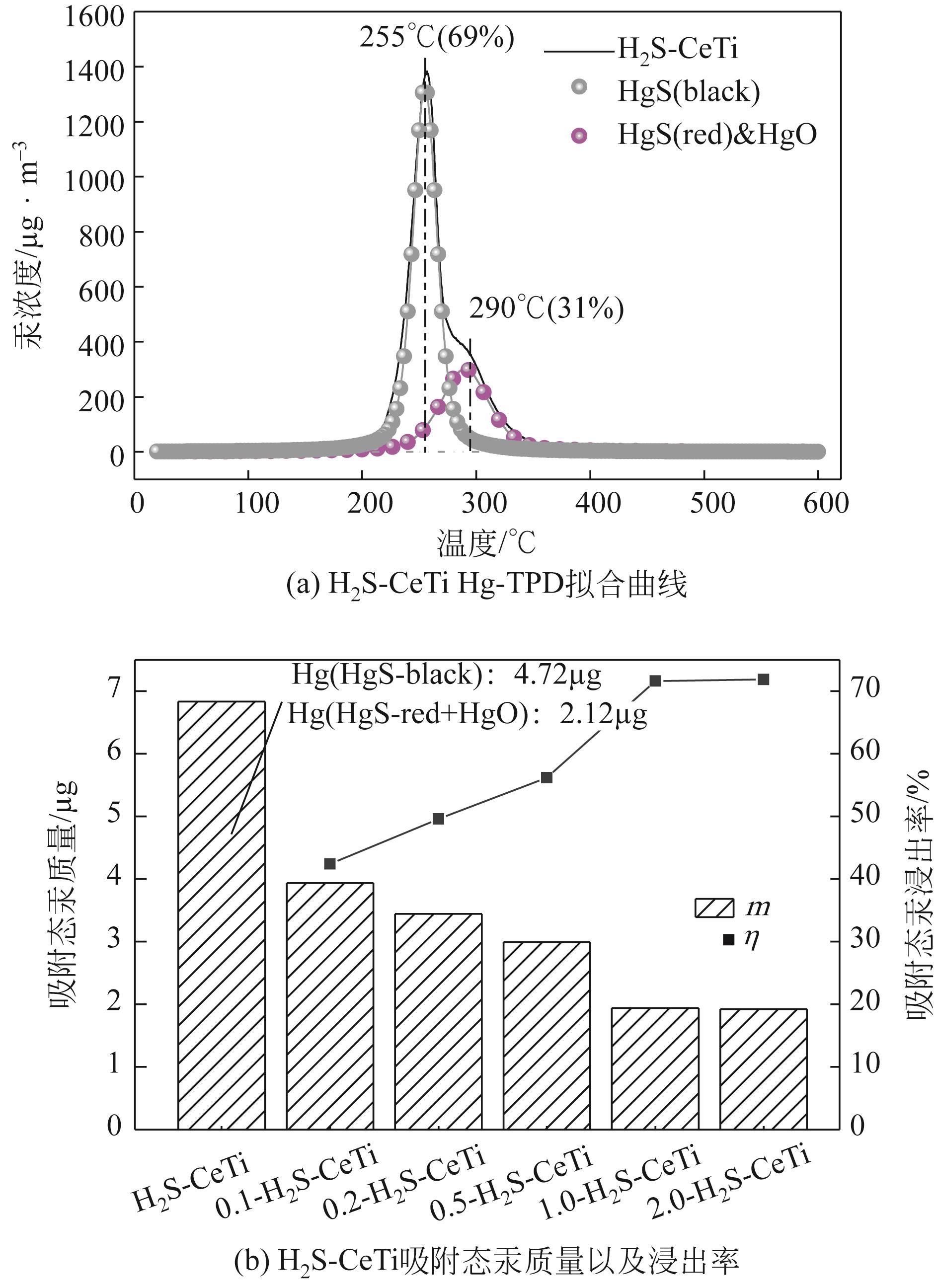
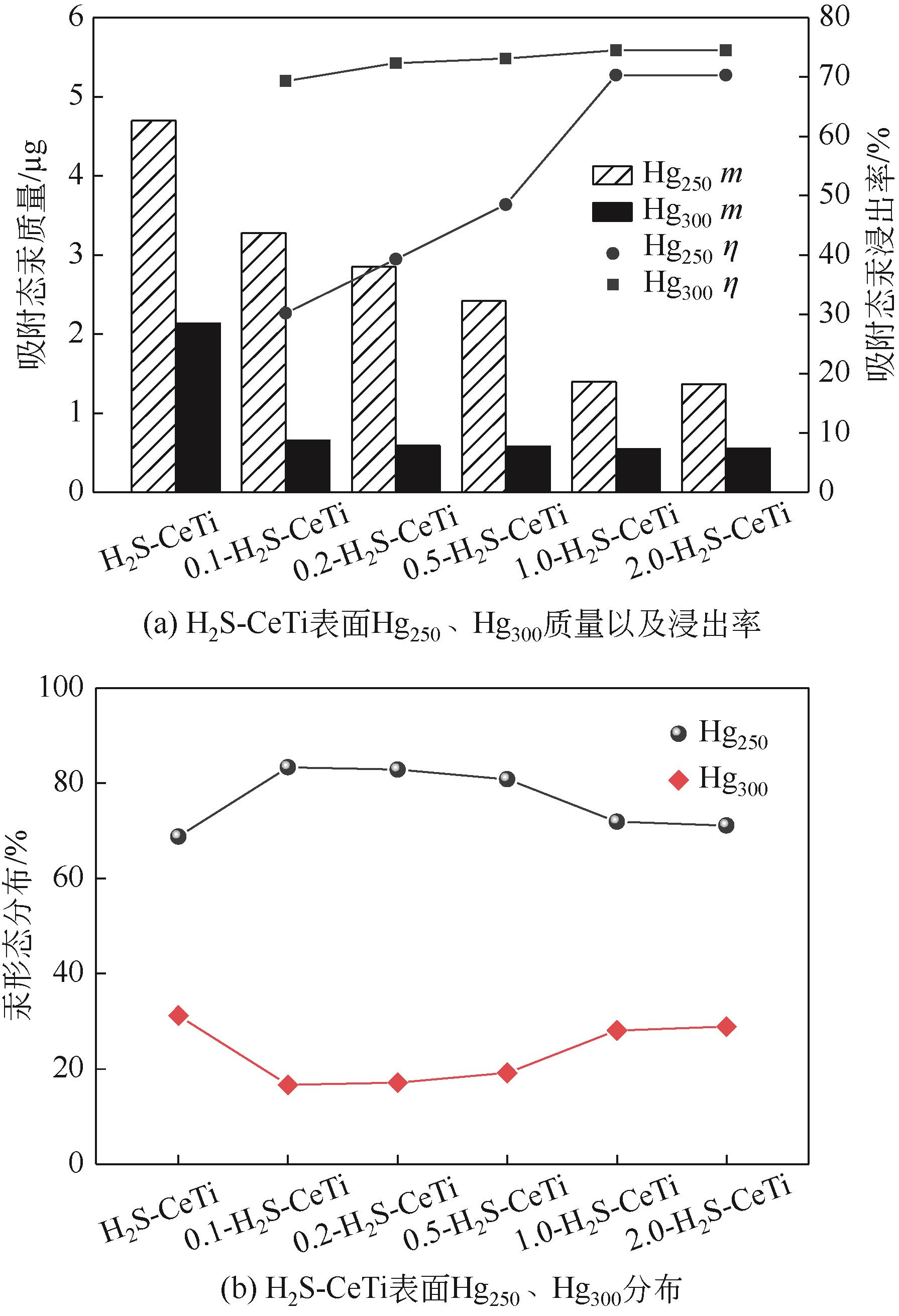
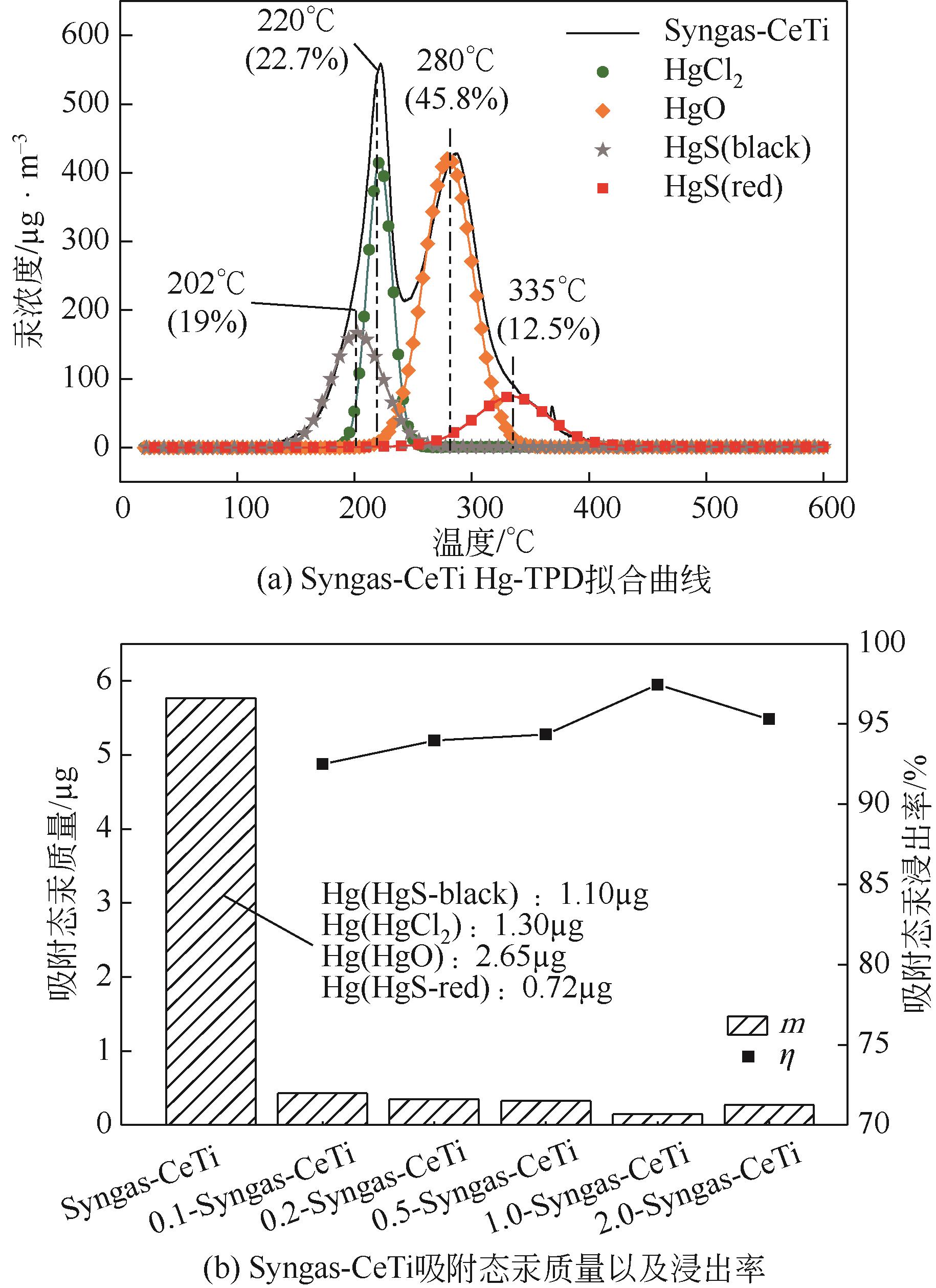
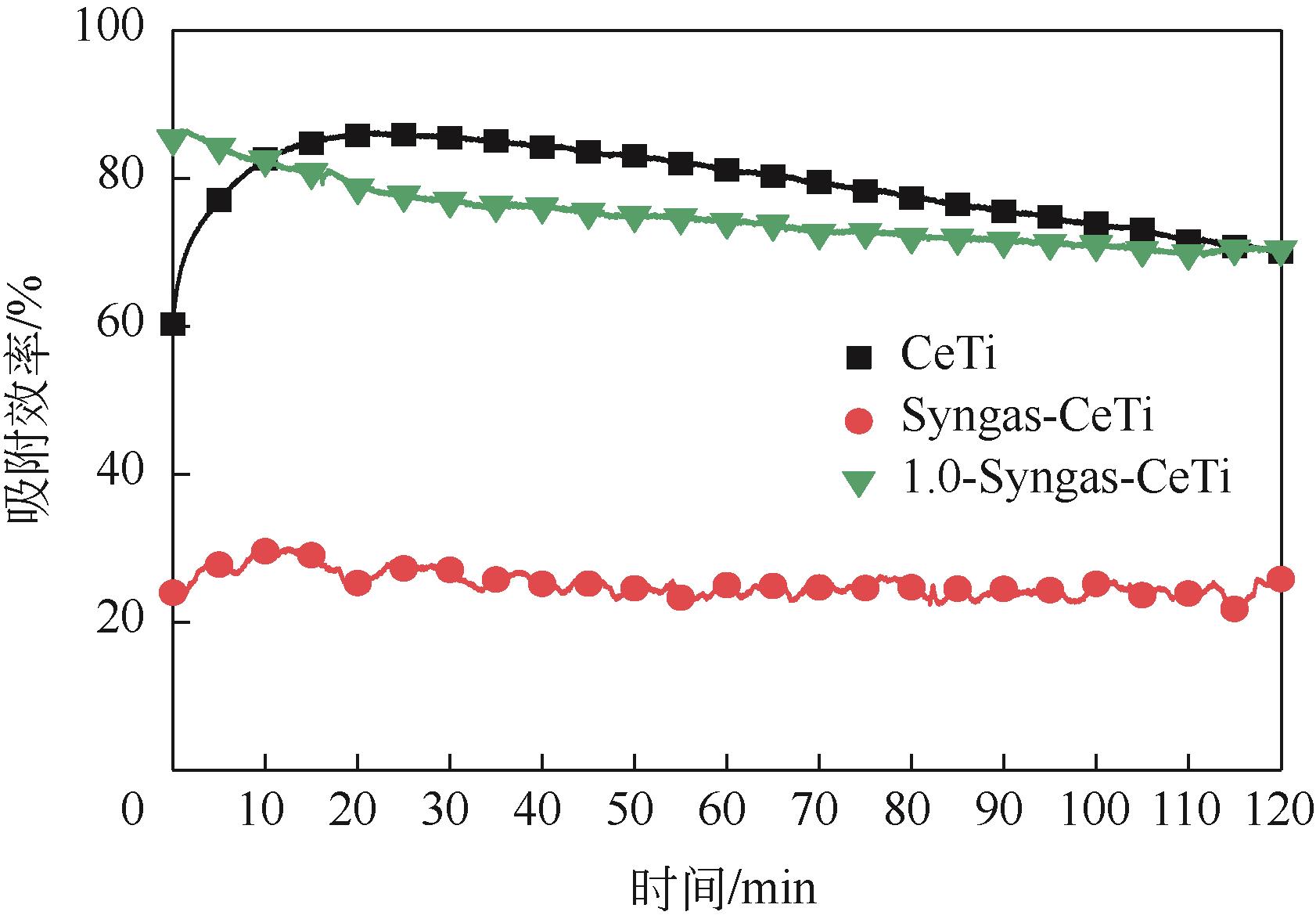
基于金属氧化物脱汞吸附剂后续无害化处理要求,本文提出采用硫代硫酸钠(Na2S2O3)溶液浸出金属氧化物脱汞产物的新思路。CeO2/TiO2吸附剂(CeTi)吸附汞后得到含汞吸附剂(Hg-CeTi),利用汞程序升温脱附实验(Hg-TPD)确定其表面吸附态汞的赋存形态。随后探究Na2S2O3溶液对于Hg-CeTi表面脱汞产物的浸出能力,着重分析不同赋存形态的汞在Na2S2O3溶液中的迁移规律。Hg-TPD结果表明,模拟煤气中脱汞产物以HgCl2、HgO和HgS为主。由于硫代硫酸根(S2O
中图分类号:
引用本文
陆洋, 周劲松, 周启昕, 王瑭, 刘壮, 李博昊, 周灵涛. CeO2/TiO2吸附剂煤气脱汞产物的浸出规律[J]. 化工进展, 2023, 42(7): 3875-3883.
LU Yang, ZHOU Jinsong, ZHOU Qixin, WANG Tang, LIU Zhuang, LI Bohao, ZHOU Lingtao. Leaching mechanism of Hg-absorption products on CeO2/TiO2 sorbentsin syngas[J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3875-3883.
| Hg-CeTi | 气体组分及体积分数 | 吸附温度/℃ |
|---|---|---|
| N2-CeTi | N2 | 120 |
| HCl-CeTi | N2+0.001% HCl | 120 |
| H2S-CeTi | N2+0.04% H2S | 120 |
| Syngas-CeTi | N2+30% H2+20% CO+0.1% NH3+ 0.001% HCl+0.04% H2S | 120 |
表1 吸附实验气氛及温度
| Hg-CeTi | 气体组分及体积分数 | 吸附温度/℃ |
|---|---|---|
| N2-CeTi | N2 | 120 |
| HCl-CeTi | N2+0.001% HCl | 120 |
| H2S-CeTi | N2+0.04% H2S | 120 |
| Syngas-CeTi | N2+30% H2+20% CO+0.1% NH3+ 0.001% HCl+0.04% H2S | 120 |
| 1 | LIU Ming, LI Fanghua, LIU Haifeng, et al. Synergistic effect on co-gasification of chicken manure and petroleum coke: An investigation of sustainable waste management[J]. Chemical Engineering Journal, 2021, 417: 128008. |
| 2 | ZHANG Xiaoyang, CUI Lin, XING Xiangwen, et al. Experimental and theoretical analysis of elemental mercury removal from syngas over Fe-Ti spinel[J]. Fuel, 2022, 324: 124430. |
| 3 | 李学谦, 周劲松, 周启昕, 等. 硫化氢对负载型钴铈双金属吸附剂脱除模拟煤气中单质汞的促进机理[J]. 化工进展, 2018, 37(11): 4493-4499. |
| LI Xueqian, ZHOU Jinsong, ZHOU Qixin, et al. Promotion mechanism of hydrogen sulfide on elemental mercury removal from simulated syngas by supported cobalt-cerium bimetallic sorbent[J]. Chemical Industry and Engineering Progress, 2018, 37(11): 4493-4499. | |
| 4 | Adnan Raza Altaf, ADEWUYI Yusuf G, TENG Haipeng, et al. Elemental mercury (Hg0) removal from coal syngas using magnetic tea-biochar: Experimental and theoretical insights[J]. Journal of Environmental Sciences, 2022, 122: 150-161. |
| 5 | 茅珏榛. 钴改性吸附剂脱除煤气中汞的机理研究[D]. 杭州: 浙江大学, 2018. |
| MAO Juezhen. Mechanism study on mercury removal by Co-based sorbents from simulated syngas[D]. Hangzhou: Zhejiang University, 2018. | |
| 6 | 侯文慧. 模拟煤气条件下金属氧化物吸附脱除单质汞的机理研究[D]. 杭州: 浙江大学, 2015. |
| HOU Wenhui. Mechanism study on the removal of elemental mercury from simulated syngas over metal oxide sorbents[D]. Hangzhou: Zhejiang University, 2015. | |
| 7 | ZHANG Huawei, LIU Xiuli, WANG Li, et al. Characteristics and stability of mercury vapor adsorption over two kinds of modified semicoke[J]. The Scientific World Journal, 2014, 2014: 260141. |
| 8 | BISSON Teresa M, XU Zhenghe. Potential hazards of brominated carbon sorbents for mercury emission control[J]. Environmental Science & Technology, 2015, 49(4): 2496-2502. |
| 9 | GRAYDON John W, ZHANG Xinzhi, KIRK Donald W, et al. Sorption and stability of mercury on activated carbon for emission control[J]. Journal of Hazardous Materials, 2009, 168(2/3): 978-982. |
| 10 | LUO Zhongyang, HU Changxing, ZHOU Jinsong, et al. Stability of mercury on three activated carbon sorbents[J]. Fuel Processing Technology, 2006, 87(8): 679-685. |
| 11 | WANG Zhen, LIU Jing, YANG Yingju, et al. AMn2O4 (A=Cu, Ni and Zn) sorbents coupling high adsorption and regeneration performance for elemental mercury removal from syngas[J]. Journal of Hazardous Materials, 2020, 388: 121738. |
| 12 | WANG Zhen, LIU Jing, YANG Yingju, et al. Regenerable Co x Mn3– x O4 spinel sorbents for elemental mercury removal from syngas: Experimental and DFT studies[J]. Fuel, 2020, 266: 117105. |
| 13 | 曹辉. 二氧化钛负载铈锰氧化物脱除煤气汞及再生的机理研究[D]. 杭州: 浙江大学, 2020. |
| CAO Hui. Mechanism study on the mercury removal from coal gas by titanium dioxide supported cerium manganese oxides and its regeneration[D]. Hangzhou: Zhejiang University, 2020. | |
| 14 | Antonia LOPEZ-ANTON M, YUAN Yang, PERRY Ron, et al. Analysis of mercury species present during coal combustion by thermal desorption[J]. Fuel, 2010, 89(3): 629-634. |
| 15 | XU Haomiao, HONG Qinyuan, LI Jiaxing, et al. Heterogeneous reaction mechanisms and functional materials for elemental mercury removal from industrial flue gas[J]. ACS ES&T Engineering, 2021, 1(10): 1383-1400. |
| 16 | DEVASENA M, NAMBI I M. Potential leaching of mercury from stable mercury sulphide[J]. International Journal of Environmental Science and Technology, 2021, 18(11): 3677-3684. |
| 17 | DEVASENA M, NAMBI Indumathi M. In situ stabilization of entrapped elemental mercury[J]. Journal of Environmental Management, 2013, 130: 185-191. |
| 18 | DIAO Xing, YUAN Chungang, WU Jingjing, et al. Mercury fractions in gypsum and estimation of mercury emission from coal-fired power plants[J]. Fuel, 2018, 226: 298-306. |
| 19 | ISSARO N, BESANCON S, BERMOND A. Thermodynamic and kinetic study of the single extraction of mercury from soil using sodium-thiosulfate[J]. Talanta, 2010, 82(5): 1659-1667. |
| 20 | HAN Chao, WANG Wei, XIE Feng, et al. Mechanism and kinetics of mercuric sulfide leaching with cuprous-thiosulfate solutions[J]. Separation and Purification Technology, 2017, 177: 223-232. |
| 21 | HAN Chao, WANG Wei, XIE Feng. Study on the leaching of mercuric oxide with thiosulfate solutions[J]. Metals, 2016, 6(9): 206. |
| 22 | LU Xixin, HUANGFU Xiaoliu, ZHANG Xiang, et al. Strong enhancement of trace mercury removal from aqueous solution with sodium thiosulfate by in situ formed Mn-(hydr)oxides[J]. Water Research, 2014, 65: 22-31. |
| 23 | XIE Feng, CHEN Junnan, WANG Jian, et al. Review of gold leaching in thiosulfate-based solutions[J]. Transactions of Nonferrous Metals Society of China, 2021, 31(11): 3506-3529. |
| 24 | 韩超. 含汞固体废物硫代硫酸盐浸出与回收技术基础研究[D]. 沈阳: 东北大学, 2017. |
| HAN Chao. A fundamental study on thiosulfate leaching and recovery of mercury-bearing solid waste[D]. Shenyang: Northeastern University, 2017. | |
| 25 | CAO Hui, ZHOU Jinsong, ZHOU Qixin, et al. Elemental mercury removal from coal gas by CeMnTi sorbents and their regeneration performance[J]. Journal of Zhejiang University-SCIENCE A, 2021, 22(3): 222-234. |
| 26 | HOU Wenhui, ZHOU Jinsong, QI Pan, et al. Effect of H2S/HCl on the removal of elemental mercury in syngas over CeO2-TiO2 [J]. Chemical Engineering Journal, 2014, 241: 131-137. |
| 27 | 孟帅琦. 汞在钯掺杂CeO2表面吸附的机理研究[D]. 杭州: 浙江大学, 2016. |
| MENG Shuaiqi. Mechanism study of mercury adsorption on Pd doped CeO2 Surface[D]. Hangzhou: Zhejiang University, 2016. | |
| 28 | LI Xueqian, ZHOU Jinsong, ZHOU Qixin, et al. Removal of elemental mercury using titania sorbents loaded with cobalt ceria oxides from syngas[J]. New Journal of Chemistry, 2018, 42(15): 12503-12510. |
| 29 | ZHOU Jinsong, HOU Wenhui, QI Pan, et al. CeO2-TiO2 sorbents for the removal of elemental mercury from syngas[J]. Environmental Science & Technology, 2013, 47(17): 10056-10062. |
| 30 | WU Xiang, DUAN Yufeng, LI Na, et al. Regenerable Ce-Mn/TiO2 catalytic sorbent for mercury removal with high resistance to SO2 [J]. Energy & Fuels, 2019, 33(9): 8835-8842. |
| 31 | LI Hailong, WU Changyu, LI Ying, et al. CeO2-TiO2 catalysts for catalytic oxidation of elemental mercury in low-rank coal combustion flue gas[J]. Environmental Science & Technology, 2011, 45(17): 7394-7400. |
| 32 | LIU Zhuang, ZHOU Jinsong, JIN Liang, et al. Mercury removal from syngas by metal oxides based adsorbent: A review[J]. Fuel, 2022, 327: 125057. |
| 33 | BARNETT Mark O, TURNER Ralph R. Bioaccessibility of mercury in soils[J]. Soil and Sediment Contamination: An International Journal, 2001, 10(3): 301-316. |
| 34 | DEAN J A. 兰氏化学手册[M]. 尚久方, 译. 13版.北京: 科学出版社, 1991: 390. |
| DEAN J A. Lange’s handbook of chemistry[M]. SHANG Jiufang, trans. 13th ed. Beijing: Science Press, 1991: 390. | |
| 35 | 陈寿椿. 重要无机化学反应[M]. 3版. 上海: 上海科学技术出版社, 1994: 79. |
| CHEN Shouchun. Important inorganic chemical reaction[M]. 3rd ed. Shanghai: Shanghai Scientific & Technical Publishers, 1994: 79. | |
| 36 | ZHAO Shilin, LUO Hui, MA Anjun, et al. Experimental study on mercury removal from coal-fired flue gas by sulfur modified biomass coke with mechanochemical method[J]. Fuel, 2022, 309: 122201. |
| 37 | RAVICHANDRAN Mahalingam, AIKEN George R, REDDY Michael M, et al. Enhanced dissolution of cinnabar (mercuric sulfide) by dissolved organic matter isolated from the Florida Everglades[J]. Environmental Science & Technology, 1998, 32(21): 3305-3311. |
| 38 | HONG Qinyuan, XU Haomiao, LIAO Yong, et al. Insight into the interfacial stability and reaction mechanism between gaseous mercury and chalcogen-based sorbents in SO2-containing flue gas[J]. Journal of Colloid and Interface Science, 2020, 577: 503-511. |
| [1] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [2] | 顾永正, 张永生. HBr改性飞灰对Hg0的动态吸附及动力学模型[J]. 化工进展, 2023, 42(S1): 498-509. |
| [3] | 汪鹏, 张洋, 范兵强, 何登波, 申长帅, 张贺东, 郑诗礼, 邹兴. 高碳铬铁盐酸浸出过程工艺及动力学[J]. 化工进展, 2023, 42(S1): 510-517. |
| [4] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [5] | 张婷婷, 潘大伟, 巨晓洁, 刘壮, 谢锐, 汪伟, 褚良银. Hg2+响应型智能凝胶检测光栅的构建与性能[J]. 化工进展, 2023, 42(8): 4143-4152. |
| [6] | 王鑫, 王兵兵, 杨威, 徐志明. 金属表面PDA/PTFE超疏水涂层抑垢与耐腐蚀性能[J]. 化工进展, 2023, 42(8): 4315-4321. |
| [7] | 张雪伟, 黄亚继, 许月阳, 程好强, 朱志成, 李金壘, 丁雪宇, 王圣, 张荣初. 碱性吸附剂对燃煤烟气中SO3的吸附特性[J]. 化工进展, 2023, 42(7): 3855-3864. |
| [8] | 吴展华, 盛敏. 绝热加速量热仪在反应安全风险评估应用中的常见问题[J]. 化工进展, 2023, 42(7): 3374-3382. |
| [9] | 谢志伟, 吴张永, 朱启晨, 蒋佳骏, 梁天祥, 刘振阳. 植物油基Ni0.5Zn0.5Fe2O4磁流体的黏度特性及磁黏特性[J]. 化工进展, 2023, 42(7): 3623-3633. |
| [10] | 杨竞莹, 施万胜, 黄振兴, 谢利娟, 赵明星, 阮文权. 改性纳米零价铁材料制备的研究进展[J]. 化工进展, 2023, 42(6): 2975-2986. |
| [11] | 王久衡, 荣鼐, 刘开伟, 韩龙, 水滔滔, 吴岩, 穆正勇, 廖徐情, 孟文佳. 水蒸气强化纤维素模板改性钙基吸附剂固碳性能及强度[J]. 化工进展, 2023, 42(6): 3217-3225. |
| [12] | 杨扬, 孙志高, 李翠敏, 李娟, 黄海峰. 静态条件下表面活性剂OP-13促进HCFC-141b水合物生成[J]. 化工进展, 2023, 42(6): 2854-2859. |
| [13] | 李卫华, 吴寅凯, 孙英杰, 尹俊权, 辛明学, 赵友杰. 垃圾焚烧飞灰重金属毒性浸出评价方法研究进展[J]. 化工进展, 2023, 42(5): 2666-2677. |
| [14] | 李玲, 马超峰, 卢春山, 于万金, 石能富, 金佳敏, 张建君, 刘武灿, 李小年. 新型含氟替代品1,1,2-三氟乙烯的合成工艺与催化剂研究进展[J]. 化工进展, 2023, 42(4): 1822-1831. |
| [15] | 阮鹏, 杨润农, 林梓荣, 孙永明. 甲烷催化部分氧化制合成气催化剂的研究进展[J]. 化工进展, 2023, 42(4): 1832-1846. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||