化工进展 ›› 2023, Vol. 42 ›› Issue (3): 1493-1507.DOI: 10.16085/j.issn.1000-6613.2022-0879
半导体光催化剂BiOCl异质结的构建及应用
胥生元1( ), 郝玮1, 王杰1, 高文生2, 谢克锋1(
), 郝玮1, 王杰1, 高文生2, 谢克锋1( )
)
- 1.兰州交通大学化学化工学院,甘肃 兰州 730070
2.兰州大学化学化工学院,甘肃 兰州 730000
-
收稿日期:2022-05-12修回日期:2022-06-22出版日期:2023-03-15发布日期:2023-04-10 -
通讯作者:谢克锋 -
作者简介:胥生元(1996—),男,硕士研究生,研究方向为功能材料。E-mail:xsyexw@163.com。 -
基金资助:国家自然科学基金青年基金(11905091);甘肃省青年科技基金(21JR7RA326);甘肃省高等学校创新基金(2021B-100)
Construction and application of BiOCl heterojunction as semiconductor photocatalyst
XU Shengyuan1( ), HAO Wei1, WANG Jie1, GAO Wensheng2, XIE Kefeng1(
), HAO Wei1, WANG Jie1, GAO Wensheng2, XIE Kefeng1( )
)
- 1.School of Chemistry and Chemical Engineering, Lanzhou Jiaotong University, Lanzhou 730070, Gansu, China
2.College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou 730000, Gansu, China
-
Received:2022-05-12Revised:2022-06-22Online:2023-03-15Published:2023-04-10 -
Contact:XIE Kefeng
摘要:
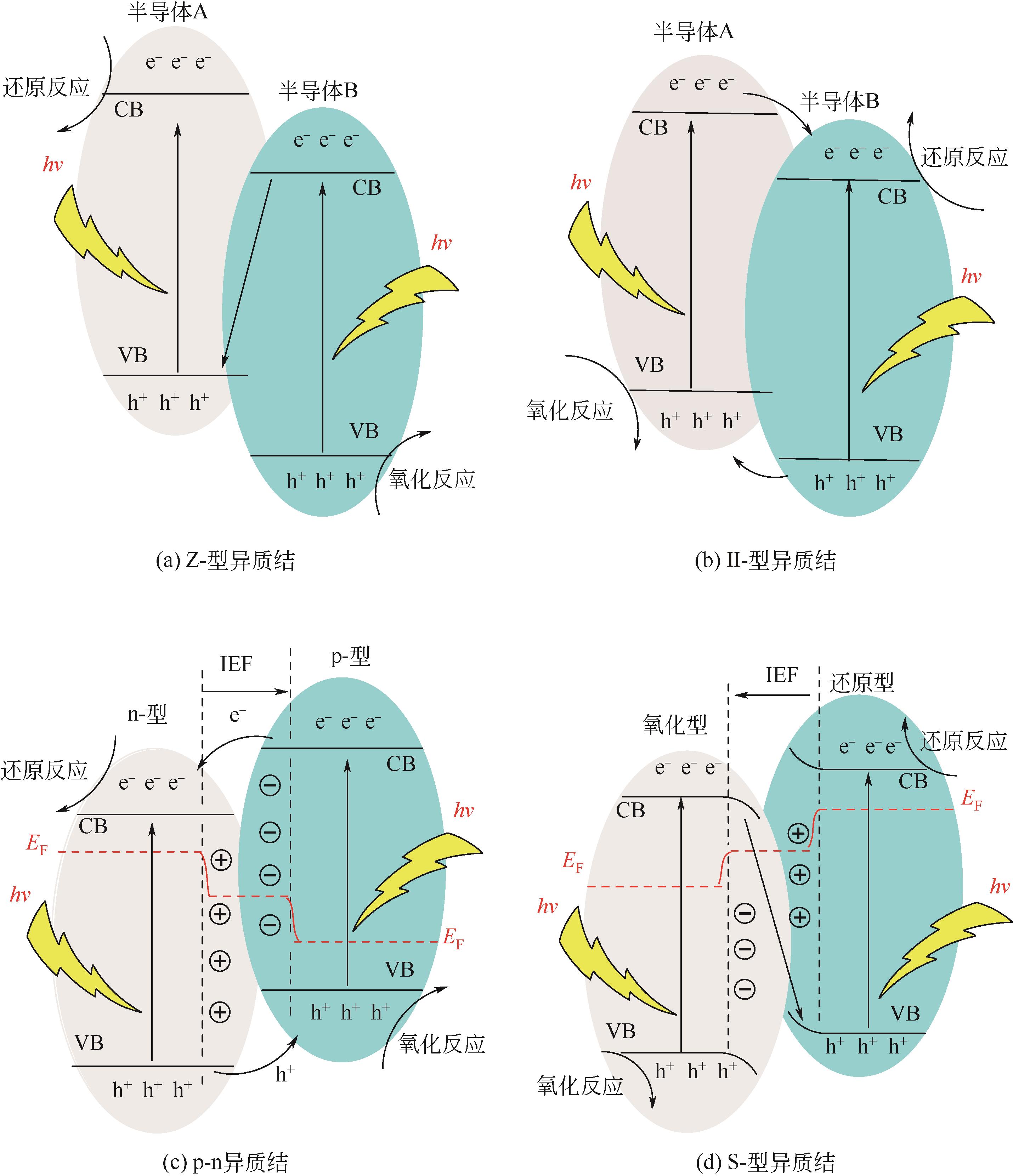
氯氧化铋(BiOCl)半导体因其独特的层状结构和可调控的电子结构,而在光催化解决环境问题和能源问题领域备受关注。为了提高BiOCl对太阳光的利用率、抑制光生电子-空穴对的高复合率和增强光电子的还原能力等,研究人员对此作出了巨大的努力。其中,构建异质结是最有效的削弱这些缺陷的方法之一。本文主要综述了Z-型、Ⅱ-型、p-n结以及S-型四类异质结的电荷传递机理以及重点介绍了一些具有优异光催化性能的BiOCl异质结。同时,对一些异质结的光催化降解性能进行了比较分析。其中,S型异质结不仅具有高效的电荷分离能力,还有强的氧化还原能力,因此其表现出优异的光催化性能。此外,本文还简述了BiOCl异质结在降解有机污染物、还原CO2、还原重金属和分解H2O等方面的应用。总结了当前BiOCl异质结遇到的一些问题。最后针对BiOCl异质结的构效关系、合成复杂等问题,提出了计算机模拟、载体负载、新技术开发的发展方向。
中图分类号:
引用本文
胥生元, 郝玮, 王杰, 高文生, 谢克锋. 半导体光催化剂BiOCl异质结的构建及应用[J]. 化工进展, 2023, 42(3): 1493-1507.
XU Shengyuan, HAO Wei, WANG Jie, GAO Wensheng, XIE Kefeng. Construction and application of BiOCl heterojunction as semiconductor photocatalyst[J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1493-1507.
| 异质结 | 制备方法 | 制备过程 | 氧化与还原反应能带与 最大吸收波长 | 文献 |
|---|---|---|---|---|
| CdS/BiOCl | 水热法 | 将CdS溶于水中,超声10min,然后在溶液中加入0.1mmol BNH①和KCl,之后把混合物转移至反应釜,在180℃下反应24h | E E | [ |
| CdS/CQDs②/BiOCl | 区域沉积法 | 将CdS量子点溶于水中,超声15min,同时,将0.5g CQDs/BiOCl分散到水中,然后将它们混合,搅拌,离心,洗涤,干燥,最后在250℃马弗炉中加热1h | E E | [ |
| Fe3O4/BiOCl/BiOI | 溶剂热法 | 将NaCl和NaI加入到BNH溶液中,再把0.28mmol Fe3O4粉末分散到混合液中,最后将混合液转移到反应釜中,并在160℃下反应12h | E E 628nm | [ |
| 2D③/2DCu2WS4/BiOCl | 混合溶剂法 | 将BiOCl和Cu2WS4溶液混合,超声3h,搅拌并使之均匀分散,然后,离心,洗涤,在60℃下干燥,即得到产物Cu2WS4/BiOCl | E E | [ |
| 2D/2DBiOCl/K+CNO④ | 水热法 | 将K+CNO纳米片、0.1mmol BNH和0.1mmol KCl通过搅拌和超声溶于水中,然后把混合物转移到反应釜中,在160℃加热24h | E E | [ |
| BiOI/BiOCl/NFs⑤ | 溶剂热法 | 将KCl、KI和BNH溶于EG⑥,然后加入PAN⑦纤维,之后把混合液全部转移至反应釜,并在160℃下反应10h | E E | [ |
| BiOI/BiOCl | 水热法 | 将[PrMIm]I⑧溶于乙酸,然后再加入0.02mol BNH并搅拌溶解,之后加入0.02mol KCl,搅拌1h。最后将悬浮液转移至反应釜,并在180℃下反应24h | E E 700nm | [ |
| OVs-Mo2S3/BiOCl | 原位模板法 | 将Mo2S3溶于EG中,然后加入5mL KCl乙二醇溶液(20g/L)和5mL BNH乙二醇溶液(48.5g/L)。将混合溶液放入微波反应器中,在160℃下反应15min | E E | [ |
| TiO2(B)⑨/BiOCl0.7I0.3-PVP | 水浴法 | 将0.267g TiO2(B)加入到溶有0.5g PVP⑩、0.007mol KCl、0.003mol KI和0.01mol BNH的水溶液中,搅拌0.5h,然后将pH调至9左右,待反应1h后,离心、洗涤和干燥 | E E | [ |
| Ag/BiOCl/AgIO3 | 光还原法 | 0.3g BiOCl溶于水中,搅拌,并用350W氙灯照射30min。然后加入0.03g AgNO3和一定量的NaIO3,再照射2.5h得到沉淀,洗涤,干燥后即得到Ag/BiOCl/AgIO3 | E E | [ |
| g-C3N4-x /BiOCl/WO2.92 | 溶剂热法 | WCl6溶于乙醇并搅拌。然后将0.3g g-C3N4-x 分散到乙醇溶液中,最后加入NaBiO3。将混合液搅拌后转移至反应釜中,在160℃下反应12h | [ | |
| Mn3O4/BiOCl | 水热法 | 4.85g BNH溶于硝酸溶液中,待分散均匀后,加入10mL、0.25mol/L MnCl2,然后用NaOH将pH调至12,最后将混合溶液转移至反应釜中,并在180℃下反应24h | E E | [ |
| 2D/2D BiOCl/g-C3N4 | 水热法 | 将0.486g BNH溶于25mL(0.1mol/L)甘露醇中,搅拌,并加入饱和NaCl。然后把g-C3N4均匀分散至混合液中,将混合溶液转移至反应釜,并在160℃下反应3h | E E | [ |
| OVs-BiOCl/BiPO4 | 溶剂热法 | 在0.005mol/L的H3PO4中加入0.13g BiOCl-OVs,搅拌1.5h,并超声10min,最后,收集沉淀,并干燥 | E E | [ |
| Cd/CdS/BiOCl | — | 将3mmol BNH溶于50mL含5mL饱和NaCl的乙醇中。同时,将3mmol柠檬酸、3mL HNO3和Cd/CdS溶于乙醇溶液中,最后将沉淀物质干燥 | [ |
表1 Z-型BiOCl异质结的构建总结
| 异质结 | 制备方法 | 制备过程 | 氧化与还原反应能带与 最大吸收波长 | 文献 |
|---|---|---|---|---|
| CdS/BiOCl | 水热法 | 将CdS溶于水中,超声10min,然后在溶液中加入0.1mmol BNH①和KCl,之后把混合物转移至反应釜,在180℃下反应24h | E E | [ |
| CdS/CQDs②/BiOCl | 区域沉积法 | 将CdS量子点溶于水中,超声15min,同时,将0.5g CQDs/BiOCl分散到水中,然后将它们混合,搅拌,离心,洗涤,干燥,最后在250℃马弗炉中加热1h | E E | [ |
| Fe3O4/BiOCl/BiOI | 溶剂热法 | 将NaCl和NaI加入到BNH溶液中,再把0.28mmol Fe3O4粉末分散到混合液中,最后将混合液转移到反应釜中,并在160℃下反应12h | E E 628nm | [ |
| 2D③/2DCu2WS4/BiOCl | 混合溶剂法 | 将BiOCl和Cu2WS4溶液混合,超声3h,搅拌并使之均匀分散,然后,离心,洗涤,在60℃下干燥,即得到产物Cu2WS4/BiOCl | E E | [ |
| 2D/2DBiOCl/K+CNO④ | 水热法 | 将K+CNO纳米片、0.1mmol BNH和0.1mmol KCl通过搅拌和超声溶于水中,然后把混合物转移到反应釜中,在160℃加热24h | E E | [ |
| BiOI/BiOCl/NFs⑤ | 溶剂热法 | 将KCl、KI和BNH溶于EG⑥,然后加入PAN⑦纤维,之后把混合液全部转移至反应釜,并在160℃下反应10h | E E | [ |
| BiOI/BiOCl | 水热法 | 将[PrMIm]I⑧溶于乙酸,然后再加入0.02mol BNH并搅拌溶解,之后加入0.02mol KCl,搅拌1h。最后将悬浮液转移至反应釜,并在180℃下反应24h | E E 700nm | [ |
| OVs-Mo2S3/BiOCl | 原位模板法 | 将Mo2S3溶于EG中,然后加入5mL KCl乙二醇溶液(20g/L)和5mL BNH乙二醇溶液(48.5g/L)。将混合溶液放入微波反应器中,在160℃下反应15min | E E | [ |
| TiO2(B)⑨/BiOCl0.7I0.3-PVP | 水浴法 | 将0.267g TiO2(B)加入到溶有0.5g PVP⑩、0.007mol KCl、0.003mol KI和0.01mol BNH的水溶液中,搅拌0.5h,然后将pH调至9左右,待反应1h后,离心、洗涤和干燥 | E E | [ |
| Ag/BiOCl/AgIO3 | 光还原法 | 0.3g BiOCl溶于水中,搅拌,并用350W氙灯照射30min。然后加入0.03g AgNO3和一定量的NaIO3,再照射2.5h得到沉淀,洗涤,干燥后即得到Ag/BiOCl/AgIO3 | E E | [ |
| g-C3N4-x /BiOCl/WO2.92 | 溶剂热法 | WCl6溶于乙醇并搅拌。然后将0.3g g-C3N4-x 分散到乙醇溶液中,最后加入NaBiO3。将混合液搅拌后转移至反应釜中,在160℃下反应12h | [ | |
| Mn3O4/BiOCl | 水热法 | 4.85g BNH溶于硝酸溶液中,待分散均匀后,加入10mL、0.25mol/L MnCl2,然后用NaOH将pH调至12,最后将混合溶液转移至反应釜中,并在180℃下反应24h | E E | [ |
| 2D/2D BiOCl/g-C3N4 | 水热法 | 将0.486g BNH溶于25mL(0.1mol/L)甘露醇中,搅拌,并加入饱和NaCl。然后把g-C3N4均匀分散至混合液中,将混合溶液转移至反应釜,并在160℃下反应3h | E E | [ |
| OVs-BiOCl/BiPO4 | 溶剂热法 | 在0.005mol/L的H3PO4中加入0.13g BiOCl-OVs,搅拌1.5h,并超声10min,最后,收集沉淀,并干燥 | E E | [ |
| Cd/CdS/BiOCl | — | 将3mmol BNH溶于50mL含5mL饱和NaCl的乙醇中。同时,将3mmol柠檬酸、3mL HNO3和Cd/CdS溶于乙醇溶液中,最后将沉淀物质干燥 | [ |
| 异质结 | 制备方法 | 制备过程 | 氧化与还原反应能带与 最大吸收波长 | 文献 |
|---|---|---|---|---|
| BiOCl/BOB① | 溶剂热法 | 将1.40g BNH均匀分散在EG②中,然后加入0.75g BOB,并超声。之后在溶液中加入0.17g NaCl,并超声1h。最后将混合物转移到反应釜,在150℃下反应12h | E E | [ |
| 1D-Bi2O2CO3/BiOCl | 水热法 | 将Bi2O2CO3多孔纳米棒均匀分散到CH2Cl2中,并搅拌均匀,然后将混合物转移至反应釜,并在160℃下反应12h | E E | [ |
| Ag/BiOCl/AgI | 光还原法 | Ag/BiOCl/AgIO3在光催化降解过程中AgIO3被还原为AgI,从而形成Ⅱ-型Ag/BiOCl/AgI | E E | [ |
| FeWO4/BiOCl | 溶剂热法 | 把1mmol BNH溶于EG,再加入1mmol KCl,并将其搅拌均匀,然后加入1mmol溶于EG的FeWO4。最后将混合物转移到反应釜中,在160℃下反应12h | E E 800nm | [ |
| 3D BiO7I/BiOCl | 原位共沉淀法 | 将200mg BiO7I分散在EG中,并超声20min,然后在溶液中加入0.248g BNH,并搅拌均匀,再加入等物质的量的KCl溶液,搅拌1h,最后将混合物转移至反应釜,并在160℃下反应12h | E E 482nm | [ |
| 2D/2D BiOCl/HTN7③ | 水热法 | 在HTN7悬浮液中加入0.2mmol BNH和0.2mmol KCl,连续搅拌。然后,将混合物转移到反应釜中,在160℃下加热24h | E E | [ |
| BiOCl/HTN5④ | 水热法 | 在HTN5悬浮液中加入0.1mmol BNH和0.1mmol KCl,并超声,然后,将混合物转移到反应釜中,在160℃下加热24h | E E | [ |
| BiOCl/Bi3NbO7 | 原位化学刻蚀法 | 将0.2g Bi3NbO7溶于5.5mL乙醇中,然后缓慢加入0.5mL HCl,待反应结束后,收集沉淀,并将其洗涤,干燥 | E E 500nm | [ |
| BiOCl/BTO⑤ | 原位水热法 | 将BiOCl NFs、BNH和C16H36O4Ti超声均匀分散在水中,然后转移至反应釜中,在160℃下反应12h | EθCB=-0.35eV; E | [ |
| BiOI/BiOCl | 无模板共沉淀法 | 将5mmol BNH溶于水中,搅拌均匀,然后加入KCl和KI,搅拌5h,洗涤,离心,干燥,即得到BiOI/BiOCl | [ | |
| Nb2O5/BiOCl | 水热法 | 将0.5g BiOCl分散于水中,然后加入Nb2O5,搅拌24h,离心,干燥,研磨,即制得Nb2O5/BiOCl样品 | E E | [ |
| BiOCl/In2S3 | 研磨法 | 将BiOCl和In2S3混合并置于研钵中,快速研磨20min,得到复合光催化剂BiOCl/In2S3 | E E 900nm | [ |
| g-C3N4/BiOCl | 水热法 | 将BNH和KCl溶于EG中,然后在溶液中加入g-C3N4,并超声30min,再将含0.1g Na2CO3的溶液加入EG中,最后将混合物转移至反应釜中,并在130℃下反应12h | E E 800nm | [ |
表2 Ⅱ-型BiOCl异质结的构建总结
| 异质结 | 制备方法 | 制备过程 | 氧化与还原反应能带与 最大吸收波长 | 文献 |
|---|---|---|---|---|
| BiOCl/BOB① | 溶剂热法 | 将1.40g BNH均匀分散在EG②中,然后加入0.75g BOB,并超声。之后在溶液中加入0.17g NaCl,并超声1h。最后将混合物转移到反应釜,在150℃下反应12h | E E | [ |
| 1D-Bi2O2CO3/BiOCl | 水热法 | 将Bi2O2CO3多孔纳米棒均匀分散到CH2Cl2中,并搅拌均匀,然后将混合物转移至反应釜,并在160℃下反应12h | E E | [ |
| Ag/BiOCl/AgI | 光还原法 | Ag/BiOCl/AgIO3在光催化降解过程中AgIO3被还原为AgI,从而形成Ⅱ-型Ag/BiOCl/AgI | E E | [ |
| FeWO4/BiOCl | 溶剂热法 | 把1mmol BNH溶于EG,再加入1mmol KCl,并将其搅拌均匀,然后加入1mmol溶于EG的FeWO4。最后将混合物转移到反应釜中,在160℃下反应12h | E E 800nm | [ |
| 3D BiO7I/BiOCl | 原位共沉淀法 | 将200mg BiO7I分散在EG中,并超声20min,然后在溶液中加入0.248g BNH,并搅拌均匀,再加入等物质的量的KCl溶液,搅拌1h,最后将混合物转移至反应釜,并在160℃下反应12h | E E 482nm | [ |
| 2D/2D BiOCl/HTN7③ | 水热法 | 在HTN7悬浮液中加入0.2mmol BNH和0.2mmol KCl,连续搅拌。然后,将混合物转移到反应釜中,在160℃下加热24h | E E | [ |
| BiOCl/HTN5④ | 水热法 | 在HTN5悬浮液中加入0.1mmol BNH和0.1mmol KCl,并超声,然后,将混合物转移到反应釜中,在160℃下加热24h | E E | [ |
| BiOCl/Bi3NbO7 | 原位化学刻蚀法 | 将0.2g Bi3NbO7溶于5.5mL乙醇中,然后缓慢加入0.5mL HCl,待反应结束后,收集沉淀,并将其洗涤,干燥 | E E 500nm | [ |
| BiOCl/BTO⑤ | 原位水热法 | 将BiOCl NFs、BNH和C16H36O4Ti超声均匀分散在水中,然后转移至反应釜中,在160℃下反应12h | EθCB=-0.35eV; E | [ |
| BiOI/BiOCl | 无模板共沉淀法 | 将5mmol BNH溶于水中,搅拌均匀,然后加入KCl和KI,搅拌5h,洗涤,离心,干燥,即得到BiOI/BiOCl | [ | |
| Nb2O5/BiOCl | 水热法 | 将0.5g BiOCl分散于水中,然后加入Nb2O5,搅拌24h,离心,干燥,研磨,即制得Nb2O5/BiOCl样品 | E E | [ |
| BiOCl/In2S3 | 研磨法 | 将BiOCl和In2S3混合并置于研钵中,快速研磨20min,得到复合光催化剂BiOCl/In2S3 | E E 900nm | [ |
| g-C3N4/BiOCl | 水热法 | 将BNH和KCl溶于EG中,然后在溶液中加入g-C3N4,并超声30min,再将含0.1g Na2CO3的溶液加入EG中,最后将混合物转移至反应釜中,并在130℃下反应12h | E E 800nm | [ |
| 异质结 | 制备方法 | 制备过程 | 氧化与还原反应能带与 最大吸收波长 | 文献 |
|---|---|---|---|---|
| 0D/2D CuO/BiOCl | 原位生长法 | BiOCl纳米片和0.05g Cu(CH3COO)2·5H2O加入到含50mL DMF①的锥形瓶中,并搅拌,然后在90℃水浴中,搅拌4h,并离心 | E E | [ |
| OVs-BiOCl/TiO2-δ | 一锅沉积法 | 将NaCl/TiO2-δ 和BNH溶于pH=10的NaOH溶液中,然后搅拌24h,即得到OVs-BiOCl/TiO2-δ | E E | [ |
| 3D/2D BiVO4/BiOCl | 原位沉积法 | 将BiVO4分散在水中,再将含0.1mmol BNH的EG溶液转移至BiVO4悬浮液中,并滴加盐酸,搅拌5h,离心,洗涤,干燥 | [ | |
| CuBi2O4/BiOCl | 一锅水热法 | 将BNH、Cu(NO3)3·3H2O和NaOH溶于水中,然后将BNH和KCl混合后,加入水溶液中,并搅拌,最后将混合物转移反应釜中,在180℃下反应24h | E E | [ |
| {110}BiOCl/ZnO | 溶剂热法 | 分别将6mmol六亚甲基四胺和Zn(CH3COO)2·3H2O溶于水,然后混合均匀,再加入BiOCl,并搅匀,最后将混合物转移至反应釜,并在90℃下反应6h | [ | |
| SCIs②-BiOCl/ZnO | 一锅水热法 | 将ZnCl2、Na2SnO3·3H2O和BNH分别溶于水中,然后混合并搅拌30min,再将混合物转移至反应釜,并在200℃下反应12h | E E | [ |
| BiOCl/ZnO | 水热法 | 在含3mmol Zn(NO3)3·6H2O的溶液中加入NaOH,生成白色沉淀后,加入KCl,并搅拌,然后加入BNH,最后将混合溶液转移至反应釜中,在100℃下反应2h | [ | |
| OVs-BiOCl/g-C3N4 | 微波辅助法 | 分别将g-C3N4、KCl和BNH溶于EG中,然后混合搅拌3min,微波加热至160℃下反应15min | E E 473nm | [ |
| α-Fe2O3/BiOCl | — | 在CH3COOH中加入0.182g BNH,再加入α-Fe2O3,搅拌30min,然后加入KCl溶液,搅拌,陈化3h,过滤,洗涤,干燥 | [ | |
| FeVO4@BiOCl | 水热法 | 将1mmol BNH和1mmol KCl溶于水中,连续搅拌,然后将FeVO4加入溶液中,最后将悬浮液转移至反应釜中,在160℃下反应18h | 650nm | [ |
| BiPO4/BiOCl | 原位化学转化法 | 2.6g BiOCl溶于乙醇中,超声15min,然后加入H3PO4,并搅拌均匀,离心即得到BiPO4/BiOCl | [ | |
| BiOCl@WO3 | 水热法 | 分别将WO3和BNH分散在乙醇中,搅拌均匀后混合,然后加入KCl,搅拌,最后将混合物转移至反应釜中,在160℃下反应20h | E E | [ |
| BiOCl/TiO2/CNFs③ | 溶剂热法 | 将5mmol Bi(NO3)3和KCl分别溶于EG中,再将它们溶于乙醇中,并搅拌1h,然后将0.015g TiO2/CNFs加入混合溶液中浸泡,最后经溶剂热处理,洗涤、过滤、干燥得到复合材料 | E E | [ |
| 0D/2D CsPbBr3/BiOCl | — | 100mg BiOCl超声分散于乙酸乙酯中,然后加入15mL CsPbBr3溶液,超声30min,并搅拌30min,干燥,得到CsPbBr3/BiOCl | E E | [ |
表3 p-n BiOCl异质结的构建总结
| 异质结 | 制备方法 | 制备过程 | 氧化与还原反应能带与 最大吸收波长 | 文献 |
|---|---|---|---|---|
| 0D/2D CuO/BiOCl | 原位生长法 | BiOCl纳米片和0.05g Cu(CH3COO)2·5H2O加入到含50mL DMF①的锥形瓶中,并搅拌,然后在90℃水浴中,搅拌4h,并离心 | E E | [ |
| OVs-BiOCl/TiO2-δ | 一锅沉积法 | 将NaCl/TiO2-δ 和BNH溶于pH=10的NaOH溶液中,然后搅拌24h,即得到OVs-BiOCl/TiO2-δ | E E | [ |
| 3D/2D BiVO4/BiOCl | 原位沉积法 | 将BiVO4分散在水中,再将含0.1mmol BNH的EG溶液转移至BiVO4悬浮液中,并滴加盐酸,搅拌5h,离心,洗涤,干燥 | [ | |
| CuBi2O4/BiOCl | 一锅水热法 | 将BNH、Cu(NO3)3·3H2O和NaOH溶于水中,然后将BNH和KCl混合后,加入水溶液中,并搅拌,最后将混合物转移反应釜中,在180℃下反应24h | E E | [ |
| {110}BiOCl/ZnO | 溶剂热法 | 分别将6mmol六亚甲基四胺和Zn(CH3COO)2·3H2O溶于水,然后混合均匀,再加入BiOCl,并搅匀,最后将混合物转移至反应釜,并在90℃下反应6h | [ | |
| SCIs②-BiOCl/ZnO | 一锅水热法 | 将ZnCl2、Na2SnO3·3H2O和BNH分别溶于水中,然后混合并搅拌30min,再将混合物转移至反应釜,并在200℃下反应12h | E E | [ |
| BiOCl/ZnO | 水热法 | 在含3mmol Zn(NO3)3·6H2O的溶液中加入NaOH,生成白色沉淀后,加入KCl,并搅拌,然后加入BNH,最后将混合溶液转移至反应釜中,在100℃下反应2h | [ | |
| OVs-BiOCl/g-C3N4 | 微波辅助法 | 分别将g-C3N4、KCl和BNH溶于EG中,然后混合搅拌3min,微波加热至160℃下反应15min | E E 473nm | [ |
| α-Fe2O3/BiOCl | — | 在CH3COOH中加入0.182g BNH,再加入α-Fe2O3,搅拌30min,然后加入KCl溶液,搅拌,陈化3h,过滤,洗涤,干燥 | [ | |
| FeVO4@BiOCl | 水热法 | 将1mmol BNH和1mmol KCl溶于水中,连续搅拌,然后将FeVO4加入溶液中,最后将悬浮液转移至反应釜中,在160℃下反应18h | 650nm | [ |
| BiPO4/BiOCl | 原位化学转化法 | 2.6g BiOCl溶于乙醇中,超声15min,然后加入H3PO4,并搅拌均匀,离心即得到BiPO4/BiOCl | [ | |
| BiOCl@WO3 | 水热法 | 分别将WO3和BNH分散在乙醇中,搅拌均匀后混合,然后加入KCl,搅拌,最后将混合物转移至反应釜中,在160℃下反应20h | E E | [ |
| BiOCl/TiO2/CNFs③ | 溶剂热法 | 将5mmol Bi(NO3)3和KCl分别溶于EG中,再将它们溶于乙醇中,并搅拌1h,然后将0.015g TiO2/CNFs加入混合溶液中浸泡,最后经溶剂热处理,洗涤、过滤、干燥得到复合材料 | E E | [ |
| 0D/2D CsPbBr3/BiOCl | — | 100mg BiOCl超声分散于乙酸乙酯中,然后加入15mL CsPbBr3溶液,超声30min,并搅拌30min,干燥,得到CsPbBr3/BiOCl | E E | [ |
| 异质结 | 制备方法 | 制备过程 | 氧化与还原反应能带与 最大吸收波长 | 文献 |
|---|---|---|---|---|
| 0D/3D Ta3N5/BiOCl | 溶剂热法 | 将BNH溶于EG中,超声后再加入Ta3N5,并超声1h,然后加入KCl,并搅拌1h,最后将混合物转移至反应釜,并在160℃下反应12h | E E 800nm | [ |
| In2O3/BiOCl | 熔盐法 | 将KNO3和LiNO3充分研磨,然后在KNO3-LiNO3中加入KCl、BNH和In2O3,研磨1h。随后将混合物放入坩埚中,在350℃的马弗炉中烧3h,冷却后,洗涤,最后在90℃下干燥8h | E E | [ |
| CdS/{001}BiOCl | 化学沉积法 | 将Cd(CH3COO)2·2H2O溶于水中,再将200mg(001)BiOCl均匀分散于溶液中,然后加入硫脲,并搅拌30min,最后在90℃下加热2.5h | E E | [ |
| BBOC/MIS① | 原位水热法 | 将Bi-BiOCl分散在乙醇和水的混合溶液中,超声,搅拌,然后加入MgCl2·6H2O、InCl3·4H2O和TAA②,并搅拌30min,最后将混合物转移到反应釜,并在180℃下反应24h | E E 697nm | [ |
| BOCl/CBO | 溶剂热法 | 将BNH溶于EG中,再加入0.2g CBO,混合搅拌,然后加入KCl,搅拌均匀后,将混合物转移至反应釜,在160℃下反应12h | E E | [ |
| 0D/2D Cu2O/BiOCl | 水热法 | 将BiOCl粉末分散在含5mmol CuCl2的水中,然后加入0.5mol/L NaOH溶液,并搅拌,再加入0.1mol/L维生素C溶液,待反应30min后,洗涤,干燥,即得到Cu2O/BiOCl | E E | [ |
| 2D/2D CIS③/BiOCl | 水热法 | 将BiOCl粉末分散在水中,再加入0.0076g TAA,并超声,然后加入In(NO3)3和Ca(NO3)2,并搅拌1h,最后将悬浮液转移至反应釜,并在160℃下反应12h | E E | [ |
| BiOCl/CdS | 水热法 | 将100mg BiOCl分散在水中,搅拌30min,然后加入NH2CSNH2和Cd(COO)2·6H2O,搅匀后转移至反应釜,并在120℃下反应4h | E E | [ |
| BiOCl/MoSe2 | 溶剂热法 | 将BiOCl、Na2MoO4·2H2O、硒粉和NaBH4加入体积比为1∶1的乙醇和水中,搅拌1h,然后将混合物转移至反应釜,并在180℃下反应12h | E E | [ |
表4 S-型BiOCl异质结的构建总结
| 异质结 | 制备方法 | 制备过程 | 氧化与还原反应能带与 最大吸收波长 | 文献 |
|---|---|---|---|---|
| 0D/3D Ta3N5/BiOCl | 溶剂热法 | 将BNH溶于EG中,超声后再加入Ta3N5,并超声1h,然后加入KCl,并搅拌1h,最后将混合物转移至反应釜,并在160℃下反应12h | E E 800nm | [ |
| In2O3/BiOCl | 熔盐法 | 将KNO3和LiNO3充分研磨,然后在KNO3-LiNO3中加入KCl、BNH和In2O3,研磨1h。随后将混合物放入坩埚中,在350℃的马弗炉中烧3h,冷却后,洗涤,最后在90℃下干燥8h | E E | [ |
| CdS/{001}BiOCl | 化学沉积法 | 将Cd(CH3COO)2·2H2O溶于水中,再将200mg(001)BiOCl均匀分散于溶液中,然后加入硫脲,并搅拌30min,最后在90℃下加热2.5h | E E | [ |
| BBOC/MIS① | 原位水热法 | 将Bi-BiOCl分散在乙醇和水的混合溶液中,超声,搅拌,然后加入MgCl2·6H2O、InCl3·4H2O和TAA②,并搅拌30min,最后将混合物转移到反应釜,并在180℃下反应24h | E E 697nm | [ |
| BOCl/CBO | 溶剂热法 | 将BNH溶于EG中,再加入0.2g CBO,混合搅拌,然后加入KCl,搅拌均匀后,将混合物转移至反应釜,在160℃下反应12h | E E | [ |
| 0D/2D Cu2O/BiOCl | 水热法 | 将BiOCl粉末分散在含5mmol CuCl2的水中,然后加入0.5mol/L NaOH溶液,并搅拌,再加入0.1mol/L维生素C溶液,待反应30min后,洗涤,干燥,即得到Cu2O/BiOCl | E E | [ |
| 2D/2D CIS③/BiOCl | 水热法 | 将BiOCl粉末分散在水中,再加入0.0076g TAA,并超声,然后加入In(NO3)3和Ca(NO3)2,并搅拌1h,最后将悬浮液转移至反应釜,并在160℃下反应12h | E E | [ |
| BiOCl/CdS | 水热法 | 将100mg BiOCl分散在水中,搅拌30min,然后加入NH2CSNH2和Cd(COO)2·6H2O,搅匀后转移至反应釜,并在120℃下反应4h | E E | [ |
| BiOCl/MoSe2 | 溶剂热法 | 将BiOCl、Na2MoO4·2H2O、硒粉和NaBH4加入体积比为1∶1的乙醇和水中,搅拌1h,然后将混合物转移至反应釜,并在180℃下反应12h | E E | [ |
| 1 | MOU Zhigang, ZHANG Hui, LIU Zeman, et al. Ultrathin BiOCl/nitrogen-doped graphene quantum dots composites with strong adsorption and effective photocatalytic activity for the degradation of antibiotic ciprofloxacin[J]. Applied Surface Science, 2019, 496: 143655. |
| 2 | NAMDARIAN Abdolhamid, GOLJANIAN TABRIZI Amin, ARSALANI Nasser, et al. Synthesis of PANi nanoarrays anchored on 2D BiOCl nanoplates for photodegradation of Congo Red in visible light region[J]. Journal of Industrial and Engineering Chemistry, 2020, 81: 228-236. |
| 3 | WU Shanshan, SU Yumei, ZHU Yi, et al. In-situ growing Bi/BiOCl microspheres on Ti3C2 nanosheets for upgrading visible-light-driven photocatalytic activity[J]. Applied Surface Science, 2020, 520: 146339. |
| 4 | LI Yilei, LIU Ying, MU Huiying, et al. The simultaneous adsorption, activation and in situ reduction of carbon dioxide over Au-loading BiOCl with rich oxygen vacancies[J]. Nanoscale, 2021, 13(4): 2585-2592. |
| 5 | SUN Liming, XIANG Li, ZHAO Xian, et al. Enhanced visible-light photocatalytic activity of BiOI/BiOCl heterojunctions: Key role of crystal facet combination[J]. ACS Catalysis, 2015, 5(6): 3540-3551. |
| 6 | TANWAR Ruchika, KAUR Bikramjit, KUMAR MANDAL Uttam. Highly efficient and visible light driven Ni0.5Zn0.5Fe2O4@PANI modified BiOCl heterocomposite catalyst for water remediation[J]. Applied Catalysis B: Environmental, 2017, 211: 305-322. |
| 7 | YAO Ling, YANG Hui, CHEN Zhongshan, et al. Bismuth oxychloride-based materials for the removal of organic pollutants in wastewater[J]. Chemosphere, 2021, 273: 128576. |
| 8 | LI Rongjie, LUAN Qingjie, DONG Cheng, et al. Light-facilitated structure reconstruction on self-optimized photocatalyst TiO2@BiOCl for selectively efficient conversion of CO2 to CH4 [J]. Applied Catalysis B: Environmental, 2021, 286: 119832. |
| 9 | ZHANG Xianlong, YUAN Long, LIANG Fengbing, et al. Water-assisted synthesis of shape-specific BiOCl nanoflowers with enhanced adsorption and photosensitized degradation of rhodamine B[J]. Environmental Chemistry Letters, 2020, 18(1): 243-249. |
| 10 | LAI Yen Ju, LEE Duu Jong. Pollutant degradation with mediator Z-scheme heterojunction photocatalyst in water: A review[J]. Chemosphere, 2021, 282: 131059. |
| 11 | WANG Huanli, ZHANG Lisha, CHEN Zhigang, et al. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances[J]. Chemical Society Reviews, 2014, 43(15): 5234-5244. |
| 12 | 龙泽清, 宋慧, 张光明. 卤氧化铋光催化剂改性及应用研究进展[J]. 材料导报, 2021, 35(5): 5067-5074. |
| LONG Zeqing, SONG Hui, ZHANG Guangming. Research progress in modification and application of bismuth oxyhalide photocatalyst[J]. Materials Reports, 2021, 35(5): 5067-5074. | |
| 13 | LIU Juan, LI Yeping, HUANG Liying, et al. Fabrication of novel narrow/wide band gap Bi4O5I2/BiOCl heterojunction with high antibacterial and degradation efficiency under LED and sunlight[J]. Applied Surface Science, 2021, 567: 150713. |
| 14 | ZHANG Li, LI Yuhan, LI Qin, et al. Recent advances on Bismuth-based Photocatalysts: Strategies and mechanisms[J]. Chemical Engineering Journal, 2021, 419: 129484. |
| 15 | CAO Jinyan, LI Jianjun, CHU Wei, et al. Facile synthesis of Mn-doped BiOCl for metronidazole photodegradation: Optimization, degradation pathway, and mechanism[J]. Chemical Engineering Journal, 2020, 400: 125813. |
| 16 | LI Zhao, MA Baojin, ZHANG Xiaofei, et al. One-pot synthesis of BiOCl nanosheets with dual functional carbon for ultra-highly efficient photocatalytic degradation of RhB[J]. Environmental Research, 2020, 182: 109077. |
| 17 | XU Min, YANG Jingkai, SUN Chaoyang, et al. Performance enhancement strategies of Bi-based photocatalysts: A review on recent progress[J]. Chemical Engineering Journal, 2020, 389: 124402. |
| 18 | LIU Wenwen, SHANG Yanyang, ZHU Anquan, et al. Enhanced performance of doped BiOCl nanoplates for photocatalysis: Understanding from doping insight into improved spatial carrier separation[J]. Journal of Materials Chemistry A, 2017, 5(24): 12542-12549. |
| 19 | XU Jingjing, FENG Bingbing, WANG Ying, et al. BiOCl decorated NaNbO3 nanocubes: A novel p-n heterojunction photocatalyst with improved activity for ofloxacin degradation[J]. Frontiers in Chemistry, 2018, 6: 393. |
| 20 | WANG Li, ZHAO Xue, Dongdong LYU, et al. Promoted photocharge separation in 2D lateral epitaxial heterostructure for visible-light-driven CO2 photoreduction[J]. Advanced Materials, 2020, 32(48): e2004311. |
| 21 | ZHA Zhenxing, LAI Jiahao, LI Ying, et al. The degradation of tetracycline by modified BiOCl nanosheets with carbon dots from the chlorella[J]. Journal of Alloys and Compounds, 2021, 855: 157454. |
| 22 | ZHANG Dan, TAN Guoqiang, WANG Min, et al. The enhanced photocatalytic activity of Ag-OVs-(001) BiOCl by separating secondary excitons under double SPR effects[J]. Applied Surface Science, 2020, 526: 146689. |
| 23 | CHEN Haijun, PENG Liming, BIAN Yucui, et al. Exerting charge transfer to stabilize Au nanoclusters for enhanced photocatalytic performance toward selective oxidation of amines[J]. Applied Catalysis B: Environmental, 2021, 284: 119704. |
| 24 | SIAO Ciao-Wei, LEE Wen-Lian William, DAI Yongming, et al. BiO x Cl y /BiO m Br n /BiO p I q /GO quaternary composites: Syntheses and application of visible-light-driven photocatalytic activities[J]. Journal of Colloid and Interface Science, 2019, 544: 25-36. |
| 25 | WANG Zhongliao, CHENG Bei, ZHANG Liuyang, et al. BiOBr/NiO S-scheme heterojunction photocatalyst for CO2 photoreduction[J]. Solar RRL, 2022, 6(1): 2100587. |
| 26 | WANG Hong, ZHANG Wendong, LI Xinwei, et al. Highly enhanced visible light photocatalysis and in situ FTIR studies on Bi metal@defective BiOCl hierarchical microspheres[J]. Applied Catalysis B: Environmental, 2018, 225: 218-227. |
| 27 | ZHOU Yunlei, YIN Huanshun, AI Shiyun. Applications of two-dimensional layered nanomaterials in photoelectrochemical sensors: A comprehensive review[J]. Coordination Chemistry Reviews, 2021, 447: 214156. |
| 28 | LIN Enzhu, HUANG Rui, WU Jiang, et al. Recyclable CoFe2O4 modified BiOCl hierarchical microspheres utilizing photo, photothermal and mechanical energy for organic pollutant degradation[J]. Nano Energy, 2021, 89: 106403. |
| 29 | SIVAKUMAR Rajamanickam, LEE Nae Yoon. Emerging bismuth-based direct Z-scheme photocatalyst for the degradation of organic dye and antibiotic residues[J]. Chemosphere, 2022, 297: 134227. |
| 30 | CAO Jinyan, CEN Wanglai, JING Yue, et al. P-doped BiOCl for visible light photodegradation of tetracycline: An insight from experiment and calculation[J]. Chemical Engineering Journal, 2022, 435: 134683. |
| 31 | WANG Chuya, ZHANG Yingjie, WANG Weikang, et al. Enhanced photocatalytic degradation of bisphenol A by Co-doped BiOCl nanosheets under visible light irradiation[J]. Applied Catalysis B: Environmental, 2018, 221: 320-328. |
| 32 | WU Haoyuan, LIU Xiangming, XU Hua, et al. Efficient photodegradation of 2-chloro-4-nitrophenol over Fe-doped BiOCl nanosheets with oxygen vacancy[J]. Catalysis Science & Technology, 2021, 11(15): 5119-5124. |
| 33 | CAI Yujie, LI Dongya, SUN Jingyu, et al. Synthesis of BiOCl nanosheets with oxygen vacancies for the improved photocatalytic properties[J]. Applied Surface Science, 2018, 439: 697-704. |
| 34 | LI Hao, LI Jie, AI Zhihui, et al. Oxygen vacancy-mediated photocatalysis of BiOCl: Reactivity, selectivity, and perspectives[J]. Angewandte Chemie International Edition, 2018, 57(1): 122-138. |
| 35 | NIU Siying, ZHANG Ruoyu, GUO Chongfeng. Oxygen vacancy induced superior visible-light-driven photo-catalytic performance in the BiOCl homojunction[J]. Materials Chemistry Frontiers, 2020, 4(8): 2314-2324. |
| 36 | YANG Cai, HE Yuanxiang, ZHONG Junbo, et al. Photocatalytic performance of rich OVs-BiOCl modified by polyphenylene sulfide[J]. Advanced Powder Technology, 2022, 33(1): 103427. |
| 37 | TIAN Cuihua, LUO Sha, SHE Jiarong, et al. Cellulose nanofibrils enable flower-like BiOCl for high-performance photocatalysis under visible-light irradiation[J]. Applied Surface Science, 2019, 464: 606-615. |
| 38 | ZHANG Jinfeng, Jiali LYU, DAI Kai, et al. One-step growth of nanosheet-assembled BiOCl/BiOBr microspheres for highly efficient visible photocatalytic performance[J]. Applied Surface Science, 2018, 430: 639-646. |
| 39 | LI Hao, ZHANG Lizhi. Photocatalytic performance of different exposed crystal facets of BiOCl[J]. Current Opinion in Green and Sustainable Chemistry, 2017, 6: 48-56. |
| 40 | SHAHID M Z, YU L, MEHMOOD R. Tailored fabrication of triple-surface-features in well-crystalline BiOCl photocatalyst and their synergistic role in catalytic processes[J]. Catalysis Science & Technology, 2020, 10(7): 2242-2253. |
| 41 | ZHANG Zhihao, ZADA Amir, CUI Nan, et al. Synthesis of Ag loaded ZnO/BiOCl with high photocatalytic performance for the removal of antibiotic pollutants[J]. Crystals, 2021, 11(8): 981. |
| 42 | 全凤娇, 石彦彪, 孙红卫, 等. 卤氧化铋光催化去除环境污染物[J]. 华中师范大学学报(自然科学版), 2021, 55(6): 925-940, 975. |
| QUAN Fengjiao, SHI Yanbiao, SUN Hongwei, et al. Photocatalytic removal of environmental pollutants by bismuth oxyhalide[J]. Journal of Central China Normal University (Natural Sciences), 2021, 55(6): 925-940, 975. | |
| 43 | YANG Xiaoli, SUN Shaodong, CUI Jie, et al. One-pot construction of robust BiOCl/ZnO p–n heterojunctions with semi-coherent interfaces toward improving charge separation for photodegradation enhancement[J]. Nanoscale Advances, 2021, 3(16): 4851-4857. |
| 44 | SUN Xu, SHI Liang, BAI Qiang, et al. Synthesis of BiOCl/Bi3NbO7 heterojunction by in situ chemical etching with enhanced photocatalytic performance for the degradation of organic pollutants[J]. Applied Surface Science, 2022, 587: 152633. |
| 45 | XU Quanlong, ZHANG Liuyang, CHENG Bei, et al. S-scheme heterojunction photocatalyst[J]. Chem, 2020, 6(7): 1543-1559. |
| 46 | ZHANG Dan, TAN Guoqiang, WANG Min, et al. The formation of direct Z-scheme Ag/BiOCl/AgIO3 heterojunction and its degradation stability[J]. Applied Surface Science, 2020, 530: 147228. |
| 47 | TANG Xiaolong, LIU Huanhuan, YANG Cai, et al. In-situ fabrication of Z-scheme CdS/BiOCl heterojunctions with largely improved photocatalytic performance[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2020, 599: 124880. |
| 48 | XIA Huan, QIN Hailan, ZHANG Yushan, et al. Modulate 1O2 by passivate oxygen vacancy to boosting the photocatalytic performance of Z-scheme Mo2S3/BiOCl heterostructure[J]. Separation and Purification Technology, 2021, 266: 118547. |
| 49 | XU Quanlong, ZHANG Liuyang, YU Jiaguo, et al. Direct Z-scheme photocatalysts: Principles, synthesis, and applications[J]. Materials Today, 2018, 21(10): 1042-1063. |
| 50 | ABDUL NASIR Jamal, MUNIR Akhtar, AHMAD Naveed, et al. Photocatalytic Z-scheme overall water splitting: Recent advances in theory and experiments[J]. Advanced Materials, 2021, 33(52): e2105195. |
| 51 | Jingxiang LOW, YU Jiaguo, JARONIEC Mietek, et al. Heterojunction photocatalysts[J]. Advanced Materials, 2017, 29(20): 1601694. |
| 52 | XU Weiwei, TIAN Wei, MENG Linxing, et al. Interfacial chemical bond-modulated Z-scheme charge transfer for efficient photoelectrochemical water splitting[J]. Advanced Energy Materials, 2021, 11(8): 2003500. |
| 53 | ZHANG Liuyang, ZHANG Jianjun, YU Huogen, et al. Emerging S-scheme photocatalyst[J]. Advanced Materials, 2022, 34(11): 2107668. |
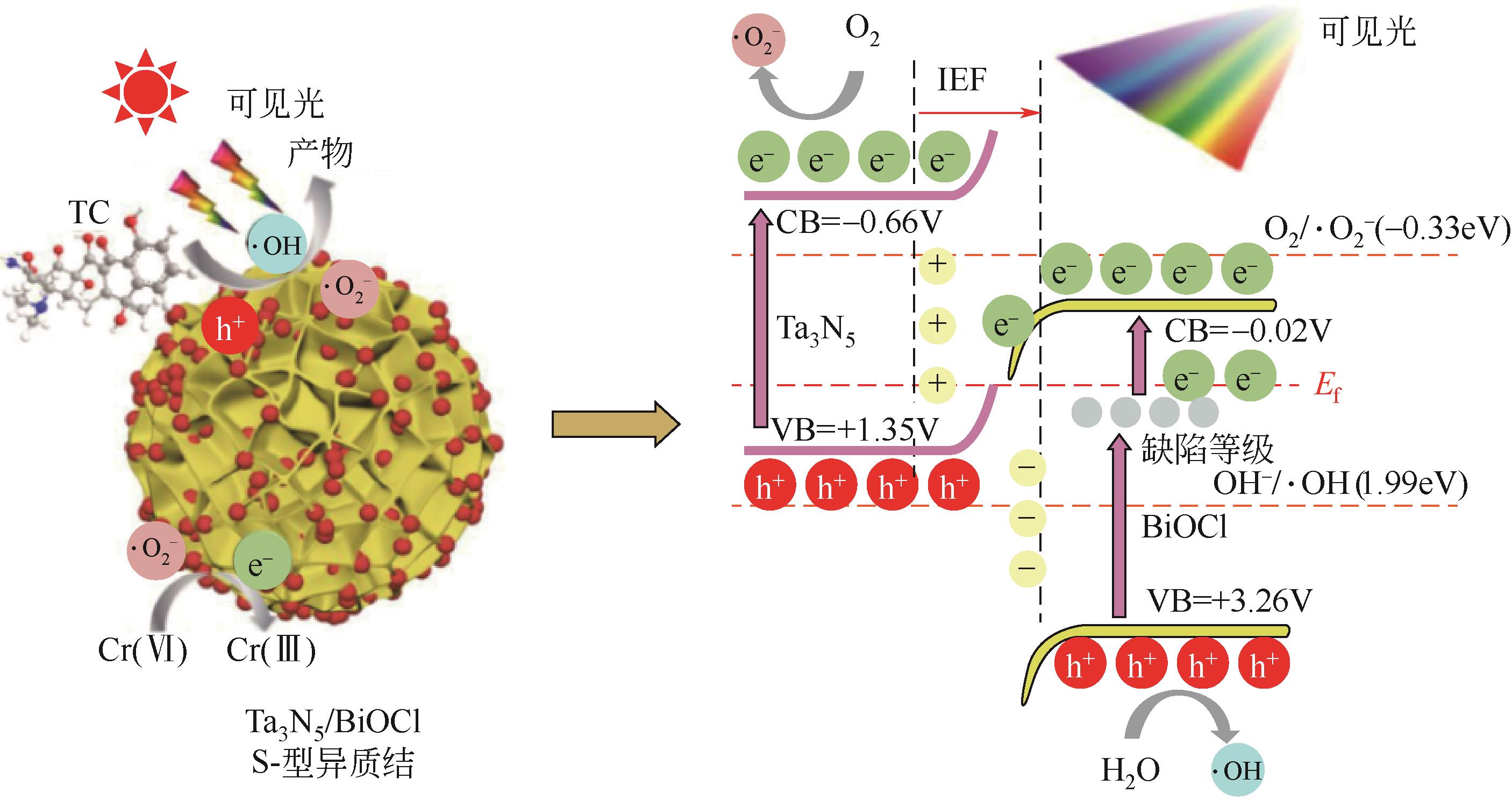
| 54 | LI Shijie, CAI Mingjie, WANG Chunchun, et al. Rationally designed Ta3N5/BiOCl S-scheme heterojunction with oxygen vacancies for elimination of tetracycline antibiotic and Cr(Ⅵ): Performance, toxicity evaluation and mechanism insight[J]. Journal of Materials Science & Technology, 2022, 123: 177-190. |
| 55 | FRISENDA Riccardo, MOLINA-MENDOZA Aday J, MUELLER Thomas, et al. Atomically thin p-n junctions based on two-dimensional materials[J]. Chemical Society Reviews, 2018, 47(9): 3339-3358. |
| 56 | QIU Juan, WANG Yingdi, LIU Xiang. One-pot hydrothermal synthesis of CuBi2O4/BiOCl p-n heterojunction with enhanced photocatalytic performance for the degradation of tetracycline hydrochloride under visible light irradiation[J]. New Journal of Chemistry, 2022, 46(6): 2898-2907. |
| 57 | XU Q L, WAGEH S, AL-GHAMDI A A, et al. Design principle of S-scheme heterojunction photocatalyst[J]. Journal of Materials Science & Technology, 2022, 124: 171-173. |
| 58 | BAO Yujie, SONG Shaoqing, YAO Guojian, et al. S-scheme photocatalytic systems[J]. Solar RRL, 2021, 5(7): 2100118. |
| 59 | GONG Shuaiqi, TENG Xue, NIU Yanli, et al. Construction of S-scheme 0D/2D heterostructures for enhanced visible-light-driven CO2 reduction[J]. Applied Catalysis B: Environmental, 2021, 298: 120521. |
| 60 | HASIJA V, KUMAR A, SUDHAIK A, et al. Step-scheme heterojunction photocatalysts for solar energy, water splitting, CO2 conversion, and bacterial inactivation: A review[J]. Environmental Chemistry Letters, 2021, 19: 2941-2966. |
| 61 | AI Changzhi, LI Jin, YANG Liang, et al. Transforming photocatalytic g-C3N4/MoSe2 into a direct Z-scheme system via boron-doping: A hybrid DFT study[J]. ChemSusChem, 2020, 13(18): 4985-4993. |
| 62 | HU Qingsong, JI Mengxia, DI Jun, et al. Ionic liquid-induced double regulation of carbon quantum dots modified bismuth oxychloride/bismuth oxybromide nanosheets with enhanced visible-light photocatalytic activity[J]. Journal of Colloid and Interface Science, 2018, 519: 263-272. |
| 63 | QI Shuyan, LIU Xueting, MA Ninglong, et al. Construction and photocatalytic properties of WS2/MoS2/BiOCl heterojunction[J]. Chemical Physics Letters, 2021, 763: 138203. |
| 64 | XU Chunping, RAVI ANUSUYADEVI Prasaanth, AYMONIER Cyril, et al. Nanostructured materials for photocatalysis[J]. Chemical Society Reviews, 2019, 48(14): 3868-3902. |
| 65 | YUAN Ye, GUO Ruitang, HONG Longfei, et al. A review of metal oxide-based Z-scheme heterojunction photocatalysts: Actualities and developments[J]. Materials Today Energy, 2021, 21: 100829. |
| 66 | PAN Jinbo, LIU Jianjun, ZUO Shengli, et al. Structure of Z-scheme CdS/CQDs/BiOCl heterojunction with enhanced photocatalytic activity for environmental pollutant elimination[J]. Applied Surface Science, 2018, 444: 177-186. |
| 67 | DANG Jingjing, GUO Jingru, WANG Liping, et al. Construction of Z-scheme Fe3O4/BiOCl/BiOI heterojunction with superior recyclability for improved photocatalytic activity towards tetracycline degradation[J]. Journal of Alloys and Compounds, 2022, 893: 162251. |
| 68 | JIANG Runren, LU Guanghua, NKOOM Matthew, et al. Mineralization and toxicity reduction of the benzophenone-1 using 2D/2D Cu2WS4/BiOCl Z-scheme system: simultaneously improved visible-light absorption and charge transfer efficiency[J]. Chemical Engineering Journal, 2020, 400: 125913. |
| 69 | LIANG Ximeng, ZHANG Yuqi, LI Di, et al. 2D/2D BiOCl/K+Ca2Nb3O10 - heterostructure with Z-scheme charge carrier transfer pathways for tetracycline degradation under simulated solar light[J]. Applied Surface Science, 2019, 466: 863-873. |
| 70 | ZHANG Pengfei, LIANG Haiou, LIU Huan, et al. A novel Z-scheme BiOI/BiOCl nanofibers photocatalyst prepared by one-pot solvothermal with efficient visible-light-driven photocatalytic activity[J]. Materials Chemistry and Physics, 2021, 272: 125031. |
| 71 | LIU Huanhuan, YANG Cai, HUANG Jiao, et al. Ionic liquid-assisted hydrothermal preparation of BiOI/BiOCl heterojunctions with enhanced separation efficiency of photo-generated charge pairs and photocatalytic performance[J]. Inorganic Chemistry Communications, 2020, 113: 107806. |
| 72 | CHENG Xueying, GUAN Renquan, CHEN Yunning, et al. The unique TiO2(B)/BiOCl0.7I0.3-P Z-scheme heterojunction effectively degrades and mineralizes the herbicide fomesafen[J]. Chemical Engineering Journal, 2022, 431: 134021. |
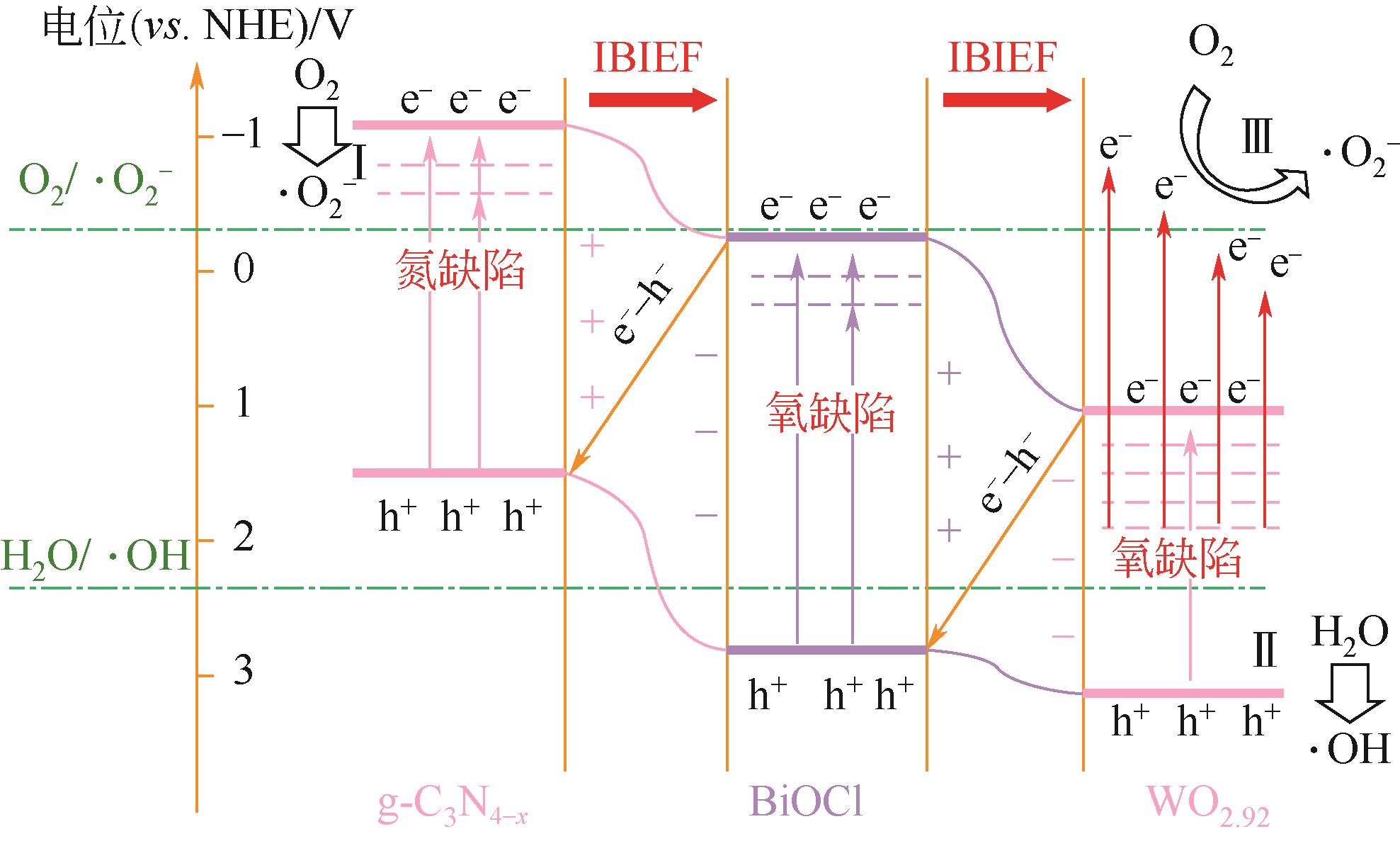
| 73 | WANG Min, TAN Guoqiang, FENG Shuaijun, et al. Defects and internal electric fields synergistically optimized g-C3N4- x /BiOCl/WO2.92 heterojunction for photocatalytic NO deep oxidation[J]. Journal of Hazardous Materials, 2021, 408: 124897. |
| 74 | SHEN Ting, SHI Xueke, GUO Jiaxiu, et al. Photocatalytic removal of NO by light-driven Mn3O4/BiOCl heterojunction photocatalyst: Optimization and mechanism[J]. Chemical Engineering Journal, 2021, 408: 128014. |
| 75 | SUN Yuwei, QI Xin, LI Ruiqi, et al. Hydrothermal synthesis of 2D/2D BiOCl/g-C3N4 Z-scheme: For TC degradation and antimicrobial activity evaluation[J]. Optical Materials, 2020, 108: 110170. |
| 76 | GAO Xiaoya, TANG Guangbei, PENG Wen, et al. Surprise in the phosphate modification of BiOCl with oxygen vacancy: in situ construction of hierarchical Z-scheme BiOCl-OV-BiPO4 photocatalyst for the degradation of carbamazepine[J]. Chemical Engineering Journal, 2019, 360: 1320-1329. |
| 77 | LI Jiajia, LU Tianyi, ZHAO Ziwei, et al. Preparation of heterostructured ternary Cd/CdS/BiOCl photocatalysts for enhanced visible-light photocatalytic degradation of organic pollutants in wastewater[J]. Inorganic Chemistry Communications, 2020, 121: 108236. |
| 78 | GUO W, JI D X, YUAN Z S, et al. Epitaxial growth of bronze phase titanium dioxide by molecular beam epitaxy[J]. AIP Advances, 2019, 9(3): 035230. |
| 79 | HEIDARI Shirin, HAGHIGHI Mohammad, SHABANI Maryam. Sunlight-activated BiOCl/BiOBr-Bi24O31Br10 photocatalyst for the removal of pharmaceutical compounds[J]. Journal of Cleaner Production, 2020, 259: 120679. |
| 80 | PENG Yin, ZHANG Qian, KAN Pengfei. Synthesis of a novel one-dimensional Bi2O2CO3-BiOCl heterostructure and its enhanced photocatalytic activity[J]. CrystEngComm, 2020, 22(41): 6822-6830. |
| 81 | SHI Xiaoqiang, WANG Lina, Achuo Anitta ZUH, et al. Photo-Fenton reaction for the degradation of tetracycline hydrochloride using a FeWO4/BiOCl nanocomposite[J]. Journal of Alloys and Compounds, 2022, 903: 163889. |
| 82 | HUANG Jialun, SHEN Jingtao, ZHANG Ganwei, et al. Visible-light-driven 3D Bi5O7I/BiOCl microsphere with enhanced photocatalytic capability: Performance, degradation pathway, antibacterium and mechanism[J]. Chemosphere, 2022, 299: 134482. |
| 83 | GAO Xin, FENG Yue, DONG Pengyu, et al. Rational design 2D/2D BiOCl/H+Ti2NbO7 - heterojunctions for enhanced photocatalytic degradation activity[J]. Applied Surface Science, 2020, 521: 146334. |
| 84 | LIU Chao, GAO Xin, ZHANGAI Caijun, et al. Layered BiOCl/H+TiNbO5 – heterojunctions for boosting visible-light-driven photocatalytic RhB degradation[J]. Sustainable Energy & Fuels, 2021, 5(18): 4680-4689. |
| 85 | NIU Siying, ZHANG Ruoyu, ZHANG Zhiyu, et al. In situ construction of the BiOCl/Bi2Ti2O7 heterojunction with enhanced visible-light photocatalytic activity[J]. Inorganic Chemistry Frontiers, 2019, 6(3): 791-798. |
| 86 | SUN Xiaoming, LU Jia, WU Jiang, et al. Enhancing photocatalytic activity on gas-phase heavy metal oxidation with self-assembled BiOI/BiOCl microflowers[J]. Journal of Colloid and Interface Science, 2019, 546: 32-42. |
| 87 | 伍书祺, 黄泽皑, 李晴川, 等. Nb2O5/BiOCl Ⅱ型异质结的构建及增强光催化还原二氧化碳[J]. 材料导报, 2021, 35(6): 6001-6007. |
| WU Shuqi, HUANG Zeai, LI Qingchuan, et al. Construction of type Ⅱ heterojunction of Nb2O5/BiOCl for enhanced photocatalytic carbon dioxide reduction[J]. Materials Reports, 2021, 35(6): 6001-6007. | |
| 88 | 姚百新, 王亚, 臧荣斌, 等. BiOCl/In2S3复合可见光催化剂制备及性能[J]. 化学通报, 2021, 84(11): 1224-1230, 1121. |
| YAO Baixin, WANG Ya, ZANG Rongbin, et al. Preparation and visible-light photocatalytic performance of BiOCl/In2S3 composite[J]. Chemistry, 2021, 84(11): 1224-1230, 1121. | |
| 89 | 吴健博, 石亮, 郑小强, 等. g-C3N4/BiOCl 复合光催化剂作为 2D/2D 异质结用于光催化降解染料性能研究[J]. 复合材料学报, 2023, 40(1): 323-333. |
| WU Jianbo, SHI Liang, ZHENG Xiaoqiang, et al. g-C3N4/BiOCl composite photocatalyst was used as 2D/2D heterojunction for photocatalytic degradation of dyes[J]. Acta Materiae Compositae Sinica, 2023, 40(1): 323-333. | |
| 90 | OUYANG Weixin, TENG Feng, FANG Xiaosheng. High performance BiOCl nanosheets/TiO2 nanotube arrays heterojunction UV photodetector: The influences of self-induced inner electric fields in the BiOCl nanosheets[J]. Advanced Functional Materials, 2018, 28(16): 1707178. |
| 91 | MAO Lebao, LIU Hao, YAO Linli, et al. Construction of a dual-functional CuO/BiOCl heterojunction for high-efficiently photoelectrochemical biosensing and photoelectrocatalytic degradation of aflatoxin B1[J]. Chemical Engineering Journal, 2022, 429: 132297. |
| 92 | ZHOU Shuai, BAO Nan, ZHANG Qingzhe, et al. Engineering hierarchical porous oxygen-deficient TiO2 fibers decorated with BiOCl nanosheets for efficient photocatalysis[J]. Applied Surface Science, 2019, 471: 96-107. |
| 93 | SU Qian, ZHU Lulu, ZHANG Mingrui, et al. Construction of a bioinspired hierarchical BiVO4/BiOCl heterojunction and its enhanced photocatalytic activity for phenol degradation[J]. ACS Applied Materials & Interfaces, 2021, 13(28): 32906-32915. |
| 94 | CHANG Junqing, ZHONG Yan, HU Chaohao, et al. Study on highly efficient BiOCl/ZnO p-n heterojunction: Synthesis, characterization and visible-light-excited photocatalytic activity[J]. Journal of Molecular Structure, 2019, 1183: 209-216. |
| 95 | 牛凤兴, 蒋帅, 高晓明, 等. BiOCl/ZnO复合光催化剂的制备及其光催化性能[J]. 功能材料, 2021, 52(6): 6190-6194, 6207. |
| NIU Fengxing, JIANG Shuai, GAO Xiaoming, et al. Preparation of BiOCl/ZnO composite photocatalyst and its photocatalytic performance[J]. Journal of Functional Materials, 2021, 52(6): 6190-6194, 6207. | |
| 96 | HOU Weidong, DENG Chengming, XU Haiming, et al. n-p BiOCl@ g-C3N4 heterostructure with rich-oxygen vacancies for photodegradation of carbamazepine[J]. ChemistrySelect, 2020, 5(9): 2767-2777. |
| 97 | ZHENG Yan, ZHANG Xiao, ZHAO Jie, et al. Assembled fabrication of α-Fe2O3/BiOCl heterojunctions with enhanced photocatalytic performance[J]. Applied Surface Science, 2018, 430: 585-594. |
| 98 | ESHAQ G, WANG Shaobin, SUN Hongqi, et al. Core/shell FeVO4@BiOCl heterojunction as a durable heterogeneous Fenton catalyst for the efficient sonophotocatalytic degradation of p-nitrophenol[J]. Separation and Purification Technology, 2020, 231: 115915. |
| 99 | ZHANG Dafeng, SU Changhua, YAO Shujuan, et al. Facile in situ chemical transformation synthesis, boosted charge separation, and increased photocatalytic activity of BiPO4/BiOCl p-n heterojunction photocatalysts under simulated sunlight irradiation[J]. Journal of Physics and Chemistry of Solids, 2020, 147: 109630. |
| 100 | WEI Yuelin, DING Yuying, CHEN Yibin, et al. Fabrication of one-dimensional visible-light-driven BiOCl@WO3 p-n heterojunction with improved photocatalytic performance[J]. Materials Science in Semiconductor Processing, 2022, 143: 106539. |
| 101 | BAO Sarenqiqige, LIANG Haiou, LI Chunping, et al. The synthesis and enhanced photocatalytic activity of heterostructure BiOCl/TiO2 nanofibers composite for tetracycline degradation in visible light[J]. Journal of Dispersion Science and Technology, 2021, 42(13): 2000-2013. |
| 102 | MA Lijiao, WU Huiqin, CHEN Biyu, et al. 0D/2D CsPbBr3 nanocrystal/BiOCl nanoplate heterostructure with enhanced photocatalytic performance[J]. Advanced Materials Interfaces, 2022, 9(16): 2102522. |
| 103 | XU Kaiqiang, SHEN Jie, ZHANG Shiying, et al. Efficient interfacial charge transfer of BiOCl-In2O3 step-scheme heterojunction for boosted photocatalytic degradation of ciprofloxacin[J]. Journal of Materials Science & Technology, 2022, 121: 236-244. |
| 104 | WANG Saisai, LIANG Xu, Yakun LYU, et al. Electric field coupling in the S-scheme CdS/BiOCl heterojunction for boosted charge transport toward photocatalytic CO2 reduction[J]. ACS Applied Energy Materials, 2022, 5(1): 1149-1158. |
| 105 | ZHANG Zhongwei, GUO Ruitang, TANG Junying, et al. Fabrication of Bi-BiOCl/MgIn2S4 heterostructure with step-scheme mechanism for carbon dioxide photoreduction into methane[J]. Journal of CO2 Utilization, 2021, 45: 101453. |
| 106 | WU Shanshan, YU Xiang, ZHANG Junlei, et al. Construction of BiOCl/CuBi2O4 S-scheme heterojunction with oxygen vacancy for enhanced photocatalytic diclofenac degradation and nitric oxide removal[J]. Chemical Engineering Journal, 2021, 411: 128555. |
| 107 | YUAN Xinxin, YANG Jieyi, YAO Yiyang, et al. Preparation, characterization and photodegradation mechanism of 0D/2D Cu2O/BiOCl S-scheme heterojunction for efficient photodegradation of tetracycline[J]. Separation and Purification Technology, 2022, 291: 120965. |
| 108 | ZHANG Zhuangzhuang, ZHANG Yuanyuan, HAN Xuanxuan, et al. Assembly of CaIn2S4 on defect-rich BiOCl for acceleration of interfacial charge separation and photocatalytic phenol degradation via S-scheme electron transfer mechanism[J]. Catalysts, 2021, 11(9): 1130. |
| 109 | YANG Lu, WANG Juan, ZHANG Yi, et al. Construction of S-scheme BiOCl/CdS composite for enhanced photocatalytic degradation of antibiotic[J]. Journal of Materials Science: Materials in Electronics, 2022, 33(16): 13303-13315. |
| 110 | HUANG Yan, CHEN Fan, GUAN Zhipeng, et al. S-scheme BiOCl/MoSe2 heterostructure with enhanced photocatalytic activity for dyes and antibiotics degradation under sunlight irradiation[J]. Sensors, 2022, 22(9): 3344. |
| 111 | TIAN Na, HUANG Hongwei, WANG Shuobo, et al. Facet-charge-induced coupling dependent interfacial photocharge separation: A case of BiOI/g-C3N4 p-n junction[J]. Applied Catalysis B: Environmental, 2020, 267: 118697. |
| 112 | REN Qiang, LIU Juming, YANG Qi, et al. A review: Photocatalysts based on BiOCl andg-C3N4 for water purification[J]. Catalysts, 2021, 11(9): 1084. |
| 113 | HE Xinghou, Tianhan KAI, DING Ping. Heterojunction photocatalysts for degradation of the tetracycline antibiotic: A review[J]. Environmental Chemistry Letters, 2021, 19(6): 4563-4601. |
| 114 | ZHOU Qin, HUANG Weiya, XU Chong, et al. Novel hierarchical carbon quantum dots-decorated BiOCl nanosheet/carbonized eggshell membrane composites for improved removal of organic contaminants from water via synergistic adsorption and photocatalysis[J]. Chemical Engineering Journal, 2021, 420: 129582. |
| 115 | CHEN Jing, REN Qifang, DING Yi, et al. Synthesis of bifunctional composites Ag/BiOCl/diatomite: Degradation of tetracycline and evaluation of antimicrobial activity[J]. Journal of Environmental Chemical Engineering, 2021, 9(6): 106476. |
| 116 | SHI Yanbiao, ZHAN Guangming, LI Hao, et al. Simultaneous manipulation of bulk excitons and surface defects for ultrastable and highly selective CO2 photoreduction[J]. Advanced Materials, 2021, 33(38): e2100143. |
| 117 | ZHANG Yajun, XU Zhongfei, WANG Qiang, et al. Unveiling the activity origin of ultrathin BiOCl nanosheets for photocatalytic CO2 reduction[J]. Applied Catalysis B: Environmental, 2021, 299: 120679. |
| 118 | WANG Genxiang, CHEN Junxiang, DING Yichun, et al. Electrocatalysis for CO2 conversion: From fundamentals to value-added products[J]. Chemical Society Reviews, 2021, 50(8): 4993-5061. |
| 119 | SONG Yang, YE Caichao, YU Xue, et al. Electron-induced enhanced interfacial interaction of the CuO/BiOCl heterostructure for boosted CO2 photoreduction performance under simulated sunlight[J]. Applied Surface Science, 2022, 583: 152463. |
| 120 | SHI Yanbiao, LI Jie, MAO Chengliang, et al. Van Der Waals gap-rich BiOCl atomic layers realizing efficient, pure-water CO2-to-CO photocatalysis[J]. Nature Communications, 2021, 12(1): 5923. |
| 121 | ZHANG Wendong, DONG Xin’an, JIA Bin, et al. 2D BiOCl/Bi12O17Cl2 nanojunction: Enhanced visible light photocatalytic NO removal and in situ DRIFTS investigation[J]. Applied Surface Science, 2018, 430: 571-577. |
| 122 | LIU Yang, XU Jingjing, CHEN Mindong. Synthesis of direct Z-Scheme Bi3NbO7/BiOCl photocatalysts with enhanced activity for CIP degradation and Cr(Ⅵ) reduction under visible light irradiation[J]. Separation and Purification Technology, 2021, 276: 119255. |
| 123 | SHI Yiqiu, XIONG Xuyang, DING Shuoping, et al. In-situ topotactic synthesis and photocatalytic activity of plate-like BiOCl/2D networks Bi2S3 heterostructures[J]. Applied Catalysis B: Environmental, 2018, 220: 570-580. |
| 124 | HUSSAIN Muhammad Bilal, MEHMOOD Rashid, AZHAR Umair, et al. BiOCl-coated UiO-66-NH2 metal-organic framework nanoparticles for visible-light photocatalytic Cr(Ⅵ) reduction[J]. ACS Applied Nano Materials, 2021, 4(4): 4037-4047. |
| 125 | ZHANG Ling, HAN Zhongkang, WANG Wenzhong, et al. Solar-light-driven pure water splitting with ultrathin BiOCl nanosheets[J]. Chemistry: A European Journal, 2015, 21(50): 18089-18094. |
| 126 | ZHAO Daming, WANG Yiqing, DONG Chungli, et al. Boron-doped nitrogen-deficient carbon nitride-based Z-scheme heterostructures for photocatalytic overall water splitting[J]. Nature Energy, 2021, 6(4): 388-397. |
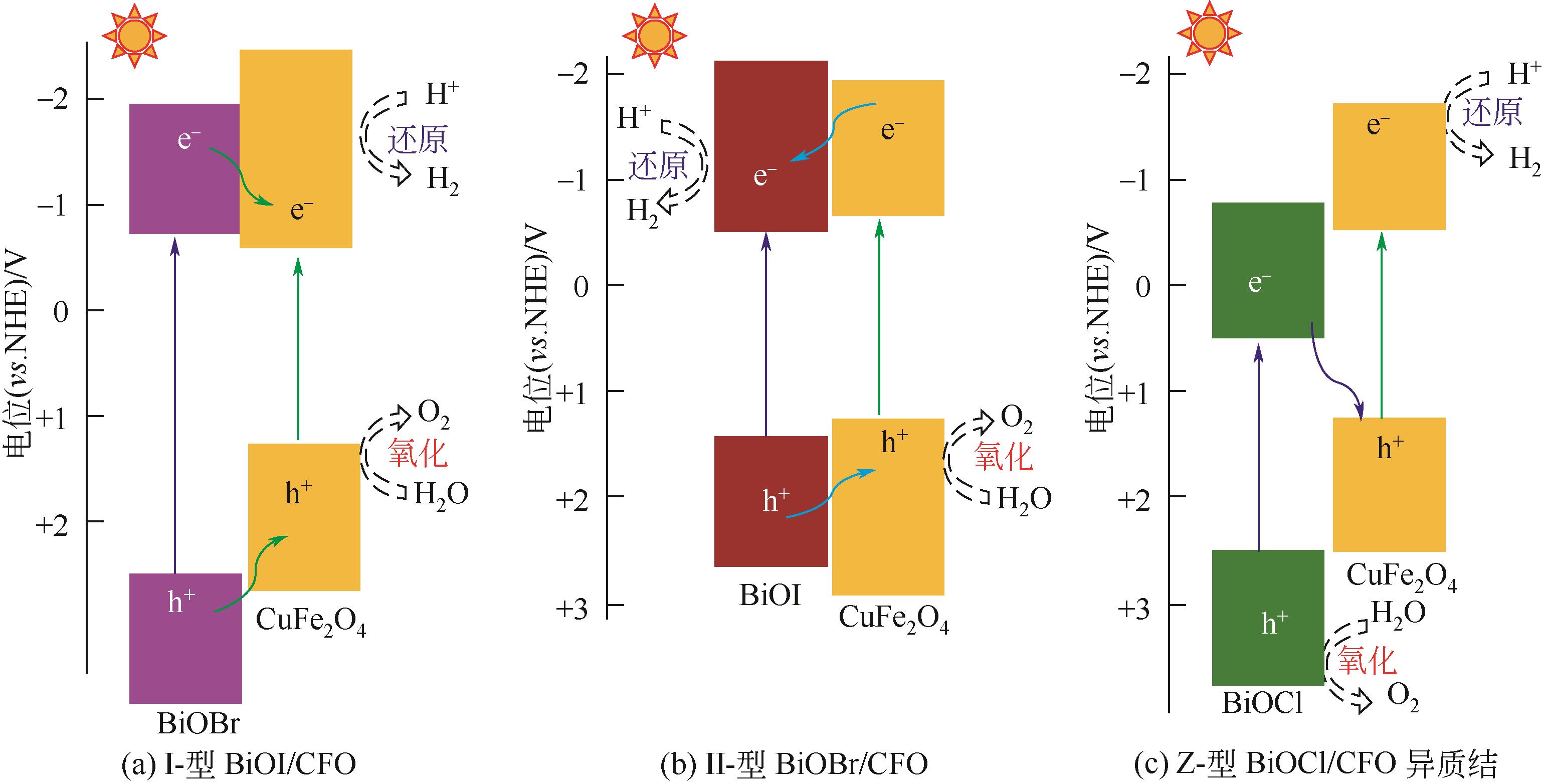
| 127 | BERA S, GHOSH S, MAIYALAGAN T, et al. Band edge engineering of BiOX/CuFe2O4 heterostructures for efficient water splitting[J]. ACS Applied Energy Materials, 2022, 5(3): 3821-3833. |
| [1] | 章萍萍, 丁书海, 高晶晶, 赵敏, 俞海祥, 刘玥宏, 谷麟. 碳量子点修饰半导体复合光催化剂降解水中有机污染物[J]. 化工进展, 2023, 42(10): 5487-5500. |
| [2] | 张鹏会, 李艳春, 胡怀生, 齐慧丽, 胡浩斌. 生物炭基光催化剂的制备、性能及环境应用研究进展[J]. 化工进展, 2022, 41(1): 1-16. |
| [3] | 李酽, 宋双, 连晓雪. MoS2/ZnO纳米复合材料的光学和光催化性能[J]. 化工进展, 2021, 40(7): 3870-3877. |
| [4] | 夏振国, 朱颖颖, 陈耿, 卢宇, 王家锋. 用于环境净化的TiO2/AC复合材料的制备及其改性研究进展[J]. 化工进展, 2021, 40(7): 3837-3846. |
| [5] | 梁一尊, 葛艳清, 王驰, 李凯, 梅毅. 低维黑磷的制备及其在光催化降解领域的应用研究进展[J]. 化工进展, 2021, 40(2): 845-858. |
| [6] | 卞俊杰, 王万圆, 满恒孝, 文成新. BiOX(Cl,Br,I)/Bi2WO6异质结型复合光催化剂用于高浓度氮氧化物的脱除[J]. 化工进展, 2021, 40(11): 6094-6101. |
| [7] | 朱恩权, 马玉花, 艾尼娃·木尼热. 红磷光催化材料的研究进展[J]. 化工进展, 2019, 38(s1): 139-143. |
| [8] | 张晓, 徐瑶华, 刘皓, 魏峰, 苑鹏. 基于金属氧化物的乙醇检测气敏材料的研究进展[J]. 化工进展, 2019, 38(07): 3207-3226. |
| [9] | 龙丹, 周俊伶, 时洪民, 王冠然, 李红双, 赵苾艺, 李贞玉. 氧化亚铜光催化剂性能提升及增强机制的研究进展[J]. 化工进展, 2019, 38(06): 2756-2767. |
| [10] | 师艳婷, 乔生莉, 张巧玲, 刘有智, 畅俊波, 秦钊. 磁性光催化剂Fe3O4/SiO2/TiO2的制备及光催化降解苯酚[J]. 化工进展, 2018, 37(11): 4322-4329. |
| [11] | 王心怡, 王志强, 张文帅, 苏进展. Sb2S3太阳能电池的研究进展[J]. 化工进展, 2018, 37(11): 4214-4225. |
| [12] | 朱宝余, 孙成勋, 王兰, 陈涛, 刘屹. 氨气检测仪研究现状[J]. 化工进展, 2017, 36(S1): 27-33. |
| [13] | 阿山, 于丹丹, 白杰, 郑家威, 李春萍. 环境领域的二氧化钛基光催化剂负载和改性技术研究进展[J]. 化工进展, 2017, 36(11): 4043-4050. |
| [14] | 黄文迪, 孙静, 申婷婷, 王西奎. Co-BiVO4异质结光催化剂的制备及其性能[J]. 化工进展, 2017, 36(11): 4080-4086. |
| [15] | 白照杲, 胡芸, 游素珍, 钟佳欣, 韦朝海. Bi2WO6-TiO2复合光催化剂对Cu-EDTA复合污染的高效光催化协同处理[J]. 化工进展, 2017, 36(06): 2164-2170. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||