化工进展 ›› 2023, Vol. 42 ›› Issue (2): 603-613.DOI: 10.16085/j.issn.1000-6613.2022-1208
• 专栏:工业污泥/精馏釜残的热化学转化利用 • 上一篇 下一篇
水热处理对含油污泥热解特性及动力学影响
- 西安交通大学能源与动力工程学院,西安市固体废物资源再生与循环利用重点实验室,中国石油集团安全环保技术研究院有限公司,陕西 西安 710049
-
收稿日期:2022-06-28修回日期:2022-08-18出版日期:2023-02-25发布日期:2023-03-13 -
通讯作者:高宁博 -
作者简介:段一航(1994—),男,博士研究生,研究方向为含油污泥资源化利用。E-mail:yihangduan@foxmail.com。 -
基金资助:陕西省重点研发计划(2021GY-114);石油石化污染物控制与处理国家重点实验室开放课题(PPC 2020002)
Effect of hydrothermal treatment on pyrolysis characteristics and kinetics of oily sludge
DUAN Yihang( ), GAO Ningbo(
), GAO Ningbo( ), QUAN Cui
), QUAN Cui
- CNPC Safety and Environmental Protection Technology Research Institute Co. , Ltd. , Xi’an Key Laboratory of Solid Waste Recycling and Resource Recovery, School of Energy and Power Engineering, Xi’an Jiaotong University, Xi’an 710049, Shaanxi, China
-
Received:2022-06-28Revised:2022-08-18Online:2023-02-25Published:2023-03-13 -
Contact:GAO Ningbo
摘要:
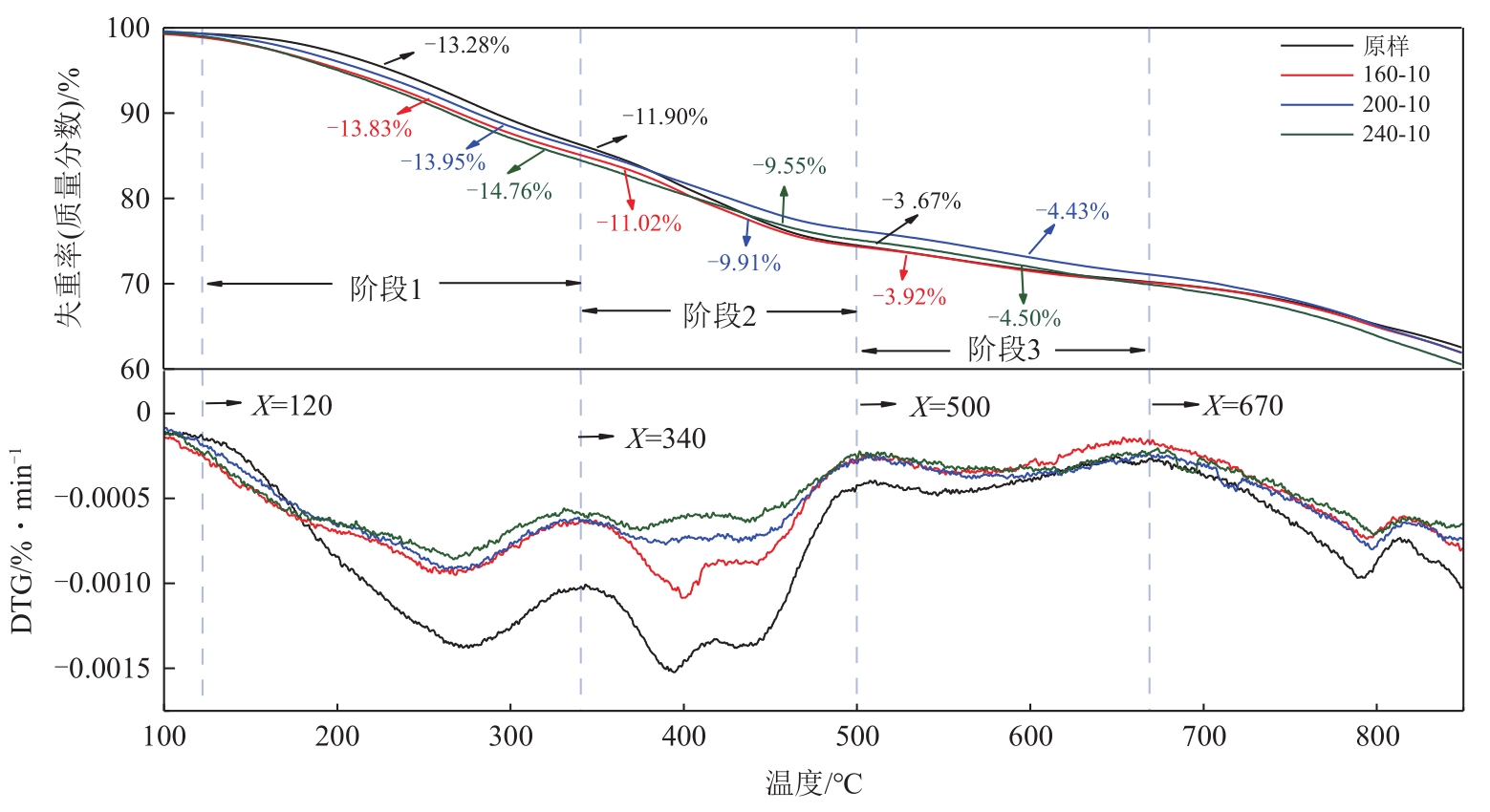
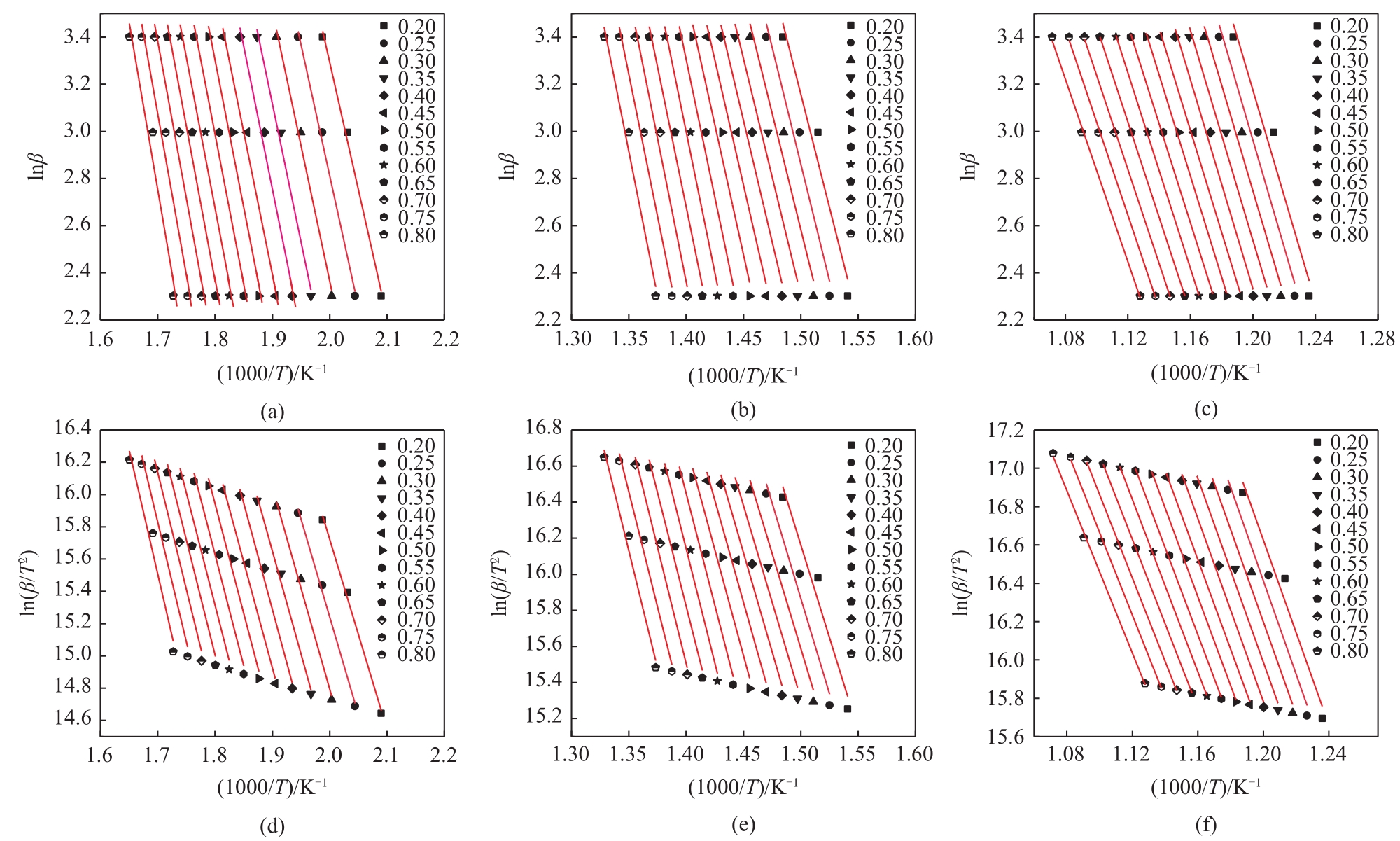
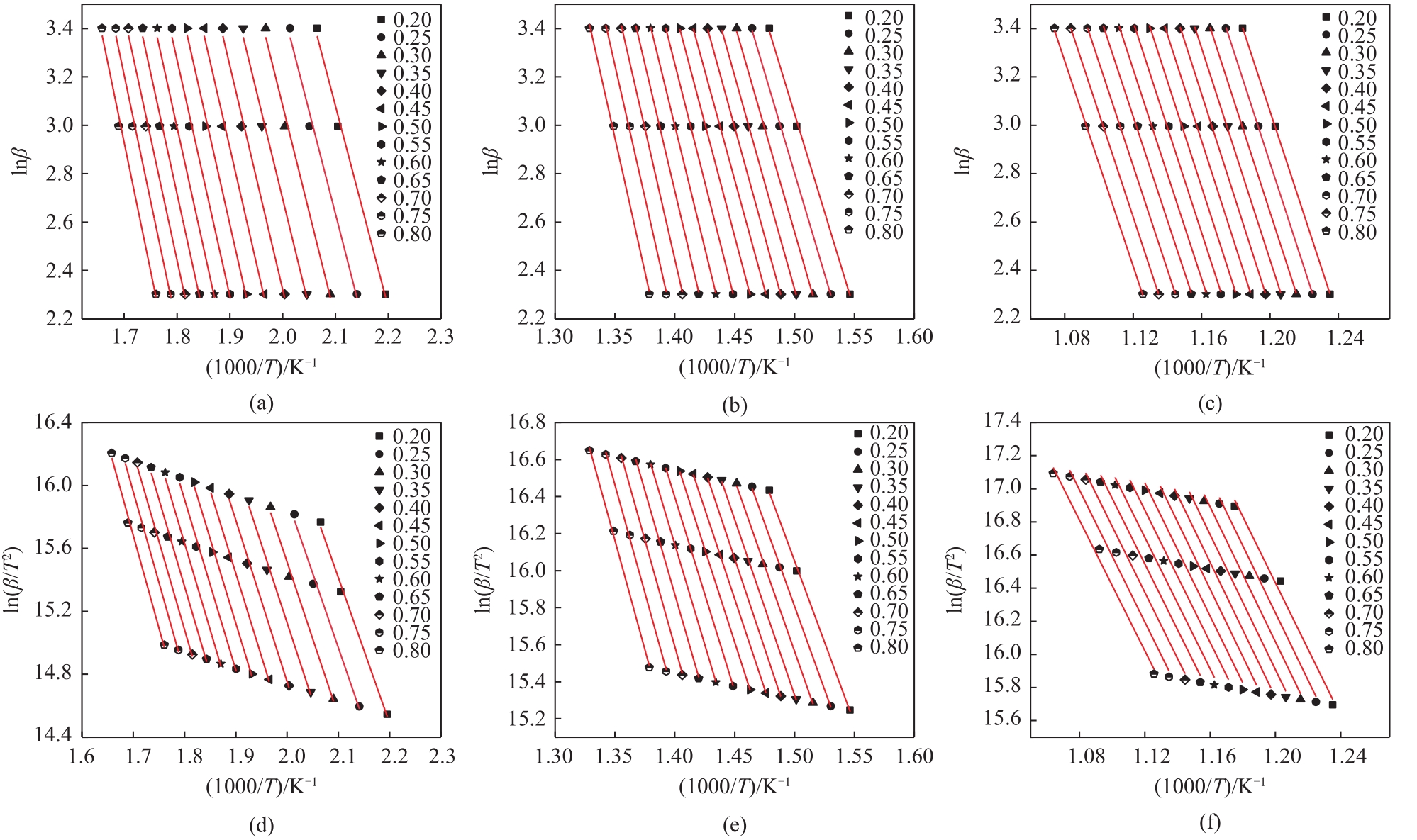
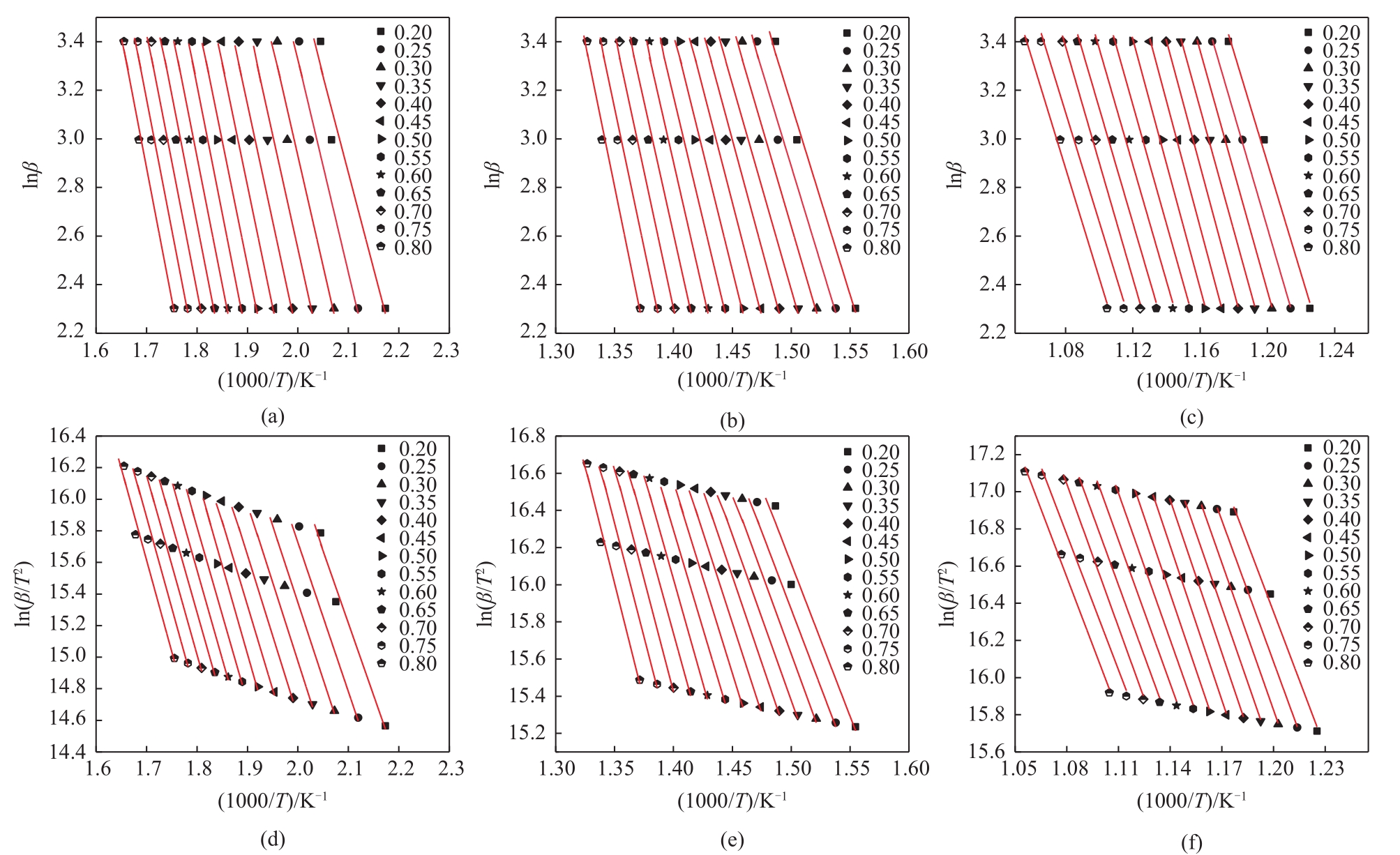
利用热重分析仪研究了水热处理对含油污泥(OS)热解特性的影响,并使用Kissinger-Akahira-Sunose(KAS)和Ozawa-Flynn-Wall(FWO)的方法对其热解动力学进行了分析,确定了经过不同水热温度处理后的含油污泥在不同热解阶段的表观活化能,考察了水热处理及其水热温度对含油污泥热解特性及动力学参数的影响。热分析的结果表明:水热处理使得含油污泥在热解不同阶段的终止温度向较低温度区间移动,在相同的转化率下经过水热处理后的OS在不同热解阶段的表观活化能均低于原样。随着水热反应温度从160℃增加到240℃,根据FWO法估算的OS在热解第一阶段的平均表观活化能从75.20kJ/mol增加到78.28kJ/mol,热解第二阶段的平均表观活化能从151.04kJ/mol降低到144.18kJ/mol,热解第三阶段的平均活化能从171.12kJ/mol增加到了192.59kJ/mol。
中图分类号:
引用本文
段一航, 高宁博, 全翠. 水热处理对含油污泥热解特性及动力学影响[J]. 化工进展, 2023, 42(2): 603-613.
DUAN Yihang, GAO Ningbo, QUAN Cui. Effect of hydrothermal treatment on pyrolysis characteristics and kinetics of oily sludge[J]. Chemical Industry and Engineering Progress, 2023, 42(2): 603-613.
| 样品 | 工业分析/% | 元素分析/% | 热值/MJ·kg-1 | 含油率/% | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 水分 | 挥发分 | 灰分 | 固定碳 | C | H | O | N | S | |||
| OS | 54.4 | 26.35 | 19.04 | 0.21 | 22.80 | 3.17 | 29.88 | 0.43 | 1.89 | 8.48 | 23.87 |
表1 含油污泥的理化性质(质量分数)
| 样品 | 工业分析/% | 元素分析/% | 热值/MJ·kg-1 | 含油率/% | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 水分 | 挥发分 | 灰分 | 固定碳 | C | H | O | N | S | |||
| OS | 54.4 | 26.35 | 19.04 | 0.21 | 22.80 | 3.17 | 29.88 | 0.43 | 1.89 | 8.48 | 23.87 |
| 样品 | β①/K·min-1 | 第一阶段 | 第二阶段 | 第三阶段 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ti1②/K | Tp1③/K | -Rp1④/%·min-1 | Ti2/K | Tp2/K | -Rp2/%·min-1 | Ti3/K | Tp3/K | -Rp3/%·min-1 | ||
| 原样 | 10 | 106.11 | 343.11 | 13.28 | 343.11 | 510.11 | 11.90 | 510.11 | 650.09 | 3.67 |
| 160-10 | 10 | 110.15 | 338.06 | 13.83 | 338.06 | 505.01 | 11.02 | 505.01 | 658.16 | 3.92 |
| 200-10 | 10 | 107.88 | 338.03 | 13.95 | 338.03 | 506.04 | 9.91 | 395.26 | 655.90 | 4.34 |
| 240-10 | 10 | 110.04 | 335.14 | 14.76 | 335.14 | 505.04 | 9.55 | 505.04 | 650.19 | 4.50 |
表2 不同样品的多元热解特征参数
| 样品 | β①/K·min-1 | 第一阶段 | 第二阶段 | 第三阶段 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ti1②/K | Tp1③/K | -Rp1④/%·min-1 | Ti2/K | Tp2/K | -Rp2/%·min-1 | Ti3/K | Tp3/K | -Rp3/%·min-1 | ||
| 原样 | 10 | 106.11 | 343.11 | 13.28 | 343.11 | 510.11 | 11.90 | 510.11 | 650.09 | 3.67 |
| 160-10 | 10 | 110.15 | 338.06 | 13.83 | 338.06 | 505.01 | 11.02 | 505.01 | 658.16 | 3.92 |
| 200-10 | 10 | 107.88 | 338.03 | 13.95 | 338.03 | 506.04 | 9.91 | 395.26 | 655.90 | 4.34 |
| 240-10 | 10 | 110.04 | 335.14 | 14.76 | 335.14 | 505.04 | 9.55 | 505.04 | 650.19 | 4.50 |
| 转化率(α) | 第一阶段 | 第二阶段 | 第三阶段 | |||
|---|---|---|---|---|---|---|
| E(FWO)/kJ·mol-1 | E(KAS)/kJ·mol-1 | E(FWO)/kJ·mol-1 | E(KAS)/kJ·mol-1 | E(FWO)/kJ·mol--1 | E(KAS)/kJ·mol-1 | |
| 0.20 | 85.35 | 97.94 | 151.89 | 145.66 | 177.58 | 200.61 |
| 0.25 | 87.80 | 100.68 | 156.48 | 149.32 | 179.48 | 202.69 |
| 0.30 | 90.65 | 103.84 | 155.76 | 153.72 | 177.34 | 200.45 |
| 0.35 | 92.61 | 106.08 | 158.29 | 157.63 | 176.08 | 199.28 |
| 0.40 | 95.71 | 109.49 | 162.56 | 160.79 | 175.13 | 198.37 |
| 0.45 | 95.31 | 109.16 | 167.86 | 161.54 | 170.94 | 194.13 |
| 0.50 | 98.63 | 112.90 | 174.18 | 167.03 | 169.44 | 192.63 |
| 0.55 | 100.13 | 114.48 | 184.45 | 173.93 | 165.88 | 189.06 |
| 0.60 | 100.92 | 115.48 | 188.80 | 179.75 | 163.91 | 186.98 |
| 0.65 | 103.37 | 118.47 | 187.62 | 186.06 | 159.87 | 182.82 |
| 0.70 | 105.51 | 120.55 | 192.20 | 191.55 | 157.66 | 180.66 |
| 0.75 | 108.11 | 123.46 | 190.07 | 193.46 | 156.01 | 179.08 |
| 0.80 | 112.38 | 128.12 | 193.62 | 195.96 | 153.08 | 176.17 |
| 均值 | 98.19 | 112.36 | 174.14 | 170.49 | 167.88 | 190.99 |
表3 通过FWO和KAS方法获得的原样在不同热解阶段的表观活化能(E)
| 转化率(α) | 第一阶段 | 第二阶段 | 第三阶段 | |||
|---|---|---|---|---|---|---|
| E(FWO)/kJ·mol-1 | E(KAS)/kJ·mol-1 | E(FWO)/kJ·mol-1 | E(KAS)/kJ·mol-1 | E(FWO)/kJ·mol--1 | E(KAS)/kJ·mol-1 | |
| 0.20 | 85.35 | 97.94 | 151.89 | 145.66 | 177.58 | 200.61 |
| 0.25 | 87.80 | 100.68 | 156.48 | 149.32 | 179.48 | 202.69 |
| 0.30 | 90.65 | 103.84 | 155.76 | 153.72 | 177.34 | 200.45 |
| 0.35 | 92.61 | 106.08 | 158.29 | 157.63 | 176.08 | 199.28 |
| 0.40 | 95.71 | 109.49 | 162.56 | 160.79 | 175.13 | 198.37 |
| 0.45 | 95.31 | 109.16 | 167.86 | 161.54 | 170.94 | 194.13 |
| 0.50 | 98.63 | 112.90 | 174.18 | 167.03 | 169.44 | 192.63 |
| 0.55 | 100.13 | 114.48 | 184.45 | 173.93 | 165.88 | 189.06 |
| 0.60 | 100.92 | 115.48 | 188.80 | 179.75 | 163.91 | 186.98 |
| 0.65 | 103.37 | 118.47 | 187.62 | 186.06 | 159.87 | 182.82 |
| 0.70 | 105.51 | 120.55 | 192.20 | 191.55 | 157.66 | 180.66 |
| 0.75 | 108.11 | 123.46 | 190.07 | 193.46 | 156.01 | 179.08 |
| 0.80 | 112.38 | 128.12 | 193.62 | 195.96 | 153.08 | 176.17 |
| 均值 | 98.19 | 112.36 | 174.14 | 170.49 | 167.88 | 190.99 |
| 转化率(α) | 第一阶段 | 第二阶段 | 第三阶段 | |||
|---|---|---|---|---|---|---|
| R2(FWO) | R2(KAS) | R2(FWO) | R2(KAS) | R2(FWO) | R2(KAS) | |
| 0.20 | 0.9957 | 0.9954 | 0.9601 | 0.9989 | 0.9653 | 0.9895 |
| 0.25 | 0.9959 | 0.9939 | 0.9671 | 0.9994 | 0.9738 | 0.9898 |
| 0.30 | 0.9936 | 0.9943 | 0.9657 | 0.9992 | 0.981 | 0.9891 |
| 0.35 | 0.9924 | 0.9939 | 0.9676 | 0.9998 | 0.9861 | 0.9878 |
| 0.40 | 0.9872 | 0.9953 | 0.9733 | 0.9999 | 0.9913 | 0.9871 |
| 0.45 | 0.9884 | 0.9959 | 0.9764 | 0.9999 | 0.9967 | 0.9858 |
| 0.50 | 0.9799 | 0.9964 | 0.971 | 0.9999 | 0.9982 | 0.9836 |
| 0.55 | 0.9759 | 0.9942 | 0.9811 | 0.9999 | 0.9989 | 0.9829 |
| 0.60 | 0.9715 | 0.994 | 0.9809 | 0.9998 | 0.9998 | 0.9841 |
| 0.65 | 0.9703 | 0.9949 | 0.9826 | 0.9992 | 0.9999 | 0.9856 |
| 0.70 | 0.9683 | 0.9963 | 0.9849 | 0.999 | 0.9996 | 0.9886 |
| 0.75 | 0.9683 | 0.9956 | 0.986 | 0.999 | 0.9987 | 0.9894 |
| 0.80 | 0.9631 | 0.9959 | 0.9844 | 0.9983 | 0.9985 | 0.9918 |
| 均值 | 0.9808 | 0.9951 | 0.9755 | 0.9994 | 0.99135 | 0.9875 |
表4 通过FWO和KAS方法获得的原样在不同热解阶段拟合曲线的相关决定系数
| 转化率(α) | 第一阶段 | 第二阶段 | 第三阶段 | |||
|---|---|---|---|---|---|---|
| R2(FWO) | R2(KAS) | R2(FWO) | R2(KAS) | R2(FWO) | R2(KAS) | |
| 0.20 | 0.9957 | 0.9954 | 0.9601 | 0.9989 | 0.9653 | 0.9895 |
| 0.25 | 0.9959 | 0.9939 | 0.9671 | 0.9994 | 0.9738 | 0.9898 |
| 0.30 | 0.9936 | 0.9943 | 0.9657 | 0.9992 | 0.981 | 0.9891 |
| 0.35 | 0.9924 | 0.9939 | 0.9676 | 0.9998 | 0.9861 | 0.9878 |
| 0.40 | 0.9872 | 0.9953 | 0.9733 | 0.9999 | 0.9913 | 0.9871 |
| 0.45 | 0.9884 | 0.9959 | 0.9764 | 0.9999 | 0.9967 | 0.9858 |
| 0.50 | 0.9799 | 0.9964 | 0.971 | 0.9999 | 0.9982 | 0.9836 |
| 0.55 | 0.9759 | 0.9942 | 0.9811 | 0.9999 | 0.9989 | 0.9829 |
| 0.60 | 0.9715 | 0.994 | 0.9809 | 0.9998 | 0.9998 | 0.9841 |
| 0.65 | 0.9703 | 0.9949 | 0.9826 | 0.9992 | 0.9999 | 0.9856 |
| 0.70 | 0.9683 | 0.9963 | 0.9849 | 0.999 | 0.9996 | 0.9886 |
| 0.75 | 0.9683 | 0.9956 | 0.986 | 0.999 | 0.9987 | 0.9894 |
| 0.80 | 0.9631 | 0.9959 | 0.9844 | 0.9983 | 0.9985 | 0.9918 |
| 均值 | 0.9808 | 0.9951 | 0.9755 | 0.9994 | 0.99135 | 0.9875 |
| 转化率(α) | 第一阶段 | 第二阶段 | 第三阶段 | |||
|---|---|---|---|---|---|---|
| E(FWO)/kJ·mol-1 | E(KAS)/kJ·mol-1 | E(FWO)/kJ·mol-1 | E(KAS)/kJ·mol-1 | E(FWO)/kJ·mol-1 | E(KAS)/kJ·mol-1 | |
| 0.20 | 65.75 | 76.99 | 128.03 | 145.66 | 170.07 | 166.53 |
| 0.25 | 67.17 | 78.65 | 131.43 | 149.32 | 171.73 | 168.69 |
| 0.30 | 69.39 | 81.23 | 135.46 | 153.72 | 172.29 | 169.27 |
| 0.35 | 70.65 | 82.72 | 139.09 | 157.63 | 172.68 | 169.44 |
| 0.40 | 73.02 | 85.38 | 142.02 | 160.79 | 174.26 | 169.27 |
| 0.45 | 75.07 | 87.71 | 142.65 | 161.54 | 173.55 | 169.19 |
| 0.50 | 76.42 | 89.29 | 147.79 | 167.03 | 172.92 | 167.11 |
| 0.55 | 77.13 | 90.21 | 154.19 | 173.93 | 172.13 | 165.86 |
| 0.60 | 78.32 | 91.54 | 159.64 | 179.75 | 170.94 | 164.95 |
| 0.65 | 78.87 | 92.28 | 165.57 | 186.06 | 169.36 | 164.70 |
| 0.70 | 79.90 | 93.53 | 170.70 | 191.55 | 169.04 | 165.61 |
| 0.75 | 82.11 | 95.94 | 172.36 | 193.46 | 169.12 | 165.61 |
| 0.80 | 83.77 | 97.85 | 174.66 | 195.96 | 167.38 | 163.78 |
| 均值 | 75.20 | 87.95 | 151.04 | 170.49 | 171.19 | 166.92 |
表5 通过FWO和KAS方法获得在160℃水热温度处理后的含油污泥在不同热解阶段的活化能(E)
| 转化率(α) | 第一阶段 | 第二阶段 | 第三阶段 | |||
|---|---|---|---|---|---|---|
| E(FWO)/kJ·mol-1 | E(KAS)/kJ·mol-1 | E(FWO)/kJ·mol-1 | E(KAS)/kJ·mol-1 | E(FWO)/kJ·mol-1 | E(KAS)/kJ·mol-1 | |
| 0.20 | 65.75 | 76.99 | 128.03 | 145.66 | 170.07 | 166.53 |
| 0.25 | 67.17 | 78.65 | 131.43 | 149.32 | 171.73 | 168.69 |
| 0.30 | 69.39 | 81.23 | 135.46 | 153.72 | 172.29 | 169.27 |
| 0.35 | 70.65 | 82.72 | 139.09 | 157.63 | 172.68 | 169.44 |
| 0.40 | 73.02 | 85.38 | 142.02 | 160.79 | 174.26 | 169.27 |
| 0.45 | 75.07 | 87.71 | 142.65 | 161.54 | 173.55 | 169.19 |
| 0.50 | 76.42 | 89.29 | 147.79 | 167.03 | 172.92 | 167.11 |
| 0.55 | 77.13 | 90.21 | 154.19 | 173.93 | 172.13 | 165.86 |
| 0.60 | 78.32 | 91.54 | 159.64 | 179.75 | 170.94 | 164.95 |
| 0.65 | 78.87 | 92.28 | 165.57 | 186.06 | 169.36 | 164.70 |
| 0.70 | 79.90 | 93.53 | 170.70 | 191.55 | 169.04 | 165.61 |
| 0.75 | 82.11 | 95.94 | 172.36 | 193.46 | 169.12 | 165.61 |
| 0.80 | 83.77 | 97.85 | 174.66 | 195.96 | 167.38 | 163.78 |
| 均值 | 75.20 | 87.95 | 151.04 | 170.49 | 171.19 | 166.92 |
| 1 | DUAN Yihang, GAO Ningbo, SIPRA Ayesha Tariq, et al. Characterization of heavy metals and oil components in the products of oily sludge after hydrothermal treatment[J]. Journal of Hazardous Materials, 2022, 424: 127293. |
| 2 | ZHOU Lingsheng, JIANG Xiumin, LIU Jianguo. Characteristics of oily sludge combustion in circulating fluidized beds[J]. Journal of Hazardous Materials, 2009, 170(1): 175-179. |
| 3 | SILVA L J DA, ALVES F C, DE FRANÇA F P. A review of the technological solutions for the treatment of oily sludges from petroleum refineries[J]. Waste Management & Research, 2012, 30(10): 1016-1030. |
| 4 | TANG Siqi, ZHENG Chunmiao, YAN Feng, et al. Product characteristics and kinetics of sewage sludge pyrolysis driven by alkaline earth metals[J]. Energy, 2018, 153: 921-932. |
| 5 | ZUBAIDY E A H, ABOUELNASR D M. Fuel recovery from waste oily sludge using solvent extraction[J]. Process Safety and Environmental Protection, 2010, 88(5): 318-326. |
| 6 | PUASA S W, ISMAIL K N, MUSMAN M Z A, et al. Enhanced oily sludge dewatering using plant-based surfactant technology[J]. Materials Today: Proceedings, 2019, 19: 1159-1165. |
| 7 | MANARA P, ZABANIOTOU A. Towards sewage sludge based biofuels via thermochemical conversion—A review[J]. Renewable and Sustainable Energy Reviews, 2012, 16(5): 2566-2582. |
| 8 | GAO Ningbo, DUAN Yihang, LI Zongyang, et al. Hydrothermal treatment combined with in situ mechanical compression for floated oily sludge dewatering[J]. Journal of Hazardous Materials, 2021, 402: 124173. |
| 9 | XU Menghan, ZHANG Jie, LIU Haifeng, et al. The resource utilization of oily sludge by co-gasification with c oal[J]. Fuel, 2014, 126: 55-61. |
| 10 | 董向元, 郭淑青, 杨继涛, 等. 玉米秸秆水热焦热解及动力学特性研究[J]. 林产化学与工业, 2018, 38(3): 83-89. |
| DONG Xiangyuan, GUO Shuqing, YANG Jitao, et al. Pyrolysis and kinetic characteristics of corn stalk char from hydrothermal carbonization[J]. Chemistry and Industry of Forest Products, 2018, 38(3): 83-89. | |
| 11 | 邢献军, 杨静, 范方宇, 等. 木屑及其水热炭的热解特性和动力学对比[J]. 农业工程学报, 2017, 33(4): 258-264. |
| XING Xianjun, YANG Jing, FAN Fangyu, et al. Comparison of pyrolysis characteristics and kinetics of sawdust and its hydrochar[J]. Transactions of the Chinese Society of Agricultural Engineering, 2017, 33(4): 258-264. | |
| 12 | WANG Shule, WEN Yuming, SHI Ziyi, et al. Effect of hydrothermal carbonization pretreatment on the pyrolysis behavior of the digestate of agricultural waste: A view on kinetics and thermodynamics[J]. Chemical Engineering Journal, 2022, 431: 133881. |
| 13 | ZHANG Jingmiao, XIA Ao, ZHU Xianqing, et al. Co-production of carbon quantum dots and biofuels via hydrothermal conversion of biomass[J]. Fuel Processing Technology, 2022, 232: 107276. |
| 14 | 高昌胜, 魏茂, 蒋文广, 等. 基于含油污泥热解残渣的路基材料制备与性能评价[J]. 硅酸盐通报, 2019, 38(6): 1895-1900. |
| GAO Changsheng, WEI Mao, JIANG Wenguang, et al. Preparation and performance evaluation of roadbed materials based on pyrolysis residue of oily sludge[J]. Bulletin of the Chinese Ceramic Society, 2019, 38(6): 1895-1900. | |
| 15 | 张秀霞, 贾宏宇, 刘炳琨. 超声处理对污泥热解特性及反应动力学影响[J]. 中国石油大学学报(自然科学版), 2022, 46(1): 171-176. |
| ZHANG Xiuxia, JIA Hongyu, LIU Bingkun. Effects of ultrasonic treatment on pyrolysis characteristics and reaction kinetics of municipal sludge[J]. Journal of China University of Petroleum (Edition of Natural Science), 2022, 46(1): 171-176. | |
| 16 | 刘承飞, 李江平, 刘大方, 等. 废旧电脑印刷电路板的热解特性及动力学分析[J]. 有色金属科学与工程, 2022, 13(1): 38-43. |
| LIU Chengfei, LI Jiangping, LIU Dafang, et al. Pyrolysis characteristics and kinetics analysis of waste computer printed circuit board[J]. Nonferrous Metals Science and Engineering, 2022, 13(1): 38-43. | |
| 17 | MIAN Inamullah, LI Xian, JIAN Yiming, et al. Kinetic study of biomass pellet pyrolysis by using distributed activation energy model and Coats Redfern methods and their comparison[J]. Bioresource Technology, 2019, 294: 122099. |
| 18 | QIN Linbo, HAN Jun, HE Xiang, et al. Recovery of energy and iron from oily sludge pyrolysis in a fluidized bed reactor[J]. Journal of Environmental Management, 2015, 154: 177-182. |
| 19 | XU Guiying, CAI Xinghui, WANG Shan, et al. Characteristics, kinetics, infrared analysis and process optimization of co-pyrolysis of waste tires and oily sludge[J]. Journal of Environmental Management, 2022, 316: 115278. |
| 20 | NAQVI Salman Raza, TARIQ Rumaisa, SHAHBAZ Muhammad, et al. Recent developments on sewage sludge pyrolysis and its kinetics: Resources recovery, thermogravimetric platforms, and innovative prospects[J]. Computers & Chemical Engineering, 2021, 150: 107325. |
| 21 | LEE Sangho, KIM Young Min, SIDDIQUI Muhammad Zain, et al. Different pyrolysis kinetics and product distribution of municipal and livestock manure sewage sludge[J]. Environmental Pollution, 2021, 285: 117197. |
| 22 | 王君. 基于热处理的含油污泥资源化利用技术[D]. 杭州: 浙江大学, 2018. |
| WANG Jun. Resource recovery from oily sludge through thermal treatment technology[D]. Hangzhou: Zhejiang University, 2018. | |
| 23 | 杨天华, 佟瑶, 李秉硕, 等. SDS联合亚临界水预处理对污泥水热液化制油的影响[J]. 太阳能学报, 2021, 42(5): 477-482. |
| YANG Tianhua, TONG Yao, LI Bingshuo, et al. Combined(SDS+subcritical water) pretreatment effect on hydro-liquefaction of municipal sludge[J]. Acta Energiae Solaris Sinica, 2021, 42(5): 477-482. | |
| 24 | Nebojša MANIĆ, Bojan JANKOVIĆ, DODEVSKI Vladimir. Model-free and model-based kinetic analysis of Poplar fluff (Populus alba) pyrolysis process under dynamic conditions[J]. Journal of Thermal Analysis and Calorimetry, 2021, 143(5): 3419-3438. |
| 25 | WANG Qiuju, ZHANG Zhao, XU Guoren, et al. Pyrolysis behaviors of antibiotic fermentation residue and wastewater sludge from penicillin production: Kinetics, gaseous products distribution, and nitrogen transformation[J]. Journal of Analytical and Applied Pyrolysis, 2021, 158: 105208. |
| 26 | MPHAHLELE Katlego, MATJIE Ratale Henry, OSIFO Peter Ogbemudia. Thermodynamics, kinetics and thermal decomposition characteristics of sewage sludge during slow pyrolysis[J]. Journal of Environmental Management, 2021, 284: 112006. |
| 27 | ZHAO Ming, RAHEEM Abdul, MEMON Zaki Mohammad, et al. Iso-conversional kinetics of low-lipid micro-algae gasification by air[J]. Journal of Cleaner Production, 2019, 207: 618-629. |
| 28 | XU G, GAI X, WANG S, et al. Characteristics, kinetics, infrared analysis and process optimization of co-pyrolysis of waste tires and oily sludge[J]. Journal of Environmental Management, 2022, 316: 115278. |
| 29 | WANG Fei, GUO Chennan, LIU Xiangyue, et al. Revealing carbon-iron interaction characteristics in sludge-derived hydrochars under different hydrothermal conditions[J]. Chemosphere, 2022, 300: 134572. |
| 30 | Gamzenur ÖZSIN, Esin APAYDıN-VAROL, Murat KıLıÇ, et al. Pyrolysis of petroleum sludge under non-isothermal conditions: Thermal decomposition behavior, kinetics, thermodynamics, and evolved gas analysis[J]. Fuel, 2021, 300: 120980. |
| 31 | Leena PAULINE A, JOSEPH Kurian. Hydrothermal carbonization of oily sludge for solid fuel recovery-investigation of chemical characteristics and combustion behaviour[J]. Journal of Analytical and Applied Pyrolysis, 2021, 157: 105235. |
| [1] | 黄益平, 李婷, 郑龙云, 戚傲, 陈政霖, 史天昊, 张新宇, 郭凯, 胡猛, 倪泽雨, 刘辉, 夏苗, 主凯, 刘春江. 三级环流反应器中气液流动与传质规律[J]. 化工进展, 2023, 42(S1): 175-188. |
| [2] | 顾永正, 张永生. HBr改性飞灰对Hg0的动态吸附及动力学模型[J]. 化工进展, 2023, 42(S1): 498-509. |
| [3] | 汪鹏, 张洋, 范兵强, 何登波, 申长帅, 张贺东, 郑诗礼, 邹兴. 高碳铬铁盐酸浸出过程工艺及动力学[J]. 化工进展, 2023, 42(S1): 510-517. |
| [4] | 刘阳, 王云刚, 修浩然, 邹立, 白彦渊. 基于动力学分析的核桃壳最佳炭化工艺[J]. 化工进展, 2023, 42(S1): 94-103. |
| [5] | 董佳宇, 王斯民. 超声强化对二甲苯结晶特性及调控机理实验[J]. 化工进展, 2023, 42(9): 4504-4513. |
| [6] | 王晋刚, 张剑波, 唐雪娇, 刘金鹏, 鞠美庭. 机动车尾气脱硝催化剂Cu-SSZ-13的改性研究进展[J]. 化工进展, 2023, 42(9): 4636-4648. |
| [7] | 邵志国, 任雯, 许世佩, 聂凡, 许毓, 刘龙杰, 谢水祥, 李兴春, 王庆吉, 谢加才. 终温对油基钻屑热解产物分布和特性影响[J]. 化工进展, 2023, 42(9): 4929-4938. |
| [8] | 李志远, 黄亚继, 赵佳琪, 于梦竹, 朱志成, 程好强, 时浩, 王圣. 污泥与聚氯乙烯共热解重金属特性[J]. 化工进展, 2023, 42(9): 4947-4956. |
| [9] | 王雪婷, 顾霞, 徐先宝, 赵磊, 薛罡, 李响. 水热预处理对餐厨垃圾厌氧发酵产戊酸的影响[J]. 化工进展, 2023, 42(9): 4994-5002. |
| [10] | 李海东, 杨远坤, 郭姝姝, 汪本金, 岳婷婷, 傅开彬, 王哲, 何守琴, 姚俊, 谌书. 炭化与焙烧温度对植物基铁碳微电解材料去除As(Ⅲ)性能的影响[J]. 化工进展, 2023, 42(7): 3652-3663. |
| [11] | 杨子育, 朱玲, 王文龙, 于超凡, 桑义敏. 阴燃法处理含油污泥的研究及应用进展[J]. 化工进展, 2023, 42(7): 3760-3769. |
| [12] | 姚丽铭, 王亚琢, 范洪刚, 顾菁, 袁浩然, 陈勇. 餐厨垃圾处理现状及其热解技术研究进展[J]. 化工进展, 2023, 42(7): 3791-3801. |
| [13] | 张杉, 仲兆平, 杨宇轩, 杜浩然, 李骞. 磷酸盐改性高岭土对生活垃圾热解过程中重金属的富集[J]. 化工进展, 2023, 42(7): 3893-3903. |
| [14] | 王俊杰, 潘艳秋, 牛亚宾, 俞路. 分子水平催化重整装置模型构建及应用[J]. 化工进展, 2023, 42(7): 3404-3412. |
| [15] | 赵毅, 杨臻, 张新为, 王刚, 杨旋. 不同裂缝损伤和愈合温度条件下沥青自愈合行为的分子模拟[J]. 化工进展, 2023, 42(6): 3147-3156. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||