化工进展 ›› 2025, Vol. 44 ›› Issue (4): 2352-2364.DOI: 10.16085/j.issn.1000-6613.2024-0544
NiCo2O4@纤蛇纹石活化过硫酸钾降解甲基橙
苏晓洁1,2( ), 严群1,2(
), 严群1,2( ), 李欣城1,2,3, 薛文慧1,2, 陈奕浩1,2
), 李欣城1,2,3, 薛文慧1,2, 陈奕浩1,2
- 1.河流源头水生态保护江西省重点实验室,江西 赣州 341000
2.赣州市赣江流域水质安全保障技术创新中心,江西 赣州 341000
3.江西理工大学资源与环境工程学院,江西 赣州 341000
-
收稿日期:2024-04-02修回日期:2024-06-20出版日期:2025-04-25发布日期:2025-05-07 -
通讯作者:严群 -
作者简介:苏晓洁(2000—),女,硕士研究生,研究方向为水污染控制高级氧化技术。E-mail:1042660813@qq.com。 -
基金资助:江西省教育厅科学技术研究项目(GJJ2200823)
Activation of potassium persulfate by NiCo2O4@chrysotile to degrade methyl orange
SU Xiaojie1,2( ), YAN Qun1,2(
), YAN Qun1,2( ), LI Xincheng1,2,3, XUE Wenhui1,2, CHEN Yihao1,2
), LI Xincheng1,2,3, XUE Wenhui1,2, CHEN Yihao1,2
- 1.Jiangxi Provincial Key Laboratory of Water Ecological Conservation at Headwater Regions, Ganzhou 341000, Jiangxi, China
2.Innovation Center for Water Quality Security Technology at Ganjiang River Basin, Ganzhou 341000, Jiangxi, China
3.School of Resource and Environmental Engineering, Jiangxi University of Science and Technology, Ganzhou 341000, Jiangxi, China
-
Received:2024-04-02Revised:2024-06-20Online:2025-04-25Published:2025-05-07 -
Contact:YAN Qun
摘要:
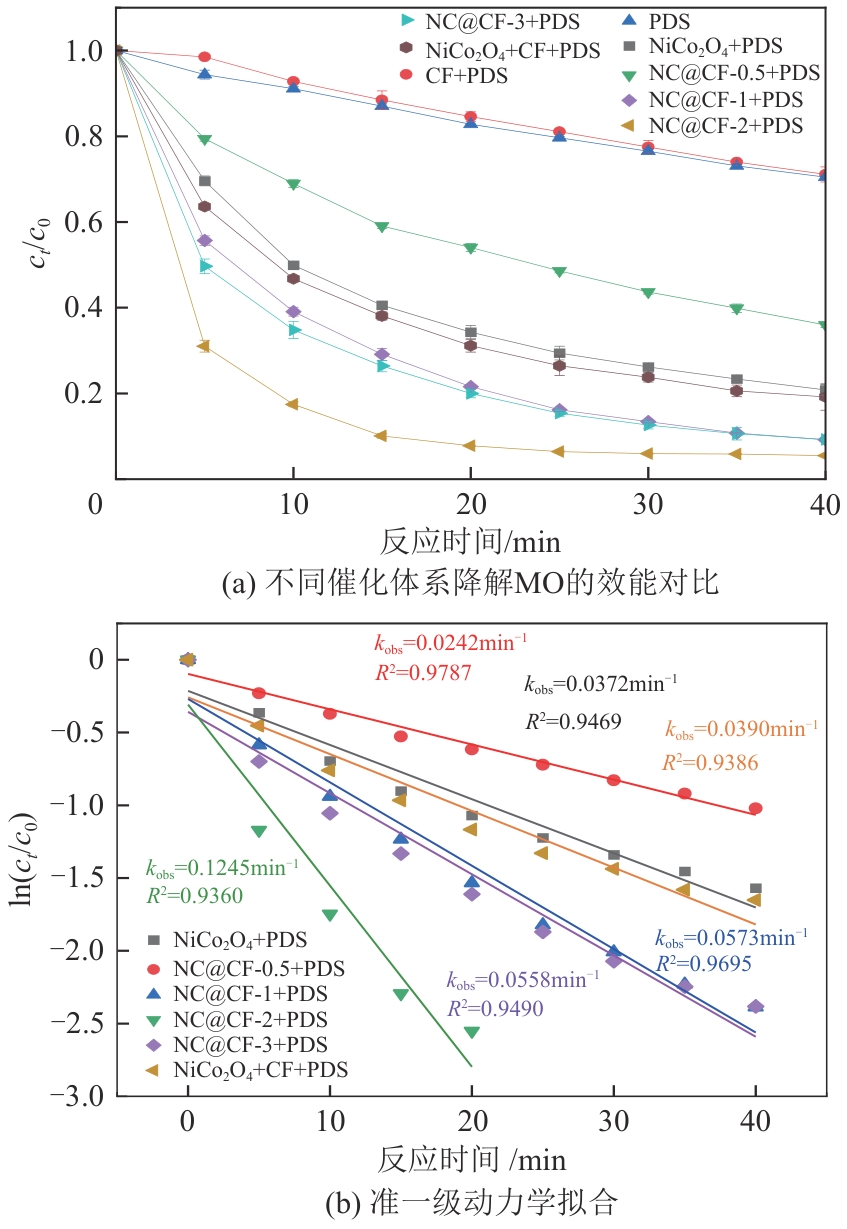
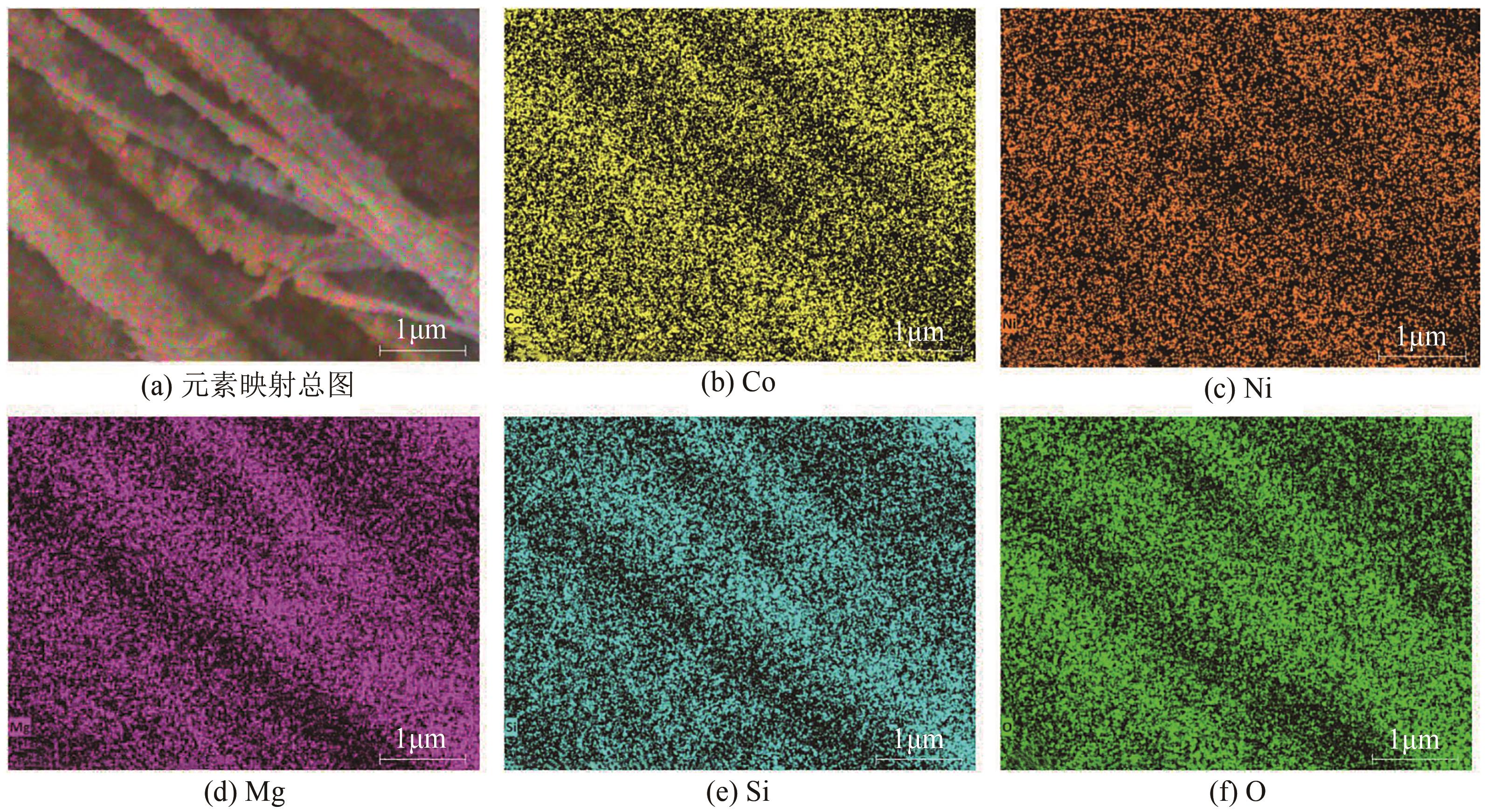
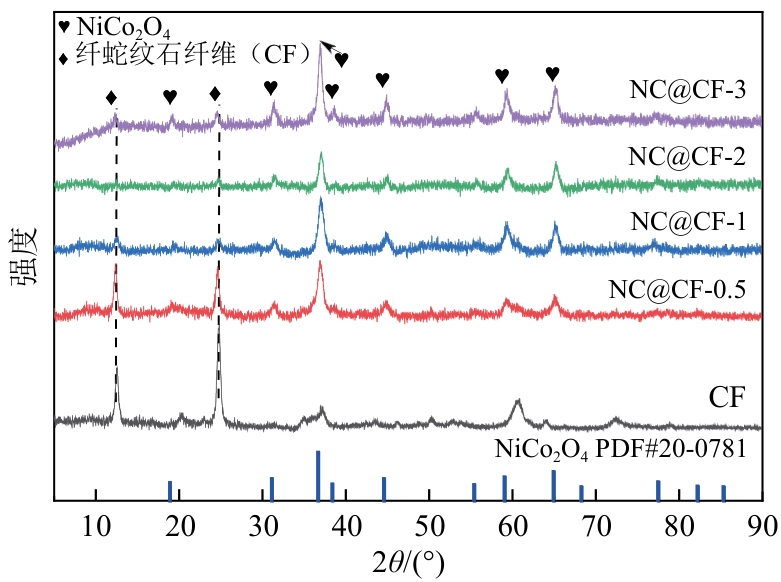
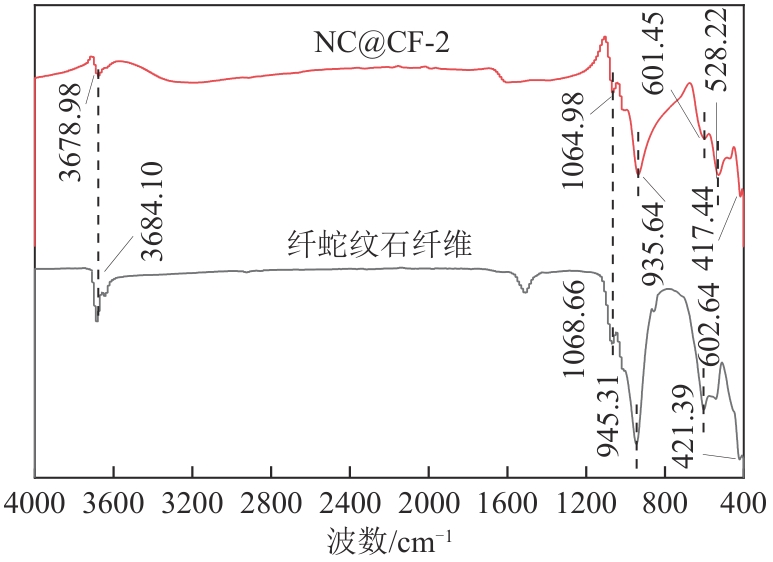
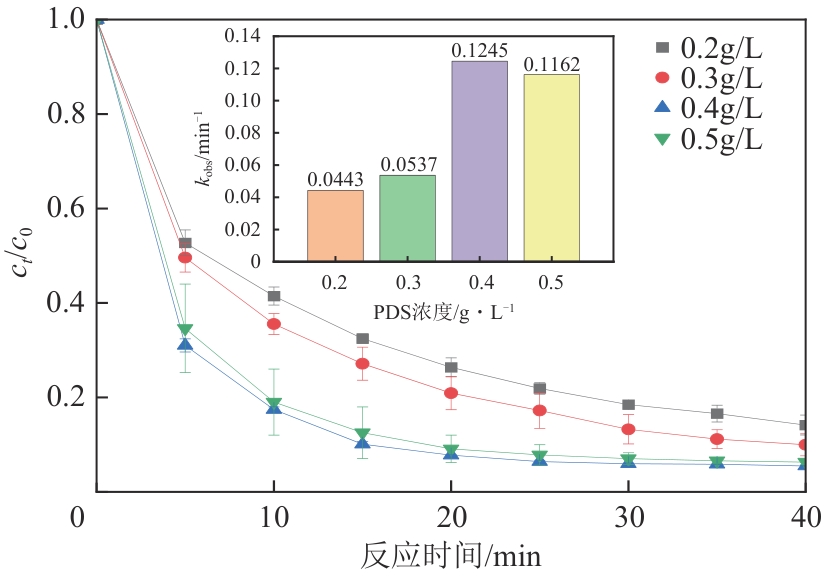
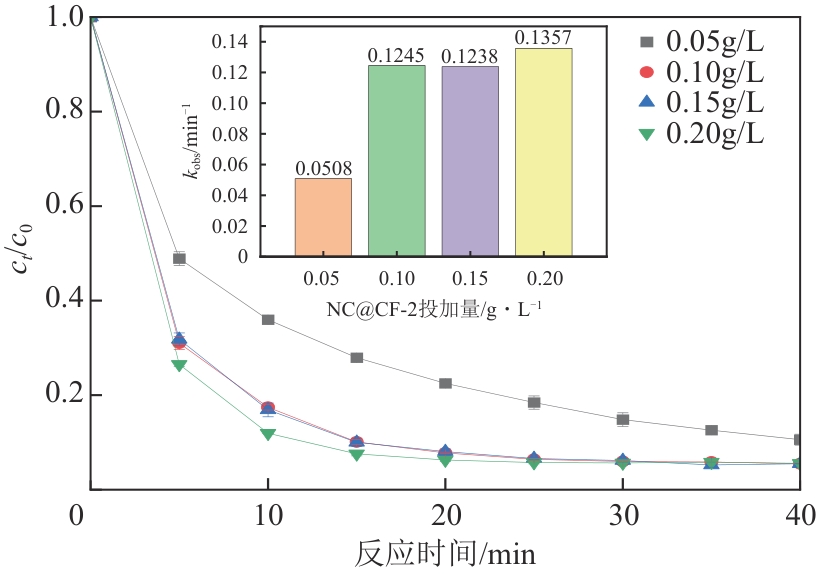
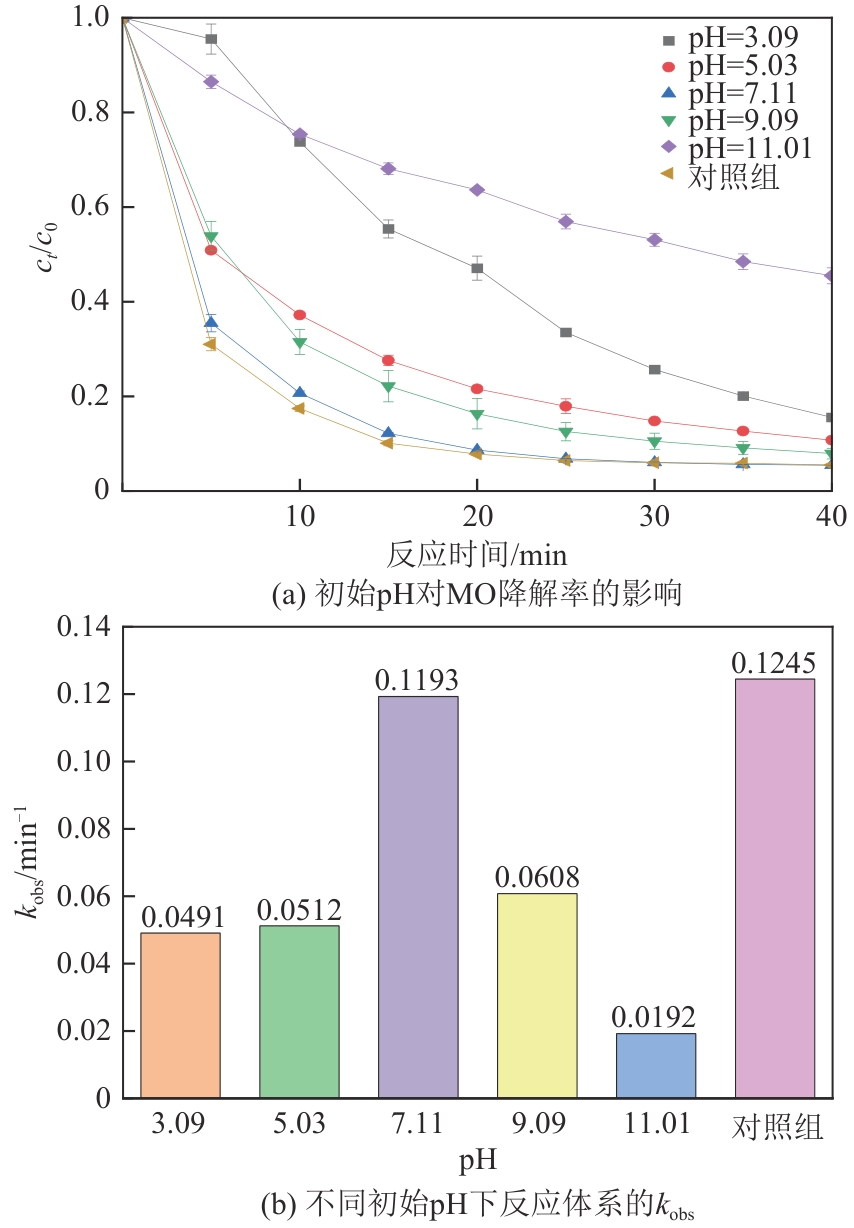
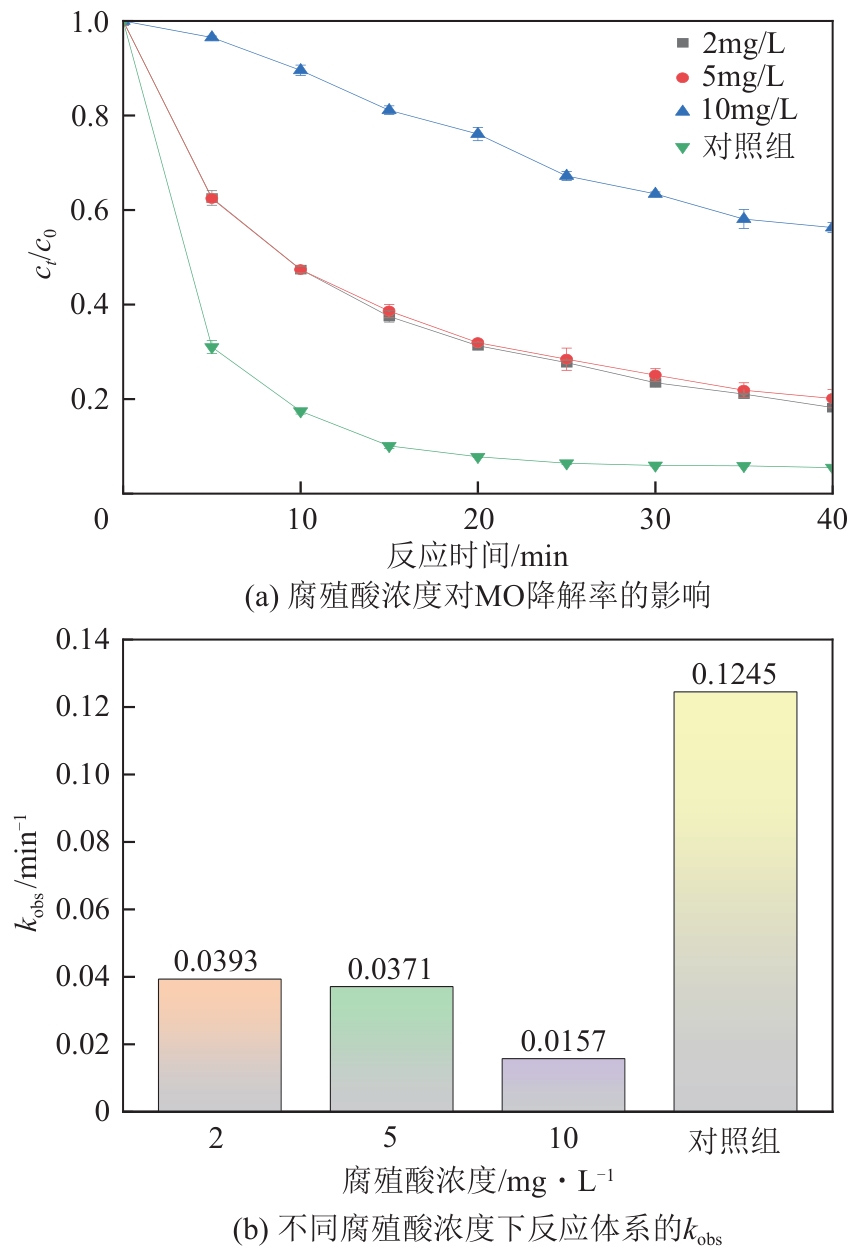
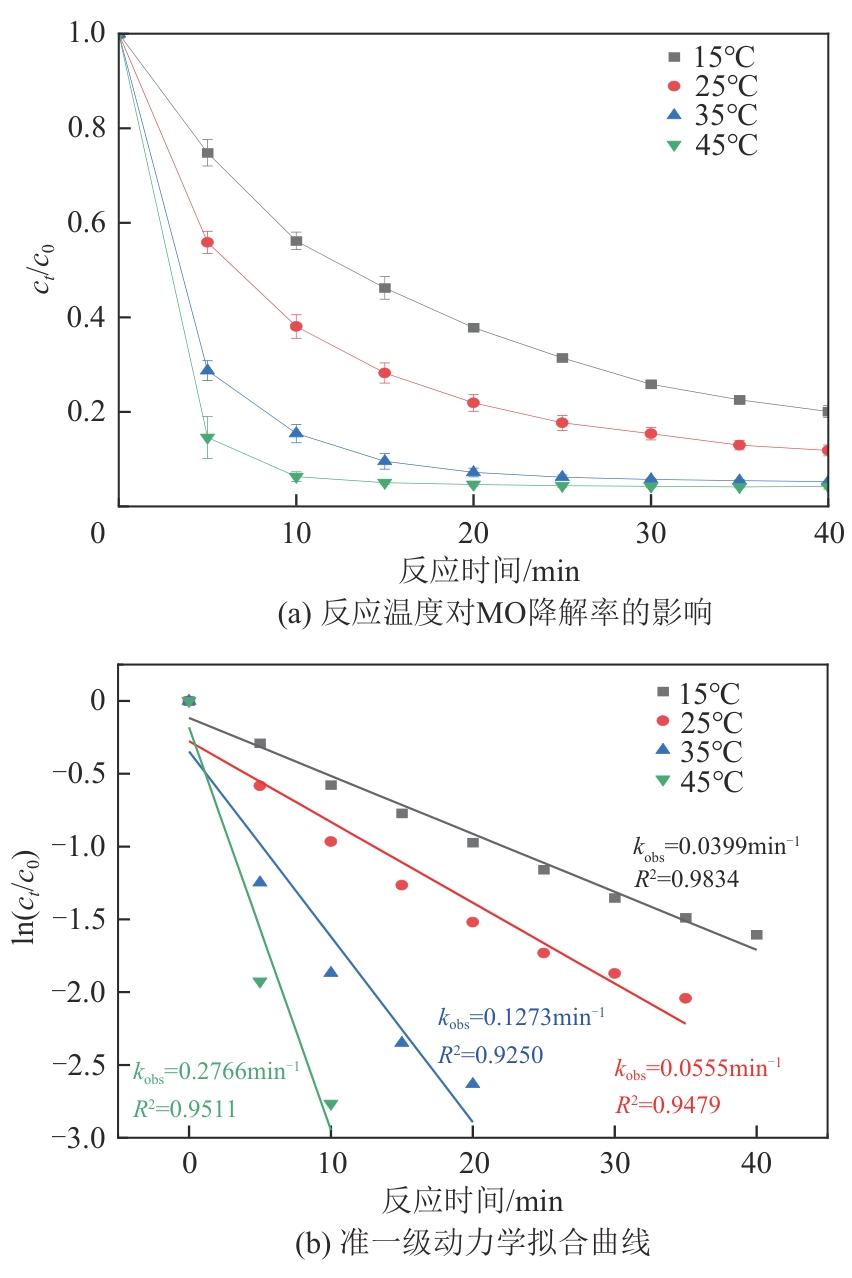
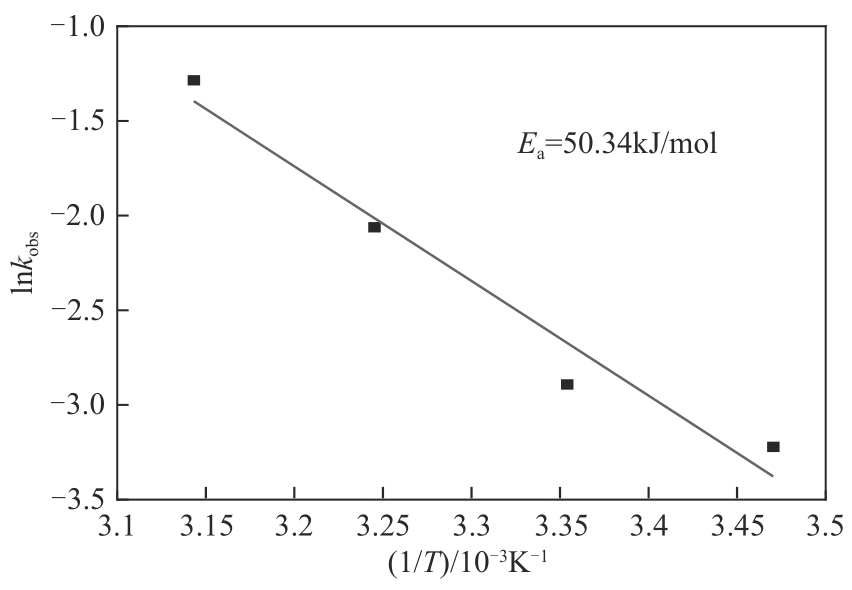
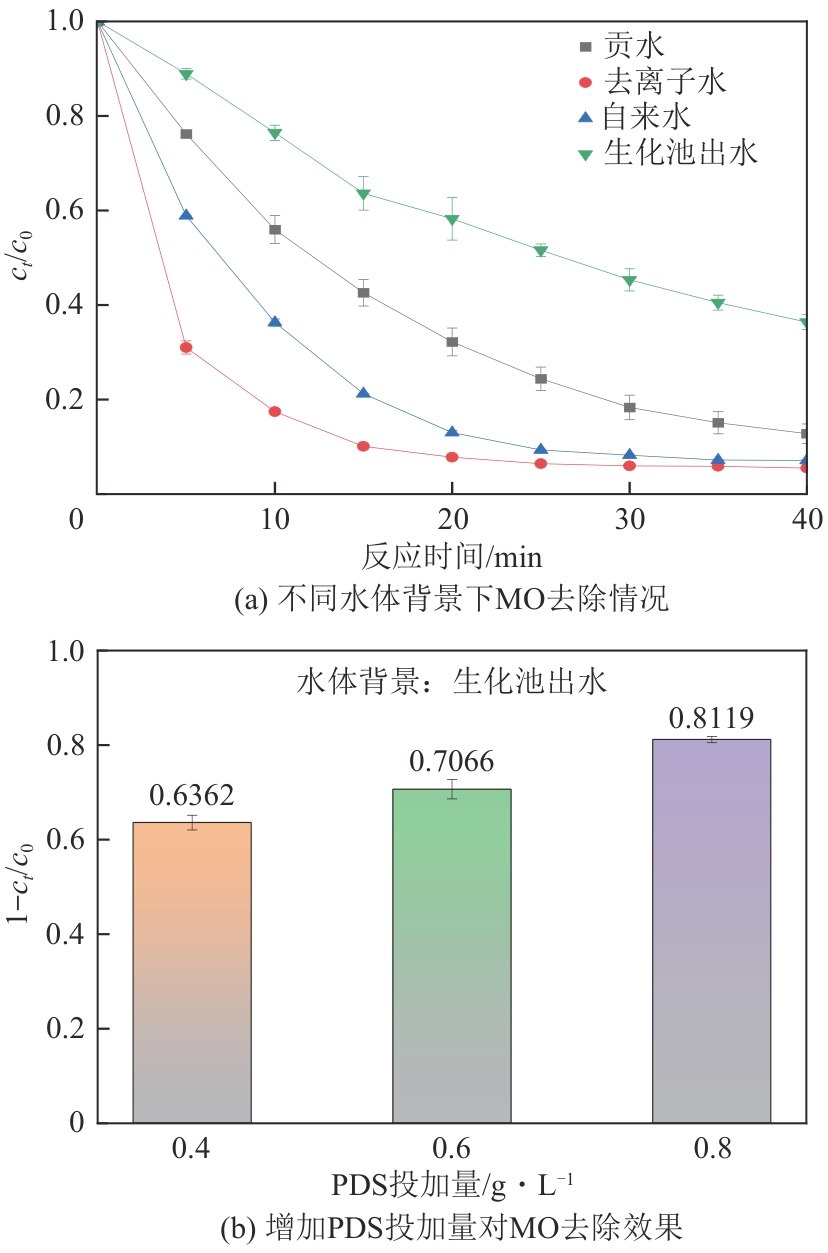
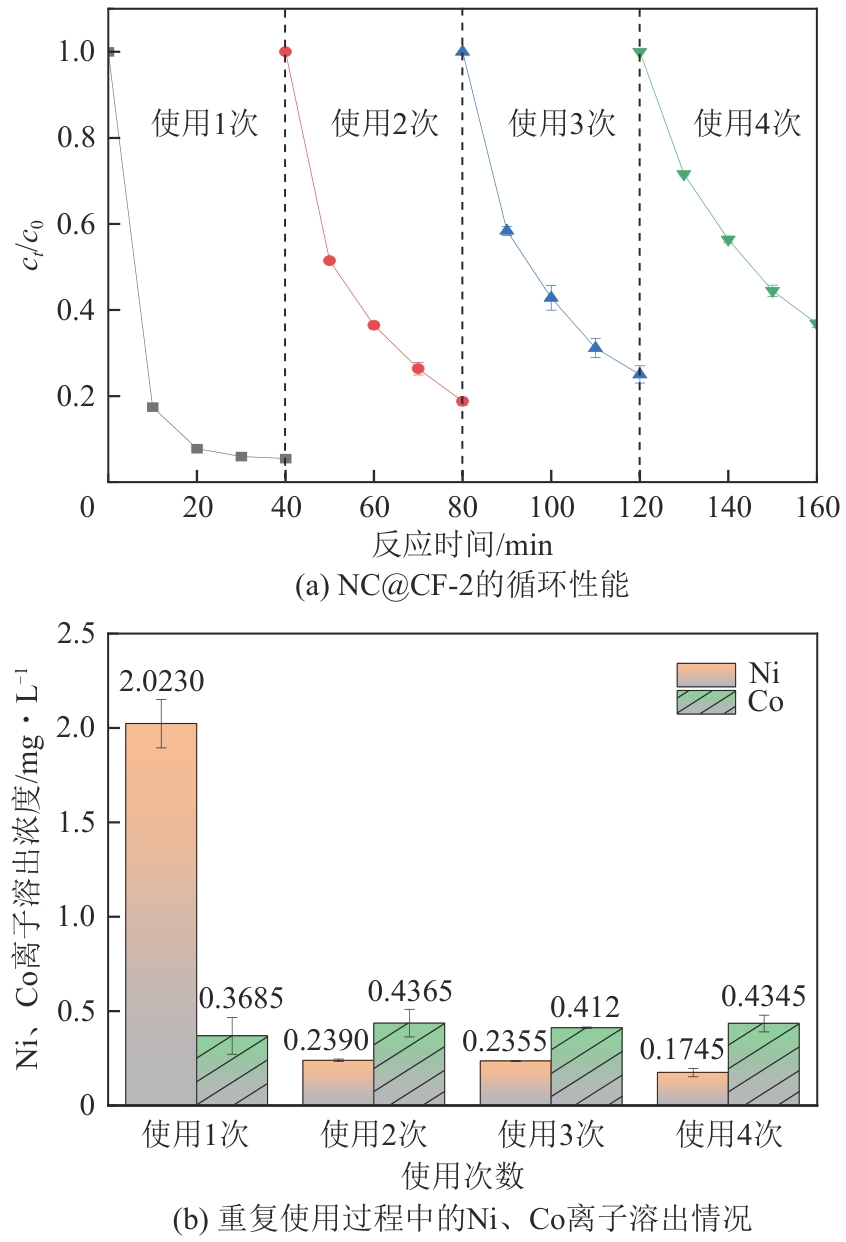
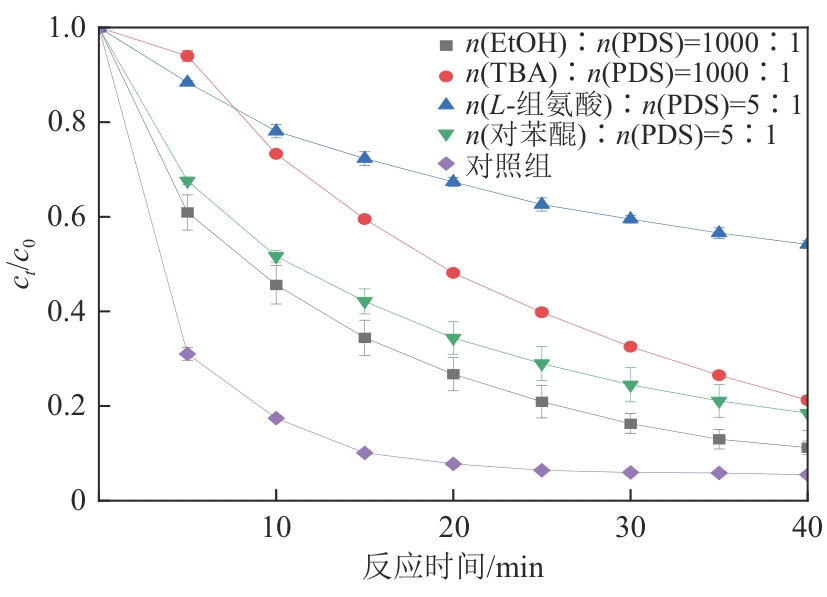
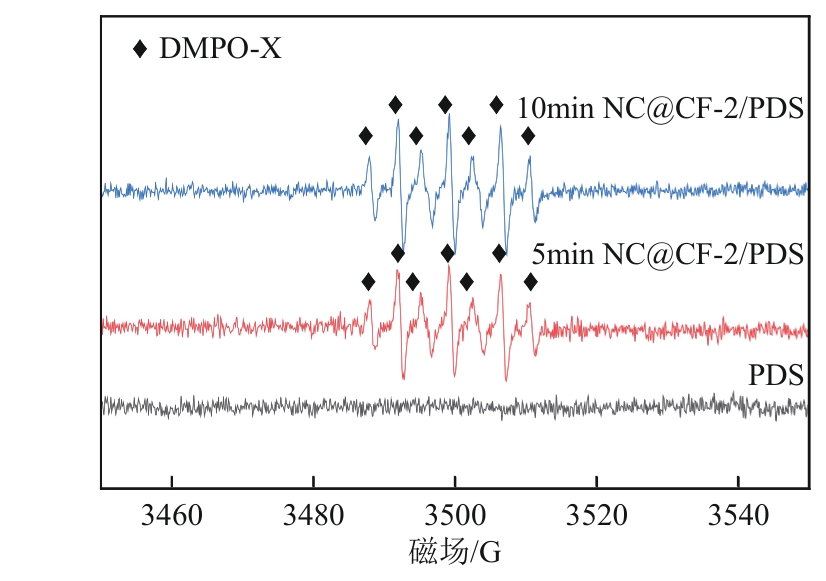
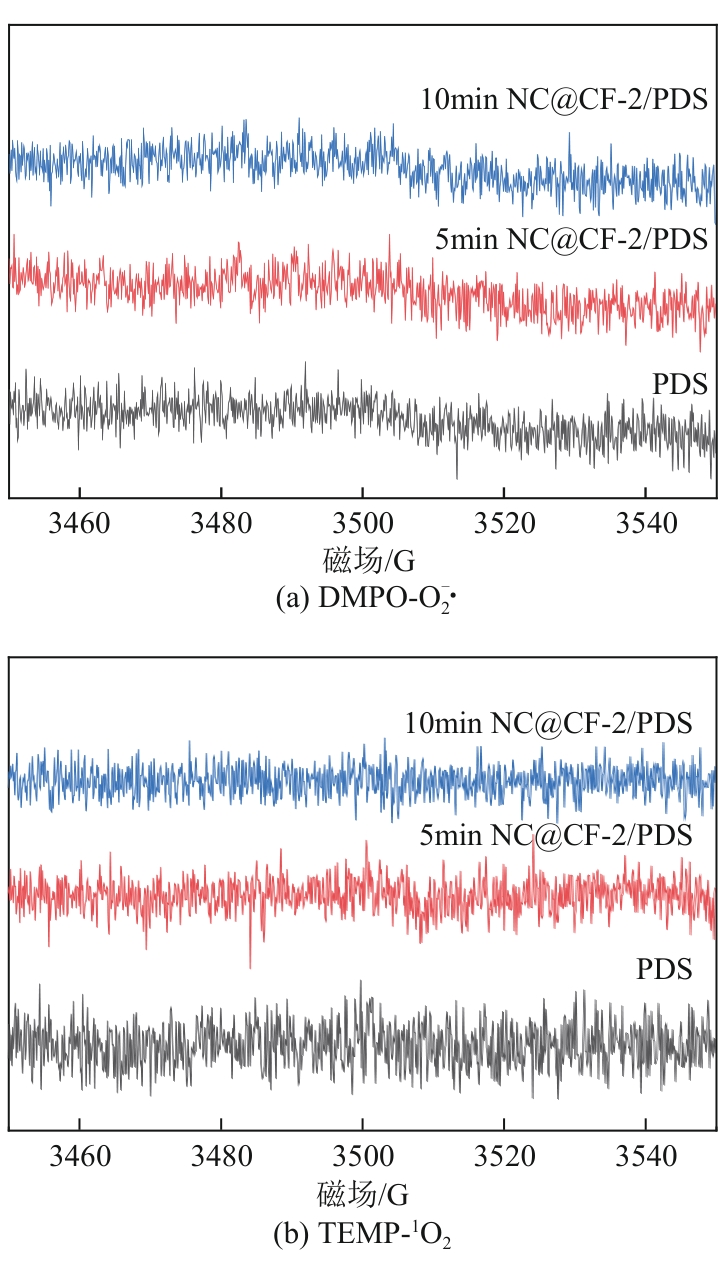
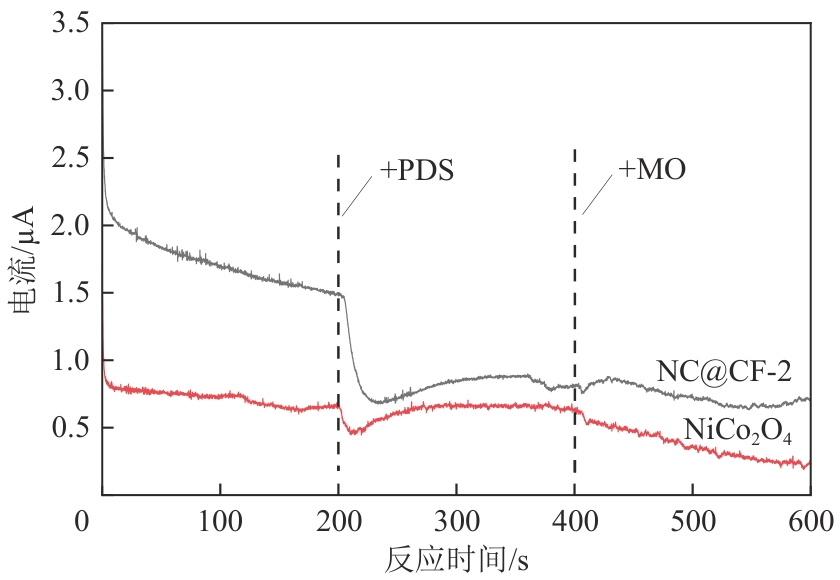
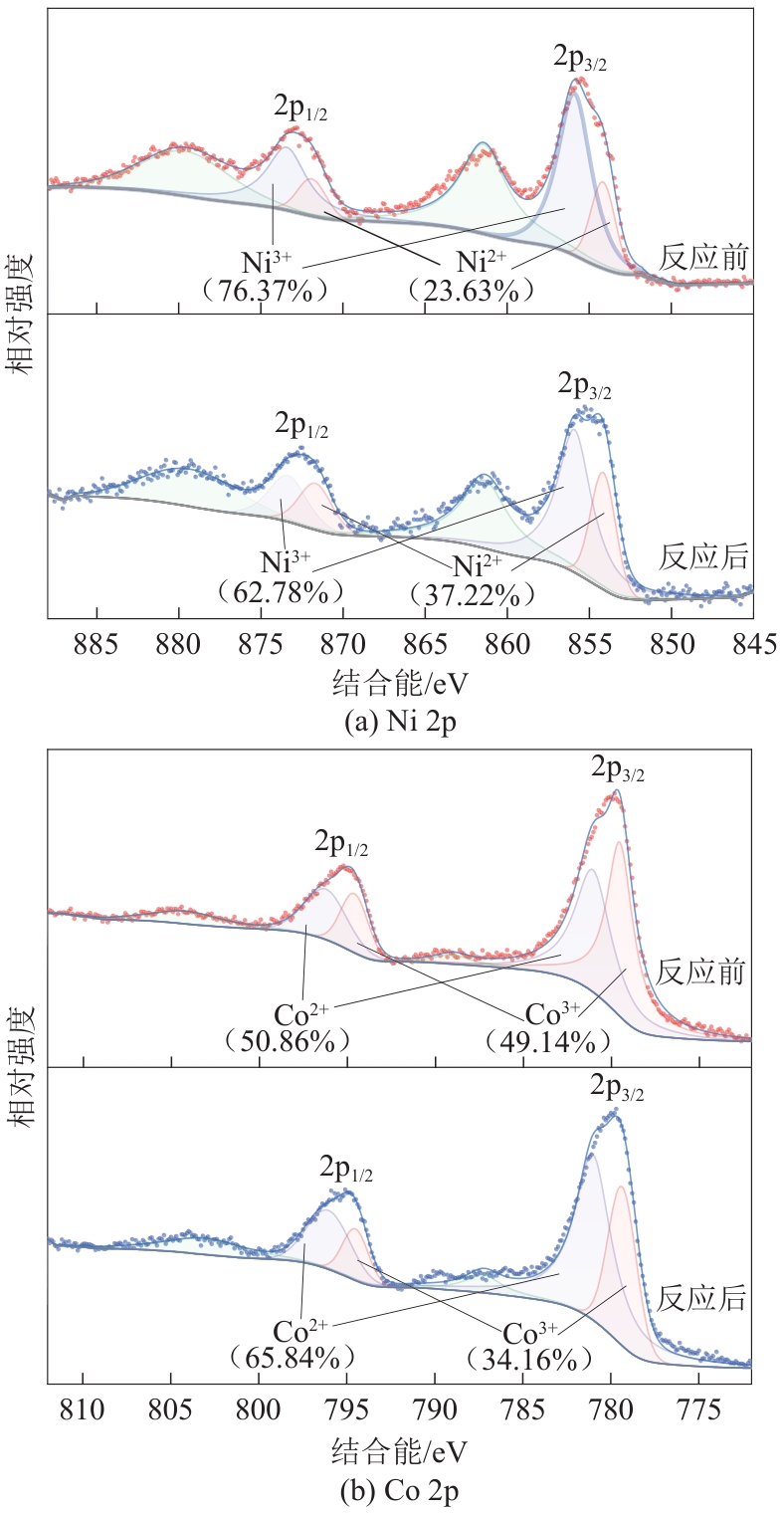
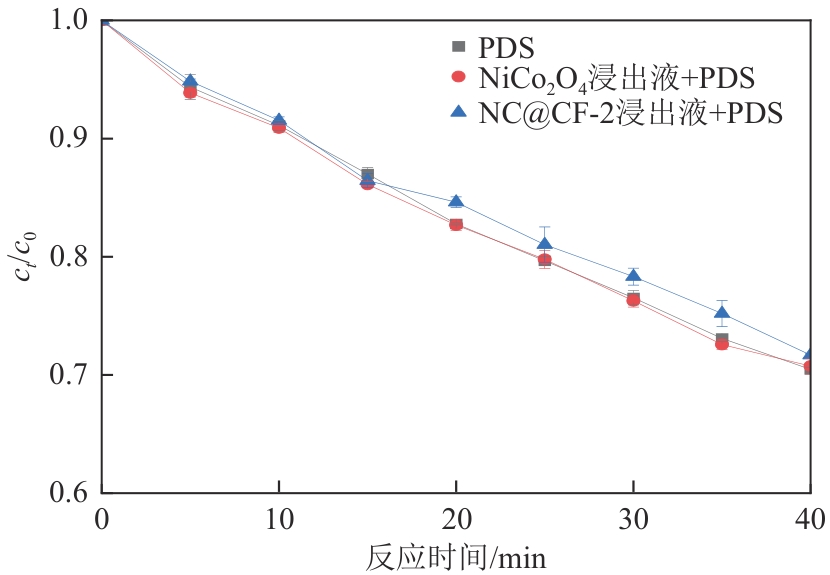
为了更好地处理水环境中的偶氮染料污染问题,充分利用我国储量丰富的天然矿物资源,实验中直接使用纤蛇纹石矿物纤维作为非均相催化剂载体处理难降解废水问题。在保证矿物资源应用经济性的同时,充分利用纤蛇纹石中的多种活性组分,以探索纤蛇纹石矿产资源利用新路径。本文通过水热-煅烧法制备具有核壳结构的复合材料NiCo2O4@纤蛇纹石纤维(NC@CF),将其用于活化过二硫酸盐(PDS)降解甲基橙(MO),并结合扫描电子显微镜、傅里叶变换红外光谱等材料表征手段对NC@CF/PDS体系降解MO的机理展开探究。结果表明:NiCo2O4和纤蛇纹石的结合在MO的降解过程中具有协同作用,负载比为2∶1的NC@CF-2/PDS体系在最优条件下降解MO,去除率可达94.50%,降解过程符合准一级动力学模型。此外,结合自由基淬灭实验、电子顺磁共振、X射线光电子能谱分析以及电化学实验表明,NC@CF-2/PDS体系中MO的降解是在·OH和SO
中图分类号:
引用本文
苏晓洁, 严群, 李欣城, 薛文慧, 陈奕浩. NiCo2O4@纤蛇纹石活化过硫酸钾降解甲基橙[J]. 化工进展, 2025, 44(4): 2352-2364.
SU Xiaojie, YAN Qun, LI Xincheng, XUE Wenhui, CHEN Yihao. Activation of potassium persulfate by NiCo2O4@chrysotile to degrade methyl orange[J]. Chemical Industry and Engineering Progress, 2025, 44(4): 2352-2364.
| 序号 | 催化剂 | 反应条件 | 污染物及去除率 | 具体情况 | NC@CF-2/PDS体系 | 参考文献 |
|---|---|---|---|---|---|---|
| 1 | 0.1g/L Co3O4/NiCo2O4双壳纳米笼 | 74μmol/L PDS催化氧化18min,T=45℃ | 8mg/L双酚A,97.3% | 酸性pH条件下显著抑制 体系催化效果,但体系具有较低的反应活化能 | 在pH为3~9时保持良好 催化效果 | [ |
| 2 | 0.8g/L 乙炔黑掺杂氧化锌(AB-ZnO) | 2mmol/L PDS光催化60min,T=30℃ | 10mg/L甲基橙,95.05% | 催化剂用量大,催化氧化条件复杂,但AB-ZnO循环利用性优异 | 在20g/L MO浓度下,催化剂投加量仅为该体系的1/8,无须添加光源,能耗较小 | [ |
| 3 | 0.5g/L CuFe2O4/硅藻土 | 0.3g/L PMS催化氧化60min,T=25℃ | 50mg/L酸性橙7,96.88% | 循环使用3次后降解率为63.95%,催化剂投加量大,重复利用性低,经济性较差 | 使用3次、4次的降解率分别为74.98%、63.18%,且反应时间较短 | [ |
表1 相关非均相催化剂/PDS体系与NC@CF-2/PDS体系对比
| 序号 | 催化剂 | 反应条件 | 污染物及去除率 | 具体情况 | NC@CF-2/PDS体系 | 参考文献 |
|---|---|---|---|---|---|---|
| 1 | 0.1g/L Co3O4/NiCo2O4双壳纳米笼 | 74μmol/L PDS催化氧化18min,T=45℃ | 8mg/L双酚A,97.3% | 酸性pH条件下显著抑制 体系催化效果,但体系具有较低的反应活化能 | 在pH为3~9时保持良好 催化效果 | [ |
| 2 | 0.8g/L 乙炔黑掺杂氧化锌(AB-ZnO) | 2mmol/L PDS光催化60min,T=30℃ | 10mg/L甲基橙,95.05% | 催化剂用量大,催化氧化条件复杂,但AB-ZnO循环利用性优异 | 在20g/L MO浓度下,催化剂投加量仅为该体系的1/8,无须添加光源,能耗较小 | [ |
| 3 | 0.5g/L CuFe2O4/硅藻土 | 0.3g/L PMS催化氧化60min,T=25℃ | 50mg/L酸性橙7,96.88% | 循环使用3次后降解率为63.95%,催化剂投加量大,重复利用性低,经济性较差 | 使用3次、4次的降解率分别为74.98%、63.18%,且反应时间较短 | [ |
| SiO2 | MgO | Fe2O3 | CaO | NiO | Cr2O3 | ZnO | SO3 | SrO | MnO | 其他 |
|---|---|---|---|---|---|---|---|---|---|---|
| 56.200 | 23.400 | 10.600 | 4.690 | 0.874 | 0.806 | 0.773 | 0.525 | 0.459 | 0.425 | 1.248 |
表2 纤蛇纹石纤维各组分质量分数(%)
| SiO2 | MgO | Fe2O3 | CaO | NiO | Cr2O3 | ZnO | SO3 | SrO | MnO | 其他 |
|---|---|---|---|---|---|---|---|---|---|---|
| 56.200 | 23.400 | 10.600 | 4.690 | 0.874 | 0.806 | 0.773 | 0.525 | 0.459 | 0.425 | 1.248 |
| 水质指标 | 贡水 | 某污水厂生化池出水 |
|---|---|---|
| pH | 6.75 | 7.05 |
| 浊度/NTU | 5.05 | 3.63 |
| UV254/cm-1 | 0.031 | 0.064 |
| DOC/mg·L-1 | 4.548 | 3.485 |
表3 不同水体的水质指标
| 水质指标 | 贡水 | 某污水厂生化池出水 |
|---|---|---|
| pH | 6.75 | 7.05 |
| 浊度/NTU | 5.05 | 3.63 |
| UV254/cm-1 | 0.031 | 0.064 |
| DOC/mg·L-1 | 4.548 | 3.485 |
| 1 | 马春燕, 沈忱思, 徐晨烨. 印染行业水污染全过程控制技术发展蓝皮书[M]. 北京: 化学工业出版社, 2021: 86. |
| MA Chunyan, SHEN Chensi, XU Chenye. Blue book on the development of water pollution control technology in printing and dyeing industry[M]. Beijing: Chemical Industry Press, 2021: 86. | |
| 2 | SIVAGAMI K, SAKTHIVEL K P, NAMBI Indumathi M. Advanced oxidation processes for the treatment of tannery wastewater[J]. Journal of Environmental Chemical Engineering, 2018, 6(3): 3656-3663. |
| 3 | TANG Xuekun, FENG Qiming, LIU Kun, et al. Fabrication of magnetic Fe3O4/silica nanofiber composites with enhanced Fenton-like catalytic performance for Rhodamine B degradation[J]. Journal of Materials Science, 2018, 53(1): 369-384. |
| 4 | LI Zishun, TANG Xuekun, LIU Kun, et al. Fabrication of novel sandwich nanocomposite as an efficient and regenerable adsorbent for methylene blue and Pb(Ⅱ) ion removal[J]. Journal of Environmental Management, 2018, 218: 363-373. |
| 5 | MIRZAEI Amir, CHEN Zhi, HAGHIGHAT Fariborz, et al. Removal of pharmaceuticals from water by homo/heterogonous Fenton-type processes—A review[J]. Chemosphere, 2017, 174: 665-688. |
| 6 | YANG Zhichao, YU Anqing, SHAN Chao, et al. Enhanced Fe(Ⅲ)-mediated Fenton oxidation of atrazine in the presence of functionalized multi-walled carbon nanotubes[J]. Water Research, 2018, 137: 37-46. |
| 7 | 李梦新. 改性过渡金属活化过硫酸盐降解酸性橙7机理研究[D]. 保定: 河北大学, 2022. |
| LI Mengxin. Study on the mechanism of modified transition metal activated persulfate to degrade Acid Orange 7[D]. Baoding: Hebei University, 2022. | |
| 8 | 王妙婷, 王可心, 杨曾, 等. 磁性硅藻土非均相Fenton催化剂制备及其催化性能[J]. 工业水处理, 2018, 38(5): 84-87. |
| WANG Miaoting, WANG Kexin, YANG Zeng, et al. Preparation of magnetic diatomite heterogeneous Fenton catalyst and its catalytic capability[J]. Industrial Water Treatment, 2018, 38(5): 84-87. | |
| 9 | 尚晓涵, 朱小彪. 中性pH条件下Fe/Cu/沸石催化非均相Fenton降解水中苯并三唑[J]. 环境工程, 2021, 39(2): 10-15. |
| SHANG Xiaohan, ZHU Xiaobiao. Heterogeneous Fenton degradation of benzotriazole in water by Fe/Cu/zeolite catalyst at neutral pH value[J]. Environmental Engineering, 2021, 39(2): 10-15. | |
| 10 | TANG Xuekun, FENG Qiming, LIU Kun, et al. Synthesis and characterization of a novel nanofibrous TiO2/SiO2 composite with enhanced photocatalytic activity[J]. Materials Letters, 2016, 183: 175-178. |
| 11 | ZHANG Wenwen, SU Yi, ZHANG Xuemei, et al. Facile synthesis of porous NiCo2O4 nanoflakes as magnetic recoverable catalysts towards the efficient degradation of RhB[J]. RSC Advances, 2016, 6(69): 64626-64633. |
| 12 | TIAN Xike, TIAN Chen, NIE Yulun, et al. Controlled synthesis of dandelion-like NiCo2O4 microspheres and their catalytic performance for peroxymonosulfate activation in humic acid degradation[J]. Chemical Engineering Journal, 2018, 331: 144-151. |
| 13 | JIANG Jingjing, WANG Xingyue, ZHANG Chongjun, et al. Porous 0D/3D NiCo2O4/g-C3N4 accelerate emerging pollutant degradation in PMS/vis system: Degradation mechanism, pathway and toxicity assessment[J]. Chemical Engineering Journal, 2020, 397: 125356. |
| 14 | ZHANG Chengji, CHEN Hong, XUE Gang, et al. A critical review of the aniline transformation fate in azo dye wastewater treatment[J]. Journal of Cleaner Production, 2021, 321: 128971. |
| 15 | WANG Meimei, CUI Yunke, CAO Hongyang, et al. Activating peroxydisulfate with Co3O4/NiCo2O4 double-shelled nanocages to selectively degrade bisphenol A—A nonradical oxidation process[J]. Applied Catalysis B: Environmental, 2021, 282: 119585. |
| 16 | WANG Lijuan, YUAN Zifan, WANG Hanzheng, et al. Acetylene black doped zinc oxide (AB-ZnO) nanorods activated peroxydisulfate for degradation of dyeing wastewater[J]. Materials Chemistry and Physics, 2024, 314: 128870. |
| 17 | 陈锦富, 严群, 龚鹏程, 等. CuFe2O4/硅藻土复合材料活化过一硫酸盐降解酸性橙[J]. 精细化工, 2023, 40(9): 2042-2051. |
| CHEN Jinfu, YAN Qun, GONG Pengcheng, et al. CuFe2O4/diatomite composite activated peroxymonosulfate for degradation of acid orange 7[J]. Fine Chemicals, 2023, 40(9): 2042-2051. | |
| 18 | GIROTTO Camila P, DE CAMPOS Sílvia D, DE CAMPOS Élvio A. Chrysotile asbestos treated with phosphoric acid as an adsorbent for ammonia nitrogen[J]. Heliyon, 2020, 6(2): e03397. |
| 19 | VALOUMA Aikaterini, VERGANELAKI Anastasia, Pagona MARAVELAKI-KALAITZAKI, et al. Chrysotile asbestos detoxification with a combined treatment of oxalic acid and silicates producing amorphous silica and biomaterial[J]. Journal of Hazardous Materials, 2016, 305: 164-170. |
| 20 | WYPYCH Fernando, SCHREINER Wido H, MATTOSO Ney, et al. Covalent grafting of phenylphosphonate groups onto layered silica derived from in situ-leached chrysotile fibers[J]. Journal of Materials Chemistry, 2003, 13(2): 304-307. |
| 21 | ZHOU Xuan, LI Xinyuan, XU Caixia, et al. A persulfate oxidation system for removing acid orange from aqueous solution: Evaluation and degradation mechanism[J]. Journal of Environmental Management, 2022, 322: 116054. |
| 22 | 黄仕元, 林森焕, 董雯, 等. 锰氮共掺杂稻壳生物炭活化过二硫酸盐降解酸性橙[J]. 复合材料学报, 2023, 40(2): 1071- 1084. |
| HUANG Shiyuan, LIN Senhuan, DONG Wen, et al. Manganese-nitrogen co-doped rice husk biochar activated peroxydisulfate to degrade acid orange[J]. Acta Materiae Compositae Sinica, 2023, 40(2): 1071-1084. | |
| 23 | 傅晓艳, 赵委托, 杜江坤, 等. 稳定化纳米零价铁活化过硫酸盐降解罗丹明B[J]. 中国给水排水, 2020, 36(11): 57-62. |
| FU Xiaoyan, ZHAO Weituo, DU Jiangkun, et al. Degradation of rhodamine B by nano-scale zero-valent iron activated persulfate[J]. China Water & Wastewater, 2020, 36(11): 57-62. | |
| 24 | QIU Yue, ZHANG Qian, WANG Zhihao, et al. Degradation of anthraquinone dye reactive blue 19 using persulfate activated with Fe/Mn modified biochar: Radical/non-radical mechanisms and fixed-bed reactor study[J]. Science of the Total Environment, 2021, 758: 143584. |
| 25 | TRAN Thi Nhung, Quoc Cuong DO, KANG Jungwan, et al. Boosted micropollutant removal over urchin-like structured hydroxyapatite-incorporated nickel magnetite catalyst via peroxydisulfate activation[J]. Water Research, 2024, 249: 120951. |
| 26 | YU Chao, YAN Chongchong, GU Jiyan, et al. In-situ Cu-loaded sludge biochar catalysts for oxidative degradation of bisphenol A from high-salinity wastewater[J]. Journal of Cleaner Production, 2023, 427: 139334. |
| 27 | 朱世俊. 给水厂铁泥基催化剂制备及活化过硫酸盐效能与机制[D]. 哈尔滨: 哈尔滨工业大学, 2021. |
| ZHU Shijun. Development of catalyst derived from drinking water treatment iron sludge for peroxymonosulfate activation and mechanisms[D]. Harbin: Harbin Institute of Technology, 2021. | |
| 28 | 吴承梓, 张巍, 万彦涛, 等. 盐酸羟胺/铁基MOFs/过硫酸盐体系降解磺胺嘧啶[J]. 中国环境科学, 2021, 41(6): 2685 -2697. |
| WU Chengzi, ZHANG Wei, WAN Yantao, et al. Degradation of sulfadiazine by hydroxylamine hydrochloride/Fe-MOFs/persulfate system[J]. China Environmental Science, 2021, 41(6): 2685-2697. | |
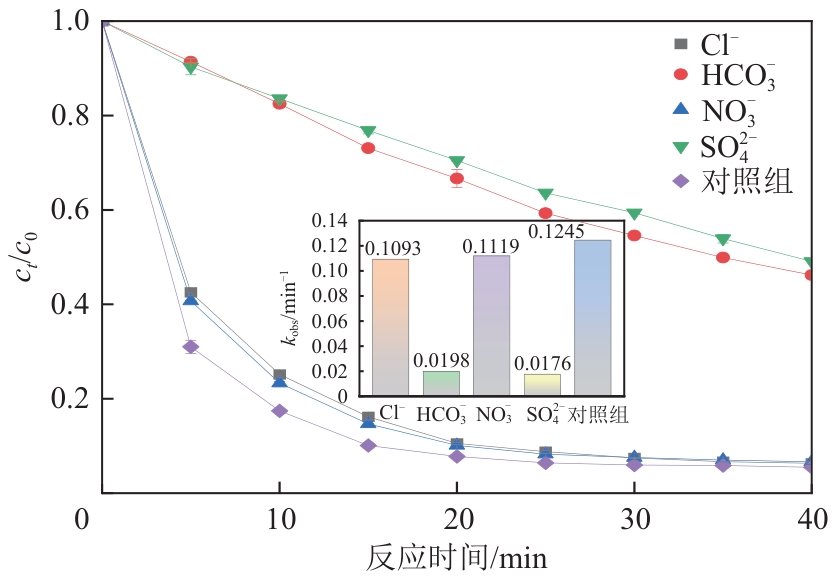
| 29 | WANG Jianlong, WANG Shizong. Effect of inorganic anions on the performance of advanced oxidation processes for degradation of organic contaminants[J]. Chemical Engineering Journal, 2021, 411: 128392. |
| 30 | WU Xiaoliang, GU Xiaogang, LU Shuguang, et al. Strong enhancement of trichloroethylene degradation in ferrous ion activated persulfate system by promoting ferric and ferrous ion cycles with hydroxylamine[J]. Separation and Purification Technology, 2015,147: 186-193. |
| 31 | 尹汉雄, 唐玉朝, 黄显怀, 等. 紫外光强化Fe(Ⅱ)-EDTA活化过硫酸盐降解直接耐酸大红4BS[J]. 环境科学研究, 2017, 30(7): 1105-1111. |
| YIN Hanxiong, TANG Yuchao, HUANG Xianhuai, et al. Decolorization effect of direct fast scarlet 4BS by Fe (Ⅱ)-EDTA activated peroxodisulfate under ultraviolet light[J]. Research of Environmental Sciences, 2017, 30(7): 1105-1111. | |
| 32 | YAO Yunjin, YANG Zeheng, SUN Hongqi, et al. Hydrothermal synthesis of Co3O4-graphene for heterogeneous activation of peroxymonosulfate for decomposition of phenol[J]. Industrial & Engineering Chemistry Research, 2012, 51(46): 14958-14965. |
| 33 | LEE Hongshin, KIM Hyoung-Il, WEON Seunghyun, et al. Activation of persulfates by graphitized nanodiamonds for removal of organic compounds[J]. Environmental Science & Technology, 2016, 50(18): 10134-10142. |
| 34 | 龚鹏程, 严群, 陈锦富, 等. 铁酸钴复合碳纳米管活化过硫酸盐降解铬黑T的性能及机理[J]. 化工进展, 2023, 42(7): 3572-3581. |
| GONG Pengcheng, YAN Qun, CHEN Jinfu, et al. Properties and mechanism of eriochrome black T degradation by carbon nanotube-cobalt ferrite composites activated persulfate[J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3572-3581. | |
| 35 | 唐学昆. 纤蛇纹石基氧化硅制备负载型高级氧化催化材料研究[D]. 长沙: 中南大学, 2021. |
| TANG Xuekun. Preparation of supported advanced oxidation catalysts from chrysotile-derived silica[D]. Changsha: Central South University, 2021. | |
| 36 | MAENO Shohei, ZHU Qianqian, SASAKI Masahide, et al. Monopersulfate oxidation of tetrabromobisphenol A by an iron(Ⅲ)- phthalocyaninetetrasulfate catalyst coordinated to imidazole functionalized silica particles[J]. Journal of Molecular Catalysis A: Chemical, 2015, 400: 56-63. |
| 37 | 裴轩瑗. 污泥基生物炭活化PDS去除抗生素抗性基因及降解双酚机制[D]. 哈尔滨: 哈尔滨工业大学, 2022. |
| PEI Xuanyuan. Mechanism for removal of antibiotic and antibiotic resistance genes and degradation of bisphenol by using sludge-derived biochar activated persulfate[D]. Harbin: Harbin Institute of Technology, 2022. | |
| 38 | TAN Chaoqun, LU Xu, CUI Xinxin, et al. Novel activation of peroxymonosulfate by an easily recyclable VC@Fe3O4 nanoparticles for enhanced degradation of sulfadiazine[J]. Chemical Engineering Journal, 2019, 363: 318-328. |
| 39 | DUAN Yan, XUE Mifeng, LIU Bin, et al. Integration of theory prediction and experimental electrooxidation of glycerol on NiCo2O4 nanosheets[J]. Chinese Journal of Catalysis, 2024, 57: 68-79. |
| 40 | BONDARCHUK Oleksandr, LAGROW Alec P, KVASHA Andriy, et al. On the X-ray photoelectron spectroscopy analysis of LiNi x Mn y Co z O2 material and electrodes[J]. Applied Surface Science, 2021, 535: 147699. |
| 41 | WU Xuanhao, KIM Jae-Hong. Outlook on single atom catalysts for persulfate-based advanced oxidation[J]. ACS ES&T Engineering, 2022, 2(10): 1776-1796. |
| [1] | 王宁, 陆诗建, 刘玲, 梁静, 刘苗苗, 孙梦圆, 康国俊. 胺吸收体系中CO2催化解吸再生技术的研究进展[J]. 化工进展, 2025, 44(1): 445-464. |
| [2] | 高雨飞, 鲁金凤. 非均相催化臭氧氧化作用机理研究进展[J]. 化工进展, 2023, 42(S1): 430-438. |
| [3] | 姚稳, 张雨晨, 滕文馨, 黎江玲. 表面活性剂对制备Ca掺杂β-In2S3微观结构的影响及其光催化降解甲基橙性能[J]. 化工进展, 2023, 42(2): 774-782. |
| [4] | 张静, 马慧玲, 曾得福, 姚潇毅. 水热催化制备绿色柴油工艺中催化剂的失活与再生[J]. 化工进展, 2022, 41(2): 682-689. |
| [5] | 何广源, 陈学敏, 王雨婷, 李发堂, 张子健, 许文浩. 钴酸镍基纳米材料在超级电容器中的研究进展[J]. 化工进展, 2021, 40(7): 3813-3825. |
| [6] | 闫雪倩, 裴向军, 杜杰, 米晓慧, 白琳嵚, 马蕊, 张明宽, 钱进. Bi2O2CO3/Fe3O4磁性复合物在环境污染治理领域中的应用[J]. 化工进展, 2021, 40(6): 3515-3525. |
| [7] | 赵媛媛,刘文静,董培,张亮,杨政伟,赵朝成. 聚苯胺中间层改性Ti/PbO2电极的制备及其降解性能[J]. 化工进展, 2019, 38(12): 5478-5486. |
| [8] | 张岩, 李渊, 赵立国, 谭小耀, 覃环, 王秋月, 黄从付. SAPO-18分子筛的快速合成及其甲醇制烯烃催化性能[J]. 化工进展, 2018, 37(05): 1815-1822. |
| [9] | 姚培, 李树白, 刘媛, 张启蒙, 季从兰, 聂华丽. HTMAC/KH550/膨润土吸附剂的制备、表征及其在甲基橙废水的吸附行为[J]. 化工进展, 2017, 36(04): 1374-1380. |
| [10] | 陈缘博, 包慧敏, 苗海龙, 李晓林, 王益庆, 郑广宇. 氧化破胶剂过硫酸钾的安全性[J]. 化工进展, 2016, 35(10): 3267-3272. |
| [11] | 杨梅梅1,周少奇1,2,3,郑永鑫1,郑 可1. 零价铁活化过硫酸钠降解甲基橙[J]. 化工进展, 2012, 31(09): 2093-2096. |
| [12] | 王 俊1,李 云1,李翠勤1,张怀志2. 铬系聚乙烯催化剂研究开发新进展[J]. 化工进展, 2012, 31(01 ): 91-97. |
| [13] | 徐 迈,王凤武,胡云虎,方文彦,朱传高. Ti/nanoTiO2-PAn复合膜电极催化降解甲基橙[J]. 化工进展, 2011, 30(11): 2554-. |
| [14] | 蒋 波,张晓东,孙 立,许 敏. 微波促进生物柴油制备的研究进展 [J]. 化工进展, 2010, 29(11): 2057-. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||