化工进展 ›› 2023, Vol. 42 ›› Issue (8): 4123-4135.DOI: 10.16085/j.issn.1000-6613.2023-0289
代谢工程改造微生物合成生物基单体的进展与挑战
- 江南大学食品科学与技术国家重点实验室,江苏 无锡 214122
-
收稿日期:2023-02-28修回日期:2023-04-08出版日期:2023-08-15发布日期:2023-09-19 -
通讯作者:刘立明 -
作者简介:高聪(1991—),男,副研究员,研究方向为微生物代谢工程。E-mail:conggao@jiangnan.edu.cn。 -
基金资助:国家重点研发计划(2021YFC2100700);国家自然科学基金青年项目(22008087)
Progress and challenges of engineering microorganisms to produce biobased monomers
GAO Cong( ), CHEN Chenghu, CHEN Xiulai, LIU Liming(
), CHEN Chenghu, CHEN Xiulai, LIU Liming( )
)
- State Key Laboratory of Food Science and Technology, Jiangnan University, Wuxi 214122, Jiangsu, China
-
Received:2023-02-28Revised:2023-04-08Online:2023-08-15Published:2023-09-19 -
Contact:LIU Liming
摘要:
单体是合成聚合物所用的小分子基础原料,目前主要来源于化石燃料。利用微生物制备生物基单体具有生产条件温和、环境友好、可持续的优势,是实现高分子材料行业绿色制造的重要途径。借助代谢工程和合成生物学元件,目前已经实现了多种单体的微生物制造,然而与石油基生产工艺相比,这些单体微生物细胞工厂的生产性能普遍较低。围绕代谢工程改造微生物合成生物基单体过程中存在的瓶颈问题,本文基于具体案例分析,从廉价底物的高效利用、提高生物基单体合成效率、强化细胞环境耐受性三个方面,总结了改造微生物合成单体的最新研究进展。同时,讨论了单体微生物细胞工厂目前存在的挑战和未来发展方向。
中图分类号:
引用本文
高聪, 陈城虎, 陈修来, 刘立明. 代谢工程改造微生物合成生物基单体的进展与挑战[J]. 化工进展, 2023, 42(8): 4123-4135.
GAO Cong, CHEN Chenghu, CHEN Xiulai, LIU Liming. Progress and challenges of engineering microorganisms to produce biobased monomers[J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4123-4135.
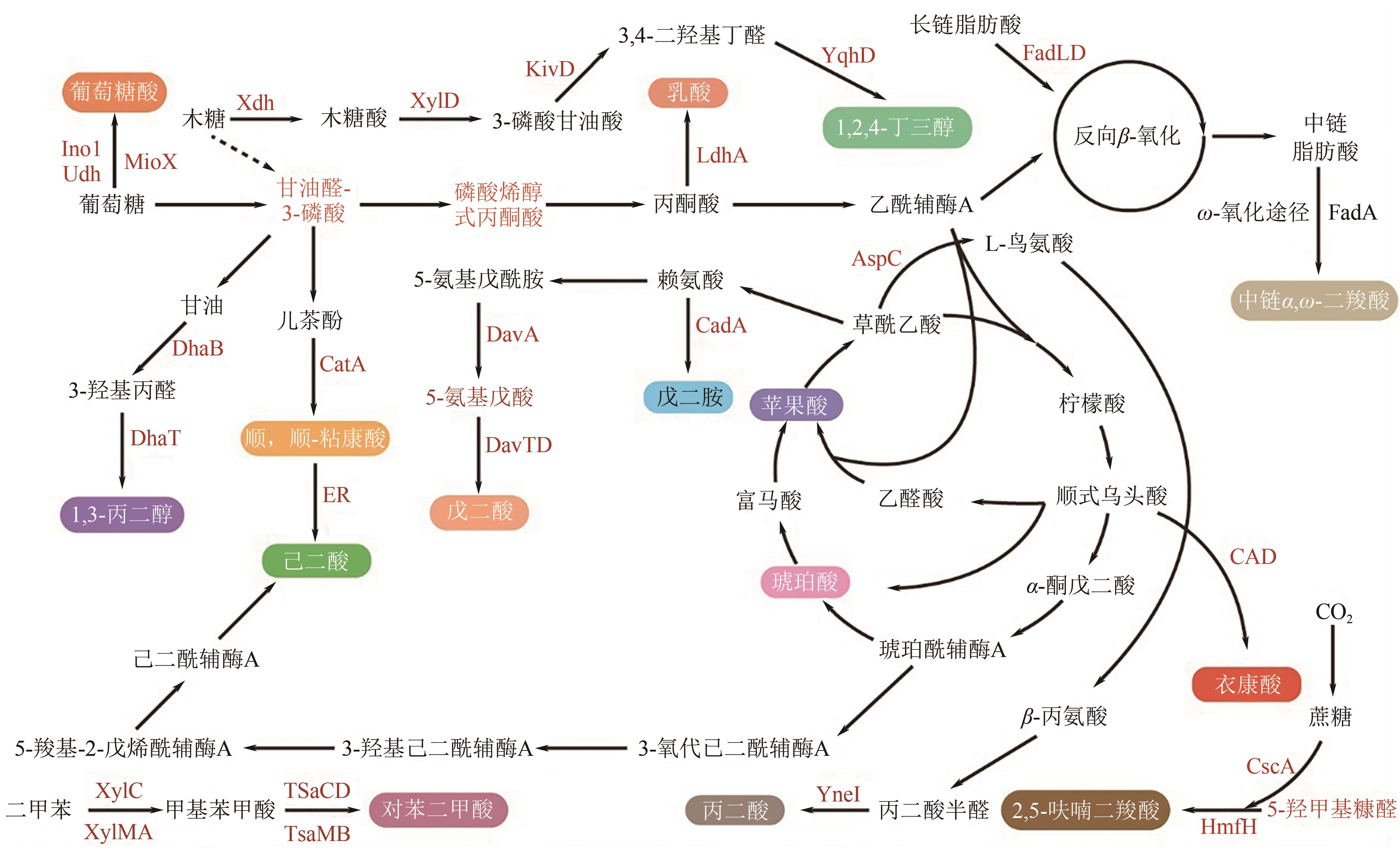
图1 微生物合成塑料单体的代谢途径CadA—赖氨酸脱羧酶I;CatA—儿茶酚1,2-双加氧酶;DavA—5-氨基戊酰胺酶;DavD—戊二酸半醛脱氢酶;DavT—5-氨基戊酸氨基转移酶;ER—烯酸还原酶;KivD—α-酮酸脱羧酶;LdhA—乳酸脱氢酶;YneI—丁二酸半醛脱氢酶;TsaC—4-CBAL脱氢酶;TsaD—4-CBA脱氢酶;TsaMB—对甲苯磺酸单加氧酶;Xdh—木糖脱氢酶;XylC—苯甲醛脱氢酶;XylD—木糖脱水酶;XylMA—二甲苯单氧酶;YqhD—乙醇脱氢酶;DhaB—甘油脱水酶;DhaT—1,3-丙二醇氧化还原酶;AspC—天冬氨酸氨基转移酶;CAD—顺式乌头酸脱羧酶;Ino1—肌醇-1-磷酸合酶;MioX—肌醇加氧酶;Udh—尿酸脱氢酶;3DGP—3-脱氧甘油戊酸酯;FadD—脂肪酰辅酶A合成酶;CscA—蔗糖-6-磷酸水解酶;HmfH—HMF/糠醛氧化还原酶
| 单体 | 市场/CNY | 菌株 | 关键调控策略 | 生产工艺 | 产量/g·L-1 | 参考文献 |
|---|---|---|---|---|---|---|
| 1,4-BDO | 126亿 | 大肠杆菌 | 代谢网络模型模拟、合成途径基因组整合 | 生物发酵 | 125.0 | [ |
| 大肠杆菌 | 全细胞催化赤藓糖醇合成 | 全细胞转化 | 4.0×10-2 | [ | ||
| 大肠杆菌 | 基因编辑调控碳流分布、抑制副产物合成 | 生物发酵 | 1.8 | [ | ||
| 大肠杆菌 | 构建谷氨酸转化途径 | 生物发酵 | 1.4 | [ | ||
| 戊二胺 | 2.20亿 | 大肠杆菌 | 蛋白质定向进化 | 全细胞转化 | 418 | [ |
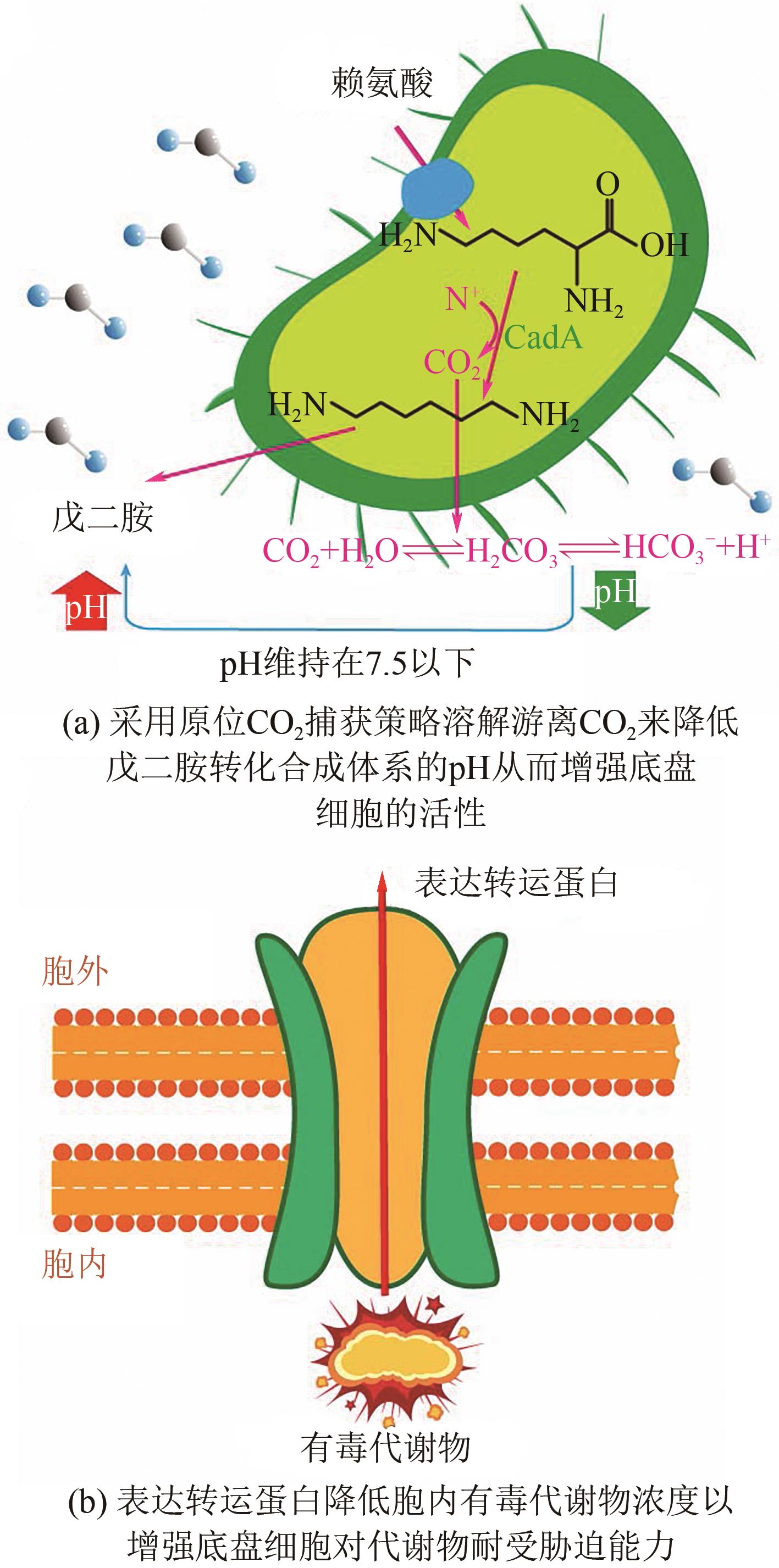
| 大肠杆菌 | 原位CO2捕集技术 | 全细胞转化 | 209.3 | [ | ||
| 大肠杆菌 | 结合定向进化和计算指导的虚拟筛选 | 全细胞转化 | 160.7 | [ | ||
| 谷棒杆菌 | 基因组整合、赖氨酸合成强化 | 生物发酵 | 125 | [ | ||
| 大肠杆菌 | 适应性进化 | 生物发酵 | 58.7 | [ | ||
| 大肠杆菌 | 温度开关设计、丁二酸联产实现碳固定 | 生物发酵 | 55.6 | [ | ||
| 大肠杆菌 | 构建抑制基因表达的sRNAs文库 | 生物发酵 | 13.7 | [ | ||
| 己二酸 | 60亿 | 大肠杆菌 | 平衡路径酶表达水平、阻断副产物积累 | 生物发酵 | 68 | [ |
| 台湾假单胞菌 | 酶挖掘、级联反应设计、代谢优化 | 全细胞转化 | 10.2 | [ | ||
| 大肠杆菌 | 化学溶剂处理和物理破碎方法 | 生物发酵 | 55.5 | [ | ||
| 酿酒酵母 | 三阶段发酵工艺优化,筛选异源酶 | 生物发酵 | 2.6×10-3 | [ | ||
| 大肠杆菌 | 建立氧依赖型动态调节系统 | 生物发酵 | 0.5 | [ | ||
| 酿酒酵母 | 过表达特异性多药耐药转运蛋白 | 生物发酵 | 0.4 | [ | ||
| 1,3-PDO | 7.76亿 | 大肠杆菌 | 阻断甘油氧化途径、引入异源途径、失活PTS | 生物发酵 | 135 | [ |
| 谷棒杆菌 | 还原力平衡、降低有毒中间代谢物积累 | 生物发酵 | 110 | [ | ||
| 罗伊氏乳杆菌 | 电子束辐照诱变 | 生物发酵 | 93.2 | [ | ||
| 肺炎克雷伯菌 | 敲除葡萄糖转运蛋白Crr | 生物发酵 | 78 | [ | ||
| 需钠弧菌 | 删除全局转录调节因子,转录组学分析 | 生物发酵 | 56.2 | [ | ||
| 丁酸梭菌 | 组合NTG和ARTP诱变筛选 | 生物发酵 | 37 | [ | ||
| 大肠杆菌 | 微调混合途径以匹配合成路径的能源需求 | 生物发酵 | 22.7 | [ | ||
| 乳酸 | 29亿 | 大肠杆菌 | 阻断副产物合成 | 生物发酵 | 142.2 | [ |
| 酿酒酵母 | 引入耐受蛋白IoGAS1 | 生物发酵 | 92 | [ | ||
| 大肠杆菌 | 筛选木糖分解代谢操纵子 | 生物发酵 | 86 | [ | ||
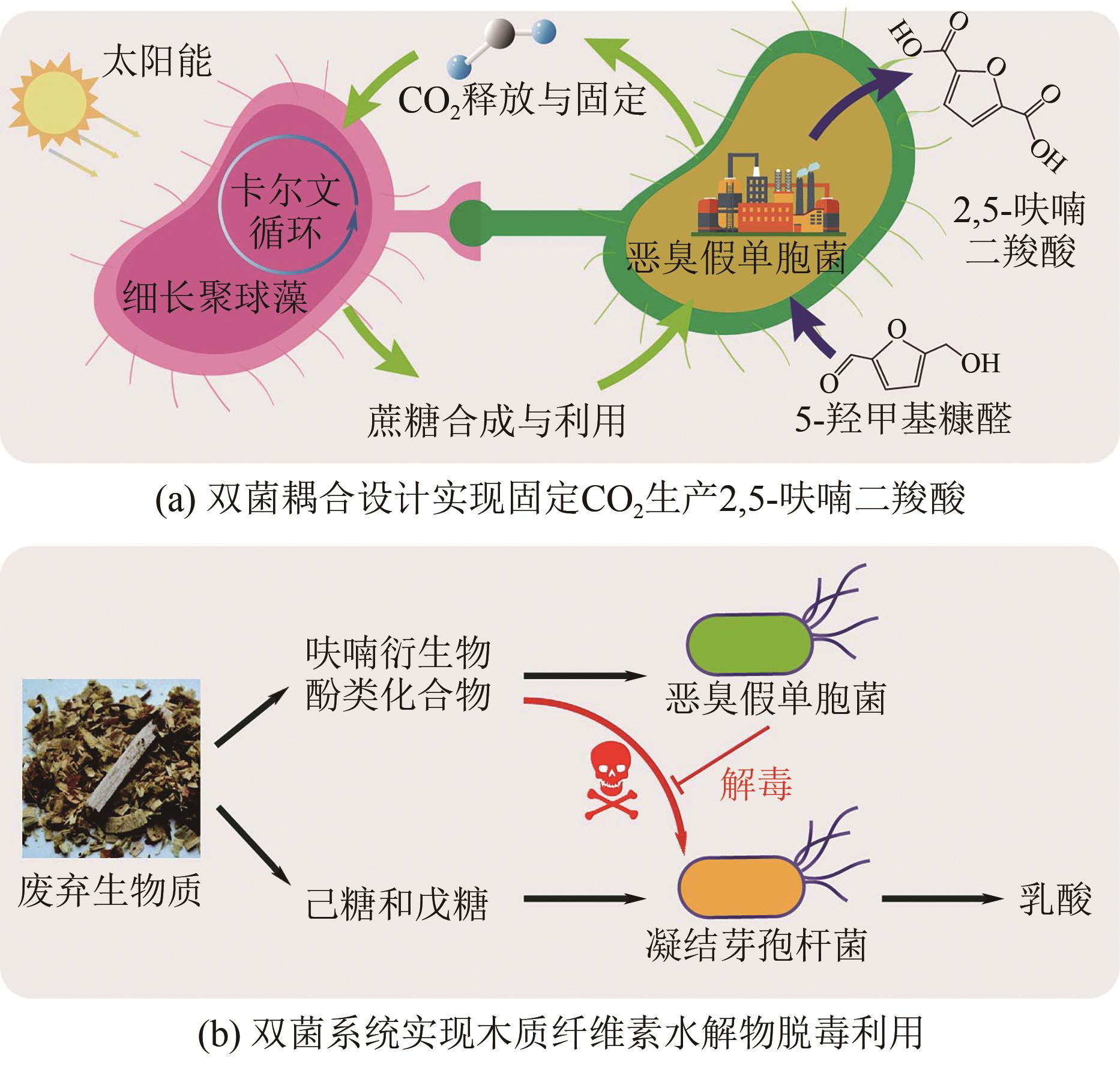
| 恶臭假单胞菌、凝结芽孢杆菌 | 合成微生物群落 | 生物发酵 | 35.8 | [ | ||
| 食木薯乳杆菌 | 聚集用于底物的同时糖化和发酵 | 生物发酵 | 18.7 | [ | ||
| 里氏木霉、戴尔根霉 | 合成微生物群落 | 生物发酵 | 4.4 | [ | ||
| 对苯二甲酸 | 495亿 | 大肠杆菌 | 基因组多拷贝整合 | 全细胞转化 | 38.3 | [ |
| 大肠杆菌 | 微生物双相转化 | 全细胞转化 | 6.9 | [ | ||
| 丁二酸 | 2.23亿 | 产琥珀酸曼氏杆菌 | 筛选不同来源的苹果酸脱氢酶 | 生物发酵 | 134.3 | [ |
| 大肠杆菌 | 溶氧动态开关 | 生物发酵 | 119 | [ | ||
| 产琥珀酸曼氏杆菌 | 改变细胞膜的流动性 | 生物发酵 | 84.2 | [ | ||
| 产琥珀酸放线杆菌 | 生物被膜的细胞固定化 | 生物发酵 | 67.3 | [ | ||
| 大肠杆菌 | 替代高能耗途径 | 生物发酵 | 56.9 | [ | ||
| 大肠杆菌 | 过表达大肠杆菌全局转录调控因子IrrE | 生物发酵 | 24.5 | [ | ||
| 产丙酸丙酸杆菌 | 原位产物分离,开发膜分离耦合发酵技术 | 生物发酵 | 20.5 | [ | ||
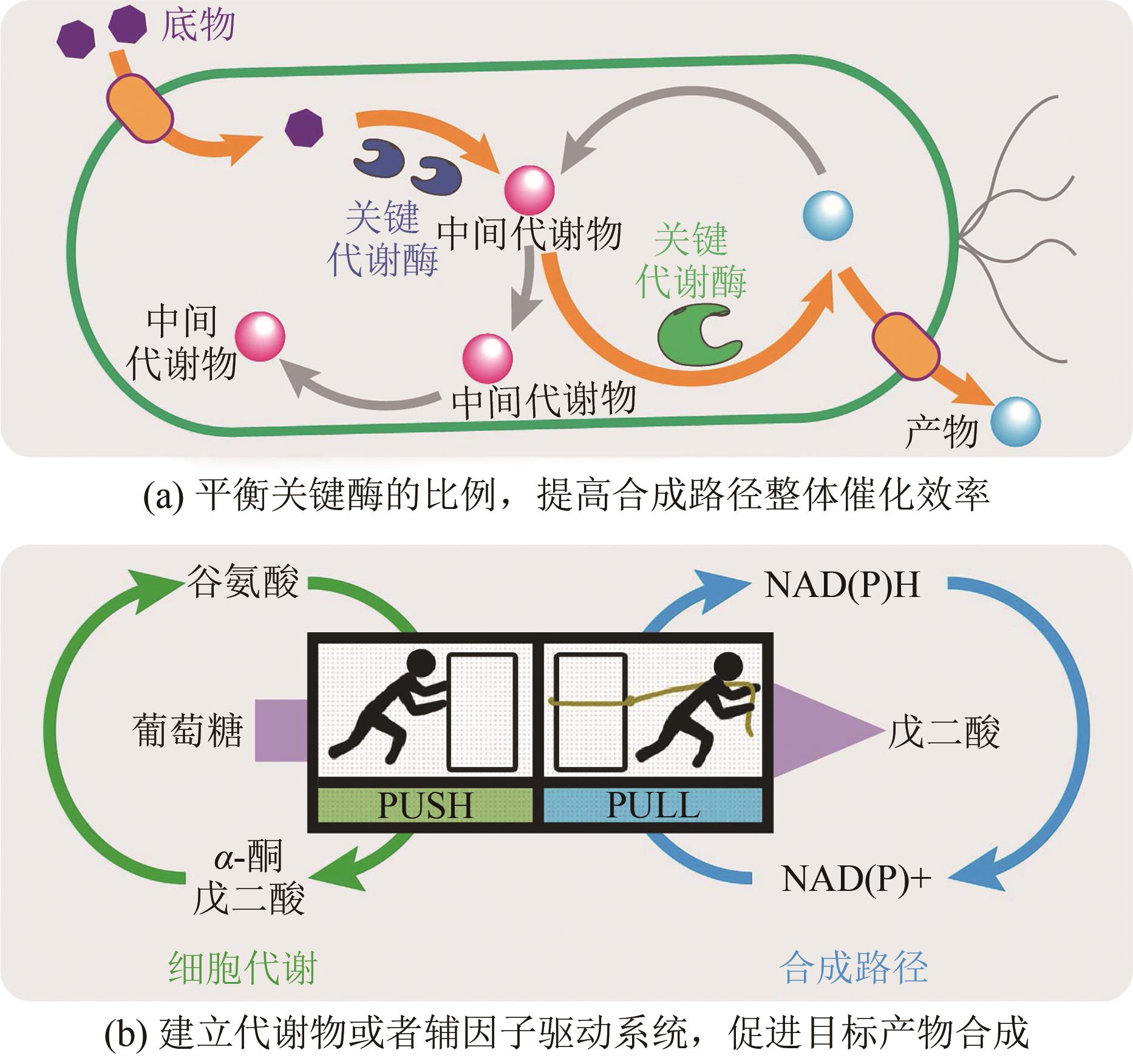
| 戊二酸 | 4100万 | 谷棒杆菌 | 基因组分析、组合优化工程 | 生物发酵 | 105.3 | [ |
| 大肠杆菌 | 质粒优化、启动子工程和核糖体结合位点工程 | 全细胞转化 | 77.6 | [ | ||
| 大肠杆菌 | 胶体几丁质细胞固定策略 | 全细胞转化 | 73.2 | [ | ||
| 大肠杆菌 | 表达戊二胺转运蛋白、耦合辅因子驱动技术 | 生物发酵 | 54.5 | [ | ||
| 大肠杆菌 | 针对副产物合成途径的自主回路 | 生物发酵 | 26 | [ | ||
| 谷棒杆菌 | 适应性进化 | 生物发酵 | 22.7 | [ | ||
| 大肠杆菌 | 引入丙二酸利用途径 | 生物发酵 | 6.3 | [ |
表1 部分塑料单体的代谢工程策略
| 单体 | 市场/CNY | 菌株 | 关键调控策略 | 生产工艺 | 产量/g·L-1 | 参考文献 |
|---|---|---|---|---|---|---|
| 1,4-BDO | 126亿 | 大肠杆菌 | 代谢网络模型模拟、合成途径基因组整合 | 生物发酵 | 125.0 | [ |
| 大肠杆菌 | 全细胞催化赤藓糖醇合成 | 全细胞转化 | 4.0×10-2 | [ | ||
| 大肠杆菌 | 基因编辑调控碳流分布、抑制副产物合成 | 生物发酵 | 1.8 | [ | ||
| 大肠杆菌 | 构建谷氨酸转化途径 | 生物发酵 | 1.4 | [ | ||
| 戊二胺 | 2.20亿 | 大肠杆菌 | 蛋白质定向进化 | 全细胞转化 | 418 | [ |
| 大肠杆菌 | 原位CO2捕集技术 | 全细胞转化 | 209.3 | [ | ||
| 大肠杆菌 | 结合定向进化和计算指导的虚拟筛选 | 全细胞转化 | 160.7 | [ | ||
| 谷棒杆菌 | 基因组整合、赖氨酸合成强化 | 生物发酵 | 125 | [ | ||
| 大肠杆菌 | 适应性进化 | 生物发酵 | 58.7 | [ | ||
| 大肠杆菌 | 温度开关设计、丁二酸联产实现碳固定 | 生物发酵 | 55.6 | [ | ||
| 大肠杆菌 | 构建抑制基因表达的sRNAs文库 | 生物发酵 | 13.7 | [ | ||
| 己二酸 | 60亿 | 大肠杆菌 | 平衡路径酶表达水平、阻断副产物积累 | 生物发酵 | 68 | [ |
| 台湾假单胞菌 | 酶挖掘、级联反应设计、代谢优化 | 全细胞转化 | 10.2 | [ | ||
| 大肠杆菌 | 化学溶剂处理和物理破碎方法 | 生物发酵 | 55.5 | [ | ||
| 酿酒酵母 | 三阶段发酵工艺优化,筛选异源酶 | 生物发酵 | 2.6×10-3 | [ | ||
| 大肠杆菌 | 建立氧依赖型动态调节系统 | 生物发酵 | 0.5 | [ | ||
| 酿酒酵母 | 过表达特异性多药耐药转运蛋白 | 生物发酵 | 0.4 | [ | ||
| 1,3-PDO | 7.76亿 | 大肠杆菌 | 阻断甘油氧化途径、引入异源途径、失活PTS | 生物发酵 | 135 | [ |
| 谷棒杆菌 | 还原力平衡、降低有毒中间代谢物积累 | 生物发酵 | 110 | [ | ||
| 罗伊氏乳杆菌 | 电子束辐照诱变 | 生物发酵 | 93.2 | [ | ||
| 肺炎克雷伯菌 | 敲除葡萄糖转运蛋白Crr | 生物发酵 | 78 | [ | ||
| 需钠弧菌 | 删除全局转录调节因子,转录组学分析 | 生物发酵 | 56.2 | [ | ||
| 丁酸梭菌 | 组合NTG和ARTP诱变筛选 | 生物发酵 | 37 | [ | ||
| 大肠杆菌 | 微调混合途径以匹配合成路径的能源需求 | 生物发酵 | 22.7 | [ | ||
| 乳酸 | 29亿 | 大肠杆菌 | 阻断副产物合成 | 生物发酵 | 142.2 | [ |
| 酿酒酵母 | 引入耐受蛋白IoGAS1 | 生物发酵 | 92 | [ | ||
| 大肠杆菌 | 筛选木糖分解代谢操纵子 | 生物发酵 | 86 | [ | ||
| 恶臭假单胞菌、凝结芽孢杆菌 | 合成微生物群落 | 生物发酵 | 35.8 | [ | ||
| 食木薯乳杆菌 | 聚集用于底物的同时糖化和发酵 | 生物发酵 | 18.7 | [ | ||
| 里氏木霉、戴尔根霉 | 合成微生物群落 | 生物发酵 | 4.4 | [ | ||
| 对苯二甲酸 | 495亿 | 大肠杆菌 | 基因组多拷贝整合 | 全细胞转化 | 38.3 | [ |
| 大肠杆菌 | 微生物双相转化 | 全细胞转化 | 6.9 | [ | ||
| 丁二酸 | 2.23亿 | 产琥珀酸曼氏杆菌 | 筛选不同来源的苹果酸脱氢酶 | 生物发酵 | 134.3 | [ |
| 大肠杆菌 | 溶氧动态开关 | 生物发酵 | 119 | [ | ||
| 产琥珀酸曼氏杆菌 | 改变细胞膜的流动性 | 生物发酵 | 84.2 | [ | ||
| 产琥珀酸放线杆菌 | 生物被膜的细胞固定化 | 生物发酵 | 67.3 | [ | ||
| 大肠杆菌 | 替代高能耗途径 | 生物发酵 | 56.9 | [ | ||
| 大肠杆菌 | 过表达大肠杆菌全局转录调控因子IrrE | 生物发酵 | 24.5 | [ | ||
| 产丙酸丙酸杆菌 | 原位产物分离,开发膜分离耦合发酵技术 | 生物发酵 | 20.5 | [ | ||
| 戊二酸 | 4100万 | 谷棒杆菌 | 基因组分析、组合优化工程 | 生物发酵 | 105.3 | [ |
| 大肠杆菌 | 质粒优化、启动子工程和核糖体结合位点工程 | 全细胞转化 | 77.6 | [ | ||
| 大肠杆菌 | 胶体几丁质细胞固定策略 | 全细胞转化 | 73.2 | [ | ||
| 大肠杆菌 | 表达戊二胺转运蛋白、耦合辅因子驱动技术 | 生物发酵 | 54.5 | [ | ||
| 大肠杆菌 | 针对副产物合成途径的自主回路 | 生物发酵 | 26 | [ | ||
| 谷棒杆菌 | 适应性进化 | 生物发酵 | 22.7 | [ | ||
| 大肠杆菌 | 引入丙二酸利用途径 | 生物发酵 | 6.3 | [ |
| 1 | LEE Y, CHO I J, CHOI S Y, et al. Systems metabolic engineering strategies for non-natural microbial polyester production[J]. Biotechnology Journal, 2019, 14(9): e1800426. |
| 2 | ROSENBOOM J G, LANGER R, TRAVERSO G. Bioplastics for a circular economy[J]. Nature Reviews Materials, 2022, 7(2): 117-137. |
| 3 | PRELL C, BUSCHE T, RUCKERT C, et al. Adaptive laboratory evolution accelerated glutarate production by Corynebacterium glutamicum [J]. Microbial Cell Factories, 2021, 20(1): 97. |
| 4 | LI Y, YANG S, MA D, et al. Microbial engineering for the production of C2-C6 organic acids[J]. Natural Product Reports, 2021, 38(8): 1518-1546. |
| 5 | DOOKERAN Z A, NIELSEN D R. Systematic engineering of Synechococcus elongatus UTEX 2973 for photosynthetic production of L-lysine, cadaverine, and glutarate[J]. ACS Synthetic Biology, 2021, 10(12): 3561-3575. |
| 6 | NGUYEN T T, LEE O K, NAIZABEKOV S, et al. Bioconversion of methane to cadaverine and lysine using an engineered type Ⅱ methanotroph, Methylosinus trichosporium OB3b[J]. Green Chemistry, 2020, 22(22): 7803-7811. |
| 7 | BRETSCHNEIDER L, HEUSCHKEL I, BUEHLER K, et al. Rational orthologous pathway and biochemical process engineering for adipic acid production using Pseudomonas taiwanensis VLB120[J]. Metabolic Engineering, 2022, 70: 206-217. |
| 8 | LI Y, CHENG Z, ZHAO C, et al. Reprogramming Escherichia coli metabolism for bioplastics synthesis from waste cooking oil[J]. ACS Synthetic Biology, 2021, 10(8): 1966-1979. |
| 9 | HU G P, LI Z H, MA D L, et al. Light-driven CO2 sequestration in Escherichia coli to achieve theoretical yield of chemicals[J]. Nature Catalysis, 2021, 4(5): 395-406. |
| 10 | ZOU W, EDROS R, AL-RUBEAI M. The relationship of metabolic burden to productivity levels in CHO cell lines[J]. Biotechnology and Applied Biochemistry, 2018, 65(2): 173-180. |
| 11 | SCHOLZ S A, GRAVES I, MINTY J J, et al. Production of cellulosic organic acids via synthetic fungal consortia[J]. Biotechnology and Bioengineering, 2018, 115(4): 1096-1100. |
| 12 | LIN T Y, WEN R C, SHEN C R, et al. Biotransformation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid by a syntrophic consortium of engineered Synechococcus elongatus and Pseudomonas putida [J]. Biotechnology Journal, 2020, 15(6): e1900357. |
| 13 | CHANDEL A K, GARLAPATI V K, SINGH A K, et al. The path forward for lignocellulose biorefineries: Bottlenecks, solutions, and perspective on commercialization[J]. Bioresource Technology, 2018, 264: 370-381. |
| 14 | LUO Z W, LEE S Y. Biotransformation of p-xylene into terephthalic acid by engineered Escherichia coli [J]. Nature Communications, 2017, 8: 15689. |
| 15 | LUO Z W, CHOI K R, LEE S Y. Improved terephthalic acid production from p-xylene using metabolically engineered Pseudomonas putida [J]. Metabolic Engineering, 2023, 76: 75-86. |
| 16 | WU M J, DI J H, GONG L, et al. Enhanced adipic acid production from sugarcane bagasse by a rapid room temperature pretreatment[J]. Chemical Engineering Journal, 2023, 452: 139320. |
| 17 | SATO R, TANAKA T, OHARA H, et al. Engineering Escherichia coli for direct production of 1,2-propanediol and 1,3-propanediol from starch[J]. Current Microbiology, 2020, 77(11): 3704-3710. |
| 18 | IMAO K, KONISHI R, KISHIDA M, et al. 1,5-Diaminopentane production from xylooligosaccharides using metabolically engineered Corynebacterium glutamicum displaying beta-xylosidase on the cell surface[J]. Bioresource Technology, 2017, 245(B): 1684-1691. |
| 19 | SINGH B, VERMA A, POOJA, et al. A biotechnological approach for degradation of inhibitory compounds present in lignocellulosic biomass hydrolysate liquor using Bordetella sp BTIITR[J]. Chemical Engineering Journal, 2017, 328: 519-526. |
| 20 | ZOU L H, OUYANG S P, HU Y L, et al. Efficient lactic acid production from dilute acid-pretreated lignocellulosic biomass by a synthetic consortium of engineered Pseudomonas putida and Bacillus coagulans [J]. Biotechnology for Biofuels, 2021, 14(1): 227. |
| 21 | QIN R, ZHU Y, AI M, et al. Reconstruction and optimization of a Pseudomonas putida-Escherichia coli microbial consortium for mcl-PHA production from lignocellulosic biomass[J]. Frontiers in Bioengineering and Biotechnology, 2022, 10: 1023325. |
| 22 | ONG K L, FICKERS P, LIN C S K. Enhancing succinic acid productivity in the yeast Yarrowia lipolytica with improved glycerol uptake rate[J]. Science of the Total Environment, 2020, 702: 134911. |
| 23 | GAO C, HOU J S, XUD P, et al. Programmable biomolecular switches for rewiring flux in Escherichia coli [J]. Nature Communications, 2019, 10(1): 3751. |
| 24 | JOHNSON C W, ABRAHAM P E, LINGER J G, et al. Eliminating a global regulator of carbon catabolite repression enhances the conversion of aromatic lignin monomers to muconate in Pseudomonas putida KT2440[J]. Metabolic Engineering Communications, 2017, 5: 19-25. |
| 25 | SIEVERT C, NIEVES L M, PANYON L A, et al. Experimental evolution reveals an effective avenue to release catabolite repression via mutations in XylR[J]. Proceedings of the National Academy of Sciences of the United States of America, 2017, 114(28): 7349-7354. |
| 26 | LU X Y, REN S L, LU J Z, et al. Enhanced 1,3-propanediol production in Klebsiella pneumoniae by a combined strategy of strengthening the TCA cycle and weakening the glucose effect[J]. Journal of Applied Microbiology, 2018, 124(3): 682-690. |
| 27 | GAO C, GUO L, DING Q, et al. Dynamic consolidated bioprocessing for direct production of xylonate and shikimate from xylan by Escherichia coli [J]. Metabolic Engineering, 2020, 60: 128-137. |
| 28 | RAWOOF S A A, KUMAR P S, DEVARAJ K, et al. Enhancement of lactic acid production from food waste through simultaneous saccharification and fermentation using selective microbial strains[J]. Biomass Conversion and Biorefinery, 2022, 12(12): 5947-5958. |
| 29 | HU G P, ZHOU J, CHEN X L, et al. Engineering synergetic CO2-fixing pathways for malate production[J]. Metabolic Engineering, 2018, 47: 496-504. |
| 30 | AHN J H, SEO H, PARK W, et al. Enhanced succinic acid production by Mannheimia employing optimal malate dehydrogenase[J]. Nature Communications, 2020, 11(1): 1970. |
| 31 | RAJ K, PARTOW S, CORREIA K, et al. Biocatalytic production of adipic acid from glucose using engineered Saccharomyces cerevisiae [J]. Metabolic Engineering Communications, 2018, 6: 28-32. |
| 32 | WANG J, GAO C, CHEN X, et al. Engineering the Cad pathway in Escherichia coli to produce glutarate from L-lysine[J]. Applied Microbiology and Biotechnology, 2021, 105(9): 3587-3599. |
| 33 | DU Y, PU Z J, KANG H, et al. Zwitterionic peptides encircling-assisted enhanced catalytic performance of lysine decarboxylase for cadaverine biotransformation and mechanism analyses[J]. Chemical Engineering Science, 2022, 251: 117447. |
| 34 | LI W, MA L, SHEN X, et al. Targeting metabolic driving and intermediate influx in lysine catabolism for high-level glutarate production[J]. Nature Communications, 2019, 10(1): 3337. |
| 35 | WANG Y J, XUE P, CAO M F, et al. Directed evolution: methodologies and applications[J]. Chemical Reviews, 2021, 121(20): 12384-12444. |
| 36 | GAO S, ZHANG A, MA D, et al. Enhancing pH stability of lysine decarboxylase via rational engineering and its application in cadaverine industrial production[J]. Biochemical Engineering Journal, 2022, 186: 108548. |
| 37 | XI Y, YE L D, YU H W. Enhanced thermal and alkaline stability of L-lysine decarboxylase CadA by combining directed evolution and computation-guided virtual screening[J]. Bioresources and Bioprocessing, 2022, 9(1): 24. |
| 38 | GAO C, WANG J P, GUO L, et al. Immobilization of microbial consortium for glutaric acid production from lysine[J]. ChemCatChem, 2021, 13(23): 5047-5055. |
| 39 | LIANG G J, ZHOU P, LU J X, et al. Dynamic regulation of membrane integrity to enhance l-malate stress tolerance in Candida glabrata [J]. Biotechnology and Bioengineering, 2021, 118(11): 4347-4359. |
| 40 | GAO C, TANG W, GUO L, et al. Improving succinate production by engineering oxygen-dependent dynamic pathway regulation in Escherichia coli [J]. Systems Microbiology and Biomanufacturing, 2022, 2(2): 331-344. |
| 41 | LI S Y, FU W X, SU R F, et al. Metabolic engineering of the malonyl-CoA pathway to efficiently produce malonate in Saccharomyces cerevisiae [J]. Metabolic Engineering, 2022, 73: 1-10. |
| 42 | GAO C, GUO L, HU G P, et al. Engineering a CRISPRi circuit for autonomous control of metabolic flux in Escherichia coli [J]. ACS Synthetic Biology, 2021, 10(10): 2661-2671. |
| 43 | JIANG G Z, YAO M D, WANG Y, et al. A “push-pull-restrain” strategy to improve citronellol production in Saccharomyces cerevisiae [J]. Metabolic Engineering, 2021, 66: 51-59. |
| 44 | SOMASUNDARAM S, JEONG J, IRISAPPAN G, et al. Enhanced production of malic acid by co-localization of phosphoenolpyruvate carboxylase and malate dehydrogenase using synthetic protein scaffold in Escherichia coli [J]. Biotechnology and Bioprocess Engineering, 2020, 25(1): 39-44. |
| 45 | DING Q, LI Z, GUO L, et al. Engineering Escherichia coli asymmetry distribution-based synthetic consortium for shikimate production[J]. Biotechnology and Bioengineering, 2022, 119(11): 3230-3240. |
| 46 | YANG Y P, LIN Y H, WANG J, et al. Sensor-regulator and RNAi based bifunctional dynamic control network for engineered microbial synthesis[J]. Nature Communications, 2018, 9(1): 3043. |
| 47 | YIN X, SHIN H D, LI J H, et al. Pgas, a Low-pH-induced promoter, as a tool for dynamic control of gene expression for metabolic engineering of Aspergillus niger [J]. Applied and Environmental Microbiology, 2017, 83(6): e03222-16. |
| 48 | HAN T, KIM G B, LEE S Y. Glutaric acid production by systems metabolic engineering of an l-lysine-overproducing Corynebacterium glutamicum [J]. Proceedings of the National Academy of Sciences of the United States of America, 2020, 117(48): 30328-30334. |
| 49 | NOH M, YOO S M, YANG D, et al. Broad-spectrum gene repression using scaffold engineering of synthetic sRNAs[J]. ACS Synthetic Biology, 2019, 8(6): 1452-1461. |
| 50 | BOECKER S, HARDER B J, KUTSCHA R, et al. Increasing ATP turnover boosts productivity of 2,3-butanediol synthesis in Escherichia coli [J]. Microbial Cell Factories, 2021, 20(1): 63. |
| 51 | ZHANG Y, LI Z, LIU Y, et al. Systems metabolic engineering of Vibrio natriegens for the production of 1,3-propanediol[J]. Metabolic Engineering, 2021, 65: 52-65. |
| 52 | YU Y, ZHU X, XU H, et al. Construction of an energy-conserving glycerol utilization pathways for improving anaerobic succinate production in Escherichia coli [J]. Metabolic Engineering, 2019, 56: 181-189. |
| 53 | LI J, LI Y, CUI Z, et al. Enhancement of succinate yield by manipulating NADH/NAD(+) ratio and ATP generation[J]. Applied Microbiology and Biotechnology, 2017, 101(8): 3153-3161. |
| 54 | DING Q, LIU Y D, HU G P, et al. Engineering Escherichia coli biofilm to increase contact surface for shikimate and L-malate production[J]. Bioresources and Bioprocessing, 2021, 8(1): 118. |
| 55 | LIU H, QI Y L, ZHOU P, et al. Microbial physiological engineering increases the efficiency of microbial cell factories[J]. Critical Reviews in Biotechnology, 2021, 41(3): 339-354. |
| 56 | WEI G, ZHANG A, LU X, et al. An environmentally friendly strategy for cadaverine bio-production: in situ utilization of CO2 self-released from L-lysine decarboxylation for pH control[J]. Journal of CO2 Utilization, 2020, 37: 278-284. |
| 57 | WANG X L, ZHOU J J, LIU S, et al. In situ carbon dioxide capture to co-produce 1,3-propanediol, biohydrogen and micro-nano calcium carbonate from crude glycerol by Clostridium butyricum [J]. Biotechnology for Biofuels and Bioproducts, 2022, 15(1): 91. |
| 58 | QI Y L, LIU H, CHEN X L, et al. Engineering microbial membranes to increase stress tolerance of industrial strains[J]. Metabolic Engineering, 2019, 53: 24-34. |
| 59 | AHN J H, LEE J A, BANG J, et al. Membrane engineering via trans-unsaturated fatty acids production improves succinic acid production in Mannheimia succiniciproducens [J]. Journal of Industrial Microbiology & Biotechnology, 2018, 45(7): 555-566. |
| 60 | ZHU Z M, YANG P S, YANG J H, et al. Comparative transcriptome analysis reveals the contribution of membrane transporters to acid tolerance in Lactococcus lactis [J]. Journal of Biotechnology, 2022, 357: 9-17. |
| 61 | YANG J H, PENG Z, ZHU Q, et al. NiFe hydrogenase accessory proteins HypB-HypC accelerate proton conversion to enhance the acid resistance and D-lactic acid production of Escherichia coli [J]. ACS Synthetic Biology, 2022, 11(4): 1521-1530. |
| 62 | BRAMEYER S, SCHUMACHER K, KUPPERMANN S, et al. Division of labor and collective functionality in Escherichia coli under acid stress[J]. Communications Biology, 2022, 5(1): 327. |
| 63 | ZHONG W, YANG M, HAO X, et al. Improvement of D-lactic acid production at low pH through expressing acid-resistant gene IoGAS1 in engineered Saccharomyces cerevisiae [J]. Journal of Chemical Technology & Biotechnology, 2020, 96(3): 732-742. |
| 64 | ZHU Z, JI X, WU Z, et al. Improved acid-stress tolerance of Lactococcus lactis NZ9000 and Escherichia coli BL21 by overexpression of the anti-acid component recT[J]. Journal of Industrial Microbiology & Biotechnology, 2018, 45(12): 1091-1101. |
| 65 | ZHANG W, TAO Y, WU M, et al. Adaptive evolution improves acid tolerance and succinic acid production in Actinobacillus succinogenes [J]. Process Biochemistry, 2020, 98: 76-82. |
| 66 | XIAO M, ZHU X, FAN F, et al. Osmotolerance in Escherichia coli is improved by activation of copper efflux genes or supplementation with sulfur-containing amino acids[J]. Applied and Environmental Microbiology, 2017, 83(7): e03050-16. |
| 67 | XIAO M, ZHU X, XU H, et al. A novel point mutation in RpoB improves osmotolerance and succinic acid production in Escherichia coli [J]. BMC Biotechnology, 2017, 17(1): 10. |
| 68 | ZHANG W M, ZHU J R, ZHU X G, et al. Expression of global regulator IrrE for improved succinate production under high salt stress by Escherichia coli [J]. Bioresource Technology, 2018, 254: 151-156. |
| 69 | LV Z, ZHOU J, ZHANG Y, et al. Techniques for enhancing the tolerance of industrial microbes to abiotic stresses: A review[J]. Biotechnology and Applied Biochemistry, 2020, 67(1): 73-81. |
| 70 | JANG B K, JU Y B, JEONG D, et al. L-lactic acid production using engineered Saccharomyces cerevisiae with improved organic acid tolerance[J]. Journal of Fungi, 2021, 7(11): 928. |
| 71 | GAO C, XU P, YE C, et al. Genetic circuit-assisted smart microbial engineering[J]. Trends in Microbiology, 2019, 27(12): 1011-1024. |
| 72 | PHAM H L, WONG A, CHUA N, et al. Engineering a riboswitch-based genetic platform for the self-directed evolution of acid-tolerant phenotypes[J]. Nature Communications, 2017, 8(1): 411. |
| 73 | YANG M M, AN Y F, ZABED H M, et al. Random mutagenesis of Clostridium butyricum strain and optimization of biosynthesis process for enhanced production of 1,3-propanediol[J]. Bioresource Technology, 2019, 284: 188-196. |
| 74 | JU J H, HEO S Y, CHOI S W, et al. Effective bioconversion of 1,3-propanediol from biodiesel-derived crude glycerol using organic acid resistance-enhanced Lactobacillus reuteri JH83[J]. Bioresource Technology, 2021, 337: 125361. |
| 75 | PEREIRA R, WEI Y J, MOHAMED E, et al. Adaptive laboratory evolution of tolerance to dicarboxylic acids in Saccharomyces cerevisiae [J]. Metabolic Engineering, 2019, 56: 130-141. |
| 76 | LI X L, WEI L Q, WANG Z Q, et al. Efficient co-production of propionic acid and succinic acid by Propionibacterium acidipropionici using membrane separation coupled technology[J]. Engineering in Life Sciences, 2021, 21(6): 429-437. |
| 77 | IIJIMA H, WATANABE A, SUKIGARA H, et al. Four-carbon dicarboxylic acid production through the reductive branch of the open cyanobacterial tricarboxylic acid cycle in Synechocystis sp. PCC 6803[J]. Metabolic Engineering, 2021, 65: 88-98. |
| 78 | LUO S S, LIN P P, NIEH L Y, et al. A cell-free self-replenishing CO2-fixing system[J]. Nature Catalysis, 2022, 5(2): 154-162. |
| 79 | MA H, ZHAO Y, HUANG W, et al. Rational flux-tuning of Halomonas bluephagenesis for co-production of bioplastic PHB and ectoine[J]. Nature Communications, 2020, 11(1): 3313. |
| 80 | ZHAO C, ZHENG T, FENG Y, et al. Engineered Halomonas spp. for production of L-lysine and cadaverine[J]. Bioresource Technology, 2022, 349: 126865. |
| 81 | TROTTER C L, BABU G S, WALLACE S. Engineering biology for sustainable 1,4-butanediol synthesis[J]. Trends in Biotechnology, 2023, 41(3): 286-288. |
| 82 | DAI L, TAI C, SHEN Y, et al. Biosynthesis of 1,4-butanediol from erythritol using whole-cell catalysis[J]. Biocatalysis and Biotransformation, 2018, 37(2): 92-96. |
| 83 | WU M Y, SUNG L Y, LI H, et al. Combining CRISPR and CRISPRi systems for metabolic engineering of E. coli and 1,4-BDO biosynthesis[J]. ACS Synthetic Biology, 2017, 6(12): 2350-2361. |
| 84 | WANG J, LI C, ZOU Y, et al. Bacterial synthesis of C3-C5 diols via extending amino acid catabolism[J]. Proc. Natl. Acad. Sci. U. S. A., 2020, 117(32): 19159-19167. |
| 85 | KIM H T, BARITUGO K A, HYUN S M, et al. Development of metabolically engineered Corynebacterium glutamicum for enhanced production of cadaverine and its use for the synthesis of bio-polyamide 510[J]. ACS Sustainable Chemistry & Engineering, 2019, 8(1): 129-138. |
| 86 | WANG X, GUO X, WANG J, et al. Ameliorating end-product inhibition to improve cadaverine production in engineered Escherichia coli and its application in the synthesis of bio-based diisocyanates[J]. Synthetic and Systems Biotechnology, 2021, 6(4): 243-253. |
| 87 | GAO S, LU J, WANG T, et al. A novel co-production of cadaverine and succinic acid based on a thermal switch system in recombinant Escherichia coli [J]. Microbial Cell Factories, 2022, 21(1): 248. |
| 88 | ZHAO M, HUANG D, ZHANG X, et al. Metabolic engineering of Escherichia coli for producing adipic acid through the reverse adipate-degradation pathway[J]. Metabolic Engineering, 2018, 47: 254-262. |
| 89 | HAO T T, LI G H, ZHOU S H, et al. Engineering the reductive TCA pathway to dynamically regulate the biosynthesis of adipic acid in Escherichia coli [J]. ACS Synthetic Biology, 2021, 10(3): 632-639. |
| 90 | ZHU F, LIU D, CHEN Z. Recent advances in biological production of 1,3-propanediol: new routes and engineering strategies[J]. Green Chemistry, 2022, 24(4): 1390-1403. |
| 91 | LI Z, DONG Y, LIU Y, et al. Systems metabolic engineering of Corynebacterium glutamicum for high-level production of 1,3-propanediol from glucose and xylose[J]. Metabolic Engineering, 2022, 70: 79-88. |
| 92 | WANG J, ZHANG R H, ZHANG J L, et al. Tunable hybrid carbon metabolism coordination for the carbon-efficient biosynthesis of 1,3-butanediol in Escherichia coli [J]. Green Chemistry, 2021, 23(21): 8694-8706. |
| 93 | LIU T, XU X, LIU Y, et al. Engineered microbial cell factories for sustainable production of L-lactic acid: A critical review[J]. Fermentation, 2022, 8(6): 279. |
| 94 | GAO H, WANG J, WU H, et al. Biofilm-integrated glycosylated membrane for biosuccinic acid production[J]. ACS Applied Bio Materials, 2021, 4(10): 7517-7523. |
| 95 | SUI X, ZHAO M, LIU Y L, et al. Enhancing glutaric acid production in Escherichia coli by uptake of malonic acid[J]. Journal of Industrial Microbiology & Biotechnology, 2020, 47(3): 311-318. |
| [1] | 孙文涛, 李春. 微生物合成植物天然产物的细胞工厂设计与构建[J]. 化工进展, 2021, 40(3): 1202-1214. |
| [2] | 郭亮, 高聪, 张丽, 陈修来, 刘立明. 人工代谢路径适配性的研究进展[J]. 化工进展, 2021, 40(3): 1252-1261. |
| [3] | 高聪, 郭亮, 胡贵鹏, 陈修来, 刘立明. 微生物细胞工厂碳流调控进展[J]. 化工进展, 2021, 40(12): 6807-6817. |
| [4] | 张正晖, 曹铭铭, 李珺, 李春, 刘护. 微生物高效分泌蛋白质的策略与应用[J]. 化工进展, 2018, 37(08): 3129-3137. |
| [5] | 郭加明,杨功勋,胡纯铿,詹美蓉,张新华. 乙醇补料发酵技术研究进展[J]. 化工进展, 2013, 32(11): 2679-2684. |
| [6] | 丁 爽1,唐崇俭1,郑 平1,方炳南2,杨翘强2. 厌氧氨氧化工艺脱氮机理和抑制因素的研究进展 [J]. 化工进展, 2010, 29(9): 1754-. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||