化工进展 ›› 2023, Vol. 42 ›› Issue (6): 3123-3129.DOI: 10.16085/j.issn.1000-6613.2022-1410
含Gemini离子液体低共熔溶剂热力学性质
- 郑州轻工业大学材料与化学工程学院,郑州市精细化学品重点实验室,河南 郑州 450001
-
收稿日期:2022-07-27修回日期:2022-09-23出版日期:2023-06-25发布日期:2023-06-29 -
通讯作者:杨许召 -
作者简介:杨许召(1978—),男,博士,副教授,主要研究方向为化学工艺、精细化工。E-mail: yangxz@zzuli.edu.cn。 -
基金资助:河南省科技攻关项目(162102210056);郑州市科技攻关项目(141PQYJS555);郑州轻工业大学博士研究基金(2020BSJJ018)
Thermodynamic properties of Gemini ionic liquid based deep eutectic solvents
YANG Xuzhao( ), LI Qing, YUAN Kangkang, ZHANG Yingying, HAN Jingli, WU Shide
), LI Qing, YUAN Kangkang, ZHANG Yingying, HAN Jingli, WU Shide
- Zhengzhou Key Laboratory of Fine Chemicals, School of Material and Chemical Engineering, Zhengzhou University of Light Industry, Zhengzhou 450001, Henan, China
-
Received:2022-07-27Revised:2022-09-23Online:2023-06-25Published:2023-06-29 -
Contact:YANG Xuzhao
摘要:
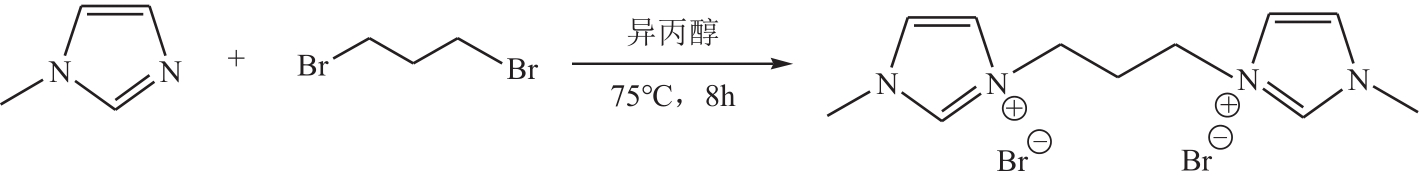
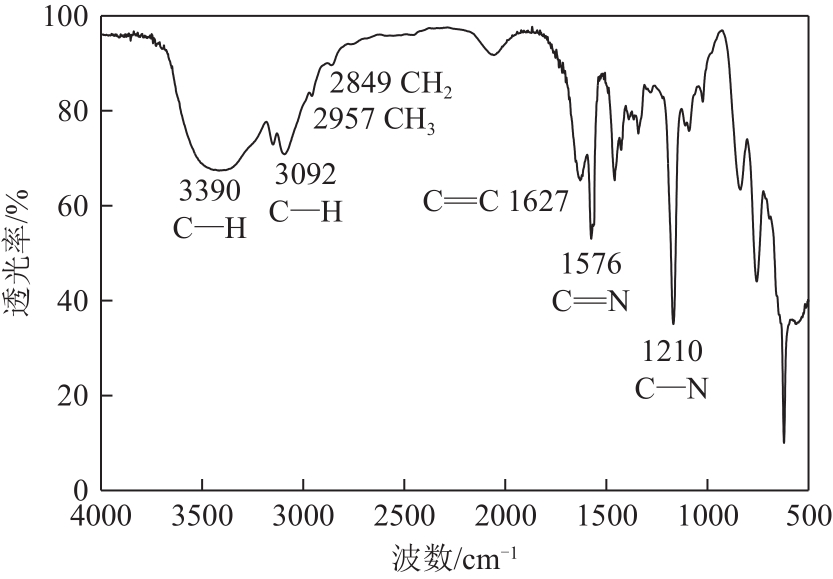
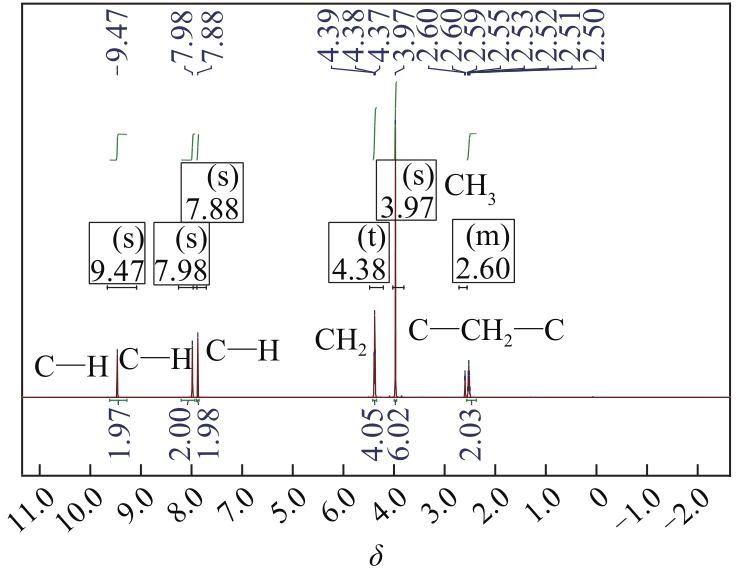
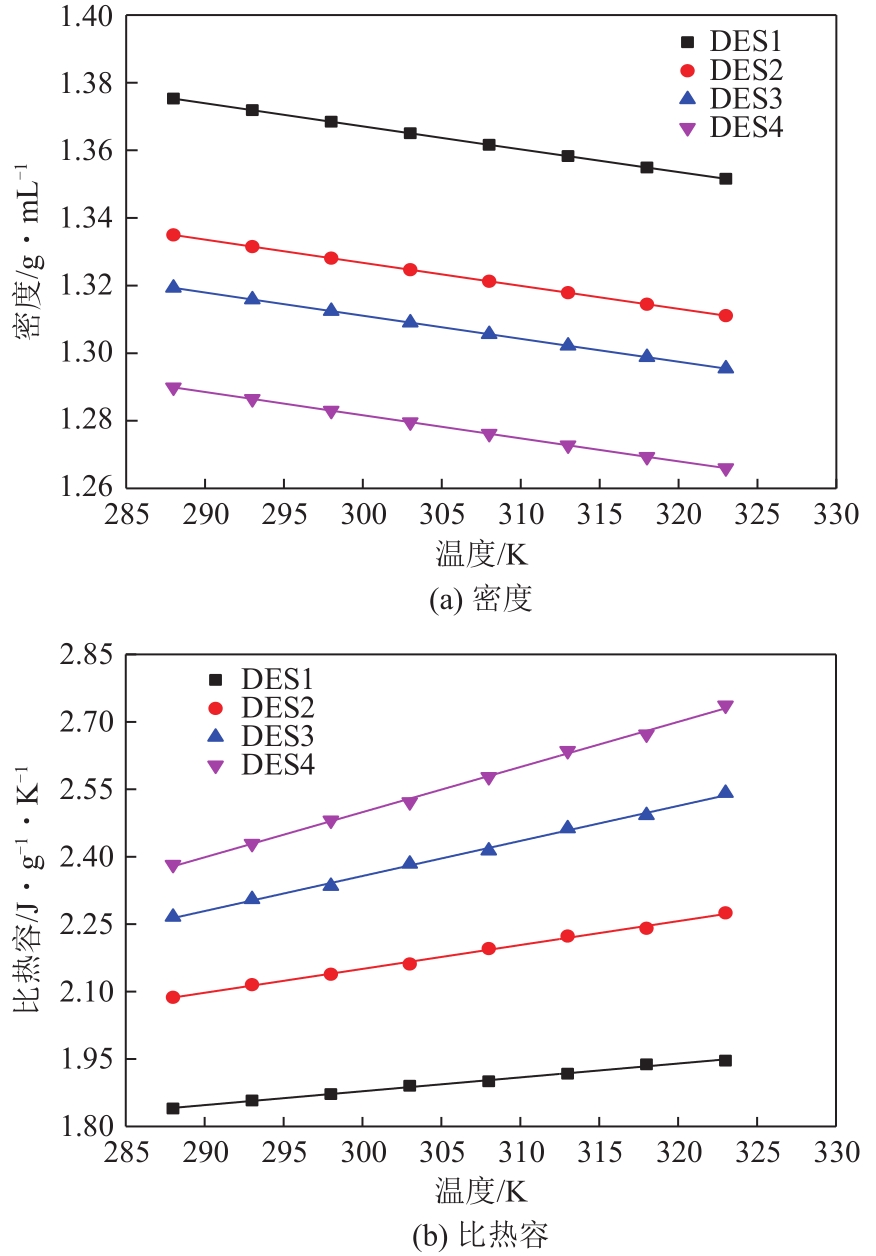
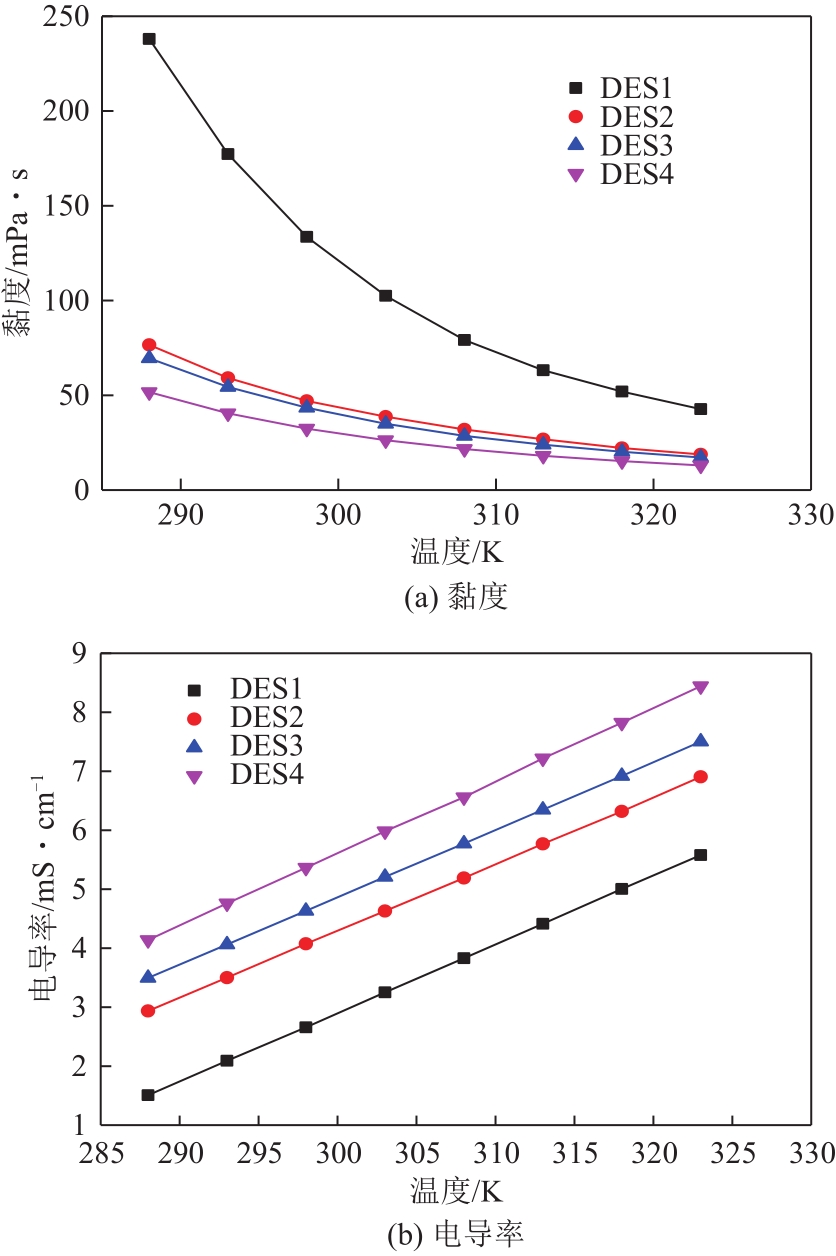
以1-甲基咪唑、1,3-二溴丙烷为原料合成Gemini离子液体1,1'-(1,3-三亚甲基)双-3-甲基咪唑二溴盐[C3(MIM)2Br2],并以不同C3(MIM)2Br2与乙二醇组成制备了一系列低共熔溶剂。熔点测试结果显示,所有的低共熔溶剂的熔点均低于-90℃,远低于C3(MIM)2Br2和乙二醇的熔点,且随着乙二醇组成的增大而降低。在288.15~323.15K条件下对该低共熔溶剂的密度、黏度、电导率、比热容等热力学性质进行了测定,结果表明,密度和黏度会随着温度的升高而降低,电导率与比热容则随着温度的升高而升高。随着乙二醇物质的量的升高,密度和黏度减小,电导率和比热容增大。密度、比热容随温度变化的线性方程的相关系数大于0.99。用VFT经验方程对黏度和电导率随温度的变化进行了拟合,结果表明,黏度与电导率的相关系数均大于0.999。
中图分类号:
引用本文
杨许召, 李庆, 袁康康, 张盈盈, 韩敬莉, 吴诗德. 含Gemini离子液体低共熔溶剂热力学性质[J]. 化工进展, 2023, 42(6): 3123-3129.
YANG Xuzhao, LI Qing, YUAN Kangkang, ZHANG Yingying, HAN Jingli, WU Shide. Thermodynamic properties of Gemini ionic liquid based deep eutectic solvents[J]. Chemical Industry and Engineering Progress, 2023, 42(6): 3123-3129.
| 热力学性质 | 实验值 | 文献值 | 相对误差/% | T/K |
|---|---|---|---|---|
| ρ/g·mL-1 | 1.1162 | 1.11676[ | -0.050 | 288.15 |
| 1.1021 | 1.1030[ | -0.082、-0.075、0.018、-0.056 | 308.15 | |
| 1.0986 | 1.0999[ | -0.118、0.457、-0.055 | 313.15 | |
| 1.0949 | 1.0978[ | -0.264、-0.055、-0.068 | 318.15 | |
| 1.0906 | 1.09105[ | -0.0412、-0.130、0.544 | 323.15 | |
| η/mPa·s | 26.0965 | 26.343[ | -0.936 | 288.15 |
| 10.0952 | 9.2122[ | 9.585、-3.598、-10.153 | 308.15 | |
| 8.6371 | 7.9605[ | 8.499、-8.155 | 313.15 | |
| 6.8190 | 6.2933[ | 8.353、-14.172、-16.084 | 318.15 | |
| 6.4921 | 6.992[ | -7.150、-6.359、-4.161、19.191 | 323.15 | |
| κ/μS·m-1 | 0.413 | 0.400[ | 3.250 | 303.15 |
| 0.516 | 0.502[ | 2.789 | 313.15 | |
| 0.609 | 0.600[ | 1.500 | 323.15 | |
| cp /J·g-1·K-1 | 2.3462 | 2.3533[ | -0.302、-1.1254 | 293.15 |
| 2.3891 | 2.3826[ | 0.273、-0.130 | 298.15 | |
| 2.4286 | 2.4160[ | 0.522、0.688、1.445 | 303.15 | |
| 2.5671 | 2.478[ | 3.596 | 323.15 |
表1 乙二醇热力学性质测定结果与文献值对比
| 热力学性质 | 实验值 | 文献值 | 相对误差/% | T/K |
|---|---|---|---|---|
| ρ/g·mL-1 | 1.1162 | 1.11676[ | -0.050 | 288.15 |
| 1.1021 | 1.1030[ | -0.082、-0.075、0.018、-0.056 | 308.15 | |
| 1.0986 | 1.0999[ | -0.118、0.457、-0.055 | 313.15 | |
| 1.0949 | 1.0978[ | -0.264、-0.055、-0.068 | 318.15 | |
| 1.0906 | 1.09105[ | -0.0412、-0.130、0.544 | 323.15 | |
| η/mPa·s | 26.0965 | 26.343[ | -0.936 | 288.15 |
| 10.0952 | 9.2122[ | 9.585、-3.598、-10.153 | 308.15 | |
| 8.6371 | 7.9605[ | 8.499、-8.155 | 313.15 | |
| 6.8190 | 6.2933[ | 8.353、-14.172、-16.084 | 318.15 | |
| 6.4921 | 6.992[ | -7.150、-6.359、-4.161、19.191 | 323.15 | |
| κ/μS·m-1 | 0.413 | 0.400[ | 3.250 | 303.15 |
| 0.516 | 0.502[ | 2.789 | 313.15 | |
| 0.609 | 0.600[ | 1.500 | 323.15 | |
| cp /J·g-1·K-1 | 2.3462 | 2.3533[ | -0.302、-1.1254 | 293.15 |
| 2.3891 | 2.3826[ | 0.273、-0.130 | 298.15 | |
| 2.4286 | 2.4160[ | 0.522、0.688、1.445 | 303.15 | |
| 2.5671 | 2.478[ | 3.596 | 323.15 |
| 溶剂 | 熔点/℃ |
|---|---|
| C3(MIM)2Br2 | 171[ |
| 乙二醇 | -13[ |
| DES1 | -98 |
| DES2 | -108 |
| DES3 | -110 |
| DES4 | -114 |
表2 离子液体和DES的熔点
| 溶剂 | 熔点/℃ |
|---|---|
| C3(MIM)2Br2 | 171[ |
| 乙二醇 | -13[ |
| DES1 | -98 |
| DES2 | -108 |
| DES3 | -110 |
| DES4 | -114 |
| 热力学性质 | 溶剂 | A | B | R2 | σ |
|---|---|---|---|---|---|
| 密度 | DES1 | 1.57066 | -6.78488×10-4 | 0.99997 | 0.00011 |
| DES2 | 1.53140 | -6.82138×10-4 | 0.99998 | 0.00011 | |
| DES3 | 1.51577 | -6.82252×10-4 | 0.99999 | 0.00011 | |
| DSE4 | 1.48676 | -6.83652×10-4 | 0.99998 | 0.00009 | |
| 比热容 | DES1 | 0.95372 | 0.00308 | 0.99766 | 0.0025 |
| DES2 | 0.55713 | 0.00531 | 0.99847 | 0.0034 | |
| DES3 | 0.01762 | 0.0078 | 0.99858 | 0.0048 | |
| DES4 | -0.51295 | 0.01004 | 0.99900 | 0.0051 |
表3 DES密度、比热容方程参数及标准差
| 热力学性质 | 溶剂 | A | B | R2 | σ |
|---|---|---|---|---|---|
| 密度 | DES1 | 1.57066 | -6.78488×10-4 | 0.99997 | 0.00011 |
| DES2 | 1.53140 | -6.82138×10-4 | 0.99998 | 0.00011 | |
| DES3 | 1.51577 | -6.82252×10-4 | 0.99999 | 0.00011 | |
| DSE4 | 1.48676 | -6.83652×10-4 | 0.99998 | 0.00009 | |
| 比热容 | DES1 | 0.95372 | 0.00308 | 0.99766 | 0.0025 |
| DES2 | 0.55713 | 0.00531 | 0.99847 | 0.0034 | |
| DES3 | 0.01762 | 0.0078 | 0.99858 | 0.0048 | |
| DES4 | -0.51295 | 0.01004 | 0.99900 | 0.0051 |
| 热力学性质 | 溶剂 | A | B | T0/K | R2 |
|---|---|---|---|---|---|
| 黏度 | DES1 | -2.08244 | 892.19761 | 170 | 0.99992 |
| DES2 | -2.21258 | 850.66318 | 158 | 0.99983 | |
| DES3 | -2.14673 | 798.55697 | 163 | 0.99998 | |
| DES4 | -2.50824 | 832.22644 | 159 | 1 | |
| 电导率 | DES1 | 3.16148 | -107.04625 | 249 | 0.99994 |
| DES2 | 3.55785 | -166.03872 | 221 | 0.99997 | |
| DES3 | 3.70920 | -191.57429 | 210 | 0.99999 | |
| DES4 | 3.85660 | -206.90876 | 203 | 0.99995 |
表4 DES黏度、电导率的VFT方程参数及相关系数
| 热力学性质 | 溶剂 | A | B | T0/K | R2 |
|---|---|---|---|---|---|
| 黏度 | DES1 | -2.08244 | 892.19761 | 170 | 0.99992 |
| DES2 | -2.21258 | 850.66318 | 158 | 0.99983 | |
| DES3 | -2.14673 | 798.55697 | 163 | 0.99998 | |
| DES4 | -2.50824 | 832.22644 | 159 | 1 | |
| 电导率 | DES1 | 3.16148 | -107.04625 | 249 | 0.99994 |
| DES2 | 3.55785 | -166.03872 | 221 | 0.99997 | |
| DES3 | 3.70920 | -191.57429 | 210 | 0.99999 | |
| DES4 | 3.85660 | -206.90876 | 203 | 0.99995 |
| 1 | ABBOTT Andrew P, CAPPER Glen, DAVIES David L, et al. Novel solvent properties of choline chloride/urea mixtures[J]. Chemical Communications, 2003, 9(1): 70-71. |
| 2 | ABBOTT A P, CAPPER G, DAVIES D L, et al. Preparation of novel, moisture-stable, Lewis-acidic ionic liquids containing quaternary ammonium salts with functional side chains[J]. Chemical Communications, 2001(19): 2010-2011. |
| 3 | SHAHBAZ K, BAROUTIAN S, MJALLI F S, et al. Densities of ammonium and phosphonium based deep eutectic solvents: prediction using artificial intelligence and group contribution techniques[J]. Thermochimica Acta, 2012, 527: 59-66. |
| 4 | ABBOTT A, BOOTHBY D, CAPPER G, et al. Deep eutectic solvents formed between choline chloride and carboxylic acids: versatile alternatives to ionic liquids[J]. Journal of the American Chemical Society, 2004, 126(29): 9142-9147. |
| 5 | PERNA Filippo Maria, VITALE Paola, CAPRIATI Vito. Deep eutectic solvents and their applications as green solvents[J]. Current Opinion in Green and Sustainable Chemistry, 2020, 21: 27-33. |
| 6 | TORRES Paulo, BALCELLS Mercè, Ramon CANELA-GARAYOA. Effect of novel deep eutectic solvents on the endo/exo ratio of Diels-Alder reactions at room temperature[J]. ACS Omega, 2021, 6(30): 19392-19399. |
| 7 | GANO Zaharaddeen S, MJALLI Farouq S, Talal AL-WAHAIBI, et al. Extractive desulfurization of liquid fuel with FeCl3-based deep eutectic solvents: experimental design and optimization by central-composite design[J]. Chemical Engineering and Processing: Process Intensification, 2015, 93: 10-20. |
| 8 | Alberto GUTIÉRREZ, APARICIO Santiago, ATILHAN Mert. Design of arginine-based therapeutic deep eutectic solvents as drug solubilization vehicles for active pharmaceutical ingredients[J]. Physical Chemistry Chemical Physics, 2019, 21(20): 10621-10634. |
| 9 | 冯善花. 低共熔溶剂的制备及在芳烃烷烃体系分离中的基础研究[D]. 北京: 北京化工大学, 2019. |
| FENG Shanhua. Preparation of the eutectic solvent and its application in separation of aromation hydrocarbon alkane systems[D]. Beijing: Beijing University of Chemical Technology, 2019. | |
| 10 | 黄文睿, 唐超凡, 陶雨峰, 等. 绿色低共熔溶剂提取野菊花中黄酮类化合物[J]. 精细化工, 2022, 39(3): 569-576. |
| HUANG Wenrui, TANG Chaofan, TAO Yufeng, et al. Extraction of flavonoids from Chrysanthemum indicum L.by green deep eutectic solvents [J]. Fine Chemicals, 2022, 39(3): 569-576. | |
| 11 | RODRIGUEZ Nerea R, GERLACH Thomas, SCHEEPERS Daniëlle, et al. Experimental determination of the LLE data of systems consisting of{hexane + benzene + deep eutectic solvent}and prediction using the conductor-like screening model for real solvents[J]. The Journal of Chemical Thermodynamics, 2017, 104: 128-137. |
| 12 | ABBOTT A P, BARRON J C, RYDER K S, et al. Eutectic-based ionic liquids with metal-containing anions and cations[J]. Chemistry, 2007, 13(22): 6495-6501. |
| 13 | ABBOTT A, CAPPER G, DAVIES D, et al. Solubility of metal oxides in deep eutectic solvents based on choline chloride[J]. Journal of Chemical & Engineering Data, 2006, 51: 1280-1282. |
| 14 | FRANCISCO M, VAN DEN BRUINHORST A, KROON M. Low-transition-temperature mixtures (LTTMs): a new generation of designer solvents[J]. Angewandte Chemie International Edition, 2013, 52(11): 3074-3085. |
| 15 | Gregorio GARCÍA, ATILHAN Mert, APARICIO Santiago. An approach for the rationalization of melting temperature for deep eutectic solvents from DFT[J]. Chemical Physics Letters, 2015, 634: 151-155. |
| 16 | AFZAL Waheed, MOHAMMADI Amir H, RICHON Dominique. Volumetric properties of mono-, di-, tri-, and polyethylene glycol aqueous solutions from (273.15 to 363.15) K: experimental measurements and correlations[J]. Journal of Chemical & Engineering Data, 2010, 54(4): 1254-1261. |
| 17 | 邓荣华, 刘迎新, 张凌伟, 等. 乙二醇-水体系的理化性质研究[J]. 内蒙古工业大学学报(自然科学版), 2009, 28(2): 106-112. |
| DENG Ronghua, LIU Yingxin, ZHANG Lingwei, et al. A study on the physicochemical properties of ethylene glycol and water system[J]. Journal of Inner Mongolia University of Technology (Natural Science), 2009, 28(2): 106-112. | |
| 18 | COMELLI F, OTTANI T S, FRANCESCONI R, et al. Excess molar enthalpies of binary mixtures containing glycols or polyglycols + dimethyl sulfoxide at 308.15K[J]. Journal of Chemical & Engineering Data, 2003, 48: 995-998. |
| 19 | GEYER H, ULBIG P, GÖRNERT M. Measurement of densities and excess molar volumes for (1,2-ethanediol, or 1,2-propanediol, or 1,2-butanediol + water) at the temperatures (278.15, 288.15, 298.15, 308.15, and 318.15) K and for (2,3-butanediol + water) at the temperatures (308.15, 313.15, and 318.15) K[J]. The Journal of Chemical Thermodynamics, 2000, 32(12): 1585-1596. |
| 20 | YANG Changsheng, MA Peisheng, JING Fengming, et al. Excess molar volumes, viscosities, and heat capacities for the mixtures of ethylene glycol + water from 273.15K to 353.15K[J]. Journal of Chemical & Engineering Data, 2003, 48(4): 836-840. |
| 21 | Milan VRANEŠ, Ivona RADOVIĆ, Siniša BIKIĆ, et al. Improving ethylene glycol transport properties by caffeine: thermodynamic and computational evidence[J]. Journal of Molecular Liquids, 2021, 333: 115918. |
| 22 | KUMAR Bhupinder, SINGH Tejwant, RAO K Srinivasa, et al. Thermodynamic and spectroscopic studies on binary mixtures of imidazolium ionic liquids in ethylene glycol[J]. The Journal of Chemical Thermodynamics, 2012, 44(1): 121-127. |
| 23 | AZIZIAN Saeid, BASHAVARD Nowrouz. Surface properties of diluted solutions of cyclohexanol and cyclopentanol in ethylene glycol[J]. Journal of Colloid and Interface Science, 2005, 282(2): 428-433. |
| 24 | AZIZIAN Saeid, HEMMATI Maryam. Surface tension of binary mixtures of ethanol + ethylene glycol from 20 to 50℃[J]. Journal of Chemical & Engineering Data, 2003, 48(3): 662-663. |
| 25 | GURUNG Bhoj Bahadur, ROY Mahendra Nath. Study of densities, viscosity deviations, and isentropic compressibilities of ternary liquid mixtures of water and ethane-1,2-diol with some monoalcohols at various temperatures[J]. Physics and Chemistry of Liquids, 2007, 45(3): 331-343. |
| 26 | QUIJADA-MALDONADO E, MEINDERSMA G W, DE HAAN A B. Viscosity and density data for the ternary system water(1)-ethanol(2)-ethylene glycol(3) between 298.15K and 328.15K[J]. The Journal of Chemical Thermodynamics, 2013, 57: 500-505. |
| 27 | 谭志诚, 沈惠华, 陈淑霞. 乙二醇及其水溶液二元体系理化性能数据的测定[J]. 化学工程, 1983, 11(1): 41-50. |
| TAN Zhicheng, SHEN Huihua, CHEN Shuxia. Determination of physical and chemical properties of ethylene glycol and its aqueous solution[J]. Chemical Engineering(China), 1983, 11 (1): 41-50. | |
| 28 | ZHOU Nianyong, FENG Hao, GUO Yixing, et al. Experimental study on the spray cooling heat transfer performance and dimensionless correlations for ethylene glycol water solution[J]. Applied Thermal Engineering Design Processes Equipment Economics, 2022, 214: 118824. |
| 29 | 王军, 张真真, 杨许召, 等. 双阳离子型离子液体的合成与性能[J]. 化学试剂, 2009, 31(9): 719-722. |
| WANG Jun, ZHANG Zhenzhen, YANG Xuzhao, et al. Study on synthesis and properties of dicationic ionic liquids[J]. Chemical Reagents, 2009, 31(9): 719-722. | |
| 30 | 杨许召. 非对称Gemini离子液体的合成及性能研究[D]. 无锡: 江南大学, 2019. |
| YANG Xuzhao. Study on synthesis and properties of asymmetrical gemini ionic liquids[D]. Wuxi: Jiangnan University, 2019. | |
| 31 | CHEMAT Fareeda, ANJUM Hirra, SHARIFF Azmi Md, et al. Thermal and physical properties of (choline chloride + urea + L-arginine) deep eutectic solvents[J]. Journal of Molecular Liquids, 2016, 218: 301-308. |
| 32 | ABBOTT Andrew P, HARRIS Robert C, RYDER Karl S, et al. Glycerol eutectics as sustainable solvent systems[J]. Green Chemistry, 2011, 13(1): 82-90. |
| 33 | Carmine D’AGOSTINO, HARRIS Robert C, ABBOTT Andrew P, et al. Molecular motion and ion diffusion in choline chloride based deep eutectic solvents studied by 1H pulsed field gradient NMR spectroscopy[J]. Physical Chemistry Chemical Physics, 2011, 13(48): 21383-21391. |
| 34 | ABBOTT Andrew P, HARRIS Robert C, RYDER Karl S. Application of hole theory to define ionic liquids by their transport properties[J]. The Journal of Physical Chemistry B, 2007, 111(18): 4910-4913. |
| [1] | 马伊, 曹世伟, 王家骏, 林立群, 邢延, 曹腾良, 卢峰, 赵振伦, 张志军. 低共熔溶剂回收废旧锂离子电池正极材料的研究进展[J]. 化工进展, 2023, 42(S1): 219-232. |
| [2] | 叶玉玺, 丁晓茜, 池华睿, 朱楷伦, 刘杨, 王凌云, 郭庆杰. 疏水性低共熔溶剂氢键交互作用调控及萃取铜性能[J]. 化工进展, 2022, 41(S1): 397-406. |
| [3] | 何晨露, 邱晨茜, 方娟, 杨旋, 赖建军, 郑新宇, 吕建华, 陈燕丹, 黄彪. 基于低共熔溶剂体系的氮掺杂超级电容炭[J]. 化工进展, 2022, 41(9): 4946-4953. |
| [4] | 程明强, 汝娟坚, 华一新, 王丁, 耿笑, 张文文, 黄皓铭, 王道祥. 低共熔溶剂在废旧锂离子电池正极材料回收中的研究进展[J]. 化工进展, 2022, 41(6): 3293-3305. |
| [5] | 解先利, 刘云云, 余强, 张宇, 张荣清, 邱雨心. 低共熔溶剂预处理提高甘草渣酶解效果优化[J]. 化工进展, 2022, 41(3): 1349-1356. |
| [6] | 陈磊, 闫兴清, 胡延伟, 于帅, 杨凯, 陈绍云, 关辉, 喻健良, HMAHGEREFTE Haroun, MARTYNOV Sergey. 二氧化碳管道意外泄漏减压过程的断裂控制研究进展[J]. 化工进展, 2022, 41(3): 1241-1255. |
| [7] | 阮佳纬, 叶香珠, 陈立芳, 漆志文. 离子液体和低共熔溶剂催化二氧化碳合成有机碳酸酯的研究进展[J]. 化工进展, 2022, 41(3): 1176-1186. |
| [8] | 谷志攀, 阳季春, 张叶, 陶乐仁, 刘泛函. 市政污泥吸附等温线模型和热力学性质[J]. 化工进展, 2022, 41(2): 998-1008. |
| [9] | 刘乾静, 陈晓淼, 王芷, 史吉平, 李保国, 刘莉. 低共熔溶剂预处理杨木水解渣拆解木质素[J]. 化工进展, 2022, 41(10): 5612-5618. |
| [10] | 张豪, 叶国华, 陈子杨, 谢禹, 左琪. 黏土钒矿直接常压活化酸浸提钒热力学分析[J]. 化工进展, 2021, 40(10): 5360-5369. |
| [11] | 易兰, 李文英, 冯杰. 离子液体/低共熔溶剂在煤基液体分离中的应用[J]. 化工进展, 2020, 39(6): 2066-2078. |
| [12] | 刘昊然, 王韵淇, 李秀萍, 赵荣祥. C6H11NO/nCF3SO3H型低共熔溶剂氧化脱除模拟油中的二苯并噻吩[J]. 化工进展, 2020, 39(5): 1632-1640. |
| [13] | 张凯,武多多,刘强,彭越,杨震,段远源. 高密度流体声速测量中脉冲回波传播时间的测定[J]. 化工进展, 2020, 39(4): 1219-1226. |
| [14] | 窦金孝,赵永奇,段晓谞,柴红宁,余江龙. 络合亚铁乙二醇-四丁基溴化铵低共熔溶剂协同吸收SO2和NO[J]. 化工进展, 2020, 39(2): 453-460. |
| [15] | 成洪业, 漆志文. 低共熔溶剂用于萃取分离的研究进展[J]. 化工进展, 2020, 39(12): 4896-4907. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||