化工进展 ›› 2022, Vol. 41 ›› Issue (8): 4498-4512.DOI: 10.16085/j.issn.1000-6613.2021-2045
废轮胎热裂解技术研究现状与进展
季炫宇1,2( ), 林伟坚1,2, 周雄1,2, 柏继松1,2, 杨宇1,2, 孔杰1,2, 廖重阳1,2
), 林伟坚1,2, 周雄1,2, 柏继松1,2, 杨宇1,2, 孔杰1,2, 廖重阳1,2
- 1.重庆科技学院,重庆 401331
2.生活垃圾资源化处理省部共建协同创新中心,重庆 401331
-
收稿日期:2021-09-29修回日期:2021-12-08出版日期:2022-08-25发布日期:2022-08-22 -
通讯作者:季炫宇 -
作者简介:季炫宇(1981—),男,博士,副教授,研究方向为清洁燃烧技术、固废资源化处理。E-mail:jixuanyu@cqust.edu.cn 。 -
基金资助:重庆市教委2020年度科学技术研究计划(KJQN202001544);生活垃圾资源化处理省部共建协同创新中心2021年度科研项目(shljzyh2021-03)
Research status and progress of waste tire pyrolysis technology
JI Xuanyu1,2( ), LIN Weijian1,2, ZHOU Xiong1,2, BAI Jisong1,2, YANG Yu1,2, KONG Jie1,2, LIAO Chongyang1,2
), LIN Weijian1,2, ZHOU Xiong1,2, BAI Jisong1,2, YANG Yu1,2, KONG Jie1,2, LIAO Chongyang1,2
- 1.Chongqing University of Science and Technology, Chongqing 401331, China
2.The Provincial and Ministerial Collaborative Innovation Center for Domestic Garbage Resource Treatment, Chongqing 401331, China
-
Received:2021-09-29Revised:2021-12-08Online:2022-08-25Published:2022-08-22 -
Contact:JI Xuanyu
摘要:
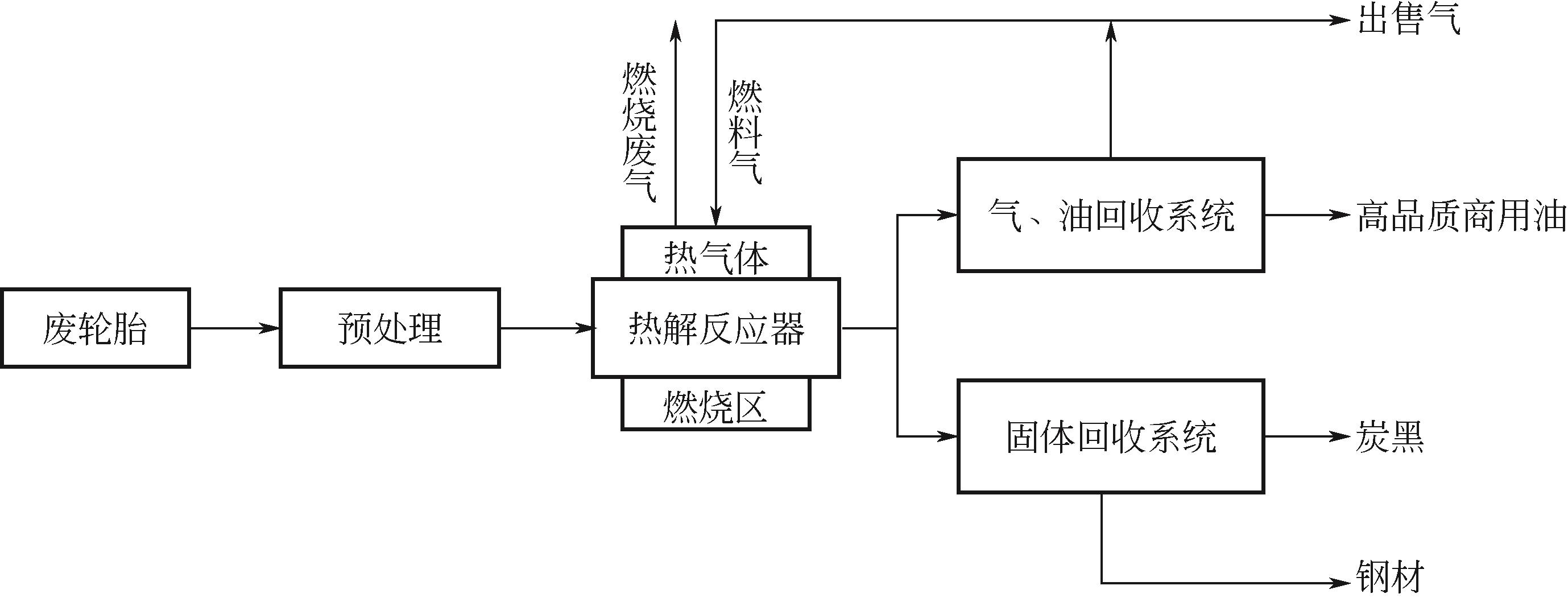
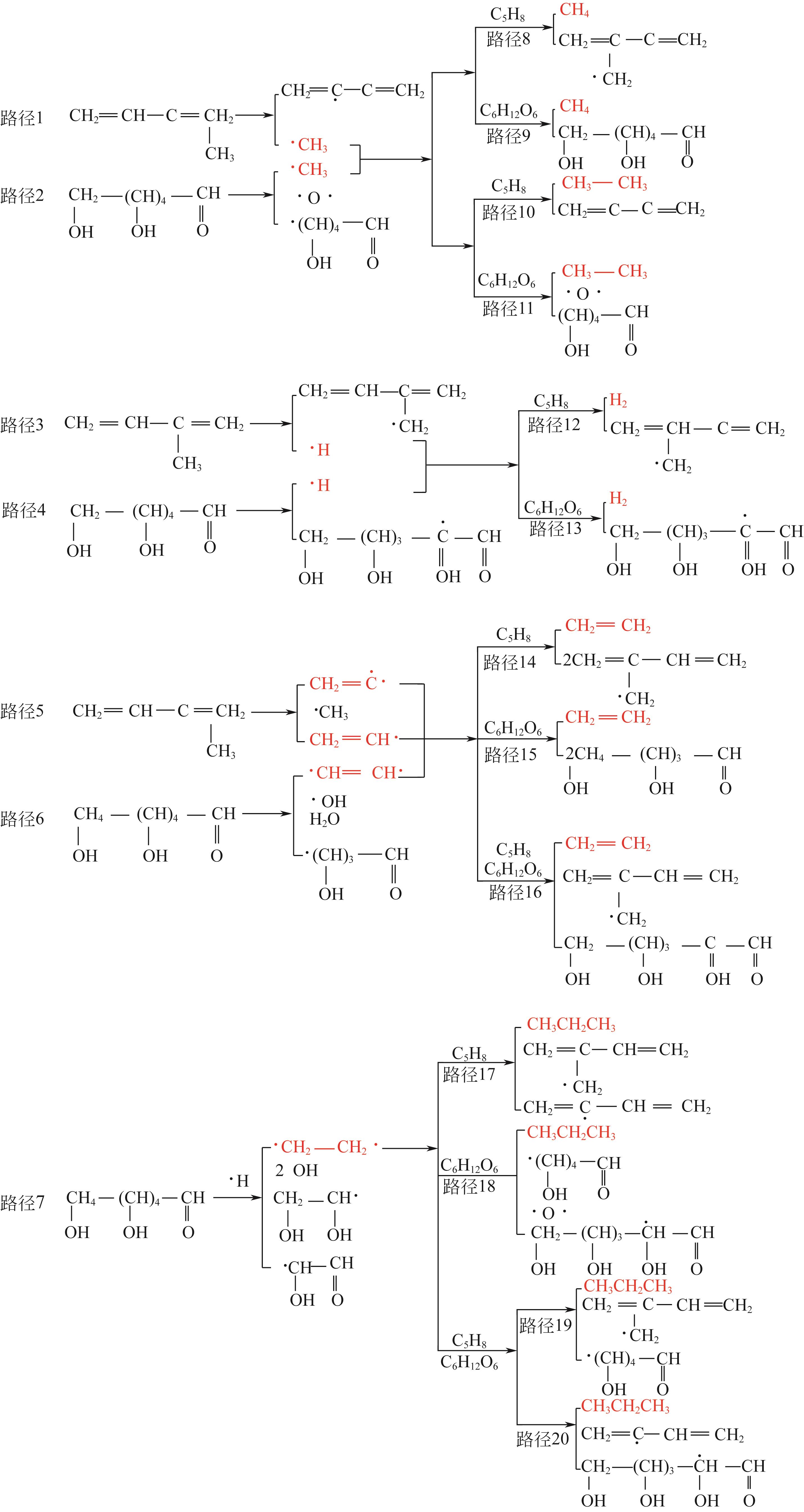
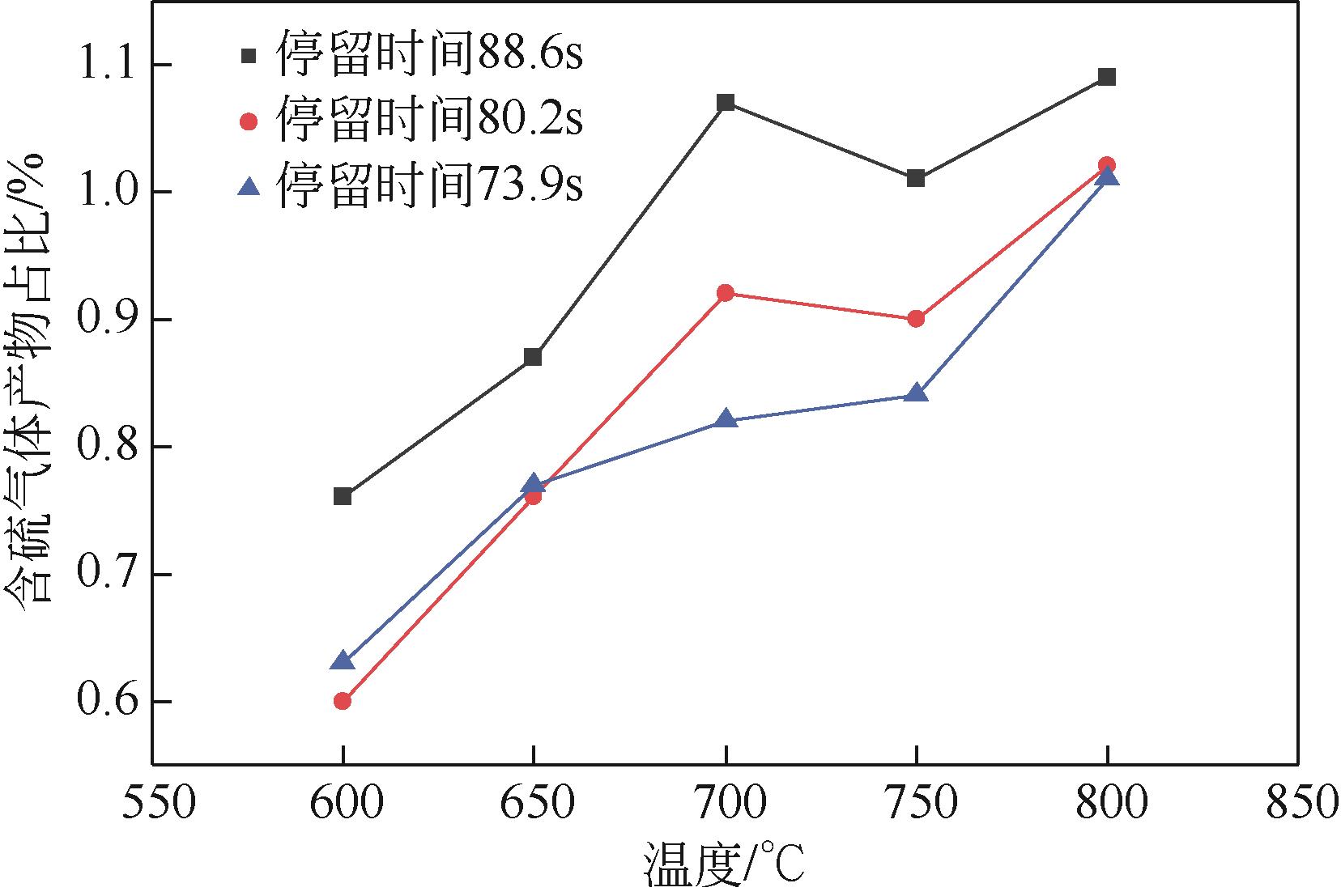
结合目前废旧轮胎资源化处理现状及研究成果,本文对热解机理、热解技术进行分析、对比,着重介绍了热解温度、升温速率、物料粒径、催化剂等工艺参数对热解产物产率的影响,分析表明Coast-Redfern积分法所得动力学模型较准确,平均反应活化能为129.5kJ/mol;现有的研究表明,热解温度对产物产率影响最大,气相产物与液相产物产率随温度升高而增加,其中液相产物产率相对较高的热解温度在500~550℃范围内,固相产物品质较高的热解温度在500~650℃范围内。其次对其固、液、气三相产物特性及应用和污染物(S、PAHs)的分布与控制方法做了归纳总结,为废旧轮胎热解技术向工业化发展提供技术依据。
中图分类号:
引用本文
季炫宇, 林伟坚, 周雄, 柏继松, 杨宇, 孔杰, 廖重阳. 废轮胎热裂解技术研究现状与进展[J]. 化工进展, 2022, 41(8): 4498-4512.
JI Xuanyu, LIN Weijian, ZHOU Xiong, BAI Jisong, YANG Yu, KONG Jie, LIAO Chongyang. Research status and progress of waste tire pyrolysis technology[J]. Chemical Industry and Engineering Progress, 2022, 41(8): 4498-4512.
物料 种类 | 元素/% | 热值 /MJ·kg-1 | ||||
|---|---|---|---|---|---|---|
| C | H | N | S | O* | ||
| 废轮胎 | 86.3 | 8.42 | 0.81 | 2.57 | 1.9 | 36.27 |
| 煤 | 88.04 | 5.52 | 1.8 | 0.42 | 4.22 | 20.34 |
| 木屑 | 49.84 | 7.73 | 2.88 | 0 | 39.55 | 15.41 |
| 塑胶 | 59.97 | 8.76 | 3.76 | 0.25 | 27.26 | 30.19 |
表1 废轮胎与煤、生物质、塑胶的元素分析及热值对比[11]
物料 种类 | 元素/% | 热值 /MJ·kg-1 | ||||
|---|---|---|---|---|---|---|
| C | H | N | S | O* | ||
| 废轮胎 | 86.3 | 8.42 | 0.81 | 2.57 | 1.9 | 36.27 |
| 煤 | 88.04 | 5.52 | 1.8 | 0.42 | 4.22 | 20.34 |
| 木屑 | 49.84 | 7.73 | 2.88 | 0 | 39.55 | 15.41 |
| 塑胶 | 59.97 | 8.76 | 3.76 | 0.25 | 27.26 | 30.19 |
| 热解技术 | 热解装置 | 液相最大产率 /% | 固相最大产率 /% | 气相最大产率 /% | 优点 | 缺点 |
|---|---|---|---|---|---|---|
| 直接热解 | 适用多种热解装置 | 45.2 | 49.53 | 14 | 热解工艺简单,成本较低 | 高价值产物回收效率较低 |
| 熔融盐热解 | 熔融盐热解装置 | 43.6 | 53.2 | 14.7 | 反应迅速;共融物可循环使用 | 对设备稳定性要求较高 |
| 等离子体热解 | 等离子体炬装置 | — | 78.4 | 65.6 | 污染极小;气体产物中H2占比较大;固相产物品质与商业炭黑一致 | 能耗高;不适用于制备热解油 |
| 催化热解 | 适用多种热解装置 | 60 | — | 24.8 | 可提升高价值目标产物产率 | 积炭结焦,活性降低;价格较昂贵 |
| 微波热解 | 微波热解炉 | 44.2 | 44.8 | 26 | 可实现整胎热解;污染小 | 实际热解温度难以测定 |
| 共热解 | 适用多种热解装置 | 48 | — | 35.4 | 最佳热解温度较低,能耗低 | 轮胎占比过高降低油品质量 |
表2 轮胎热解技术对比[25-26,31,36,39,41,43]
| 热解技术 | 热解装置 | 液相最大产率 /% | 固相最大产率 /% | 气相最大产率 /% | 优点 | 缺点 |
|---|---|---|---|---|---|---|
| 直接热解 | 适用多种热解装置 | 45.2 | 49.53 | 14 | 热解工艺简单,成本较低 | 高价值产物回收效率较低 |
| 熔融盐热解 | 熔融盐热解装置 | 43.6 | 53.2 | 14.7 | 反应迅速;共融物可循环使用 | 对设备稳定性要求较高 |
| 等离子体热解 | 等离子体炬装置 | — | 78.4 | 65.6 | 污染极小;气体产物中H2占比较大;固相产物品质与商业炭黑一致 | 能耗高;不适用于制备热解油 |
| 催化热解 | 适用多种热解装置 | 60 | — | 24.8 | 可提升高价值目标产物产率 | 积炭结焦,活性降低;价格较昂贵 |
| 微波热解 | 微波热解炉 | 44.2 | 44.8 | 26 | 可实现整胎热解;污染小 | 实际热解温度难以测定 |
| 共热解 | 适用多种热解装置 | 48 | — | 35.4 | 最佳热解温度较低,能耗低 | 轮胎占比过高降低油品质量 |
| T/℃ | 废轮胎热解气组分/% | 热值/MJ·kg-1 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CH 4 | H 2 | CO | CO 2 | C 2 H 4 | C 3 H 6 | C 3 H 9 | 其他 | ||
| 400 | 24.73 | 12.07 | 13.07 | 18.51 | 4.84. | 7.95 | 5.98 | 9.24 | 32.04 |
| 450 | 28.25 | 13.39 | 11.69 | 12.17 | 5.44 | 8.25 | 6.47 | 9.31 | 35.44 |
| 500 | 32.83 | 15.58 | 7.58 | 7.17 | 6.54 | 7.87 | 6.23 | 8.57 | 37.75 |
| 550 | 34.84 | 15.18 | 7.18 | 7.18 | 6.45 | 7.04 | 6.07 | 7.81 | 37.54 |
| 600 | 37.01 | 16.00 | 6.34 | 6.31 | 6.00 | 6.07 | 5.33 | 9.55 | 36.49 |
| 650 | 40.16 | 17.06 | 5.43 | 5.50 | 5.50 | 5.25 | 4.55 | 9.80 | 35.46 |
表3 不同温度条件下热解气组分[55]
| T/℃ | 废轮胎热解气组分/% | 热值/MJ·kg-1 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CH 4 | H 2 | CO | CO 2 | C 2 H 4 | C 3 H 6 | C 3 H 9 | 其他 | ||
| 400 | 24.73 | 12.07 | 13.07 | 18.51 | 4.84. | 7.95 | 5.98 | 9.24 | 32.04 |
| 450 | 28.25 | 13.39 | 11.69 | 12.17 | 5.44 | 8.25 | 6.47 | 9.31 | 35.44 |
| 500 | 32.83 | 15.58 | 7.58 | 7.17 | 6.54 | 7.87 | 6.23 | 8.57 | 37.75 |
| 550 | 34.84 | 15.18 | 7.18 | 7.18 | 6.45 | 7.04 | 6.07 | 7.81 | 37.54 |
| 600 | 37.01 | 16.00 | 6.34 | 6.31 | 6.00 | 6.07 | 5.33 | 9.55 | 36.49 |
| 650 | 40.16 | 17.06 | 5.43 | 5.50 | 5.50 | 5.25 | 4.55 | 9.80 | 35.46 |
| 油品特性 | 国际标准(商业柴油) | 热解油 | 检测方法 | |
|---|---|---|---|---|
| 最小值 | 最大值 | |||
| 十六烷值 | 45 | 55 | 47 | ISO 5156 |
| 密度 (15℃)/kg·m-3 | 840 | 880 | 847 | ISO 3675 |
| 黏度 (40℃)/mm2·s-1 | 2.0 | 5.5 | 2.4 | ISO 3104 |
| S质量分数/% | 0.1 | 0.5 | 0.38 | ISO/DIS 14596 |
| 闪点/℃ | 52 | — | 48 | EN 22719 |
表4 轮胎热解油与商业柴油性质的比较[71]
| 油品特性 | 国际标准(商业柴油) | 热解油 | 检测方法 | |
|---|---|---|---|---|
| 最小值 | 最大值 | |||
| 十六烷值 | 45 | 55 | 47 | ISO 5156 |
| 密度 (15℃)/kg·m-3 | 840 | 880 | 847 | ISO 3675 |
| 黏度 (40℃)/mm2·s-1 | 2.0 | 5.5 | 2.4 | ISO 3104 |
| S质量分数/% | 0.1 | 0.5 | 0.38 | ISO/DIS 14596 |
| 闪点/℃ | 52 | — | 48 | EN 22719 |
| 1 | 苏瑞景, 关杰, 梁波. 废轮胎资源化利用现状[J]. 上海第二工业大学学报, 2016, 33(1): 20-26. |
| SU Ruijing, GUAN Jie, LIANG Bo. Research progress in recycling of waste tyre[J]. Journal of Shanghai Second Polytechnic University, 2016, 33(1): 20-26. | |
| 2 | WILLIAMS P T. Pyrolysis of waste tyres: a review[J]. Waste Management, 2013, 33(8): 1714-1728. |
| 3 | 曾毅夫, 吴志恒, 何曦, 等. 废旧轮胎热解资源化技术进展[J]. 橡胶工业, 2020, 67(12): 949-953. |
| ZENG Yifu, WU Zhiheng, HE Xi, et al. Technical progress of pyrolysis and recycling of waste tires[J]. China Rubber Industry, 2020, 67(12): 949-953. | |
| 4 | 张珊珊. 核桃壳木醋液的生物活性与化学成分研究[D]. 杨凌: 西北农林科技大学, 2010. |
| ZHANG Shanshan. Biological activities and chemical profiles of pyroligneous acids from walnut shell[D]. Yangling: Northwest A & F University, 2010. | |
| 5 | CRESPO J E, PARRES F, NADAL A, et al. Improved tire adhesion of laminates (GTR) by incorporating reclaimed rubber (NRR)[J]. Annals of the Oradea University Fascicle of Management and Technological Engineering, 2011, 3(3): DOI: 10.15660/AUOFMTE.2011-3.2340. |
| 6 | 黄菊文, 李光明, 贺文智, 等. 废旧轮胎热解资源化技术研究进展[J]. 化工进展, 2010, 29(11): 2159-2164. |
| HUANG Juwen, LI Guangming, HE Wenzhi, et al. Research progress in recovery technology of waste tyre by pyrolysis[J]. Chemical Industry and Engineering Progress, 2010, 29(11): 2159-2164. | |
| 7 | 孙岳红, 雷国安, 路丽珠, 等. 废旧橡胶循环利用技术进展[J]. 橡胶科技, 2020, 18(2): 77-80. |
| SUN Yuehong, LEI Guoan, LU Lizhu, et al. Technology progress of waste rubber recycling[J]. Rubber Science and Technology, 2020, 18(2): 77-80. | |
| 8 | 高若峰, 王迎雪, 彭少贤, 等. 废旧橡胶回收利用再生剂研究进展[J]. 弹性体, 2015, 25(4): 74-77. |
| GAO Ruofeng, WANG Yingxue, PENG Shaoxian, et al. Progress in reclaiming agent of waste rubber regeneration[J]. China Elastomerics, 2015, 25(4): 74-77. | |
| 9 | REN Qiangqiang, WU Ziyue, HU Song, et al. Sulfur self-doped char with high specific capacitance derived from waste tire: effects of pyrolysis temperature[J]. Science of the Total Environment, 2020, 741: 140193. |
| 10 | 齐涛, 王娜, 张纪刚. 废橡胶与煤共热解的工艺研究[J]. 煤炭加工与综合利用, 2018(12): 22-25. |
| QI Tao, WANG Na, ZHANG Jigang. Study on the co-pyrolysis process of waste rubber and coal[J]. Coal Processing & Comprehensive Utilization, 2018(12): 22-25. | |
| 11 | 戴贤明. 废轮胎热解过程及产物特性试验研究[D]. 武汉: 华中科技大学, 2009. |
| DAI Xianming. Experimental research on pyrolysis process of waste tyres and characteristics of pyrolytic products[D]. Wuhan: Huazhong University of Science and Technology, 2009. | |
| 12 | 隋莹. 废旧轮胎热解的动力学研究与特性分析[J]. 橡塑技术与装备, 2017, 43(13): 39-44. |
| SUI Ying. Dynamic research and characteristics analysis of pyrolysis of waste tires[J]. China Rubber/Plastics Technology and Equipment, 2017, 43(13): 39-44. | |
| 13 | 赵巍, 汪琦, 邹宗树, 等. 塑料和橡胶垃圾的热解机理及动力学分析[J]. 工程热物理学报, 2008, 29(11): 1977-1979. |
| ZHAO Wei, WANG Qi, ZOU Zongshu, et al. Pyrolysis mechanism and kinetics of plastic and rubber waste[J]. Journal of Engineering Thermophysics, 2008, 29(11): 1977-1979. | |
| 14 | ARABIOURRUTIA M, LOPEZ G, ELORDI G, et al. Product distribution obtained in the pyrolysis of tyres in a conical spouted bed reactor[J]. Chemical Engineering Science, 2007, 62(18/19/20): 5271-5275. |
| 15 | MURILLO R, ARANDA A, AYLÓN E, et al. Process for the separation of gas products from waste tire pyrolysis[J]. Industrial & Engineering Chemistry Research, 2006, 45(5): 1734-1738. |
| 16 | JUMA M, KOREŇOVÁ Z, MARKOŠ J, et al. Pyrolysis and combustion of scrap tire[J]. Petroleum & Coal, 2006, 48(1):15-26. |
| 17 | 陈波宇, 顾瑛, 陈生, 等. 热重分析法测定橡胶并用比[J]. 轮胎工业, 2021, 41(6): 395-398. |
| CHEN Boyu, GU Ying, CHEN Sheng, et al. Determination of rubber blend ratio by thermogravimetric analysis method[J]. Tire Industry, 2021, 41(6): 395-398. | |
| 18 | LEUNG D Y C, WANG C L. Kinetic study of scrap tyre pyrolysis and combustion[J]. Journal of Analytical and Applied Pyrolysis, 1998, 45(2): 153-169. |
| 19 | 王林郁. 废轮胎热解动力学分析[J]. 山东化工, 2011, 40(12): 56-58. |
| WANG Linyu. Pyrolysis kinetics analysis of waste tire[J]. Shandong Chemical Industry, 2011, 40(12): 56-58. | |
| 20 | 苏亚欣, 张先中, 赵兵涛. 废轮胎粉的热解特性及其动力学模型[J]. 东华大学学报(自然科学版), 2008, 34(6): 740-743, 751. |
| SU Yaxin, ZHANG Xianzhong, ZHAO Bingtao. Pyrolysis of waste tire powder and its dynamic model[J]. Journal of Donghua University (Natural Science), 2008, 34(6): 740-743, 751. | |
| 21 | 孙蓉, 朱宝忠, 孙运兰. 热分析-质谱法研究汽车废轮胎热解行为及反应动力学[J]. 过程工程学报, 2016, 16(6): 966-971. |
| SUN Rong, ZHU Baozhong, SUN Yunlan. Pyrolysis and reaction kinetics of automobile waste tire with TG-DSC-MS technique[J]. The Chinese Journal of Process Engineering, 2016, 16(6): 966-971. | |
| 22 | 闫大海. 废轮胎回转窑中试热解产物应用及热解机理和动力学模型研究[D]. 杭州: 浙江大学, 2006. |
| YAN Dahai. Study on the utilization of pyrolytic products from pyrolysis of used tyre in a pilot-scale rotary kiln and the mechanism and kinetic model of rubber pyrolysis[D]. Hangzhou: Zhejiang University, 2006. | |
| 23 | MIRANDA M, PINTO F, GULYURTLU I, et al. Pyrolysis of rubber tyre wastes: a kinetic study[J]. Fuel, 2013, 103: 542-552. |
| 24 | YAO Erren, WANG Yuzhuo, YANG Qirong, et al. Co-pyrolysis mechanism of natural rubber and cellulose based on thermogravimetry-gas chromatography and molecular dynamics simulation[J]. Energy & Fuels, 2019, 33(12): 12450-12458. |
| 25 | LARESGOITI M F, CABALLERO B M, DE MARCO I, et al. Characterization of the liquid products obtained in tyre pyrolysis[J]. Journal of Analytical and Applied Pyrolysis, 2004, 71(2): 917-934. |
| 26 | 张会亮, 范晓旭, 刘彦丰, 等. 块状废轮胎固定床热解特性实验研究[J]. 可再生能源, 2015, 33(1): 149-153. |
| ZHANG Huiliang, FAN Xiaoxu, LIU Yanfeng, et al. Experimental study on pyrolysis of blocky tires in a fixed-bed reactor[J]. Renewable Energy Resources, 2015, 33(1): 149-153. | |
| 27 | 李鑫. 废轮胎流化床热解特性研究[D]. 杭州: 浙江大学, 2003. |
| LI Xin. Studies on characteristics of pyrolysis for waste tires in fluidized bed[D]. Hangzhou: Zhejiang University, 2003. | |
| 28 | 张兴华, 常杰, 王铁军, 等. 碱性条件下废轮胎真空热裂解研究[J]. 燃料化学学报, 2005, 33(6): 713-716. |
| ZHANG Xinghua, CHANG Jie, WANG Tiejun, et al. Vacuum pyrolysis of waste tires with basic additives[J]. Journal of Fuel Chemistry and Technology, 2005, 33(6): 713-716. | |
| 29 | LOPEZ G, OLAZAR M, AGUADO R, et al. Vacuum pyrolysis of waste tires by continuously feeding into a conical spouted bed reactor[J]. Industrial & Engineering Chemistry Research, 2010, 49(19): 8990-8997. |
| 30 | CHAMBERS R W. Scrap-tire feeding and coking process: US4030984[P]. 1977-06-21. |
| 31 | STELMACHOWSKI M. Conversion of waste rubber to the mixture of hydrocarbons in the reactor with molten metal[J]. Energy Conversion and Management, 2009, 50(7): 1739-1745. |
| 32 | 赵增立, 李海滨, 吴创之, 等. 生物质等离子体气化研究[J]. 太阳能学报, 2005, 26(4): 468-472. |
| ZHAO Zengli, LI Haibin, WU Chuangzhi, et al. The study on the plasma gasification of biomass[J]. Acta Energiae Solaris Sinica, 2005, 26(4): 468-472. | |
| 33 | HUANG H, TANG L. Pyrolysis treatment of waste tire powder in a capacitively coupled RF plasma reactor[J]. Energy Conversion and Management, 2009, 50(3): 611-617. |
| 34 | CHANG J S, GU B W, LOOY P C, et al. Thermal plasma pyrolysis of used old tires for production of syngas[J]. Fuel and Energy Abstracts, 1997, 38(1): 41-42. |
| 35 | 杜长明, 吴焦, 黄娅妮. 等离子体热解气化有机废弃物制氢的关键技术分析[J]. 中国环境科学, 2016, 36(11): 3429-3440. |
| DU Changming, WU Jiao, HUANG Yani. Analysis of critical technology for hydrogen production in plasma pyrolysis and gasification of organic waste[J]. China Environmental Science, 2016, 36(11): 3429-3440. | |
| 36 | ALSALEH A, SATTLER M L. Waste tire pyrolysis: influential parameters and product properties[J]. Current Sustainable/Renewable Energy Reports, 2014, 1(4): 129-135. |
| 37 | DŨNG N A, WONGKASEMJIT S, JITKARNKA S. Effects of pyrolysis temperature and Pt-loaded catalysts on polar-aromatic content in tire-derived oil[J]. Applied Catalysis B: Environmental, 2009, 91(1/2): 300-307. |
| 38 | KAR T, KELEŞ S, KAYGUSUZ K. Comparison of catalytic and noncatalytic pyrolysis and product yields of some waste biomass species[J]. International Journal of Energy Research, 2019, 43(6): 2032-2043. |
| 39 | WILLIAMS P T, BRINDLE A J. Aromatic chemicals from the catalytic pyrolysis of scrap tyres[J]. Journal of Analytical and Applied Pyrolysis, 2003, 67(1): 143-164. |
| 40 | LIDSTRÖM P, TIERNEY J, WATHEY B, et al. Microwave assisted organic synthesis—A review[J]. Tetrahedron, 2001, 57(45): 9225-9283. |
| 41 | 杨亚青. 废轮胎微波热解过程及产物分布特性试验研究[D]. 济南: 山东大学, 2017. |
| YANG Yaqing. Experimental study on process and product distribution of waste tire microwave pyrolysis[D]. Jinan: Shandong University, 2017. | |
| 42 | UNDRI A, MEINI S, ROSI L, et al. Microwave pyrolysis of polymeric materials: Waste tires treatment and characterization of the value-added products[J]. Journal of Analytical and Applied Pyrolysis, 2013, 103: 149-158. |
| 43 | HOSOYA T, KAWAMOTO H, SAKA S. Solid/liquid- and vapor-phase interactions between cellulose- and lignin-derived pyrolysis products[J]. Journal of Analytical and Applied Pyrolysis, 2009, 85(1/2): 237-246. |
| 44 | SHAH S A Y, ZEESHAN M, FAROOQ M Z, et al. Co-pyrolysis of cotton stalk and waste tire with a focus on liquid yield quantity and quality[J]. Renewable Energy, 2019, 130: 238-244. |
| 45 | 刘岗. 生物质与废轮胎共热解及催化对液体产物的影响[D]. 太原: 太原理工大学, 2007. |
| LIU Gang. Influence of co-pyrolysis and catalysis of biomass with waste tires on the liquid properties[D]. Taiyuan: Taiyuan University of Technology, 2007. | |
| 46 | 吕全伟, 林顺洪, 李玉, 等. TG-FTIR研究污泥掺混废轮胎共热解特性[J]. 应用化工, 2017, 46(11): 2195-2198, 2203. |
| Quanwei LYU, LIN Shunhong, LI Yu, et al. Study on the co-pyrolysis characteristic of sewage sludge and waste tires using TG-FTIR[J]. Applied Chemical Industry, 2017, 46(11): 2195-2198, 2203. | |
| 47 | 杨金鑫. 废轮胎与煤焦油共热解制备燃料油和炭黑工艺研究[D]. 赣州: 江西理工大学, 2014. |
| YANG Jinxin. The copyrolysis process study of waste tire with coal tar for fuel oil and carbon black[D]. Ganzhou: Jiangxi University of Science and Technology, 2014. | |
| 48 | 刘海兵. 尾煤和废轮胎共热解研究[D]. 北京: 中国矿业大学(北京), 2012. |
| LIU Haibing. Research on co-pyrolysis of coal taillings and waste tire[D]. Beijing: China University of Mining & Technology, Beijing, 2012. | |
| 49 | 刘阳生, 白庆中, 李迎霞, 等. 废轮胎的热解及其产物分析[J]. 环境科学, 2000, 21(6): 85-88. |
| LIU Yangsheng, BAI Qingzhong, LI Yingxia, et al. The waste tire pyrolysis and its yields analysis[J]. Chinese Journal of Enviromental Science, 2000, 21(6): 85-88. | |
| 50 | 钟浩文, 杨启容, 姚尔人, 等. 丁苯橡胶热解过程的分子动力学研究[J]. 青岛大学学报(工程技术版), 2019, 34(2): 95-100. |
| ZHONG Haowen, YANG Qirong, YAO Erren, et al. Molecular dynamics study on the pyrolysis of styrene butadiene rubber[J]. Journal of Qingdao University (Engineering & Technology Edition), 2019, 34(2): 95-100. | |
| 51 | 付兴民, 张玉秀, 刘海兵, 等. 初始温度对废轮胎热解的影响[J]. 环境工程学报, 2013, 7(5): 1907-1912. |
| FU Xingmin, ZHANG Yuxiu, LIU Haibing, et al. Influences of initial temperature on pyrolysis of scrap tire[J]. Chinese Journal of Environmental Engineering, 2013, 7(5): 1907-1912. | |
| 52 | 陈汉平, 隋海清, 王贤华, 等. 废轮胎热解多联产过程中温度对产物品质的影响[J]. 中国电机工程学报, 2012, 32(23): 119-125, 159. |
| CHEN Hanping, SUI Haiqing, WANG Xianhua, et al. Effects of temperature on the product property during multi-cogeneration based on waste tyre pyrolysis[J]. Proceedings of the CSEE, 2012, 32(23): 119-125, 159. | |
| 53 | 王慧. 废轮胎热解油的资源化利用研究[D]. 上海: 华东理工大学, 2011. |
| WANG Hui. Research on utilization of used tire pyrolysis oil[D]. Shanghai: East China University of Science and Technology, 2011. | |
| 54 | 张志霄, 池涌, 高雅丽, 等. 废轮胎热解油的成分分析及二次热解反应[J]. 工程热物理学报, 2005, 26(1): 159-162. |
| ZHANG Zhixiao, CHI Yong, GAO Yali, et al. Characteristics of pyrolytic oil derived from pilot-scale pyrolysis of scrap tires and the secondary pyrolysis[J]. Journal of Engineering Thermophysics, 2005, 26(1): 159-162. | |
| 55 | NISAR J, ALI G, ULLAH N, et al. Pyrolysis of waste tire rubber: Influence of temperature on pyrolysates yield[J]. Journal of Environmental Chemical Engineering, 2018, 6(2): 3469-3473. |
| 56 | SEIFALI ABBAS-ABADI M, NEKOOMANESH HAGHIGHI M. The consideration of different effective zeolite based catalysts and heating rate on the pyrolysis of styrene butadiene rubber (SBR) in a stirred reactor[J]. Energy & Fuels, 2017, 31(11): 12358-12363. |
| 57 | MKHIZE N M, VAN DER GRYP P, DANON B, et al. Effect of temperature and heating rate on limonene production from waste tyre pyrolysis[J]. Journal of Analytical and Applied Pyrolysis, 2016, 120: 314-320. |
| 58 | 张义烽, 薛勇, 蒋东燕, 等. 粒径、升温速率及催化剂对废轮胎胶粉热解特性的影响[J]. 西南科技大学学报, 2013, 28(1): 65-69. |
| ZHANG Yifeng, XUE Yong, JIANG Dongyan, et al. Influence of tire powder's pyrolysis by particle size/heating rate and catalyst and its thermogravimetric analysis[J]. Journal of Southwest University of Science and Technology, 2013, 28(1): 65-69. | |
| 59 | 邓飞虎, 王黎. 轮胎粒径对热解产物的影响[J]. 应用化工, 2019, 48(6): 1382-1384. |
| DENG Feihu, WANG Li. Influences of tire size on pyrolysis products[J]. Applied Chemical Industry, 2019, 48(6): 1382-1384. | |
| 60 | 蒋智慧, 刘洋, 宋永猛, 等. 废旧轮胎热解及热解产物研究展望[J]. 化工进展, 2021, 40(1): 515-525. |
| JIANG Zhihui, LIU Yang, SONG Yongmeng, et al. Review of pyrolysis for waste tires and research prospects of pyrolysis products[J]. Chemical Industry and Engineering Progress, 2021, 40(1): 515-525. | |
| 61 | MA Sijie, LEONG H, HE Limo, et al. Effects of pressure and residence time on limonene production in waste tires pyrolysis process[J]. Journal of Analytical and Applied Pyrolysis, 2020, 151: 104899. |
| 62 | KORDOGHLI S, PARASCHIV M, KUNCSER R, et al. Catalysts’ influence on thermochemical decomposition of waste tires[J]. Environmental Progress & Sustainable Energy, 2017, 36(5): 1560-1567. |
| 63 | 帅坤, 闫雨瑗, 崔小龙, 等. 废轮胎热解催化剂及工艺研究进展[J]. 过程工程学报, 2017, 17(1): 193-200. |
| SHUAI Kun, YAN Yuyuan, CUI Xiaolong, et al. Research advances in pyrolysis processes and catalysts of waste tires[J]. The Chinese Journal of Process Engineering, 2017, 17(1): 193-200. | |
| 64 | SALMASI S S Z, ABBAS-ABADI M S, HAGHIGHI M N, et al. The effect of different zeolite based catalysts on the pyrolysis of poly butadiene rubber[J]. Fuel, 2015, 160: 544-548. |
| 65 | SHAH J, JAN M R, MABOOD F. Recovery of value-added products from the catalytic pyrolysis of waste tyre[J]. Energy Conversion and Management, 2009, 50(4): 991-994. |
| 66 | 张兴华, 常杰, 王铁军, 等. 真空条件下金属氧化物催化废轮胎热解研究[J]. 能源工程, 2006(1): 41-45. |
| ZHANG Xinghua, CHANG Jie, WANG Tiejun, et al. A study on metal-oxide catalyzed pyrolysis of waste tires under vacuum conditions[J]. Energy Engineering, 2006(1): 41-45. | |
| 67 | ZHANG Yeshui, WU Chunfei, NAHIL M A, et al. Pyrolysis–catalytic reforming/gasification of waste tires for production of carbon nanotubes and hydrogen[J]. Energy & Fuels, 2015, 29(5): 3328-3334. |
| 68 | PORTOFINO S, CASU S, IOVANE P, et al. Optimizing H2 production from waste tires via combined steam gasification and catalytic reforming[J]. Energy & Fuels, 2011, 25(5): 2232-2241. |
| 69 | ROFIQUL ISLAM M, HANIU H, RAFIQUL ALAM BEG M. Liquid fuels and chemicals from pyrolysis of motorcycle tire waste: product yields, compositions and related properties[J]. Fuel, 2008, 87(13/14): 3112-3122. |
| 70 | DE MARCO RODRIGUEZ I, LARESGOITI M F, CABRERO M A, et al. Pyrolysis of scrap tyres[J]. Fuel Processing Technology, 2001, 72(1): 9-22. |
| 71 | KARAGÖZ M, AĞBULUT Ü, SARıDEMIR S. Waste to energy: Production of waste tire pyrolysis oil and comprehensive analysis of its usability in diesel engines[J]. Fuel, 2020, 275: 117844. |
| 72 | MURUGAN S, RAMASWAMY M C, NAGARAJAN G. The use of tyre pyrolysis oil in diesel engines[J]. Waste Management, 2008, 28(12): 2743-2749. |
| 73 | 徐宗平, 郭庆民. 废轮胎热解回收中的废气综合利用[J]. 再生资源与循环经济, 2017, 10(4): 34-37. |
| XU Zongping, GUO Qingmin. The multipurpose utilization of waste gas in waste tire pyrolysis recycling[J]. Recyclable Resources and Circular Economy, 2017, 10(4): 34-37. | |
| 74 | XU Junqing, YU Jiaxue, XU Jianglin, et al. High-value utilization of waste tires: A review with focus on modified carbon black from pyrolysis[J]. Science of the Total Environment, 2020, 742: 140235. |
| 75 | 朱小涛. 表面改性活性炭对土壤中铅的吸附与稳定性能研究[D]. 武汉: 武汉科技大学, 2016. |
| ZHU Xiaotao. Effect of surface modified activated carbon on adsorption and stabilization of lead in soil[D]. Wuhan: Wuhan University of Science and Technology, 2016. | |
| 76 | 吴英亮. 化学药品活化法生产活性炭(中)[J]. 生意通, 2011(4):116-120. |
| WU Yingliang. Production of activated carbon by chemical activation method[J]. Business News, 2011(4):116-120. | |
| 77 | TANG L, HUANG H. Thermal plasma pyrolysis of used tires for carbon black recovery[J]. Journal of Materials Science, 2005, 40(14): 3817-3819. |
| 78 | 沈伯雄, 鲁锋, 朱国营, 等. 废轮胎热解炭黑及其改性后的特性研究[J]. 环境工程学报, 2010, 4(7): 1615-1618. |
| SHEN Boxiong, LU Feng, ZHU Guoying, et al. Characterization of modified and original carbon blacks from pyrolysis of scrap tires[J]. Chinese Journal of Environmental Engineering, 2010, 4(7): 1615-1618. | |
| 79 | ROY C, CHAALA A, DARMSTADT H. The vacuum pyrolysis of used tires: end-uses for oil and carbon black products[J]. Journal of Analytical and Applied Pyrolysis, 1999, 51(1/2): 201-221. |
| 80 | 刘俊, 陈云嫩, 聂锦霞. 废轮胎热解炭黑制备活性炭及处理染料废水[J]. 中国环境科学, 2018, 38(10): 3795-3800. |
| LIU Jun, CHEN Yunnen, NIE Jinxia. Preparation of activated carbon from waste tire pyrolysis carbon black and its treatment of dyeing wastewater[J]. China Environmental Science, 2018, 38(10): 3795-3800. | |
| 81 | OGASAWARA S, KURODA M, WAKAO N. Preparation of activated carbon by thermal decomposition of used automotive tires[J]. Industrial & Engineering Chemistry Research, 1987, 26(12): 2552-2556. |
| 82 | SUSA D, HAYDARY J. Sulphur distribution in the products of waste tire pyrolysis[J]. Chemical Papers, 2013, 67(12): 1521-1526. |
| 83 | LI Wei, HUANG Chuanfeng, LI Dapeng, et al. Derived oil production by catalytic pyrolysis of scrap tires[J]. Chinese Journal of Catalysis, 2016, 37(4): 526-532. |
| 84 | 金余其, 陆王琳, 池涌, 等. 废轮胎热解油加氢精制硫氮脱除特性研究[J]. 燃料化学学报, 2007, 35(6): 772-776. |
| JIN Yuqi, LU Wanglin, CHI Yong, et al. Research on sulfur and nitrogen removal characteristics of scrap-tire pyrolytic oil by hydrofining[J]. Journal of Fuel Chemistry and Technology, 2007, 35(6): 772-776. | |
| 85 | 吴丹, 周洁, 俞天明, 等. 废轮胎热解衍生油非加氢脱硫[J]. 环境工程学报, 2013, 7(8): 3153-3157. |
| WU Dan, ZHOU Jie, YU Tianming, et al. Non-hydrogenation desulfurization of derived pyrolytic oil from scrap tires[J]. Chinese Journal of Environmental Engineering, 2013, 7(8): 3153-3157. | |
| 86 | 岳敏, 谷学新, 邹洪, 等. 多环芳烃的危害与防治[J]. 首都师范大学学报(自然科学版), 2003, 24(3): 40-44, 31. |
| YUE Min, GU Xuexin, ZOU Hong, et al. Killer of health—polycyclic aromatic hydrocarbons[J]. Journal of Capital Normal University, 2003, 24(3): 40-44, 31. | |
| 87 | CHEN S J, SU H B, CHANG J E, et al. Emissions of polycyclic aromatic hydrocarbons (PAHs) from the pyrolysis of scrap tires[J]. Atmospheric Environment, 2007, 41(6): 1209-1220. |
| 88 | LEVENDIS Y A, ATAL A, CARLSON J B. On the correlation of CO and PAH emissions from the combustion of pulverized coal and waste tires[J]. Environmental Science & Technology, 1998, 32(23): 3767-3777. |
| [1] | 王家庆, 宋广伟, 李强, 郭帅成, DAI Qingli. 橡胶混凝土界面改性方法及性能提升路径[J]. 化工进展, 2023, 42(S1): 328-343. |
| [2] | 邵博识, 谭宏博. 锯齿波纹板对挥发性有机物低温脱除过程强化模拟分析[J]. 化工进展, 2023, 42(S1): 84-93. |
| [3] | 邵志国, 任雯, 许世佩, 聂凡, 许毓, 刘龙杰, 谢水祥, 李兴春, 王庆吉, 谢加才. 终温对油基钻屑热解产物分布和特性影响[J]. 化工进展, 2023, 42(9): 4929-4938. |
| [4] | 李志远, 黄亚继, 赵佳琪, 于梦竹, 朱志成, 程好强, 时浩, 王圣. 污泥与聚氯乙烯共热解重金属特性[J]. 化工进展, 2023, 42(9): 4947-4956. |
| [5] | 李海东, 杨远坤, 郭姝姝, 汪本金, 岳婷婷, 傅开彬, 王哲, 何守琴, 姚俊, 谌书. 炭化与焙烧温度对植物基铁碳微电解材料去除As(Ⅲ)性能的影响[J]. 化工进展, 2023, 42(7): 3652-3663. |
| [6] | 姚丽铭, 王亚琢, 范洪刚, 顾菁, 袁浩然, 陈勇. 餐厨垃圾处理现状及其热解技术研究进展[J]. 化工进展, 2023, 42(7): 3791-3801. |
| [7] | 张杉, 仲兆平, 杨宇轩, 杜浩然, 李骞. 磷酸盐改性高岭土对生活垃圾热解过程中重金属的富集[J]. 化工进展, 2023, 42(7): 3893-3903. |
| [8] | 王俊杰, 潘艳秋, 牛亚宾, 俞路. 分子水平催化重整装置模型构建及应用[J]. 化工进展, 2023, 42(7): 3404-3412. |
| [9] | 李若琳, 何少林, 苑宏英, 刘伯约, 纪冬丽, 宋阳, 刘博, 余绩庆, 徐英俊. 原位热解对油页岩物性及地下水水质影响探索[J]. 化工进展, 2023, 42(6): 3309-3318. |
| [10] | 李瑞东, 黄辉, 同国虎, 王跃社. 原油精馏塔中铵盐吸湿特性及其腐蚀行为[J]. 化工进展, 2023, 42(6): 2809-2818. |
| [11] | 李栋先, 王佳, 蒋剑春. 皂脚热解-催化气态加氢制备生物燃料[J]. 化工进展, 2023, 42(6): 2874-2883. |
| [12] | 王志伟, 郭帅华, 吴梦鸽, 陈颜, 赵俊廷, 李辉, 雷廷宙. 生物质与塑料催化共热解技术研究进展[J]. 化工进展, 2023, 42(5): 2655-2665. |
| [13] | 梁贻景, 马岩, 卢展烽, 秦福生, 万骏杰, 王志远. La1-x Sr x MnO3钙钛矿涂层的抗结焦性能[J]. 化工进展, 2023, 42(4): 1769-1778. |
| [14] | 葛伟童, 廖亚龙, 李明原, 嵇广雄, 郗家俊. Pd-Fe/MWCNTs双金属催化剂制备及其脱氯动力学[J]. 化工进展, 2023, 42(4): 1885-1894. |
| [15] | 刘静, 林琳, 张健, 赵峰. 生物质基炭材料孔径调控及电化学性能研究进展[J]. 化工进展, 2023, 42(4): 1907-1916. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||