化工进展 ›› 2022, Vol. 41 ›› Issue (2): 998-1008.DOI: 10.16085/j.issn.1000-6613.2021-0519
市政污泥吸附等温线模型和热力学性质
谷志攀1,2( ), 阳季春2, 张叶2, 陶乐仁1,3(
), 阳季春2, 张叶2, 陶乐仁1,3( ), 刘泛函2
), 刘泛函2
- 1.上海理工大学能源与动力工程学院制冷与低温研究所,上海 200093
2.嘉兴学院建筑工程学院,浙江 嘉兴 314001
3.上海市动力工程多相流动与传热重点实验室,上海 200093
-
收稿日期:2021-03-15修回日期:2021-05-23出版日期:2022-02-05发布日期:2022-02-23 -
通讯作者:陶乐仁 -
作者简介:谷志攀(1983—),男,讲师,博士研究生,研究方向为多孔介质传热传质。E-mail:guzhipan418@126.com 。 -
基金资助:国家自然科学基金(51568060);浙江省自然科学基金青年科学基金(LQ19E080014);浙江省基础公益研究计划(LGG19E060005);上海市动力工程多相流动与传热重点实验室项目(1N-15-301-101)
Mathematical modelling of water sorption isotherms and thermodynamic properties of municipal sewage sludge
GU Zhipan1,2( ), YANG Jichun2, ZHANG Ye2, TAO Leren1,3(
), YANG Jichun2, ZHANG Ye2, TAO Leren1,3( ), LIU Fanhan2
), LIU Fanhan2
- 1.Instiute of Refrigeration and Cryogenics, School of Energy and Power, University of Shanghai for Science and Technology, Shanghai 200093, China
2.School of Architecture and Civil Engineering, Jiaxing University, Jiaxing 314001, Zhejiang, China
3.Shanghai Key Laboratory of Multiphase Flow and Heat Transfer in Power Engineering, University of Shanghai for Science and Technology, Shanghai 200093, China
-
Received:2021-03-15Revised:2021-05-23Online:2022-02-05Published:2022-02-23 -
Contact:TAO Leren
摘要:
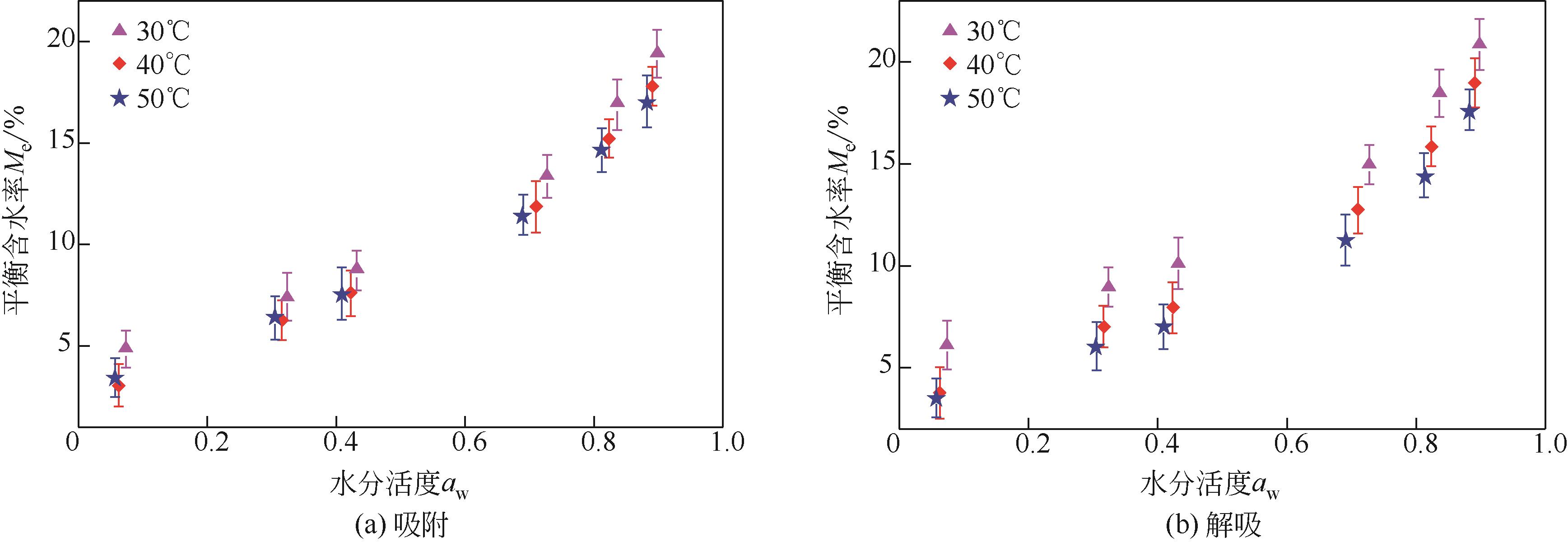
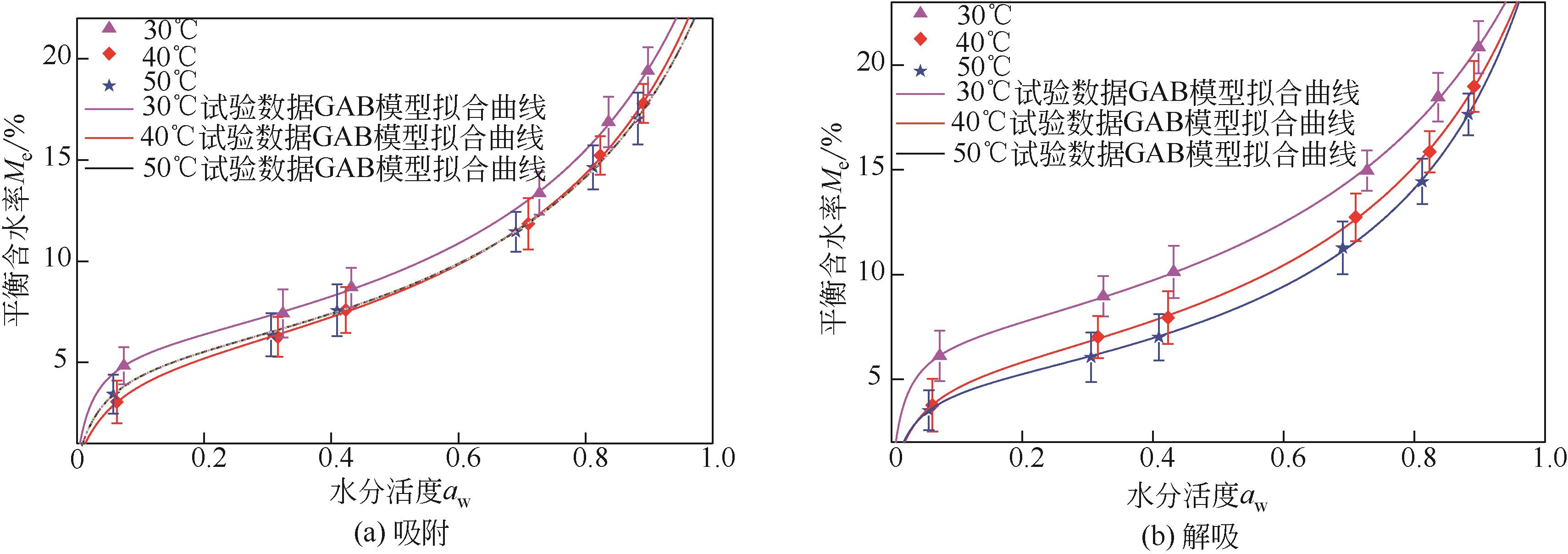
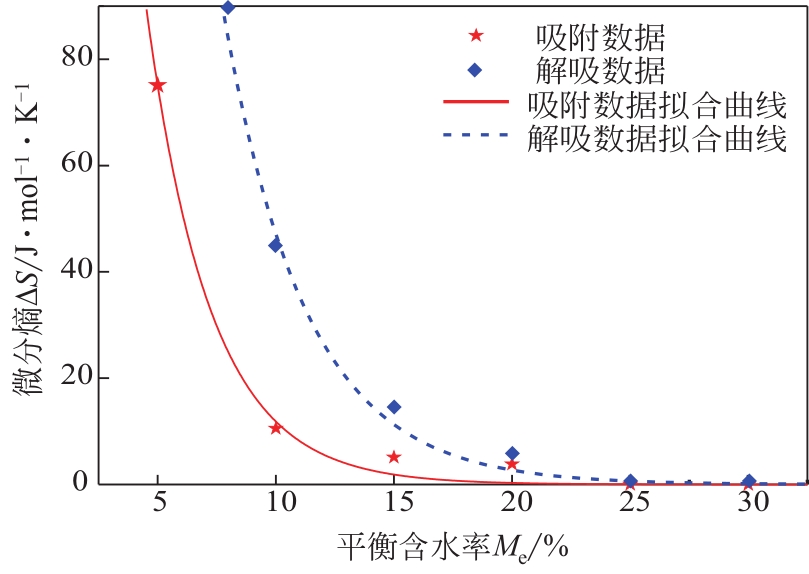
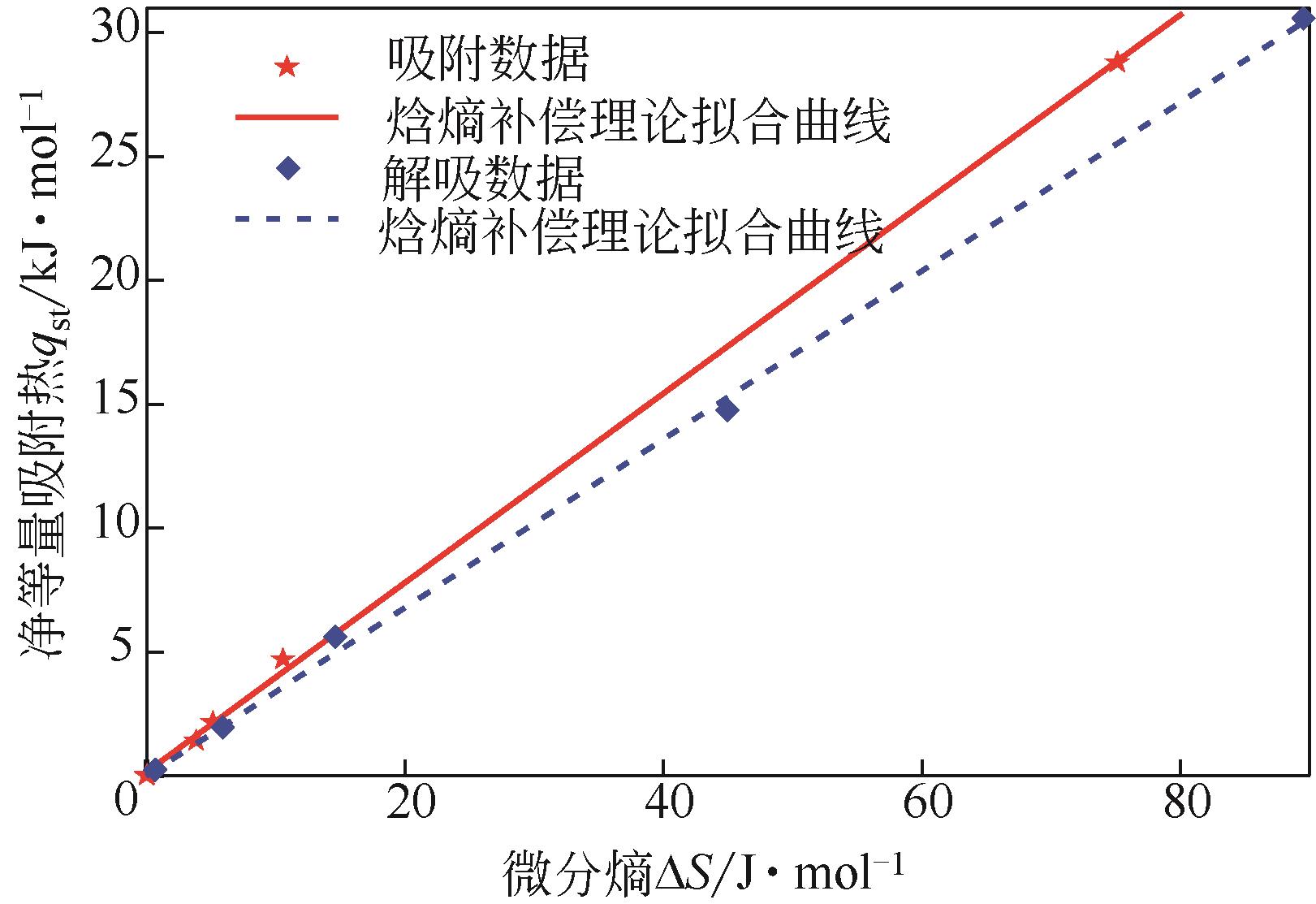
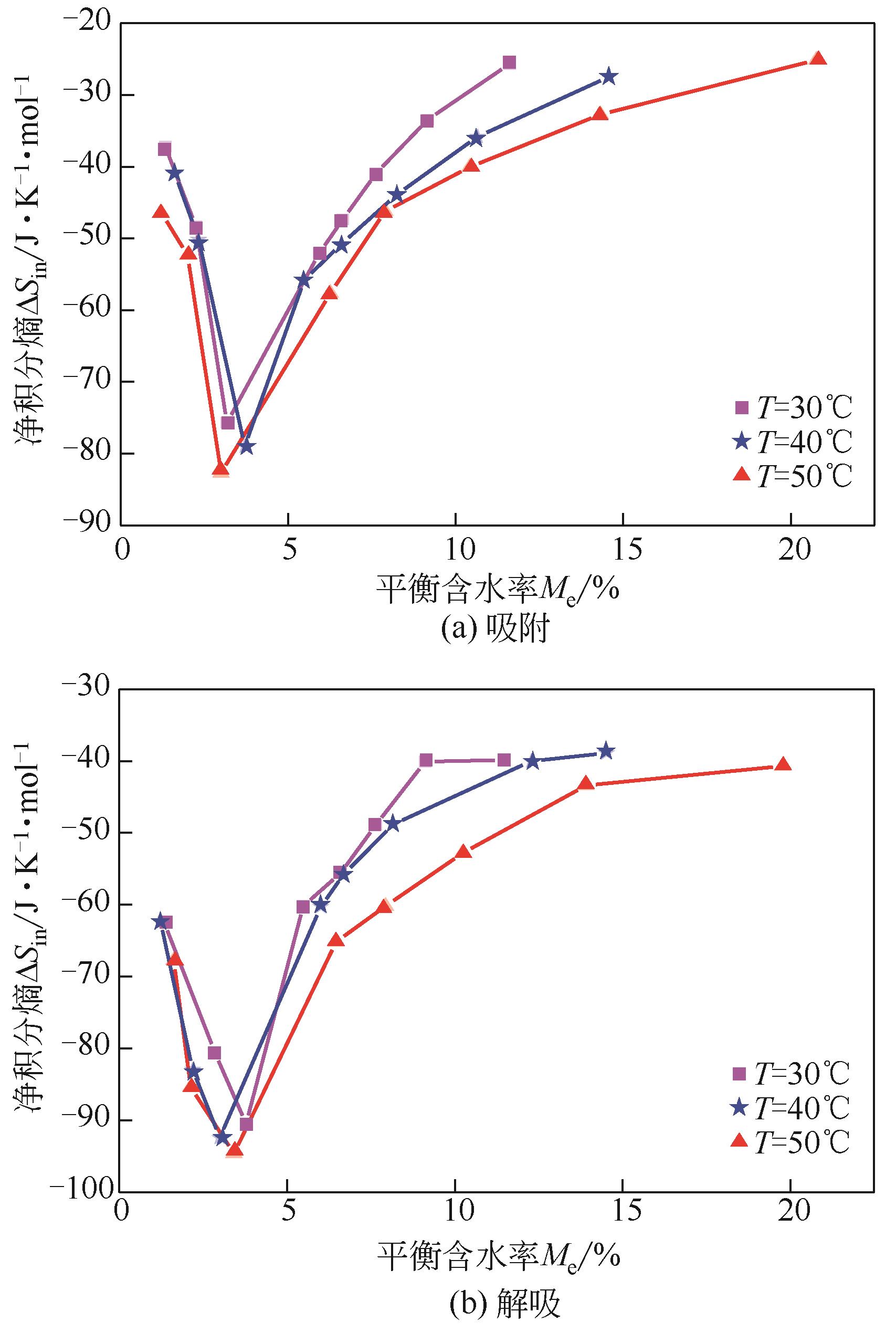
采用静态重量法测定了市政污泥在30℃、40℃、50℃下的吸附等温线,选用11个常见的数学模型对实验数据进行了拟合并对最佳模型进行了解析,通过净等量吸附热qst、微分熵ΔS、扩散压力π、净积分焓qin和净积分熵ΔSin等指标评价污泥的热力学性质。试验结果表明,在温度恒定时,等温曲线属于Ⅱ型,GAB模型拟合效果最佳,能较好地反映平衡含水量随水分活度的变化。应用Clausius-Clapeyron方程,利用等温线模型计算净等量吸附热和微分熵,随着平衡含水率的增加,净等量吸附热和微分熵明显降低,调和平均温度Thm与等速温度Tl不等,焓-熵补偿理论成立。在一定的水活度下,扩散压力随温度的升高而减小,在温度恒定的情况下,扩张压力随水分活度增大而升高。净积分焓随平衡含水率的增加而减小,而净积分熵在低平衡含水率时随平衡含水率的增加而减小,在30℃、40℃和50℃时分别达到最小值-75.698J/(K?mol)、-78.987J/(K?mol)和-82.687J/(K?mol),然后呈上升趋势。
中图分类号:
引用本文
谷志攀, 阳季春, 张叶, 陶乐仁, 刘泛函. 市政污泥吸附等温线模型和热力学性质[J]. 化工进展, 2022, 41(2): 998-1008.
GU Zhipan, YANG Jichun, ZHANG Ye, TAO Leren, LIU Fanhan. Mathematical modelling of water sorption isotherms and thermodynamic properties of municipal sewage sludge[J]. Chemical Industry and Engineering Progress, 2022, 41(2): 998-1008.
| 盐 | 水活性 | ||
|---|---|---|---|
| 30℃ | 40℃ | 50℃ | |
| KOH | 0.074 | 0.063 | 0.057 |
| LiCl | 0.11 | 0.11 | 0.11 |
| MgCl2 | 0.32 | 0.31 | 0.30 |
| K2CO3 | 0.43 | 0.43 | 0.42 |
| NaBr | 0.56 | 0.53 | 0.51 |
| NaNO3 | 0.73 | 0.71 | 0.58 |
| NaCl | 0.75 | 0.75 | 0.71 |
| KCl | 0.84 | 0.82 | 0.77 |
| BaCl2 | 0.90 | 0.89 | 0.88 |
表1 饱和盐溶液及其相应的水活性
| 盐 | 水活性 | ||
|---|---|---|---|
| 30℃ | 40℃ | 50℃ | |
| KOH | 0.074 | 0.063 | 0.057 |
| LiCl | 0.11 | 0.11 | 0.11 |
| MgCl2 | 0.32 | 0.31 | 0.30 |
| K2CO3 | 0.43 | 0.43 | 0.42 |
| NaBr | 0.56 | 0.53 | 0.51 |
| NaNO3 | 0.73 | 0.71 | 0.58 |
| NaCl | 0.75 | 0.75 | 0.71 |
| KCl | 0.84 | 0.82 | 0.77 |
| BaCl2 | 0.90 | 0.89 | 0.88 |
| 模型 | 函数表达式 |
|---|---|
| Halsey | |
| Henderson | |
| Oswin | |
| Smith | |
| Lespam | |
| Modified Halsey | |
| Modified Henderson | |
| Modified Oswin | |
| Enderby | |
| GAB | |
| Peleg |
表2 等温线模型
| 模型 | 函数表达式 |
|---|---|
| Halsey | |
| Henderson | |
| Oswin | |
| Smith | |
| Lespam | |
| Modified Halsey | |
| Modified Henderson | |
| Modified Oswin | |
| Enderby | |
| GAB | |
| Peleg |
| 模型 | 参数 | 解吸 | 吸附 | ||||
|---|---|---|---|---|---|---|---|
| 30℃ | 40℃ | 50℃ | 30℃ | 40℃ | 50℃ | ||
| Enerby | k1 | 164.6200 | 154.6625 | 97.5232 | 228.5969 | 67.6927 | 41.9163 |
| k2 | 4.3777 | 4.1455 | 3.2694 | 4.1245 | 3.4799 | 2.8263 | |
| n1 | -21.4747 | -27.5731 | -16.7879 | -35.8708 | -10.4499 | -5.5114 | |
| n2 | 0.7922 | 0.8081 | 0.8657 | 0.8080 | 0.8355 | 0.8684 | |
| R2 | 0.9987 | 0.9918 | 0.9914 | 0.9994 | 0.9992 | 0.9936 | |
| SSE | 6.7642×10-5 | 2.2067×10-5 | 2.2948×10-4 | 5.3219×10-5 | 5.7626×10-5 | 1.8101×10-4 | |
| GAB | Mm | 6.9489 | 5.7250 | 4.9885 | 5.8989 | 5.5057 | 5.4636 |
| K | 0.74617 | 0.78664 | 0.81484 | 0.7791 | 0.78411 | 0.77744 | |
| C | 38.6475 | 38.8496 | 30.2742 | 61.2461 | 19.2301 | 13.1561 | |
| R2 | 0.9986 | 0.9993 | 0.9981 | 0.9996 | 0.9987 | 0.9979 | |
| SSE | 7.0842×10-5 | 5.4423×10-5 | 8.0862×10-5 | 4.5813×10-5 | 6.7642×10-5 | 8.5269×10-5 | |
| Henderson | a | 0.004480 | 0.009660 | 0.01752 | 0.006690 | 0.01658 | 0.02156 |
| b | 2.0713 | 1.8695 | 1.6997 | 1.9860 | 1.7112 | 1.6387 | |
| R2 | 0.9834 | 0.9784 | 0.9784 | 0.9695 | 0.9915 | 0.9944 | |
| SSE | 6.6675×10-4 | 1.0083×10-3 | 1.0083×10-3 | 1.6067×10-3 | 2.2751×10-4 | 1.6334×10-4 | |
| Lespam | k1 | 4.1040 | 2.7496 | 2.3542 | 1.8656 | 4.3583 | 6.6839 |
| k2 | 87.0296 | 102.9505 | 109.1127 | 120.1665 | 82.3949 | 64.8542 | |
| b | 0.8122 | 1.31745 | 0.95785 | 3.09977 | -1.5383 | -4.6904 | |
| R2 | 0.9871 | 0.9881 | 0.9789 | 0.9907 | 0.9812 | 0.9762 | |
| SSE | 4.2235×10-4 | 3.5621×10-4 | 9.7470×10-4 | 2.4491×10-4 | 8.2005×10-4 | 1.1562×10-3 | |
| Modified Halsey | a | 11.6936 | 7.2225 | 7.8648 | 5.1827 | 10.5786 | 4.0135 |
| b | -1.50581 | -0.67996 | -0.92382 | -0.11143 | -1.51745 | -0.03796 | |
| c | 2.3746 | 2.1245 | 2.0419 | 2.2667 | 2.0878 | 2.0452 | |
| R2 | 0.9876 | 0.9906 | 0.9908 | 0.9964 | 0.9840 | 09864 | |
| SSE | 3.8973×10-4 | 2.4711×10-4 | 2.4270×10-4 | 1.1932×10-4 | 6.3179×10-4 | 4.7042×10-4 | |
| Modified Henderson | a | 2.749×10-5 | 7.2117×10-5 | 8.4859×10-5 | 3.92302×10-5 | 9.31591×10-5 | 1.12525×10-4 |
| b | -153.8850 | -197.6900 | -157.8362 | -155.6501 | -160.9387 | -160.8820 | |
| c | 2.1207 | 1.9468 | 1.8093 | 2.0644 | 1.7860 | 1.7145 | |
| R2 | 0.9878 | 0.9831 | 0.9844 | 0.9738 | 0.9947 | 0.9958 | |
| SSE | 3.7629×10-4 | 6.9230×10-4 | 6.0489×10-4 | 1.317×10-3 | 1.5677×10-4 | 1.3253×10-4 | |
| Modified Oswin | a | -11.3030 | -12.0411 | -12.5870 | -11.8017 | -12.5025 | -12.7686 |
| b | 0.5534 | 0.5350 | 0.5216 | 0.5410 | 0.5235 | 0.5168 | |
| n | 0.3064 | 0.3387 | 0.3736 | 0.3181 | 0.3654 | 0.3854 | |
| R2 | 0.9879 | 0.9980 | 0.9979 | 0.9955 | 0.9982 | 0.9933 | |
| SSE | 3.6957×10-4 | 8.4066×10-5 | 8.6269×10-5 | 1.3914×10-4 | 7.9659×10-5 | 1.8762×10-4 | |
| Peleg | k1 | 13.9311 | 14.6001 | 15.3302 | 14.9750 | 13.2518 | 12.9026 |
| k2 | 12.8412 | 10.8048 | 10.3439 | 10.6098 | 11.7910 | 12.5854 | |
| n1 | 4.5828 | 4.5950 | 5.3950 | 4.4285 | 5.6993 | 7.0189 | |
| n2 | 0.3601 | 0.3618 | 0.4305 | 0.2973 | 0.5238 | 0.6412 | |
| R2 | 0.9899 | 0.9988 | 0.9978 | 0.9983 | 0.9980 | 0.9899 | |
| SSE | 2.6253×10-4 | 6.6439×10-5 | 8.8472×10-5 | 7.7456×10-5 | 8.4066×10-5 | 2.3509×10-4 | |
| Halsey | k | 458.71 | 87.4532 | 53.1284 | 159.9017 | 62.3501 | 77.6911 |
| n | 2.7239 | 2.23048 | 2.0917 | 2.4367 | 2.1552 | 2.2392 | |
| R2 | 0.9890 | 0.9850 | 0.9930 | 0.9890 | 0.9768 | 0.9818 | |
| SSE | 5.8658×10-4 | 8.0070×10-4 | 3.7246×10-4 | 5.865×10-4 | 1.239×10-3 | 9.7199×10-4 | |
| Oswin | k | 11.37929 | 9.2118 | 8.3955 | 9.7888 | 8.4931 | 8.6816 |
| n | 0.2821 | 0.3469 | 0.3676 | 0.3189 | 0.3608 | 0.3410 | |
| R2 | 0.9942 | 0.9985 | 0.9965 | 0.9954 | 0.9972 | 0.9980 | |
| SSE | 3.0823×10-4 | 7.8053×10-5 | 1.8511×10-4 | 2.4399×10-4 | 1.4764×10-4 | 1.0481×10-4 | |
| Smith | a | 6.1447 | 3.9708 | 3.4089 | 4.7343 | 3.4131 | 3.7764 |
| b | -6.6351 | -6.8745 | -6.6602 | -6.5677 | -6.6802 | -6.3820 | |
| R2 | 0.9946 | 0.9942 | 0.9987 | 0.9967 | 0.9902 | 0.9906 | |
| SSE | 2.8681×10-4 | 3.0823×10-4 | 6.7347×10-4 | 1.7440×10-4 | 5.2348×10-4 | 5.0093×10-4 | |
表3 污泥3吸附、解吸等温线模型参数及精度
| 模型 | 参数 | 解吸 | 吸附 | ||||
|---|---|---|---|---|---|---|---|
| 30℃ | 40℃ | 50℃ | 30℃ | 40℃ | 50℃ | ||
| Enerby | k1 | 164.6200 | 154.6625 | 97.5232 | 228.5969 | 67.6927 | 41.9163 |
| k2 | 4.3777 | 4.1455 | 3.2694 | 4.1245 | 3.4799 | 2.8263 | |
| n1 | -21.4747 | -27.5731 | -16.7879 | -35.8708 | -10.4499 | -5.5114 | |
| n2 | 0.7922 | 0.8081 | 0.8657 | 0.8080 | 0.8355 | 0.8684 | |
| R2 | 0.9987 | 0.9918 | 0.9914 | 0.9994 | 0.9992 | 0.9936 | |
| SSE | 6.7642×10-5 | 2.2067×10-5 | 2.2948×10-4 | 5.3219×10-5 | 5.7626×10-5 | 1.8101×10-4 | |
| GAB | Mm | 6.9489 | 5.7250 | 4.9885 | 5.8989 | 5.5057 | 5.4636 |
| K | 0.74617 | 0.78664 | 0.81484 | 0.7791 | 0.78411 | 0.77744 | |
| C | 38.6475 | 38.8496 | 30.2742 | 61.2461 | 19.2301 | 13.1561 | |
| R2 | 0.9986 | 0.9993 | 0.9981 | 0.9996 | 0.9987 | 0.9979 | |
| SSE | 7.0842×10-5 | 5.4423×10-5 | 8.0862×10-5 | 4.5813×10-5 | 6.7642×10-5 | 8.5269×10-5 | |
| Henderson | a | 0.004480 | 0.009660 | 0.01752 | 0.006690 | 0.01658 | 0.02156 |
| b | 2.0713 | 1.8695 | 1.6997 | 1.9860 | 1.7112 | 1.6387 | |
| R2 | 0.9834 | 0.9784 | 0.9784 | 0.9695 | 0.9915 | 0.9944 | |
| SSE | 6.6675×10-4 | 1.0083×10-3 | 1.0083×10-3 | 1.6067×10-3 | 2.2751×10-4 | 1.6334×10-4 | |
| Lespam | k1 | 4.1040 | 2.7496 | 2.3542 | 1.8656 | 4.3583 | 6.6839 |
| k2 | 87.0296 | 102.9505 | 109.1127 | 120.1665 | 82.3949 | 64.8542 | |
| b | 0.8122 | 1.31745 | 0.95785 | 3.09977 | -1.5383 | -4.6904 | |
| R2 | 0.9871 | 0.9881 | 0.9789 | 0.9907 | 0.9812 | 0.9762 | |
| SSE | 4.2235×10-4 | 3.5621×10-4 | 9.7470×10-4 | 2.4491×10-4 | 8.2005×10-4 | 1.1562×10-3 | |
| Modified Halsey | a | 11.6936 | 7.2225 | 7.8648 | 5.1827 | 10.5786 | 4.0135 |
| b | -1.50581 | -0.67996 | -0.92382 | -0.11143 | -1.51745 | -0.03796 | |
| c | 2.3746 | 2.1245 | 2.0419 | 2.2667 | 2.0878 | 2.0452 | |
| R2 | 0.9876 | 0.9906 | 0.9908 | 0.9964 | 0.9840 | 09864 | |
| SSE | 3.8973×10-4 | 2.4711×10-4 | 2.4270×10-4 | 1.1932×10-4 | 6.3179×10-4 | 4.7042×10-4 | |
| Modified Henderson | a | 2.749×10-5 | 7.2117×10-5 | 8.4859×10-5 | 3.92302×10-5 | 9.31591×10-5 | 1.12525×10-4 |
| b | -153.8850 | -197.6900 | -157.8362 | -155.6501 | -160.9387 | -160.8820 | |
| c | 2.1207 | 1.9468 | 1.8093 | 2.0644 | 1.7860 | 1.7145 | |
| R2 | 0.9878 | 0.9831 | 0.9844 | 0.9738 | 0.9947 | 0.9958 | |
| SSE | 3.7629×10-4 | 6.9230×10-4 | 6.0489×10-4 | 1.317×10-3 | 1.5677×10-4 | 1.3253×10-4 | |
| Modified Oswin | a | -11.3030 | -12.0411 | -12.5870 | -11.8017 | -12.5025 | -12.7686 |
| b | 0.5534 | 0.5350 | 0.5216 | 0.5410 | 0.5235 | 0.5168 | |
| n | 0.3064 | 0.3387 | 0.3736 | 0.3181 | 0.3654 | 0.3854 | |
| R2 | 0.9879 | 0.9980 | 0.9979 | 0.9955 | 0.9982 | 0.9933 | |
| SSE | 3.6957×10-4 | 8.4066×10-5 | 8.6269×10-5 | 1.3914×10-4 | 7.9659×10-5 | 1.8762×10-4 | |
| Peleg | k1 | 13.9311 | 14.6001 | 15.3302 | 14.9750 | 13.2518 | 12.9026 |
| k2 | 12.8412 | 10.8048 | 10.3439 | 10.6098 | 11.7910 | 12.5854 | |
| n1 | 4.5828 | 4.5950 | 5.3950 | 4.4285 | 5.6993 | 7.0189 | |
| n2 | 0.3601 | 0.3618 | 0.4305 | 0.2973 | 0.5238 | 0.6412 | |
| R2 | 0.9899 | 0.9988 | 0.9978 | 0.9983 | 0.9980 | 0.9899 | |
| SSE | 2.6253×10-4 | 6.6439×10-5 | 8.8472×10-5 | 7.7456×10-5 | 8.4066×10-5 | 2.3509×10-4 | |
| Halsey | k | 458.71 | 87.4532 | 53.1284 | 159.9017 | 62.3501 | 77.6911 |
| n | 2.7239 | 2.23048 | 2.0917 | 2.4367 | 2.1552 | 2.2392 | |
| R2 | 0.9890 | 0.9850 | 0.9930 | 0.9890 | 0.9768 | 0.9818 | |
| SSE | 5.8658×10-4 | 8.0070×10-4 | 3.7246×10-4 | 5.865×10-4 | 1.239×10-3 | 9.7199×10-4 | |
| Oswin | k | 11.37929 | 9.2118 | 8.3955 | 9.7888 | 8.4931 | 8.6816 |
| n | 0.2821 | 0.3469 | 0.3676 | 0.3189 | 0.3608 | 0.3410 | |
| R2 | 0.9942 | 0.9985 | 0.9965 | 0.9954 | 0.9972 | 0.9980 | |
| SSE | 3.0823×10-4 | 7.8053×10-5 | 1.8511×10-4 | 2.4399×10-4 | 1.4764×10-4 | 1.0481×10-4 | |
| Smith | a | 6.1447 | 3.9708 | 3.4089 | 4.7343 | 3.4131 | 3.7764 |
| b | -6.6351 | -6.8745 | -6.6602 | -6.5677 | -6.6802 | -6.3820 | |
| R2 | 0.9946 | 0.9942 | 0.9987 | 0.9967 | 0.9902 | 0.9906 | |
| SSE | 2.8681×10-4 | 3.0823×10-4 | 6.7347×10-4 | 1.7440×10-4 | 5.2348×10-4 | 5.0093×10-4 | |
| 参数 | 吸附 | 解吸 |
|---|---|---|
| Mm0 | 0.02996 | 1.6379 |
| C0 | 1.1321 | 2.5146×10-12 |
| K0 | 3.0870 | 0.7566 |
| ΔH/kJ·mol-1 | 13.7074 | 3.2062 |
| HM-HN/kJ·mol-1 | 8.9807 | 77.6208 |
| HL-HN/kJ·mol-1 | -3.5705 | 0.07985 |
表4 GAB模型参数
| 参数 | 吸附 | 解吸 |
|---|---|---|
| Mm0 | 0.02996 | 1.6379 |
| C0 | 1.1321 | 2.5146×10-12 |
| K0 | 3.0870 | 0.7566 |
| ΔH/kJ·mol-1 | 13.7074 | 3.2062 |
| HM-HN/kJ·mol-1 | 8.9807 | 77.6208 |
| HL-HN/kJ·mol-1 | -3.5705 | 0.07985 |
| 1 | MOU X Z, CHEN Z Q. Experimental study on the effect of sludge thickness on the characteristics of ultrasound-assisted hot air convective drying municipal sewage sludge[J]. Drying Technology, 2021, 39(6): 752-764. |
| 2 | 陈思思, 杨殿海, 庞维海, 等. 我国剩余污泥厌氧转化的主要影响因素及影响机制研究进展[J]. 化工进展, 2020, 39(4): 1511-1520. |
| CHEN Sisi, YANG Dianhai, PANG Weihai, et al. Main influencing factors and mechanisms of anaerobic transformation of excess sludge in China[J]. Chemical Industry and Engineering Progress, 2020, 39(4): 1511-1520. | |
| 3 | ERIKSSON E, CHRISTENSEN N, EJBYE SCHMIDT J, et al. Potential priority pollutants in sewage sludge[J]. Desalination, 2008, 226(1/2/3): 371-388. |
| 4 | 宋文婷, 郭静, 杨倩倩, 等. 污泥有机污染物降解研究进展[J]. 化工进展, 2020, 39(1): 380-386. |
| SONG Wenting, GUO Jing, YANG Qianqian, et al. Research progress on degradation of sludge organic pollutants[J]. Chemical Industry and Engineering Progress, 2020, 39(1): 380-386. | |
| 5 | 张俊杰, 邵敬爱, 黄河洵, 等. 利用污泥制备活性炭及其吸附特性的研究进展[J]. 化工进展, 2017, 36(10): 3876-3886. |
| ZHANG Junjie, SHAO Jing’ai, HUANG Hexun, et al. Review on the preparation of activated carbon from sludge and its adsorption characteristics[J]. Chemical Industry and Engineering Progress, 2017, 36(10): 3876-3886. | |
| 6 | 陈丹丹, 窦昱昊, 卢平, 等. 污泥深度脱水技术研究进展[J]. 化工进展, 2019, 38(10): 4722-4746. |
| CHEN Dandan, DOU Yuhao, LU Ping, et al. A review on sludge deep dewatering technology[J]. Chemical Industry and Engineering Progress, 2019, 38(10): 4722-4746. | |
| 7 | 米琼, 戴世金, 张瑞娜, 等. 干污泥高维填埋的堆体边坡稳定性模拟与分析[J]. 中国环境科学, 2018, 38(4): 1397-1402. |
| MI Qiong, DAI Shijin, ZHANG Ruina, et al. Slope stability simulation of dry sludge pile in high-dimensional landfill operation[J]. China Environmental Science, 2018, 38(4): 1397-1402. | |
| 8 | 王子文, 曹蓉, 杨艳坤, 等. 聚季铵盐调理污泥深度脱水过程与中试效能[J]. 化工进展, 2019, 38(7): 3458-3464. |
| WANG Ziwen, CAO Rong, YANG Yankun, et al. Performance and pilot-scale efficiency of polyquaternary ammonium in sludge deep dewatering process[J]. Chemical Industry and Engineering Progress, 2019, 38(7): 3458-3464. | |
| 9 | WU B R, DAI X H, CHAI X L. Critical review on dewatering of sewage sludge: Influential mechanism, conditioning technologies and implications to sludge re-utilizations[J]. Water Research, 2020, 180: 115912. |
| 10 | SYED-HASSAN S S A, WANG Y, HU S, et al. Thermochemical processing of sewage sludge to energy and fuel: fundamentals, challenges and considerations[J]. Renewable and Sustainable Energy Reviews, 2017, 80: 888-913. |
| 11 | CHAN W P, WANG J Y. Comprehensive characterisation of sewage sludge for thermochemical conversion processes—Based on Singapore survey[J]. Waste Management, 2016, 54: 131-142. |
| 12 | 郑晓园, 蒋正伟, 陈伟, 等. 污水污泥水热炭化过程中磷的迁移转化特性[J]. 化工进展, 2020, 39(5): 2017-2025. |
| ZHENG Xiaoyuan, JIANG Zhengwei, CHEN Wei, et al. Migration and transformation of phosphorus in sewage sludge during hydrothermal carbonization process[J]. Chemical Industry and Engineering Progress, 2020, 39(5): 2017-2025. | |
| 13 | 范海宏, 武亚磊, 李斌斌, 等. 市政污泥干化动力学研究[J]. 环境工程学报, 2015, 9(9): 4488-4494. |
| FAN Haihong, WU Yalei, LI Binbin, et al. Investigation of drying kinetic of municipal dewater sewage sludge[J]. Chinese Journal of Environmental Engineering, 2015, 9(9): 4488-4494. | |
| 14 | 张绪坤, 刘胜平, 吴青荣, 等. 污泥低温干燥动力学特性及干燥参数优化[J]. 农业工程学报, 2017, 33(17): 216-223. |
| ZHANG Xukun, LIU Shengping, WU Qingrong, et al. Drying kinetics and parameters optimization of sludge drying at low temperature[J]. Transactions of the Chinese Society of Agricultural Engineering, 2017, 33(17): 216-223. | |
| 15 | 李斌斌, 范海宏, 任洋明, 等. 城市污泥薄层干燥特性及动力学研究[J]. 环境工程, 2016, 34(10): 103-107. |
| LI Binbin, FAN Haihong, REN Yangming, et al. Municipal sludge drying characteristics and dynamics based on thin layer drying model[J]. Environmental Engineering, 2016, 34(10): 103-107. | |
| 16 | 郑龙, 伍健东, 周兴求, 等. 温度和相对湿度对污泥低温干燥速率的影响[J]. 环境工程学报, 2016, 10(2): 922-928. |
| ZHENG Long, WU Jiandong, ZHOU Xingqiu, et al. Effects of temperature and relative humidity on drying rate of sludge at low temperature[J]. Chinese Journal of Environmental Engineering, 2016, 10(2): 922-928. | |
| 17 | 邓文义, 梅静, 刘亚军, 等. CaO和木屑对市政污泥干化过程中黏滞特性的影响[J]. 化工进展, 2017, 36(5): 1933-1939. |
| DENG Wenyi, MEI Jing, LIU Yajun, et al. Effect of CaO and sawdust on sticky properties of municipal sewage sludge during drying process[J]. Chemical Industry and Engineering Progress, 2017, 36(5): 1933-1939. | |
| 18 | 刘亚军, 王爱春, 邓文义. 市政污泥热力干化过程中黏滞特性研究进展[J]. 化工进展, 2018, 37(6): 2378-2385. |
| LIU Yajun, WANG Aichun, DENG Wenyi. Progress in sticky characteristics of sewage sludge during thermal drying process[J]. Chemical Industry and Engineering Progress, 2018, 37(6): 2378-2385. | |
| 19 | 郑玲玲, 程榕, 郑燕萍, 等. MVR空心桨叶干燥污泥的特性及动力学[J]. 化工进展, 2016, 35(S1): 53-57. |
| ZHENG Lingling, CHENG Rong, ZHENG Yanping, et al. Drying properties and kinetics of MVR technology applied to the hollow blade dryer drying sludge[J]. Chemical Industry and Engineering Progress, 2016, 35(S1): 53-57. | |
| 20 | LÉONARD A, BLACHER S, MARCHOT P, et al. Measurement of shrinkage and cracks associated to convective drying of soft materials by X-ray microtomography[J]. Drying Technology, 2004, 22(7): 1695-1708. |
| 21 | FONT R, GOMEZ-RICO M F, FULLANA A. Skin effect in the heat and mass transfer model for sewage sludge drying[J]. Separation and Purification Technology, 2011, 77(1): 146-161. |
| 22 | HUANG Y W, CHEN M Q. Thin-layer isothermal drying kinetics of municipal sewage sludge based on two falling rate stages during hot-air-forced convection[J]. Journal of Thermal Analysis and Calorimetry, 2017, 129(1): 567-575. |
| 23 | GUO J, CHEN M Q, HUANG Y W, et al. Salinity effects on ultrasound-assisted hot air drying kinetics of sewage sludge[J]. Thermochimica Acta, 2019, 678: 178298. |
| 24 | EOM H, JANG Y H, LEE D Y, et al. Optimization of a hybrid sludge drying system with flush drying and microwave drying technology[J]. Chemical Engineering Research and Design, 2019, 148: 68-74. |
| 25 | DI FRAIA S, FIGAJ R D, MASSAROTTI N, et al. An integrated system for sewage sludge drying through solar energy and a combined heat and power unit fuelled by biogas[J]. Energy Conversion and Management, 2018, 171: 587-603. |
| 26 | WANG P L, MOHAMMED D, ZHOU P, et al. Roof solar drying processes for sewage sludge within sandwich-like chamber bed[J]. Renewable Energy, 2019, 136: 1071-1081. |
| 27 | HASSINE N BEN, CHESNEAU X, LAATAR A H, et al. Modelisation and simulation of heat and mass transfers during solar drying of sewage sludge with introduction of real climatic conditions[J]. Journal of Applied Fluid Mechanics, 2017, 10(2): 651-659. |
| 28 | JIANG J, DANG L P, YUENSIN C, et al. Simulation of microwave thin layer drying process by a new theoretical model[J]. Chemical Engineering Science, 2017, 162: 69-76. |
| 29 | BELLUR S R, CORONELLA C J, VÁSQUEZ V R. Analysis of biosolids equilibrium moisture and drying[J]. Environmental Progress & Sustainable Energy, 2009, 28(2): 291-298. |
| 30 | REMINGTON C, BOURGAULT C, DOREA C C. Measurement and modelling of moisture sorption isotherm and heat of sorption of fresh feces[J]. Water, 2020, 12(2): 323. |
| 31 | BOUGAYR E H, LAKHAL E K, IDLIMAM A, et al. Experimental study of hygroscopic equilibrium and thermodynamic properties of sewage sludge[J]. Applied Thermal Engineering, 2018, 143: 521-531. |
| 32 | FAKHFAKH R, MIHOUBI D, KECHAOU N. Moisture sorption isotherms and thermodynamic properties of bovine leather[J]. Heat and Mass Transfer, 2018, 54(4): 1163-1176. |
| 33 | 杨昭, 李想, 陶志超. 豌豆种子吸附等温线与热力学性质研究[J]. 农业机械学报, 2017, 48(10): 323-329. |
| YANG Zhao, LI Xiang, TAO Zhichao. Sorption isotherms and thermodynamic properties of pea seed[J]. Transactions of the Chinese Society for Agricultural Machinery, 2017, 48(10): 323-329. | |
| 34 | NOURHÈNE B, NEILA B, MOHAMMED K, et al. Sorptions isotherms and isosteric heats of sorption of olive leaves (Chemlali variety): experimental and mathematical investigations[J]. Food and Bioproducts Processing, 2008, 86(3): 167-175. |
| 35 | BERTOLIN C, DE FERRI L, STROJECKI M. Application of the Guggenheim, Anderson, de Boer (GAB) equation to sealing treatments on pine wood[J]. Procedia Structural Integrity, 2020, 26: 147-154. |
| 36 | POLATOĞLU B, BEŞE A V, KAYA M, et al. Moisture adsorption isotherms and thermodynamics properties of sucuk (Turkish dry-fermented sausage)[J]. Food and Bioproducts Processing, 2011, 89(4): 449-456. |
| 37 | MGHAZLI S, IDLIMAM A, MAHROUZ M, et al. Comparative moisture sorption isotherms, modelling and isosteric heat of sorption of controlled and irradiated Moroccan rosemary leaves[J]. Industrial Crops and Products, 2016, 88: 28-35. |
| 38 | OUERTANI S, AZZOUZ S, HASSINI L, et al. Moisture sorption isotherms and thermodynamic properties of Jack pine and palm wood: comparative study[J]. Industrial Crops and Products, 2014, 56: 200-210. |
| 39 | IGLESIAS H A, CHIRIFE J. Prediction of the effect of temperature on water sorption isotherms of food material[J]. International Journal of Food Science & Technology, 1976, 11(2): 109-116. |
| 40 | KAYA S, KAHYAOGLU T. Moisture sorption and thermodynamic properties of safflower petals and tarragon[J]. Journal of Food Engineering, 2007, 78(2): 413-421. |
| [1] | 徐晨阳, 都健, 张磊. 基于图神经网络的化学反应优劣评价[J]. 化工进展, 2023, 42(S1): 205-212. |
| [2] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [3] | 崔守成, 徐洪波, 彭楠. 两种MOFs材料用于O2/He吸附分离的模拟分析[J]. 化工进展, 2023, 42(S1): 382-390. |
| [4] | 陈崇明, 陈秋, 宫云茜, 车凯, 郁金星, 孙楠楠. 分子筛基CO2吸附剂研究进展[J]. 化工进展, 2023, 42(S1): 411-419. |
| [5] | 许春树, 姚庆达, 梁永贤, 周华龙. 共价有机框架材料功能化策略及其对Hg(Ⅱ)和Cr(Ⅵ)的吸附性能研究进展[J]. 化工进展, 2023, 42(S1): 461-478. |
| [6] | 顾永正, 张永生. HBr改性飞灰对Hg0的动态吸附及动力学模型[J]. 化工进展, 2023, 42(S1): 498-509. |
| [7] | 郭强, 赵文凯, 肖永厚. 增强流体扰动强化变压吸附甲硫醚/氮气分离的数值模拟[J]. 化工进展, 2023, 42(S1): 64-72. |
| [8] | 陈林, 徐培渊, 张晓慧, 陈杰, 徐振军, 陈嘉祥, 密晓光, 冯永昌, 梅德清. 液化天然气绕管式换热器壳侧混合工质流动及传热特性[J]. 化工进展, 2023, 42(9): 4496-4503. |
| [9] | 张帆, 陶少辉, 陈玉石, 项曙光. 基于改进恒热传输模型的精馏模拟初始化[J]. 化工进展, 2023, 42(9): 4550-4558. |
| [10] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [11] | 杨莹, 侯豪杰, 黄瑞, 崔煜, 王兵, 刘健, 鲍卫仁, 常丽萍, 王建成, 韩丽娜. 利用煤焦油中酚类物质Stöber法制备碳纳米球用于CO2吸附[J]. 化工进展, 2023, 42(9): 5011-5018. |
| [12] | 张振, 李丹, 陈辰, 吴菁岚, 应汉杰, 乔浩. 吸附树脂对唾液酸的分离纯化[J]. 化工进展, 2023, 42(8): 4153-4158. |
| [13] | 张智琛, 朱云峰, 成卫戍, 马守涛, 姜杰, 孙冰, 周子辰, 徐伟. 高压聚乙烯失控分解研究进展:反应机理、引发体系与模型[J]. 化工进展, 2023, 42(8): 3979-3989. |
| [14] | 姜晶, 陈霄宇, 张瑞妍, 盛光遥. 载锰生物炭制备及其在环境修复中应用研究进展[J]. 化工进展, 2023, 42(8): 4385-4397. |
| [15] | 于静文, 宋璐娜, 刘砚超, 吕瑞东, 武蒙蒙, 冯宇, 李忠, 米杰. 一种吲哚基超交联聚合物In-HCP对水中碘的吸附作用[J]. 化工进展, 2023, 42(7): 3674-3683. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||