化工进展 ›› 2021, Vol. 40 ›› Issue (8): 4205-4222.DOI: 10.16085/j.issn.1000-6613.2020-1801
CH4在原油体系中溶解规律及影响机理
- 中国石油大学(华东),山东省油气储运安全省级重点实验室,山东 青岛 266580
-
收稿日期:2020-09-08出版日期:2021-08-05发布日期:2021-08-12 -
通讯作者:刘刚 -
作者简介:李秉繁(1991—),男,博士研究生,研究方向为油气长距离管输技术。E-mail:670264522@qq.com 。 -
基金资助:国家自然科学基金(51774315)
Dissolution of CH4 in the crude oil system: behaviors and mechanisms
LI Bingfan( ), LIU Gang(
), LIU Gang( ), CHEN Lei
), CHEN Lei
- University of Petroleum(East China), Shandong Provincial Key Laboratory of Oil & Gas Storage and Transportation Safety, Qingdao 266580, Shandong, China
-
Received:2020-09-08Online:2021-08-05Published:2021-08-12 -
Contact:LIU Gang
摘要:
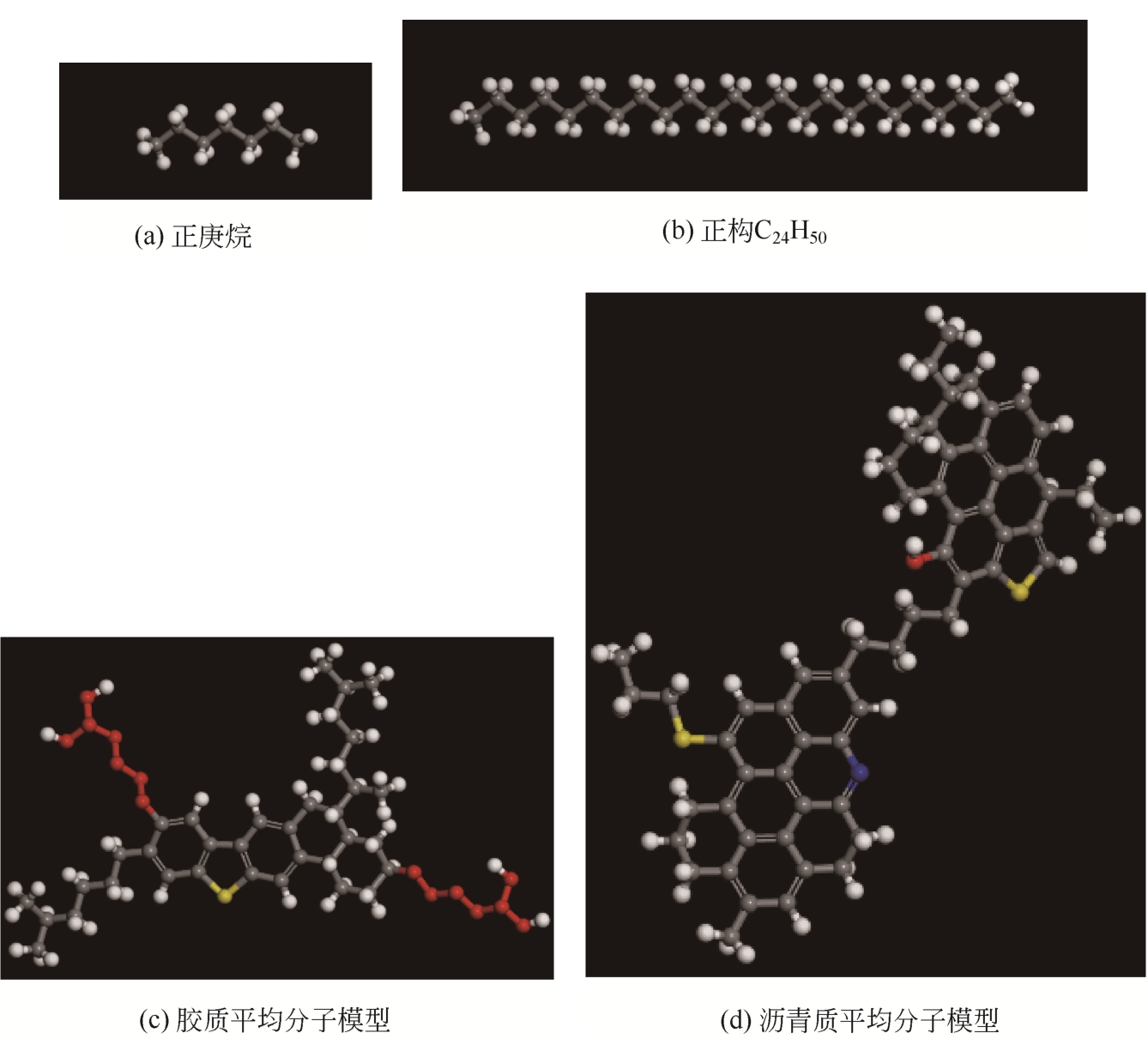
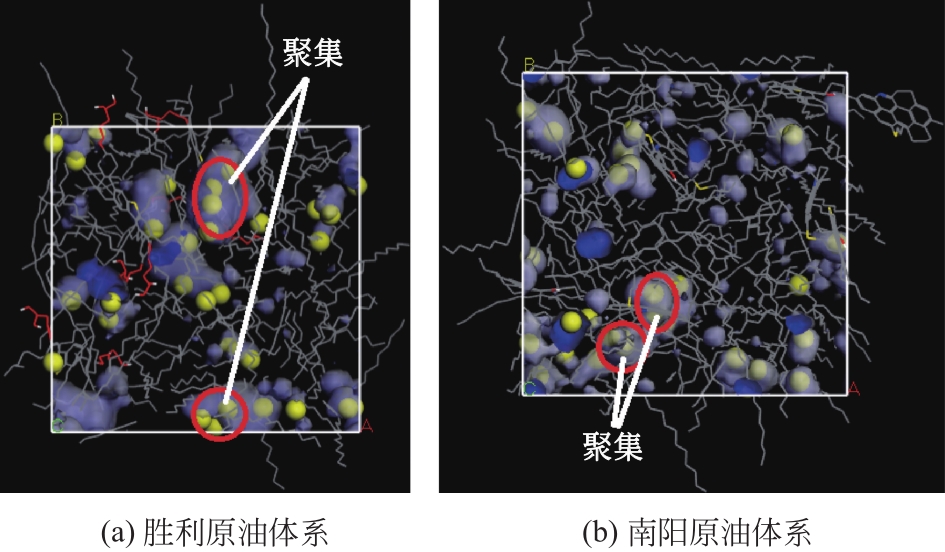
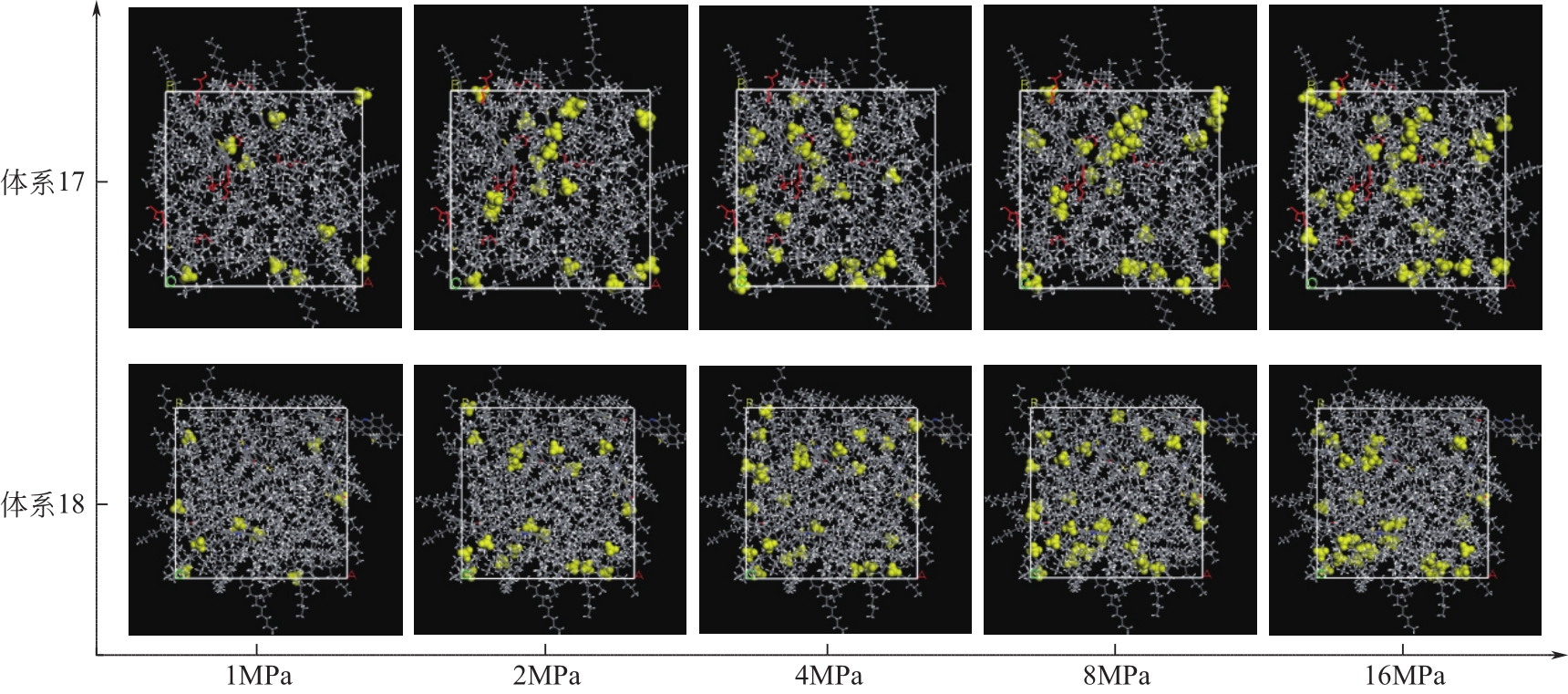
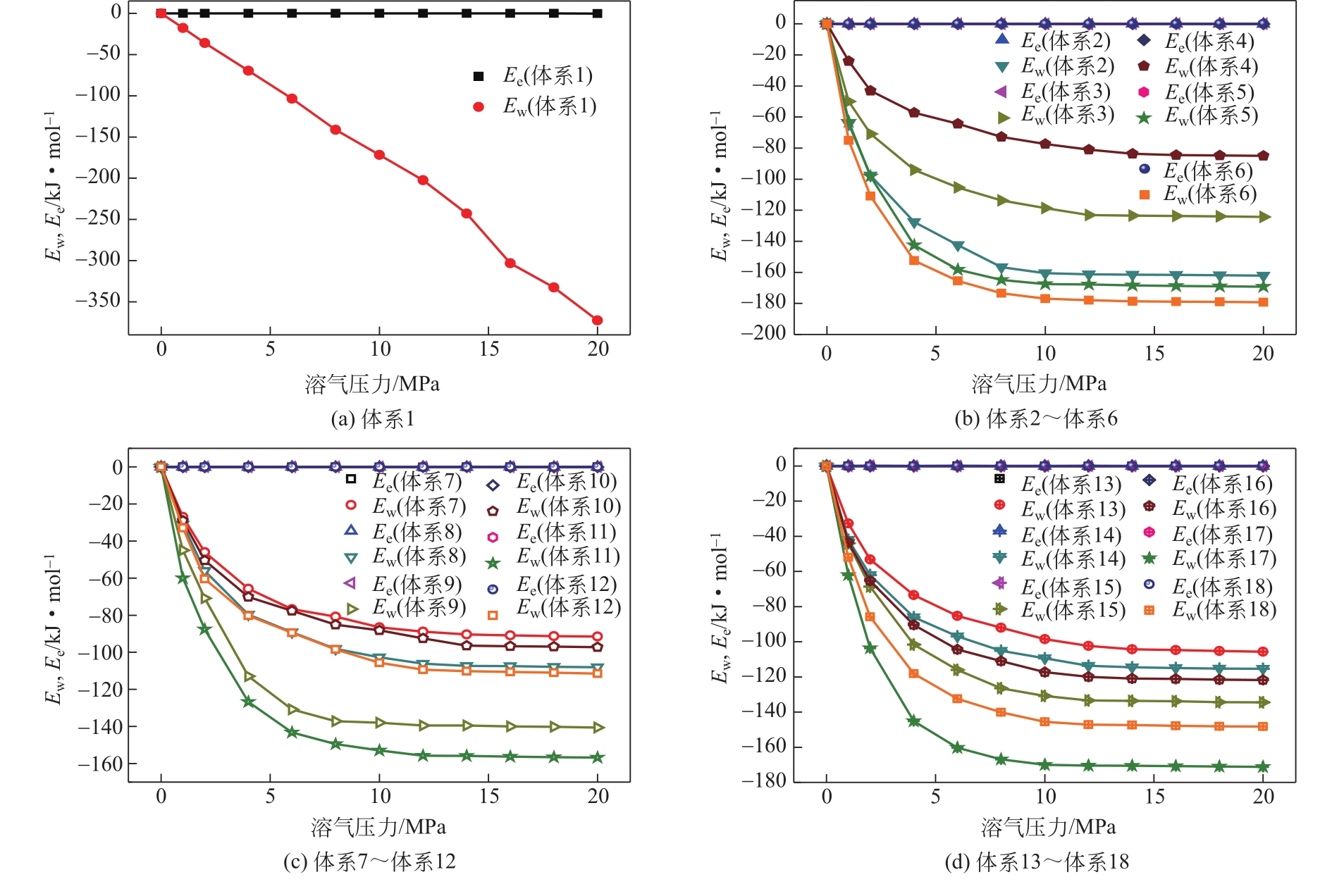
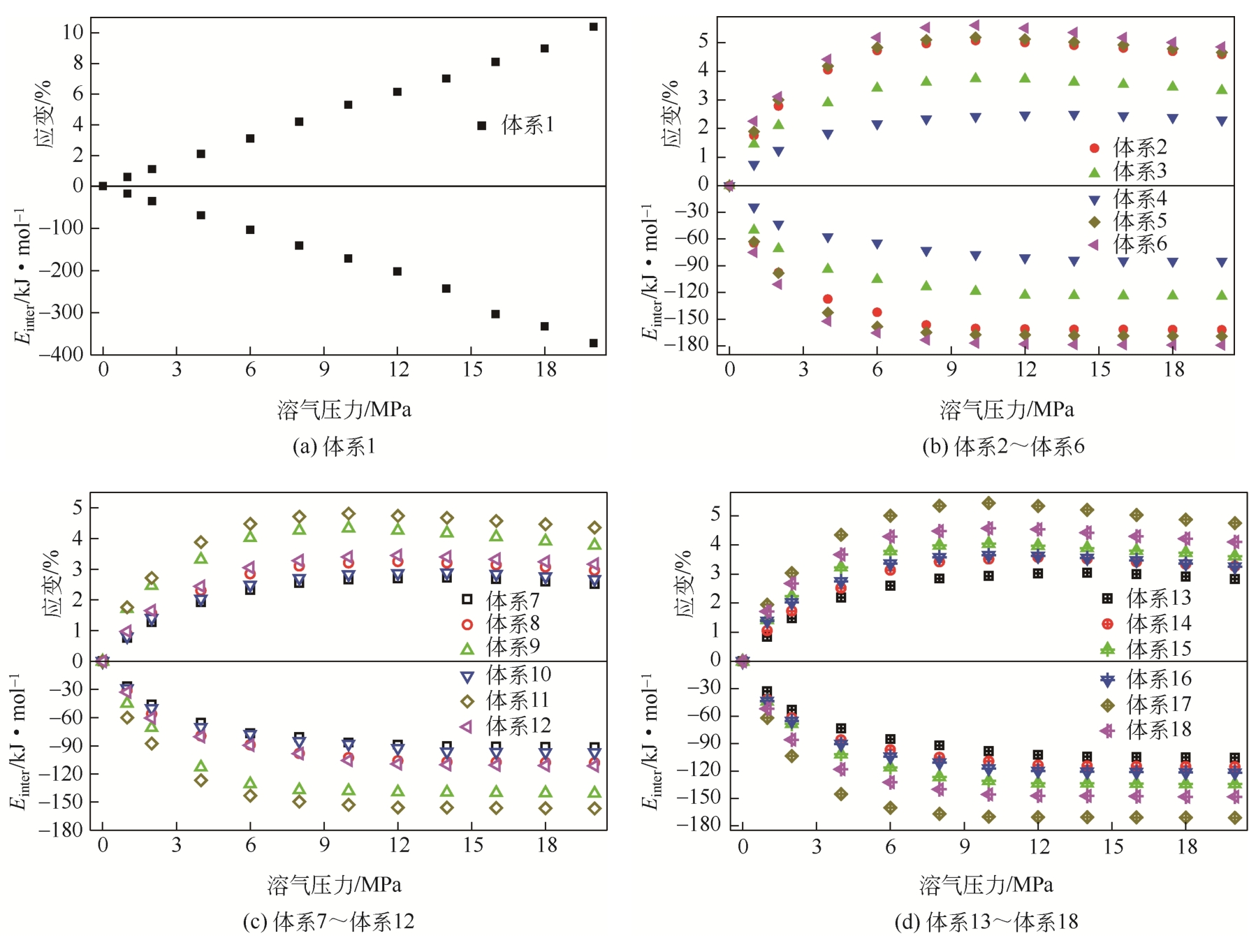
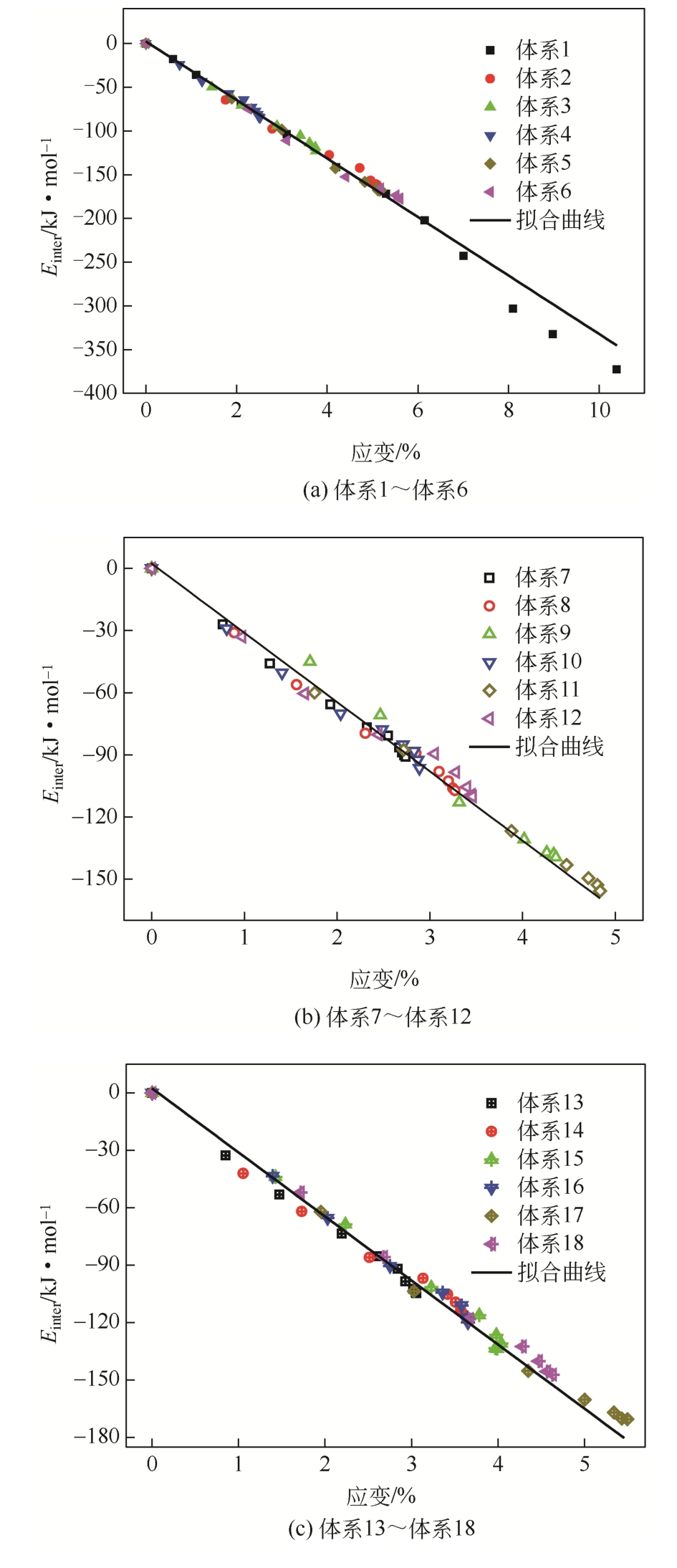
为了研究甲烷(CH4)在原油体系中的溶解过程,本文采用CH4溶解度测试和分子模拟相结合的方式,系统研究了不同温度、压力条件下CH4在原油体系中的溶解行为。以正庚烷为基础油,蜡、胶质、沥青质含量为影响因素,按照L16(43)正交表构建相应的原油体系,并构建胜利原油和南阳原油两种特定比例的原油体系,共18种原油体系。利用分子动力学方法模拟了CH4分子在不同原油体系中的溶解过程,并利用试验结果验证了模拟的可靠性。结果表明:在泡点压力范围内,随着溶气压力的增加,CH4溶解量逐渐增大,但其溶解位置可能发生变化;当溶气压力较低时,CH4分子较为分散地分布在原油体系中,随着CH4溶解量的增多,部分CH4分子会发生聚集;CH4在原油体系中的溶解为物理溶解过程,且CH4溶解过程中范德华力为主要的力学作用方式;原油体系自由体积分数越大,能够为CH4提供的溶解空间越大;原油大分子对CH4溶解量影响程度依次为:蜡<胶质<沥青质,且原油大分子未改变CH4溶解的力学作用方式,并通过力学模型揭示了相互作用能与原油体系体积变化量的线性关系,促进对CH4在原油体系中溶解的分子级理解。
中图分类号:
引用本文
李秉繁, 刘刚, 陈雷. CH4在原油体系中溶解规律及影响机理[J]. 化工进展, 2021, 40(8): 4205-4222.
LI Bingfan, LIU Gang, CHEN Lei. Dissolution of CH4 in the crude oil system: behaviors and mechanisms[J]. Chemical Industry and Engineering Progress, 2021, 40(8): 4205-4222.
| 油样 | 20℃密度 /kg·m-3 | 凝点 /℃ | 析蜡点 /℃ | 含蜡量 /% | 胶质 质量分数/% | 沥青质 质量分数/% |
|---|---|---|---|---|---|---|
| 胜利原油 | 878.2 | 34 | 47 | 24.5 | 18.03 | 0.12 |
| 南阳原油 | 875.0 | 47 | 62 | 38.2 | — | 15 |
表1 常压条件下油样基本物性
| 油样 | 20℃密度 /kg·m-3 | 凝点 /℃ | 析蜡点 /℃ | 含蜡量 /% | 胶质 质量分数/% | 沥青质 质量分数/% |
|---|---|---|---|---|---|---|
| 胜利原油 | 878.2 | 34 | 47 | 24.5 | 18.03 | 0.12 |
| 南阳原油 | 875.0 | 47 | 62 | 38.2 | — | 15 |
试验 压力 | 胜利原油 | 南阳原油 | ||
|---|---|---|---|---|
表观黏度(10s-1) /Pa·s | 平均降黏率 /% | 表观黏度(10s-1) /Pa·s | 平均降黏率 /% | |
| 常压 | 1.65 | — | 32.18 | — |
| 1 | 1.52 | 7.88 | 30.84 | 4.16 |
| 2 | 1.40 | 15.15 | 29.55 | 8.17 |
表2 不同溶气压力条件下原油平均降黏率(30℃)
试验 压力 | 胜利原油 | 南阳原油 | ||
|---|---|---|---|---|
表观黏度(10s-1) /Pa·s | 平均降黏率 /% | 表观黏度(10s-1) /Pa·s | 平均降黏率 /% | |
| 常压 | 1.65 | — | 32.18 | — |
| 1 | 1.52 | 7.88 | 30.84 | 4.16 |
| 2 | 1.40 | 15.15 | 29.55 | 8.17 |
| 体系 | 组合情况 | 体系内分子个数 | ||
|---|---|---|---|---|
| 蜡分子 | 胶质 | 沥青质 | ||
| 1 | 111 | 0 | 0 | 0 |
| 2 | 122 | 0 | 5 | 5 |
| 3 | 133 | 0 | 11 | 11 |
| 4 | 144 | 0 | 25 | 25 |
| 5 | 212 | 5 | 0 | 5 |
| 6 | 221 | 5 | 5 | 0 |
| 7 | 234 | 5 | 11 | 25 |
| 8 | 243 | 5 | 25 | 11 |
| 9 | 313 | 11 | 0 | 11 |
| 10 | 324 | 11 | 5 | 25 |
| 11 | 331 | 11 | 11 | 0 |
| 12 | 342 | 11 | 25 | 5 |
| 13 | 414 | 25 | 0 | 25 |
| 14 | 423 | 25 | 5 | 11 |
| 15 | 432 | 25 | 11 | 5 |
| 16 | 441 | 25 | 25 | 0 |
| 17 | 胜利原油 | 13 | 4 | 0 |
| 18 | 南阳原油 | 25 | 0 | 4 |
表3 原油体系
| 体系 | 组合情况 | 体系内分子个数 | ||
|---|---|---|---|---|
| 蜡分子 | 胶质 | 沥青质 | ||
| 1 | 111 | 0 | 0 | 0 |
| 2 | 122 | 0 | 5 | 5 |
| 3 | 133 | 0 | 11 | 11 |
| 4 | 144 | 0 | 25 | 25 |
| 5 | 212 | 5 | 0 | 5 |
| 6 | 221 | 5 | 5 | 0 |
| 7 | 234 | 5 | 11 | 25 |
| 8 | 243 | 5 | 25 | 11 |
| 9 | 313 | 11 | 0 | 11 |
| 10 | 324 | 11 | 5 | 25 |
| 11 | 331 | 11 | 11 | 0 |
| 12 | 342 | 11 | 25 | 5 |
| 13 | 414 | 25 | 0 | 25 |
| 14 | 423 | 25 | 5 | 11 |
| 15 | 432 | 25 | 11 | 5 |
| 16 | 441 | 25 | 25 | 0 |
| 17 | 胜利原油 | 13 | 4 | 0 |
| 18 | 南阳原油 | 25 | 0 | 4 |
| 体系 | 组分 | 密度/g·cm-3 | 相对误差/% | |
|---|---|---|---|---|
| 模拟值 | 试验值 | |||
| 1 | 111 | 0.662 | 0.684 | 3.22 |
| 2 | 122 | 0.85 | 0.87 | 2.30 |
| 3 | 133 | 0.885 | 0.933 | 5.14 |
| 4 | 144 | 1.035 | 1.012 | 2.2 |
| 5 | 212 | 0.843 | 0.857 | 1.63 |
| 6 | 221 | 0.777 | 0.798 | 2.63 |
| 7 | 234 | 0.994 | 0.996 | 0.20 |
| 8 | 243 | 0.951 | 0.973 | 2.26 |
| 9 | 313 | 0.862 | 0.899 | 4.12 |
| 10 | 324 | 0.97 | 0.986 | 1.62 |
| 11 | 331 | 0.854 | 0.874 | 2.29 |
| 12 | 342 | 0.938 | 0.969 | 3.20 |
| 13 | 414 | 0.966 | 0.98 | 1.43 |
| 14 | 423 | 0.925 | 0.946 | 2.22 |
| 15 | 432 | 0.875 | 0.915 | 4.37 |
| 16 | 441 | 0.905 | 0.942 | 3.93 |
| 17 | 胜利原油 | 0.862 | 0.878 | 1.82 |
| 18 | 南阳原油 | 0.842 | 0.875 | 3.77 |
表4 模拟密度与试验参考密度对比
| 体系 | 组分 | 密度/g·cm-3 | 相对误差/% | |
|---|---|---|---|---|
| 模拟值 | 试验值 | |||
| 1 | 111 | 0.662 | 0.684 | 3.22 |
| 2 | 122 | 0.85 | 0.87 | 2.30 |
| 3 | 133 | 0.885 | 0.933 | 5.14 |
| 4 | 144 | 1.035 | 1.012 | 2.2 |
| 5 | 212 | 0.843 | 0.857 | 1.63 |
| 6 | 221 | 0.777 | 0.798 | 2.63 |
| 7 | 234 | 0.994 | 0.996 | 0.20 |
| 8 | 243 | 0.951 | 0.973 | 2.26 |
| 9 | 313 | 0.862 | 0.899 | 4.12 |
| 10 | 324 | 0.97 | 0.986 | 1.62 |
| 11 | 331 | 0.854 | 0.874 | 2.29 |
| 12 | 342 | 0.938 | 0.969 | 3.20 |
| 13 | 414 | 0.966 | 0.98 | 1.43 |
| 14 | 423 | 0.925 | 0.946 | 2.22 |
| 15 | 432 | 0.875 | 0.915 | 4.37 |
| 16 | 441 | 0.905 | 0.942 | 3.93 |
| 17 | 胜利原油 | 0.862 | 0.878 | 1.82 |
| 18 | 南阳原油 | 0.842 | 0.875 | 3.77 |
| 正庚烷(25℃) | 胜利原油(40℃) | 南阳原油(40℃) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
压力 /MPa | 试验值 /mol·mol-1 | 计算值 /mol·mol-1 | 相对误差 /% | 压力 /MPa | 试验值 /mmol·g-1 | 计算值 /mmol·g-1 | 相对误差 /% | 压力 /MPa | 试验值 /mmol·g-1 | 计算值 /mmol·g-1 | 相对误差 /% | ||
| 3.67 | 0.163 | 0.161 | 4.294 | 0.5 | 0.262 | 0.263 | 0.382 | 0.5 | 0.236 | 0.179 | 24.175 | ||
| 6.96 | 0.276 | 2.657 | 7.246 | 1 | 0.433 | 0.498 | 15.067 | 1 | 0.399 | 0.422 | 5.793 | ||
| 10.3 | 0.376 | 0.355 | 7.181 | 1.5 | 0.629 | 0.695 | 10.576 | 1.5 | 0.530 | 0.602 | 13.518 | ||
| 13.7 | 0.470 | 0.455 | 5.319 | 2 | 0.758 | 0.837 | 10.490 | 2 | 0.633 | 0.712 | 12.405 | ||
| 17.01 | 0.539 | 0.525 | 4.453 | — | — | — | — | — | — | — | — | ||
表5 模拟溶解度值与试验值对比
| 正庚烷(25℃) | 胜利原油(40℃) | 南阳原油(40℃) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
压力 /MPa | 试验值 /mol·mol-1 | 计算值 /mol·mol-1 | 相对误差 /% | 压力 /MPa | 试验值 /mmol·g-1 | 计算值 /mmol·g-1 | 相对误差 /% | 压力 /MPa | 试验值 /mmol·g-1 | 计算值 /mmol·g-1 | 相对误差 /% | ||
| 3.67 | 0.163 | 0.161 | 4.294 | 0.5 | 0.262 | 0.263 | 0.382 | 0.5 | 0.236 | 0.179 | 24.175 | ||
| 6.96 | 0.276 | 2.657 | 7.246 | 1 | 0.433 | 0.498 | 15.067 | 1 | 0.399 | 0.422 | 5.793 | ||
| 10.3 | 0.376 | 0.355 | 7.181 | 1.5 | 0.629 | 0.695 | 10.576 | 1.5 | 0.530 | 0.602 | 13.518 | ||
| 13.7 | 0.470 | 0.455 | 5.319 | 2 | 0.758 | 0.837 | 10.490 | 2 | 0.633 | 0.712 | 12.405 | ||
| 17.01 | 0.539 | 0.525 | 4.453 | — | — | — | — | — | — | — | — | ||
| 体系 | 原油体系体积/?3 | 体系 | 原油体系体积/?3 |
|---|---|---|---|
| 体系1 | 22777.56 | 体系10 | 68951.63 |
| 体系2 | 38182.82 | 体系11 | 45008.31 |
| 体系3 | 50821.36 | 体系12 | 66071.80 |
| 体系4 | 80052.22 | 体系13 | 73341.38 |
| 体系5 | 33501.69 | 体系14 | 53874.80 |
| 体系6 | 32650.47 | 体系15 | 62628.34 |
| 体系7 | 77681.58 | 体系16 | 63042.01 |
| 体系8 | 74932.23 | 体系17 | 36543.51 |
| 体系9 | 43913.76 | 体系18 | 41314.62 |
表6 原油体系体积
| 体系 | 原油体系体积/?3 | 体系 | 原油体系体积/?3 |
|---|---|---|---|
| 体系1 | 22777.56 | 体系10 | 68951.63 |
| 体系2 | 38182.82 | 体系11 | 45008.31 |
| 体系3 | 50821.36 | 体系12 | 66071.80 |
| 体系4 | 80052.22 | 体系13 | 73341.38 |
| 体系5 | 33501.69 | 体系14 | 53874.80 |
| 体系6 | 32650.47 | 体系15 | 62628.34 |
| 体系7 | 77681.58 | 体系16 | 63042.01 |
| 体系8 | 74932.23 | 体系17 | 36543.51 |
| 体系9 | 43913.76 | 体系18 | 41314.62 |
| 体系 | 参数 | 20℃ | 40℃ | 60℃ | 80℃ | 体系 | 参数 | 20℃ | 40℃ | 60℃ | 80℃ |
|---|---|---|---|---|---|---|---|---|---|---|---|
2 | VL | 1.817 | 1.675 | 1.582 | 1.511 | 11 | VL | 1.756 | 1.656 | 1.568 | 1.515 |
| PL | 1.870 | 1.904 | 2.157 | 2.250 | PL | 1.906 | 2.111 | 2.302 | 2.568 | ||
| R2 | 0.997 | 0.997 | 0.996 | 0.995 | R2 | 0.992 | 0.987 | 0.988 | 0.985 | ||
3 | VL | 1.479 | 1.403 | 1.330 | 1.258 | 12 | VL | 1.277 | 1.186 | 1.103 | 1.058 |
| PL | 2.020 | 2.258 | 2.438 | 2.595 | PL | 2.335 | 2.573 | 2.593 | 2.894 | ||
| R2 | 0.998 | 0.998 | 0.998 | 0.997 | R2 | 0.997 | 0.998 | 0.999 | 0.998 | ||
4 | VL | 0.945 | 0.900 | 0.859 | 0.797 | 13 | VL | 1.122 | 1.052 | 1.008 | 0.965 |
| PL | 2.910 | 3.263 | 3.556 | 3.908 | PL | 2.572 | 2.714 | 3.020 | 3.306 | ||
| R2 | 0.997 | 0.998 | 0.999 | 0.998 | R2 | 0.998 | 0.998 | 0.995 | 0.995 | ||
5 | VL | 1.855 | 1.725 | 1.608 | 1.527 | 14 | VL | 1.564 | 1.450 | 1.380 | 1.331 |
| PL | 1.863 | 1.899 | 1.926 | 2.064 | PL | 1.962 | 2.118 | 2.381 | 2.578 | ||
| R2 | 0.992 | 0.991 | 0.989 | 0.989 | R2 | 0.998 | 0.996 | 0.993 | 0.993 | ||
6 | VL | 1.885 | 1.751 | 1.672 | 1.603 | 15 | VL | 1.391 | 1.289 | 1.205 | 1.150 |
| PL | 1.436 | 1.561 | 1.619 | 1.659 | PL | 2.225 | 2.376 | 2.541 | 2.820 | ||
| R2 | 0.995 | 0.991 | 0.991 | 0.990 | R2 | 0.998 | 0.999 | 0.998 | 0.999 | ||
7 | VL | 0.992 | 0.926 | 0.858 | 0.822 | 16 | VL | 1.443 | 1.345 | 1.262 | 1.174 |
| PL | 2.694 | 3.007 | 3.194 | 3.431 | PL | 2.196 | 2.344 | 2.488 | 2.622 | ||
| R2 | 0.999 | 0.997 | 0.998 | 0.999 | R2 | 0.997 | 0.997 | 0.997 | 0.995 | ||
8 | VL | 1.233 | 1.158 | 1.099 | 1.056 | 17 | VL | 1.869 | 1.734 | 1.652 | 1.586 |
| PL | 2.406 | 2.696 | 3.014 | 3.207 | PL | 1.627 | 1.710 | 1.836 | 2.047 | ||
| R2 | 0.998 | 0.997 | 0.997 | 0.996 | R2 | 0.991 | 0.988 | 0.989 | 0.986 | ||
9 | VL | 1.702 | 1.612 | 1.510 | 1.421 | 18 | VL | 1.757 | 1.673 | 1.578 | 1.493 |
| PL | 2.016 | 2.164 | 2.391 | 2.582 | PL | 1.924 | 2.117 | 2.358 | 2.572 | ||
| R2 | 0.984 | 0.983 | 0.983 | 0.978 | R2 | 0.997 | 0.995 | 0.997 | 0.995 | ||
10 | VL | 1.025 | 0.935 | 0.860 | 0.824 | ||||||
| PL | 2.620 | 2.778 | 3.086 | 3.404 | |||||||
| R2 | 0.998 | 0.996 | 0.993 | 0.993 |
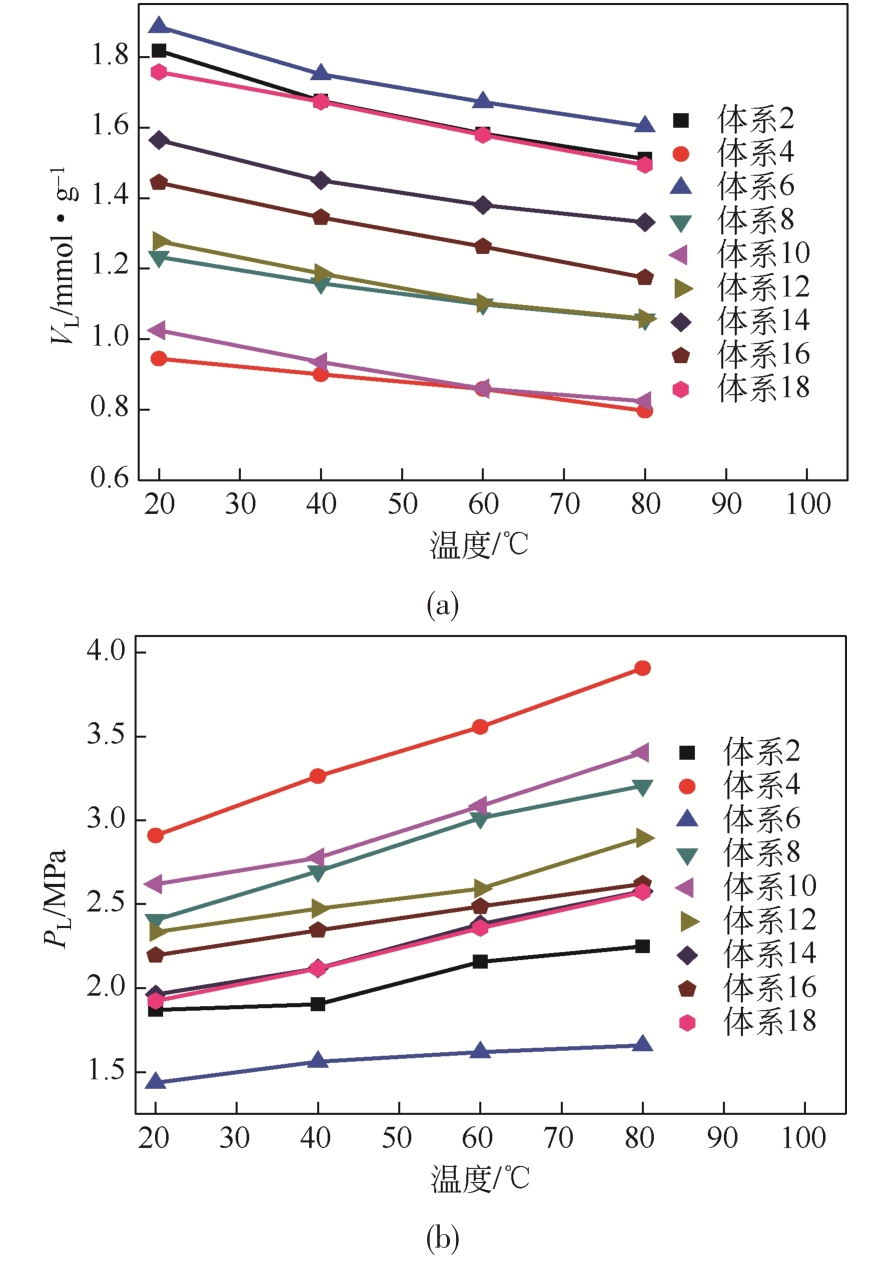
表7 不同温度下Langmuir方程拟合参数
| 体系 | 参数 | 20℃ | 40℃ | 60℃ | 80℃ | 体系 | 参数 | 20℃ | 40℃ | 60℃ | 80℃ |
|---|---|---|---|---|---|---|---|---|---|---|---|
2 | VL | 1.817 | 1.675 | 1.582 | 1.511 | 11 | VL | 1.756 | 1.656 | 1.568 | 1.515 |
| PL | 1.870 | 1.904 | 2.157 | 2.250 | PL | 1.906 | 2.111 | 2.302 | 2.568 | ||
| R2 | 0.997 | 0.997 | 0.996 | 0.995 | R2 | 0.992 | 0.987 | 0.988 | 0.985 | ||
3 | VL | 1.479 | 1.403 | 1.330 | 1.258 | 12 | VL | 1.277 | 1.186 | 1.103 | 1.058 |
| PL | 2.020 | 2.258 | 2.438 | 2.595 | PL | 2.335 | 2.573 | 2.593 | 2.894 | ||
| R2 | 0.998 | 0.998 | 0.998 | 0.997 | R2 | 0.997 | 0.998 | 0.999 | 0.998 | ||
4 | VL | 0.945 | 0.900 | 0.859 | 0.797 | 13 | VL | 1.122 | 1.052 | 1.008 | 0.965 |
| PL | 2.910 | 3.263 | 3.556 | 3.908 | PL | 2.572 | 2.714 | 3.020 | 3.306 | ||
| R2 | 0.997 | 0.998 | 0.999 | 0.998 | R2 | 0.998 | 0.998 | 0.995 | 0.995 | ||
5 | VL | 1.855 | 1.725 | 1.608 | 1.527 | 14 | VL | 1.564 | 1.450 | 1.380 | 1.331 |
| PL | 1.863 | 1.899 | 1.926 | 2.064 | PL | 1.962 | 2.118 | 2.381 | 2.578 | ||
| R2 | 0.992 | 0.991 | 0.989 | 0.989 | R2 | 0.998 | 0.996 | 0.993 | 0.993 | ||
6 | VL | 1.885 | 1.751 | 1.672 | 1.603 | 15 | VL | 1.391 | 1.289 | 1.205 | 1.150 |
| PL | 1.436 | 1.561 | 1.619 | 1.659 | PL | 2.225 | 2.376 | 2.541 | 2.820 | ||
| R2 | 0.995 | 0.991 | 0.991 | 0.990 | R2 | 0.998 | 0.999 | 0.998 | 0.999 | ||
7 | VL | 0.992 | 0.926 | 0.858 | 0.822 | 16 | VL | 1.443 | 1.345 | 1.262 | 1.174 |
| PL | 2.694 | 3.007 | 3.194 | 3.431 | PL | 2.196 | 2.344 | 2.488 | 2.622 | ||
| R2 | 0.999 | 0.997 | 0.998 | 0.999 | R2 | 0.997 | 0.997 | 0.997 | 0.995 | ||
8 | VL | 1.233 | 1.158 | 1.099 | 1.056 | 17 | VL | 1.869 | 1.734 | 1.652 | 1.586 |
| PL | 2.406 | 2.696 | 3.014 | 3.207 | PL | 1.627 | 1.710 | 1.836 | 2.047 | ||
| R2 | 0.998 | 0.997 | 0.997 | 0.996 | R2 | 0.991 | 0.988 | 0.989 | 0.986 | ||
9 | VL | 1.702 | 1.612 | 1.510 | 1.421 | 18 | VL | 1.757 | 1.673 | 1.578 | 1.493 |
| PL | 2.016 | 2.164 | 2.391 | 2.582 | PL | 1.924 | 2.117 | 2.358 | 2.572 | ||
| R2 | 0.984 | 0.983 | 0.983 | 0.978 | R2 | 0.997 | 0.995 | 0.997 | 0.995 | ||
10 | VL | 1.025 | 0.935 | 0.860 | 0.824 | ||||||
| PL | 2.620 | 2.778 | 3.086 | 3.404 | |||||||
| R2 | 0.998 | 0.996 | 0.993 | 0.993 |
| 体系 | 组合 情况 | 分子个数 | 溶解量 /mmol·g-1 | ||
|---|---|---|---|---|---|
| 蜡分子 | 胶质 | 沥青质 | |||
| 1 | 111 | 0 | 0 | 0 | 13.959 |
| 2 | 122 | 0 | 5 | 5 | 1.817 |
| 3 | 133 | 0 | 11 | 11 | 1.479 |
| 4 | 144 | 0 | 25 | 25 | 0.945 |
| 5 | 212 | 5 | 0 | 5 | 1.855 |
| 6 | 221 | 5 | 5 | 0 | 1.885 |
| 7 | 234 | 5 | 11 | 25 | 0.992 |
| 8 | 243 | 5 | 25 | 11 | 1.233 |
| 9 | 313 | 11 | 0 | 11 | 1.702 |
| 10 | 324 | 11 | 5 | 25 | 1.025 |
| 11 | 331 | 11 | 11 | 0 | 1.756 |
| 12 | 342 | 11 | 25 | 5 | 1.277 |
| 13 | 414 | 25 | 0 | 25 | 1.122 |
| 14 | 423 | 25 | 5 | 11 | 1.564 |
| 15 | 432 | 25 | 11 | 5 | 1.391 |
| 16 | 441 | 25 | 25 | 0 | 1.443 |
| 水平K1 | 4.550 | 4.660 | 4.761 | — | |
| 水平K2 | 1.491 | 1.573 | 1.585 | — | |
| 水平K3 | 1.440 | 1.405 | 1.495 | — | |
| 水平K4 | 1.380 | 1.225 | 1.021 | — | |
| 极差R | 3.170 | 3.435 | 3.740 | — | |
| 因子主次 | 3 | 2 | 1 | — | |
表8 正交实验方案
| 体系 | 组合 情况 | 分子个数 | 溶解量 /mmol·g-1 | ||
|---|---|---|---|---|---|
| 蜡分子 | 胶质 | 沥青质 | |||
| 1 | 111 | 0 | 0 | 0 | 13.959 |
| 2 | 122 | 0 | 5 | 5 | 1.817 |
| 3 | 133 | 0 | 11 | 11 | 1.479 |
| 4 | 144 | 0 | 25 | 25 | 0.945 |
| 5 | 212 | 5 | 0 | 5 | 1.855 |
| 6 | 221 | 5 | 5 | 0 | 1.885 |
| 7 | 234 | 5 | 11 | 25 | 0.992 |
| 8 | 243 | 5 | 25 | 11 | 1.233 |
| 9 | 313 | 11 | 0 | 11 | 1.702 |
| 10 | 324 | 11 | 5 | 25 | 1.025 |
| 11 | 331 | 11 | 11 | 0 | 1.756 |
| 12 | 342 | 11 | 25 | 5 | 1.277 |
| 13 | 414 | 25 | 0 | 25 | 1.122 |
| 14 | 423 | 25 | 5 | 11 | 1.564 |
| 15 | 432 | 25 | 11 | 5 | 1.391 |
| 16 | 441 | 25 | 25 | 0 | 1.443 |
| 水平K1 | 4.550 | 4.660 | 4.761 | — | |
| 水平K2 | 1.491 | 1.573 | 1.585 | — | |
| 水平K3 | 1.440 | 1.405 | 1.495 | — | |
| 水平K4 | 1.380 | 1.225 | 1.021 | — | |
| 极差R | 3.170 | 3.435 | 3.740 | — | |
| 因子主次 | 3 | 2 | 1 | — | |
| 体系 | 碳占比 | 体系 | 碳占比 |
|---|---|---|---|
| 体系1 | 7.00 | 体系10 | 18.54 |
| 体系2 | 10.64 | 体系11 | 11.06 |
| 体系3 | 14.21 | 体系12 | 15.13 |
| 体系4 | 20.33 | 体系13 | 18.50 |
| 体系5 | 10.14 | 体系14 | 15.06 |
| 体系6 | 9.05 | 体系15 | 14.04 |
| 体系7 | 19.01 | 体系16 | 14.50 |
| 体系8 | 16.62 | 体系17 | 9.85 |
| 体系9 | 13.22 | 体系18 | 11.91 |
表9 不同原油体系的碳占比
| 体系 | 碳占比 | 体系 | 碳占比 |
|---|---|---|---|
| 体系1 | 7.00 | 体系10 | 18.54 |
| 体系2 | 10.64 | 体系11 | 11.06 |
| 体系3 | 14.21 | 体系12 | 15.13 |
| 体系4 | 20.33 | 体系13 | 18.50 |
| 体系5 | 10.14 | 体系14 | 15.06 |
| 体系6 | 9.05 | 体系15 | 14.04 |
| 体系7 | 19.01 | 体系16 | 14.50 |
| 体系8 | 16.62 | 体系17 | 9.85 |
| 体系9 | 13.22 | 体系18 | 11.91 |
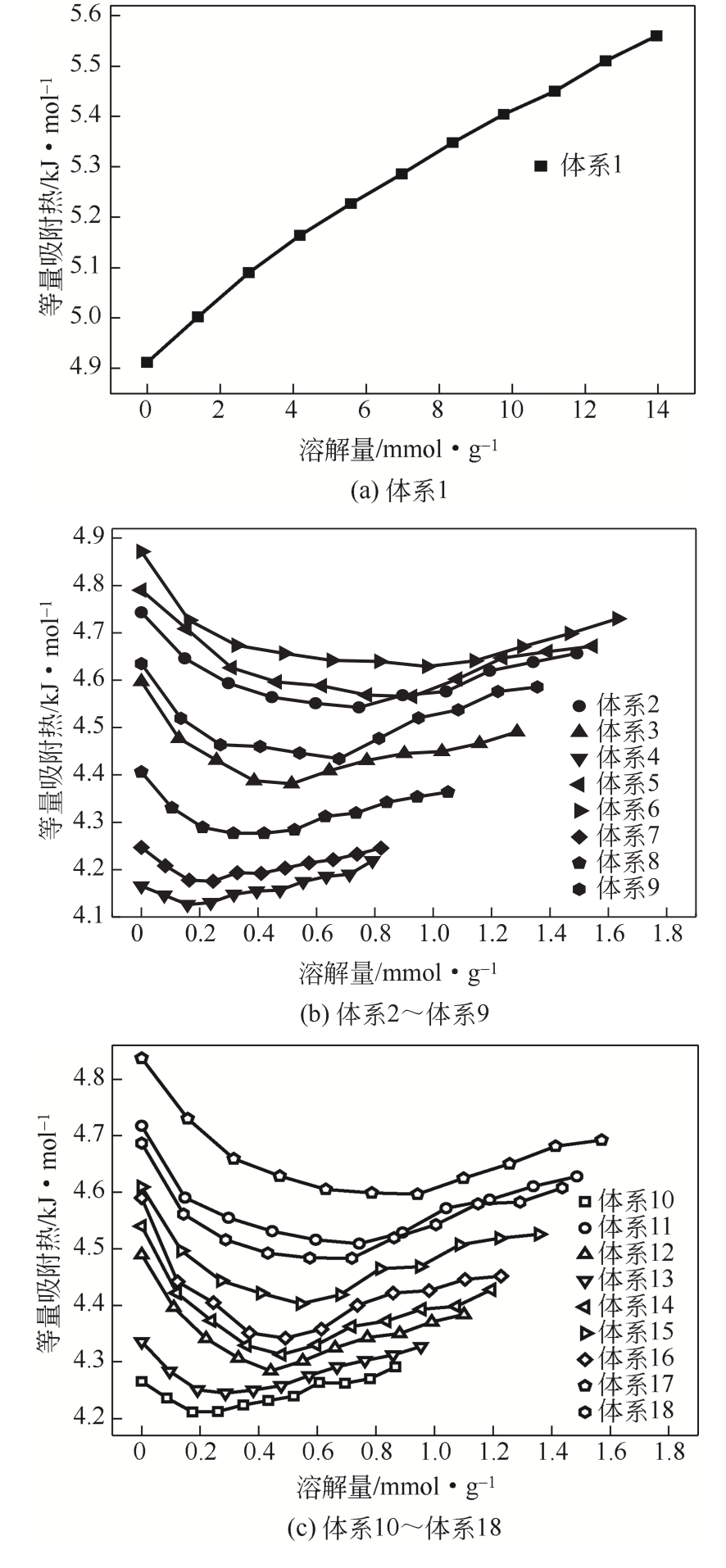
| 体系 | 等量吸附热/kJ·mol-1 | 平均值/kJ·mol-1 |
|---|---|---|
| 体系1 | 4.912~5.564 | 5.268 |
| 体系2 | 4.543~4.743 | 4.609 |
| 体系3 | 4.381~4.597 | 4.451 |
| 体系4 | 4.126~4.219 | 4.163 |
| 体系5 | 4.565~4.790 | 4.639 |
| 体系6 | 4.629~4.872 | 4.689 |
| 体系7 | 4.175~4.247 | 4.210 |
| 体系8 | 4.277~4.406 | 4.323 |
| 体系9 | 4.434~4.635 | 4.514 |
| 体系10 | 4.211~4.291 | 4.246 |
| 体系11 | 4.509~4.718 | 4.577 |
| 体系12 | 4.284~4.490 | 4.354 |
| 体系13 | 4.245~4.336 | 4.285 |
| 体系14 | 4.313~4.540 | 4.387 |
| 体系15 | 4.403~4.609 | 4.480 |
| 体系16 | 4.342~4.590 | 4.421 |
| 体系17 | 4.597~4.837 | 4.664 |
| 体系18 | 4.48~4.687 | 4.551 |
表10 20℃、0~20MPa条件下原油体系的等量吸附热
| 体系 | 等量吸附热/kJ·mol-1 | 平均值/kJ·mol-1 |
|---|---|---|
| 体系1 | 4.912~5.564 | 5.268 |
| 体系2 | 4.543~4.743 | 4.609 |
| 体系3 | 4.381~4.597 | 4.451 |
| 体系4 | 4.126~4.219 | 4.163 |
| 体系5 | 4.565~4.790 | 4.639 |
| 体系6 | 4.629~4.872 | 4.689 |
| 体系7 | 4.175~4.247 | 4.210 |
| 体系8 | 4.277~4.406 | 4.323 |
| 体系9 | 4.434~4.635 | 4.514 |
| 体系10 | 4.211~4.291 | 4.246 |
| 体系11 | 4.509~4.718 | 4.577 |
| 体系12 | 4.284~4.490 | 4.354 |
| 体系13 | 4.245~4.336 | 4.285 |
| 体系14 | 4.313~4.540 | 4.387 |
| 体系15 | 4.403~4.609 | 4.480 |
| 体系16 | 4.342~4.590 | 4.421 |
| 体系17 | 4.597~4.837 | 4.664 |
| 体系18 | 4.48~4.687 | 4.551 |
| 体系 | 液体分子所占有的体积/?3 | 自由的体积/?3 | 自由体积分数/% |
|---|---|---|---|
| 体系1 | 21210.66 | 1966.90 | 8.486 |
| 体系2 | 36588.23 | 1594.59 | 4.176 |
| 体系3 | 48823.01 | 1998.35 | 3.932 |
| 体系4 | 78514.80 | 1537.42 | 1.921 |
| 体系5 | 32011.95 | 1489.74 | 4.447 |
| 体系6 | 30797.41 | 1853.06 | 5.675 |
| 体系7 | 76103.78 | 1577.80 | 2.031 |
| 体系8 | 73082.61 | 1849.62 | 2.468 |
| 体系9 | 42138.28 | 1775.48 | 4.043 |
| 体系10 | 67452.92 | 1498.71 | 2.174 |
| 体系11 | 43092.17 | 1916.14 | 4.257 |
| 体系12 | 64227.34 | 1844.46 | 2.792 |
| 体系13 | 71665.13 | 1676.25 | 2.286 |
| 体系14 | 52325.56 | 1549.24 | 2.876 |
| 体系15 | 60674.83 | 1953.51 | 3.119 |
| 体系16 | 60820.33 | 2221.68 | 3.524 |
| 体系17 | 34698.63 | 1844.88 | 5.048 |
| 体系18 | 39594.58 | 1720.04 | 4.163 |
表11 原油体系结构模型的表征参数
| 体系 | 液体分子所占有的体积/?3 | 自由的体积/?3 | 自由体积分数/% |
|---|---|---|---|
| 体系1 | 21210.66 | 1966.90 | 8.486 |
| 体系2 | 36588.23 | 1594.59 | 4.176 |
| 体系3 | 48823.01 | 1998.35 | 3.932 |
| 体系4 | 78514.80 | 1537.42 | 1.921 |
| 体系5 | 32011.95 | 1489.74 | 4.447 |
| 体系6 | 30797.41 | 1853.06 | 5.675 |
| 体系7 | 76103.78 | 1577.80 | 2.031 |
| 体系8 | 73082.61 | 1849.62 | 2.468 |
| 体系9 | 42138.28 | 1775.48 | 4.043 |
| 体系10 | 67452.92 | 1498.71 | 2.174 |
| 体系11 | 43092.17 | 1916.14 | 4.257 |
| 体系12 | 64227.34 | 1844.46 | 2.792 |
| 体系13 | 71665.13 | 1676.25 | 2.286 |
| 体系14 | 52325.56 | 1549.24 | 2.876 |
| 体系15 | 60674.83 | 1953.51 | 3.119 |
| 体系16 | 60820.33 | 2221.68 | 3.524 |
| 体系17 | 34698.63 | 1844.88 | 5.048 |
| 体系18 | 39594.58 | 1720.04 | 4.163 |
| 体系 | 原油体系体积模量/GPa | 体积膨胀系数 |
|---|---|---|
| 体系1 | 1.925 | 17.382 |
| 体系2 | 2.195 | 15.243 |
| 体系3 | 2.526 | 13.240 |
| 体系4 | 4.001 | 8.361 |
| 体系5 | 2.102 | 15.917 |
| 体系6 | 1.972 | 16.966 |
| 体系7 | 3.731 | 8.966 |
| 体系8 | 2.966 | 11.280 |
| 体系9 | 2.378 | 14.068 |
| 体系10 | 3.725 | 8.980 |
| 体系11 | 2.304 | 14.516 |
| 体系12 | 2.867 | 11.667 |
| 体系13 | 3.449 | 9.700 |
| 体系14 | 2.803 | 11.933 |
| 体系15 | 2.448 | 13.667 |
| 体系16 | 2.625 | 12.746 |
| 体系17 | 2.052 | 16.301 |
| 体系18 | 2.308 | 14.493 |
表12 原油体系的体积模量、体积膨胀系数值
| 体系 | 原油体系体积模量/GPa | 体积膨胀系数 |
|---|---|---|
| 体系1 | 1.925 | 17.382 |
| 体系2 | 2.195 | 15.243 |
| 体系3 | 2.526 | 13.240 |
| 体系4 | 4.001 | 8.361 |
| 体系5 | 2.102 | 15.917 |
| 体系6 | 1.972 | 16.966 |
| 体系7 | 3.731 | 8.966 |
| 体系8 | 2.966 | 11.280 |
| 体系9 | 2.378 | 14.068 |
| 体系10 | 3.725 | 8.980 |
| 体系11 | 2.304 | 14.516 |
| 体系12 | 2.867 | 11.667 |
| 体系13 | 3.449 | 9.700 |
| 体系14 | 2.803 | 11.933 |
| 体系15 | 2.448 | 13.667 |
| 体系16 | 2.625 | 12.746 |
| 体系17 | 2.052 | 16.301 |
| 体系18 | 2.308 | 14.493 |
| 1 | SHIRYAEVA R, KUDASHEVA F, GIMAEV R, et al. Rheological properties of crude oils with a high resin and asphaltene content: effect of an electromagnetic field and modifiers[J]. Chemistry and Technology of Fuels and Oils, 2006, 42(3): 202-205. |
| 2 | LI Bingfan, LIU Gang, XING Xiao, et al. Molecular dynamics simulation of CO2 dissolution in heavy oil resin-asphaltene[J]. Journal of CO2 Utilization, 2019, 33: 303-310. |
| 3 | HINAI Nasser M, MYERS Matthew B, DEHGHANI Ali M, et al. Effects of oligomers dissolved in CO2 or associated gas on IFT and miscibility pressure with a gas-light crude oil system[J]. Journal of Petroleum Science and Engineering, 2019, 181: 106210. |
| 5 | YANG Hongqun, XU Zhenghe, FAN Maohong, et al. Progress in carbon dioxide separation and capture: a review[J]. Journal of Environmental Sciences, 2008, 20 (1): 14-27. |
| 6 | LEUNG Dennis Y, CARAMANNA Giorgio, MAROTO Mercedes M. An overview of current status of carbon dioxide capture and storage technologies[J]. Renewable and Sustainable Energy Reviews, 2014, 39: 426-443. |
| 7 | SABOORIAN-JOOYBARI H. A novel methodology for simultaneous estimation of gas diffusivity and solubility in bitumens and heavy oils[C]//SPE Heavy Oil Conference, Canada: Society of Petroleum Engineers, 2012. |
| 8 | YANG Fei, LI Chuanxian, XIA Binghuan, et al. Solubility and rheological properties of live crude oils[J]. Advanced Materials Research, 2012, 524-527: 1881-1888. |
| 9 | 鲁彦伯. 溶气原油流变性研究及模型的建立[D]. 东营: 中国石油大学(华东), 2011. |
| LU Yanbo. Research on peological property of live crude oil and foundation of model[D]. Dongying: China University of Petroleum (East China), 2011. | |
| 10 | 李保平. 溶气原油溶气规律及流变规律研究[D]. 青岛: 中国石油大学(华东), 2012. |
| LI Baoping. Research on resolve law and reological law of live crude oil[D]. Qingdao: China University of Petroleum (East China), 2012. | |
| 11 | 夏炳焕. 天然气在原油中的溶解性及溶气原油流变性研究[D]. 青岛: 中国石油大学(华东), 2010. |
| XIA Binghuan. Research on natural gas solubility in crude oil and rheological property of live crude oil[D]. Qingdao: China University of Petroleum (East China), 2010. | |
| 12 | 李传宪, 阎孔尧, 杨爽, 等. CO2溶胀和CH4协同作用下长庆原油流动性的改善[J]. 石油化工高等学校学报, 2017, 30(5): 88-94. |
| LI Chuanxian, YAN Kongyao, YANG Shuang, et al. CO2 swelling and synergistic effect of CH4 on rheological improvement of Changqing crude oil[J]. Journal of Petrochemical Universities, 2017, 30(5): 88-94. | |
| 13 | RABITZ Herschel. Strong-arming molecular dynamics[J]. Science, 2006, 314 (5797): 264-265. |
| 14 | DONG Zejiao, LIU Zhiyang, WANG Peng, et al. Nanostructure characterization of asphalt-aggregate interface through molecular dynamics simulation and atomic force microscopy[J]. Fuel, 2017, 189: 155-163. |
| 15 | XU Meng, YI Junyan, FENG Decheng, et al. Analysis of adhesive characteristics of asphalt based on atomic force microscopy and molecular dynamics simulation[J]. ACS Applied Materials & Interfaces, 2016, 8 (19): 12393-12403. |
| 16 | YANG Xiaoning, ZHANG Cuijuan. Structure and diffusion behavior of dense carbon dioxide fluid in clay-like slit pores by molecular dynamics simulation[J]. Chemical Physics Letters, 2005, 407(4/5/6): 427-432. |
| 17 | JIN Zhehui, FIROOZABADI Abbas. Effect of water on methane and carbon dioxide sorption in clay minerals by Monte Carlo simulations[J]. Fluid Phase Equilibria, 2014, 382: 10-20. |
| 18 | BROCHARD Laurent, VANDAMME Matthieu, PELLENQ Roland, et al. Adsorption-induced deformation of microporous materials: coal swelling induced by CO2-CH4 competitive adsorption[J]. Langmuir, 2012, 28(5): 2659-2670. |
| 19 | OTTIGER Stefan, PINI Ronny, STORTI Giuseppe, et al. Competitive adsorption equilibria of CO2 and CH4 on a dry coal[J]. Adsorption, 2008, 14(4/5): 539-556. |
| 20 | GEORGE J D ST, BARAKAT M A. The change in effective stress associated with shrinkage from gas desorption in coal[J]. International Journal of Coal Geology, 2001, 45(2/3): 105-113. |
| 21 | FAURE Francois, ROUSSEAU Bernard, VERONIQUE Lachet, et al. Molecular simulation of the solubility and diffusion of carbon dioxide and hydrogen sulfide in polyethylene melts[J]. Fluid Phase Equilibria, 2007, 261(1/2): 168-175. |
| 22 | ZHANG Junfang, PAN Zhejun, LIU Keyu, et al. Molecular simulation of CO2 solubility and its effect on octane swelling[J]. Energy & Fuels, 2013, 27 (5): 2741-2747. |
| 23 | 刘沺. 溶气原油流变性研究及流变模型的建立[D]. 东营: 中国石油大学(华东), 2015. |
| LIU Tian. Research on reological property of live oil and establishment of reological model[D]. Dongying: China University of Petroleum (East China), 2015. | |
| 24 | 白帆. 原油组成对结蜡规律影响的研究[D]. 东营: 中国石油大学(华东), 2014. |
| BAI Fan. Effect of crude oil composition on wax deposition[D]. Dongying: China University of Petroleum (East China), 2014. | |
| 25 | JENNINGS P W. Binder characterization and evaluation by nuclear magnetic resonance spectroscopy[R]. Washington D C: Strategic Highway Research Program of National Research Council, 1993: 1-21. |
| 26 | BOUHADDA Y, BORMANN D, SHEU E, et al. Characterization of Algerian Hassi-Messaoud asphaltene structure using Raman spectrometry and X-ray diffraction[J]. Fuel, 2007, 86 (12/13): 1855-1864. |
| 27 | KOWALEWSKI I, VANDENBROUCKE M, HUC A Y, et al. Preliminary results on molecular modeling of asphaltenes using structure elucidation programs in conjunction with molecular simulation programs[J]. Energy & Fuels, 1996, 10 (1): 97-107. |
| 28 | CLEVER H L, YOMG C L. Solubility data series v27/28[M]. Oxford: The International Union of Pure and Applied Chemistry, Pergamon Press, 1987. |
| 29 | EVERETT Douglas H, POWL John C. Adsorption in slit-like and cylindrical micropores in the henry's law region. A model for the microporosity of carbons[J]. Journal of the Chemical Society, Faraday Transactions 1, 1976, 72: 619-636. |
| 30 | 田力. CH4、CO2在烟煤结构模型中吸附的分子模拟研究[D]. 东营: 中国石油大学(华东), 2014. |
| TIAN Li. Molecular simulation study on adsorption of CH4 and CO2 in bituminous coal structure model[D]. Dongying: China University of Petroleum (East China), 2014. | |
| 31 | BILLEMONT Pierre, COASNE Benoit, DE WEIRELD Guy. An experimental and molecular simulation study of the adsorption of carbon dioxide and methane in nanoporous carbons in the presence of water[J]. Langmuir, 2011, 27(3): 1015-1024. |
| 32 | BILLEMONT Pierre, COASNE Benoit, DE WEIRELD Guy. Adsorption of carbon dioxide, methane, and their mixtures in porous carbons: effect of surface chemistry, water content, and pore disorder[J]. Langmuir, 2013, 29(10): 3328-3338. |
| 33 | NODZENSKI Adam. Sorption and desorption of gases (CH4, CO2) on hard coal and active carbon at elevated pressures[J]. Fuel, 1998, 77(11): 1243-1246. |
| 34 | 傅献彩, 沈文霞, 姚天扬. 物理化学[M]. 北京: 高等教育出版牡, 2006. |
| FU Xiancai, SHEN Wenxia, YAO Tianyang. Physical chemistry[M]. Beijing: Higher Education Press, 2006. | |
| 35 | Thomas G FOX, FLORY Paul J. Viscosity-molecular eight and viscosity-temperature relationships for polystyrene and polyisobutylene1, 2[J]. Journal of the American Chemical Society, 1948, 70(7): 2384-2395. |
| 36 | HE Yabin, ZHOU Wei, QIAN Guodong, et al. Methane storage in metal-organic frameworks[J]. Chemical Society Reviews, 2014, 43 (16): 5657-5678. |
| 37 | LI Ben, PAN Fusheng, FANG Zhiping, et al. Molecular dynamics simulation of diffusion behavior of benzene/water in PDMS-Calix[4]arene hybrid pervaporation membranes[J]. Industrial & Engineering Chemistry Research, 2008, 47 (13): 4440-4447. |
| 38 | COUSSY O. Poromechanics[M]. Chichester, UK:John Wiley & Sons, 2004. |
| 39 | BROCHARD L, VANDAMME M, PELLENQ J M. Poromechanics of microporous media[J]. Journal of the Mechanics and Physics of Solids, 2012, 60(4): 606-622. |
| 40 | SONG J, CURTIN W A. Atomic mechanism and prediction of hydrogen embrittlement in iron[J]. Nature Materials, 2013, 12(2): 145-151. |
| 41 | ZHOU Xiao, MARCHANG Daniel, MCDOWELL David L, et al. Chemomechanical origin of hydrogen trapping at grain boundaries in fcc metals[J]. Physical Review Letters, 2016, 116(7): 075502. |
| [1] | 崔守成, 徐洪波, 彭楠. 两种MOFs材料用于O2/He吸附分离的模拟分析[J]. 化工进展, 2023, 42(S1): 382-390. |
| [2] | 王琦, 寇丽红, 王冠宇, 王吉坤, 刘敏, 李兰廷, 王昊. 焦化废水生物出水中可溶解性有机物的分子识别[J]. 化工进展, 2023, 42(9): 4984-4993. |
| [3] | 赖诗妮, 江丽霞, 李军, 黄宏宇, 小林敬幸. 含碳掺氨燃料的研究进展[J]. 化工进展, 2023, 42(9): 4603-4615. |
| [4] | 黄玉飞, 李子怡, 黄杨强, 金波, 罗潇, 梁志武. 光催化CO2和CH4重整催化剂研究进展[J]. 化工进展, 2023, 42(8): 4247-4263. |
| [5] | 赵健, 卓泽文, 董航, 高文健. 含蜡原油及其乳状液体系微观结构观测的新方法[J]. 化工进展, 2023, 42(8): 4372-4384. |
| [6] | 吴亚, 赵丹, 方荣苗, 李婧瑶, 常娜娜, 杜春保, 王文珍, 史俊. 用于复杂原油乳液的高效破乳剂开发及应用研究进展[J]. 化工进展, 2023, 42(8): 4398-4413. |
| [7] | 奚永兰, 王成成, 叶小梅, 刘洋, 贾昭炎, 曹春晖, 韩挺, 张应鹏, 田雨. 微纳米气泡在厌氧消化中的应用研究进展[J]. 化工进展, 2023, 42(8): 4414-4423. |
| [8] | 欧阳素芳, 周道伟, 黄伟, 贾凤. 新型耐迁移橡胶防老剂的研究进展[J]. 化工进展, 2023, 42(7): 3708-3719. |
| [9] | 刘洋, 叶小梅, 苗晓, 王成成, 贾昭炎, 曹春晖, 奚永兰. 农村有机生活垃圾干发酵氨胁迫下中试工艺[J]. 化工进展, 2023, 42(7): 3847-3854. |
| [10] | 张凯, 吕秋楠, 李刚, 李小森, 莫家媚. 南海海泥中甲烷水合物的形貌及赋存特性[J]. 化工进展, 2023, 42(7): 3865-3874. |
| [11] | 孙征楠, 李洪晶, 荆国林, 张福宁, 颜飚, 刘晓燕. EVA及其改性聚合物在原油降凝剂领域的应用[J]. 化工进展, 2023, 42(6): 2987-2998. |
| [12] | 赵毅, 杨臻, 张新为, 王刚, 杨旋. 不同裂缝损伤和愈合温度条件下沥青自愈合行为的分子模拟[J]. 化工进展, 2023, 42(6): 3147-3156. |
| [13] | 杨发容, 顾丽莉, 刘洋, 李伟雪, 蔡洁云, 王惠平. 计算机模拟辅助特丁津分子印迹聚合物的制备及应用[J]. 化工进展, 2023, 42(6): 3157-3166. |
| [14] | 李瑞东, 黄辉, 同国虎, 王跃社. 原油精馏塔中铵盐吸湿特性及其腐蚀行为[J]. 化工进展, 2023, 42(6): 2809-2818. |
| [15] | 马源, 肖晴月, 岳君容, 崔彦斌, 刘姣, 许光文. CeO2-Al2O3复合载体负载Ni基催化剂催化CO x 共甲烷化性能[J]. 化工进展, 2023, 42(5): 2421-2428. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||