化工进展 ›› 2021, Vol. 40 ›› Issue (4): 2048-2059.DOI: 10.16085/j.issn.1000-6613.2020-1411
过氧化氢及其基本有机化学品绿色合成技术
史延强( ), 夏玥穜, 温朗友, 郜亮, 徐广通, 宗保宁(
), 夏玥穜, 温朗友, 郜亮, 徐广通, 宗保宁( )
)
- 中国石油化工股份有限公司石油化工科学研究院,北京 100083
-
收稿日期:2020-07-22出版日期:2021-04-05发布日期:2021-04-14 -
通讯作者:宗保宁 -
作者简介:史延强(1988—),男,博士研究生,研究方向为催化材料与有机化学品合成。E-mail:shiyq.ripp@sinopec.com 。 -
基金资助:国家自然科学基金(U19B6002)
Hydrogen peroxide and its green synthesis of basic organic chemicals
SHI Yanqiang( ), XIA Yuetong, WEN Langyou, GAO Liang, XU Guangtong, ZONG Baoning(
), XIA Yuetong, WEN Langyou, GAO Liang, XU Guangtong, ZONG Baoning( )
)
- Research Institute of Petroleum Processing, SINOPEC, Beijing 100083, China
-
Received:2020-07-22Online:2021-04-05Published:2021-04-14 -
Contact:ZONG Baoning
摘要:
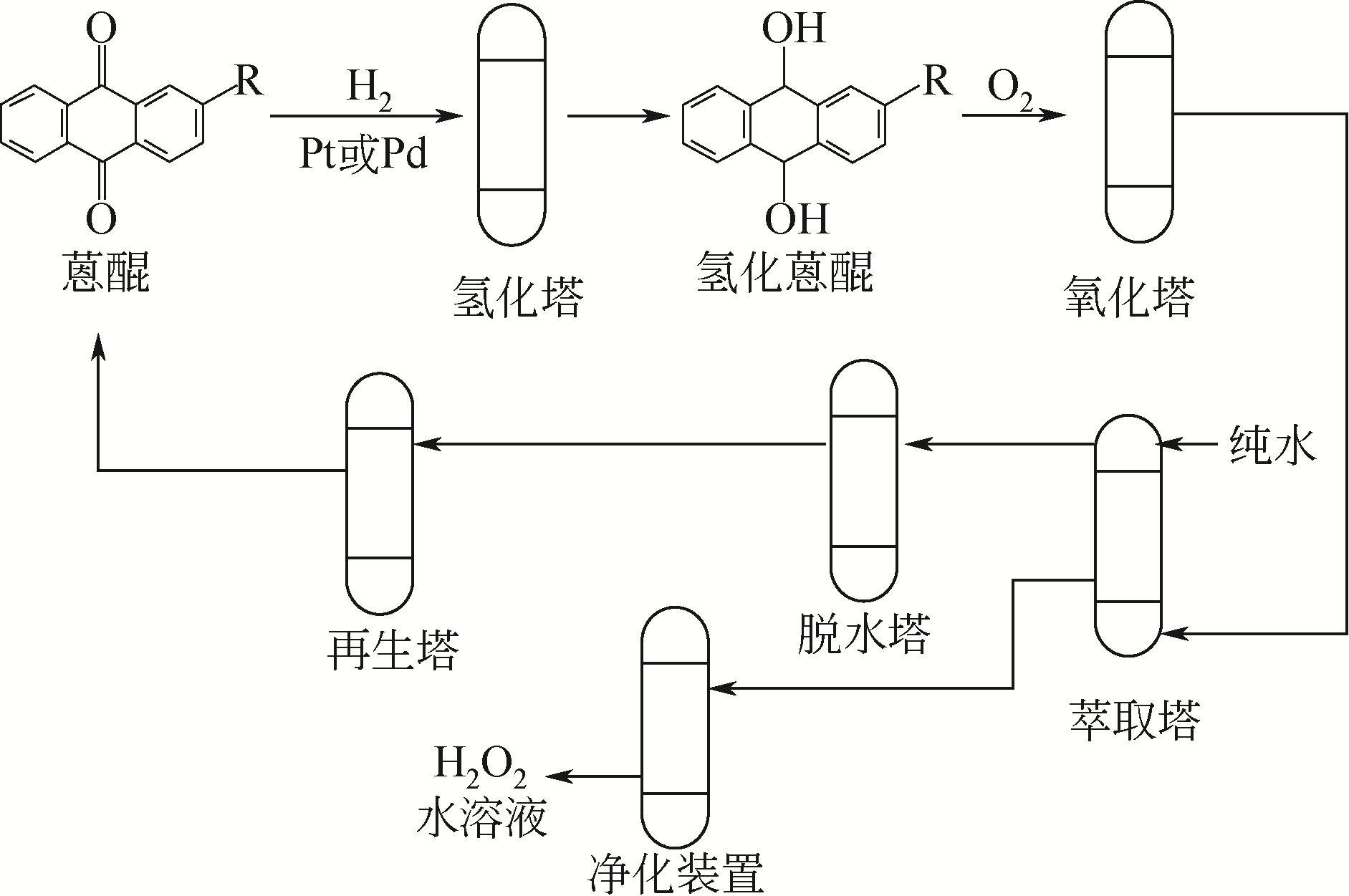
烃类氧化与氮化反应是生产基础有机化学品和高附加值产品的主要反应,在满足和丰富人类物质需求方面贡献巨大,传统的工业氧化与氮化工艺导致严重的环境问题,亟需绿色化转型。作为公认的绿色氧化剂,过氧化氢在烃类氧化和氮化的绿色生产工艺中应用广泛。文章简要介绍了国内外过氧化氢生产现状,重点介绍了中国石化石油化工科学研究院浆态床过氧化氢生产技术,以己内酰胺、环氧丙烷和环氧氯丙烷为例,介绍了过氧化氢在绿色烃类氧化和氮化反应中的应用,汇报了石油化工科学研究院近年来在绿色化工方面的主要研究进展及工业实践结果。多个成套绿色化工技术的成功开发突破了国外对我国的技术封锁,为多个化工生产基地提供全流程绿色生产技术,有力保障了我国化工行业的绿色化转型。
中图分类号:
引用本文
史延强, 夏玥穜, 温朗友, 郜亮, 徐广通, 宗保宁. 过氧化氢及其基本有机化学品绿色合成技术[J]. 化工进展, 2021, 40(4): 2048-2059.
SHI Yanqiang, XIA Yuetong, WEN Langyou, GAO Liang, XU Guangtong, ZONG Baoning. Hydrogen peroxide and its green synthesis of basic organic chemicals[J]. Chemical Industry and Engineering Progress, 2021, 40(4): 2048-2059.
| 聚合物 | 能耗 /MJ·(kg产品)-1 | 碳排放 /MJ·(kg产品)-1 |
|---|---|---|
| 尼龙-6 | 129.1 | 6.7 |
| 尼龙-66 | 139.5 | 6.4 |
| 聚碳酸酯(PC) | 104.3 | 3.4 |
| 高抗冲聚苯乙烯(HIPS) | 86.4 | 2.4 |
| 通用聚苯乙烯(GPPS) | 82.3 | 2.3 |
| 涤纶树脂(PET) | 69.6 | 2.2 |
| 低密度聚乙烯(LDPE) | 81.5 | 1.9 |
| 高密度聚乙烯(HDPE) | 79.4 | 1.8 |
| 线型低密度聚乙烯(LLDPE) | 78.3 | 1.8 |
| 聚丙烯(PP) | 77.1 | 1.6 |
表1 单位聚合物产品能耗和碳排放[12]
| 聚合物 | 能耗 /MJ·(kg产品)-1 | 碳排放 /MJ·(kg产品)-1 |
|---|---|---|
| 尼龙-6 | 129.1 | 6.7 |
| 尼龙-66 | 139.5 | 6.4 |
| 聚碳酸酯(PC) | 104.3 | 3.4 |
| 高抗冲聚苯乙烯(HIPS) | 86.4 | 2.4 |
| 通用聚苯乙烯(GPPS) | 82.3 | 2.3 |
| 涤纶树脂(PET) | 69.6 | 2.2 |
| 低密度聚乙烯(LDPE) | 81.5 | 1.9 |
| 高密度聚乙烯(HDPE) | 79.4 | 1.8 |
| 线型低密度聚乙烯(LLDPE) | 78.3 | 1.8 |
| 聚丙烯(PP) | 77.1 | 1.6 |
| 项目 | 工作液组成 | 蒽醌溶解度/g·L-1 | Pd质量分数/% | 催化剂 | 氢化反应器 | 氢化效率/g·L-1 | 萃取液H2O2质量分数/% |
|---|---|---|---|---|---|---|---|
| 国内技术 | AR+TOP+EAQ | 125~140 | 0.3 | Pd/Al2O3 | 固定床 | 7~8 | 27.5~35 |
| FMC | AR+TOP+EAQ | 160~180 | 0.3 | — | 固定床 | 10~12 | 27.5~35 |
| MGC | AR+TBU+AAQ | 250~300 | 1~2 | Pd/SiO2 | 浆态床 | 15~18 | 45~48 |
| Solvay | AR+TBU+EAQ | 160~180 | 1~2 | Pd/Al2O3-ZrO2 | 浆态床 | 12~15 | 43~46 |
| Degussa | AR+TOP+EAQ | 160~180 | 1~2 | Pd | 浆态床 | 11~15 | 40~45 |
| Arkema | AR+2-MCHA+EAQ | 160~180 | 1~2 | — | 浆态床 | 11~14 | 40~45 |
表2 国内外主要公司过氧化氢生产工艺及参数[15,27]
| 项目 | 工作液组成 | 蒽醌溶解度/g·L-1 | Pd质量分数/% | 催化剂 | 氢化反应器 | 氢化效率/g·L-1 | 萃取液H2O2质量分数/% |
|---|---|---|---|---|---|---|---|
| 国内技术 | AR+TOP+EAQ | 125~140 | 0.3 | Pd/Al2O3 | 固定床 | 7~8 | 27.5~35 |
| FMC | AR+TOP+EAQ | 160~180 | 0.3 | — | 固定床 | 10~12 | 27.5~35 |
| MGC | AR+TBU+AAQ | 250~300 | 1~2 | Pd/SiO2 | 浆态床 | 15~18 | 45~48 |
| Solvay | AR+TBU+EAQ | 160~180 | 1~2 | Pd/Al2O3-ZrO2 | 浆态床 | 12~15 | 43~46 |
| Degussa | AR+TOP+EAQ | 160~180 | 1~2 | Pd | 浆态床 | 11~15 | 40~45 |
| Arkema | AR+2-MCHA+EAQ | 160~180 | 1~2 | — | 浆态床 | 11~14 | 40~45 |
| 项目 | 工作液组成 | 氢化效率 /g·L-1 | 工作液损失 /kg·(tH2O2)-1 | 能耗 /kW·h·(tH2O2)-1 | 废气 /m3·(tH2O2)-1 | 废水 /m3·(tH2O2)-1 | 后处理程序 | 单套装置最高产能/104t·a-1 |
|---|---|---|---|---|---|---|---|---|
| 浆态床 | AR+TOP+EAQ | 12~13 | 2.03 | 600 | 0 | 0.105 | 全酸性环境,本质安全 | 12 |
| 固定床 | AR+TOP+EAQ | 7~8 | 2.54 | 742 | 1000 | 0.15 | 酸碱切换,易爆炸 | 5 |
表3 固定床与浆态床工艺对比
| 项目 | 工作液组成 | 氢化效率 /g·L-1 | 工作液损失 /kg·(tH2O2)-1 | 能耗 /kW·h·(tH2O2)-1 | 废气 /m3·(tH2O2)-1 | 废水 /m3·(tH2O2)-1 | 后处理程序 | 单套装置最高产能/104t·a-1 |
|---|---|---|---|---|---|---|---|---|
| 浆态床 | AR+TOP+EAQ | 12~13 | 2.03 | 600 | 0 | 0.105 | 全酸性环境,本质安全 | 12 |
| 固定床 | AR+TOP+EAQ | 7~8 | 2.54 | 742 | 1000 | 0.15 | 酸碱切换,易爆炸 | 5 |
| 技术 | 反应器 | 环己酮转化率 /% | 环己酮肟选择性 /% | H2O2利用率 /% | 催化剂微反评价寿命 /h | 单位催化剂产能 /g酮·(g催化剂)-1 |
|---|---|---|---|---|---|---|
| 中国石化技术 | 单釜 | 99.5 | 99.5 | 92.0 | 132 | 936 |
| 国外对比技术 | 双釜串联 | 99.4 | 98.2 | 89.0 | 70 | 424 |
表4 中国石化与国外环己酮氨肟化技术对比
| 技术 | 反应器 | 环己酮转化率 /% | 环己酮肟选择性 /% | H2O2利用率 /% | 催化剂微反评价寿命 /h | 单位催化剂产能 /g酮·(g催化剂)-1 |
|---|---|---|---|---|---|---|
| 中国石化技术 | 单釜 | 99.5 | 99.5 | 92.0 | 132 | 936 |
| 国外对比技术 | 双釜串联 | 99.4 | 98.2 | 89.0 | 70 | 424 |
| 技术商 | 甲醇/双氧水(摩尔比) | 丙烯/双氧水(摩尔比) | 反应温度/℃ | 反应压力/MPa | H2O2转化率/% | 环氧丙烷选择性/% |
|---|---|---|---|---|---|---|
| 中国石化 | 7.0 | 2.0 | 40 | 2.0 | 98.8 | 96.0 |
| 国外技术1 | 9.6 | 2.0 | 40 | 2.1 | 96.0 | 95.0 |
| 国外技术2 | 15.6 | 2.0 | 65 | 1.6 | 96.8 | 94.5 |
表5 HPPO技术对比
| 技术商 | 甲醇/双氧水(摩尔比) | 丙烯/双氧水(摩尔比) | 反应温度/℃ | 反应压力/MPa | H2O2转化率/% | 环氧丙烷选择性/% |
|---|---|---|---|---|---|---|
| 中国石化 | 7.0 | 2.0 | 40 | 2.0 | 98.8 | 96.0 |
| 国外技术1 | 9.6 | 2.0 | 40 | 2.1 | 96.0 | 95.0 |
| 国外技术2 | 15.6 | 2.0 | 65 | 1.6 | 96.8 | 94.5 |
| 生产方法 | 原材料 | ECH 收率/% | 催化剂 | 剂耗 /t·(tECH)-1 | 能耗 | 废渣量 /t·(tECH)-1 | 废水 排放量 /t·(tECH)-1 | 预计 投资 /亿元 | 预计环保 投资 /亿元 | 环保费用 占总投资 比例/% |
|---|---|---|---|---|---|---|---|---|---|---|
| 丙烯高温氯化法 | 丙烯,Cl2,Ca(OH)2 | 70~75 | — | — | 高 | 0.8~1.2 | 40 | 1.67 | 0.7 | 20 |
| 甘油法 | 甘油,干燥HCl,Ca(OH)2 | 85~90 | ZnCl2 | 0.0005 | 较低 | 0.4~0.6 | 15~17 | 1.62 | 0.5 | 10 |
| 氯丙烯直接环氧化法 | 氯丙烯,H2O2 | 95 | 钛硅分子筛 | 0.00016 | 较低 | 0 | 2-3 | 0.57 | 0.062 | 3 |
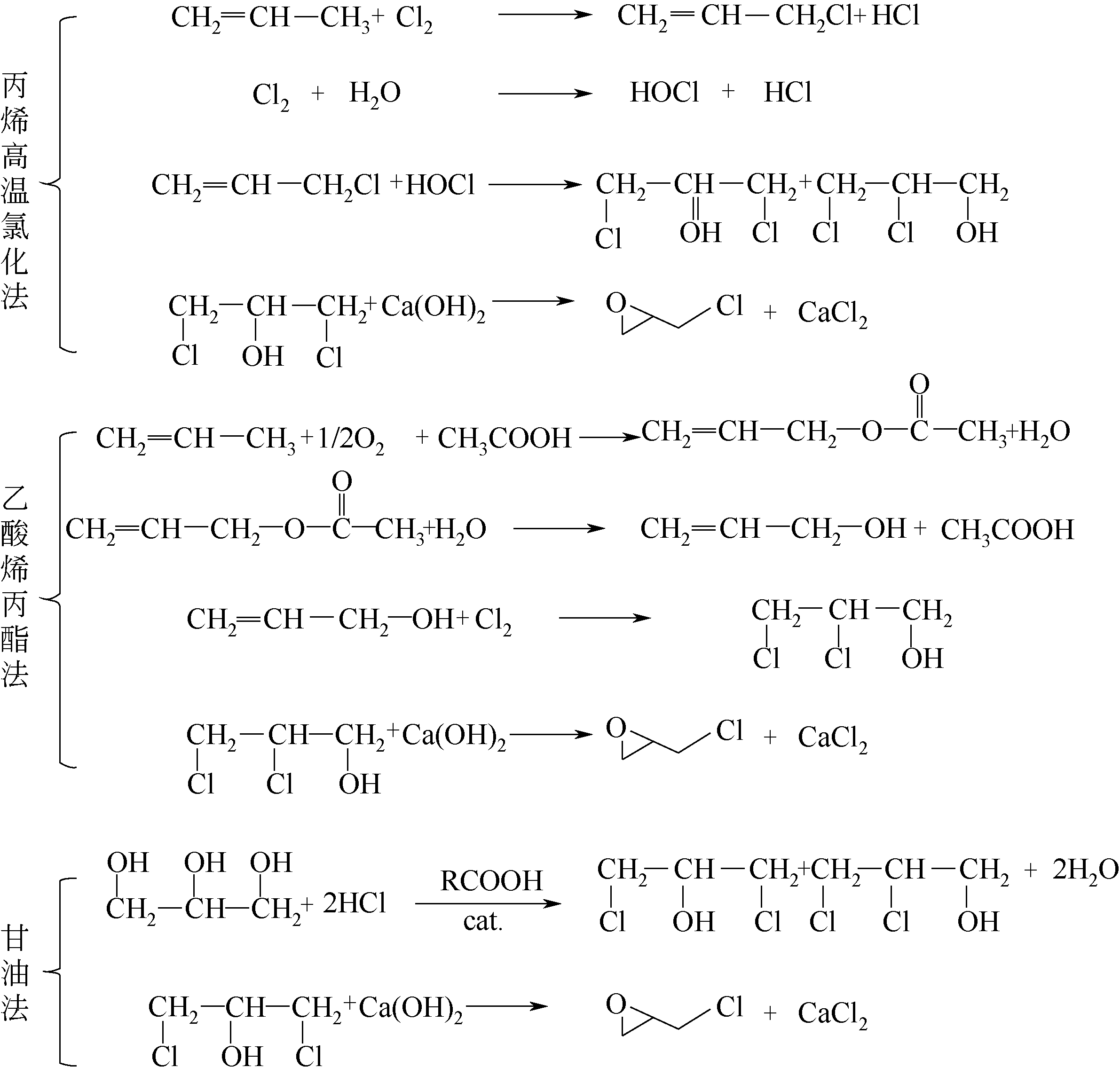
表6 二氯丙醇皂化法与氯丙烯H2O2直接环氧化法对比[70-71]
| 生产方法 | 原材料 | ECH 收率/% | 催化剂 | 剂耗 /t·(tECH)-1 | 能耗 | 废渣量 /t·(tECH)-1 | 废水 排放量 /t·(tECH)-1 | 预计 投资 /亿元 | 预计环保 投资 /亿元 | 环保费用 占总投资 比例/% |
|---|---|---|---|---|---|---|---|---|---|---|
| 丙烯高温氯化法 | 丙烯,Cl2,Ca(OH)2 | 70~75 | — | — | 高 | 0.8~1.2 | 40 | 1.67 | 0.7 | 20 |
| 甘油法 | 甘油,干燥HCl,Ca(OH)2 | 85~90 | ZnCl2 | 0.0005 | 较低 | 0.4~0.6 | 15~17 | 1.62 | 0.5 | 10 |
| 氯丙烯直接环氧化法 | 氯丙烯,H2O2 | 95 | 钛硅分子筛 | 0.00016 | 较低 | 0 | 2-3 | 0.57 | 0.062 | 3 |
| 1 | PUNNIYAMURTHY T, VELUSAMY S, IQBAL J. Recent advances in transition metal catalyzed oxidation of organic substrates with molecular oxygen[J]. Chemical Reviews, 2005, 105(6): 2329-2363. |
| 2 | SCHRANCK J, TLILI A, BELLER M. More sustainable formation of C-N and C-C bonds for the synthesis of N-heterocycles[J]. Angewandte Chemie: International Edition, 2013, 52(30): 7642-7644. |
| 3 | JAGADEESH R V, JUNGE H, BELLER M. Green synthesis of nitriles using non-noble metal oxides-based nanocatalysts[J]. Nature Communications, 2014, 5: 4123. |
| 4 | SHELDON R A, AREMDS I W C E, BRINK G T, et al. Green, catalytic oxidations of alcohols[J]. Accounts of Chemical Research, 2002, 35(9): 774-781. |
| 5 | DAVIS S E, IDE M S, DAVIS R J. Selective oxidation of alcohols and aldehydes over supported metal nanoparticles[J]. Green Chemistry, 2013, 15(1): 17-45. |
| 6 | SHELDON R A, DOWNING R S. Heterogeneous catalytic transformations for environmentally friendly production[J]. Applied Catalysis A: General, 1999, 189(2): 163-183. |
| 7 | ISUPOVA L A, IVANOVA Y A. Removal of nitrous oxide in nitric acid production[J]. Kinetics and Catalysis, 2019, 60(6): 744-760. |
| 8 | 高丽雅, 檀学军, 张东升, 等. 羟胺(盐)的合成及其应用研究进展[J]. 化工进展, 2012, 31(9): 2043-2048. |
| GAO Liya, TAN Xuejun, ZHANG Dongsheng, et al. Progress of synthesis and application of hydroxylamine(salts)[J]. Chemical Industry and Engineering Progress, 2012, 31(9): 2043-2048. | |
| 9 | CHEN J G, CROOKS R M, SEEFELDT L C, et al. Beyond fossil fuel-driven nitrogen transformations[J]. Science, 2018, 360(6391): eaar6611. |
| 10 | SEAYAD J, TILLACK A, HARTUNG C, et al. Base-catalyzed hydroamination of olefins: an environmentally friendly route to amines[J]. Advanced Synthesis and Catalysis, 2002, 344(8): 795-813. |
| 11 | ANASTAS P T, LANKEY R L. Life cycle assessment and green chemistry: the yin and yang of industrial ecology[J]. Green Chemistry, 2000, 2: 289-295. |
| 12 | Eco-profiles of plastics[R]. Brussels, Belgium: Association of Plastics Manufacturers in Europe (PlasticsEurope), 2012—2019. |
| 13 | NOYORI R, AOKI M, SATO K. Green oxidation with aqueous hydrogen peroxide[J]. Chemical Communications, 2003(16): 1977-1986. |
| 14 | PENG Ling, LIU Chan, LI Na, et al. Direct cyclohexanone oxime synthesis via oxidation-oximization of cyclohexane with ammonium acetate[J]. Chemical Communications, 2020, 56(9): 1436-1439. |
| 15 | GAO Guohua, TIAN Yanan, GONG Xiaoxiao, et al. Advances in the production technology of hydrogen peroxide[J]. Chinese Journal of Catalysis, 2020, 41(7): 1039-1047. |
| 16 | 王松林, 程义, 张晓昕, 等. 蒽醌法生产过氧化氢加氢催化剂的研究进展[J]. 化工进展, 2017, 36(11): 4057-4063. |
| WANG Songlin, CHENG Yi, ZHANG Xiaoxin, et al. Advances in hydrogenation catalyst for the production of hydrogen peroxide through the anthroquinone route[J]. Chemical Industry and Engineering Progress, 2017, 36(11): 4057-4063. | |
| 17 | CAMPOS-MARTIN J M, BLANCO-BRIEVA G, FIERRO J L. Hydrogen peroxide synthesis: an outlook beyond the anthraquinone process[J]. Angewandte Chemie International Edition, 2006, 45(42): 6962-6984. |
| 18 | YASUHIRO S, SHUNSUKE K, YUSUKE K, et al. Sunlight-driven hydrogen peroxide production from water and molecular oxygen by metal-free photocatalysts[J]. Angewandte Chemie International Edition, 2014, 53(49): 13454-13459. |
| 19 | 姚冬龄. 中国过氧化氢生产现状及展望[J]. 无机盐工业, 2013, 45(9): 1-4. |
| YAO Dongling. Current situation and outlook of hydrogen peroxide production in China[J]. Inorganic Chemicals Industry, 2013, 45(9): 1-4. | |
| 20 | 潘智勇, 高国华, 杨克勇, 等. 浆态床过氧化氢生产技术[J]. 中国科学: 化学, 2015, 45(5):541-546. |
| PAN Zhiyong, GAO Guohua, YANG Keyong, et al. H2O2 production technology with slurry reactor[J]. Scientia Sinica Chimica, 2015, 45(5):541-546. | |
| 21 | CHEN Qunlai. Development of an anthraquinone process for the production of hydrogen peroxide in a trickle bed reactor—From bench scale to industrial scale[J]. Chemical Engineering & Processing Process Intensification, 2008, 47(5): 787-792. |
| 22 | 刘航, 方向晨, 贾立明, 等. 蒽醌法生产H2O2工作液的改进[J]. 石油学报(石油加工), 2015, 31(1): 72-77. |
| LIU Hang, FANG Xiangchen, JIA Liming, et al. Improvement of working solution for H2O2 production by anthraquinone method[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2015, 31(1): 72-77. | |
| 23 | 贾雪婷, 杨益辰, 刘国柱, 等. 2-乙基和 2-戊基蒽醌在 TMB/DIBC 中溶解度的测定与关联[J]. 高校化学工程学报, 2014, 28(6): 1183-1189. |
| JIA Xueting, YANG Yichen, LIU Guozhu, et al. Measurement of the solubilities of 2-ethylanthraquinone and 2-amylanthraquinone in TMB/DIBC mixed solvents and their correlation with thermodynamic equations[J]. Journal of Chemical Engineering of Chinese Universities, 2014, 28(6): 1183-1189. | |
| 24 | 姜华, 马敬洪. 蒽醌法新型工作液加氢反应器特性研究[J]. 化工设计通讯, 2015, 41(1): 82-84. |
| JIANG Hua, MA Jinghong. Characteristic research on new-style working solution hydrogenation reactor in hydrogen peroxide plant by anthraquinone process[J]. Chemical Engineering Design Communications, 2015, 41(1): 82-84. | |
| 25 | 杨秀娜, 卿光宗, 付骐, 等. 蒽醌法双氧水生产装置的优化改造[J]. 无机盐工业, 2013, 45(11): 37, 49. |
| YANG Xiuna, QING Guangzong, FU Qi, et al. Optimization and reforming for hydrogen peroxide production unit by anthraquinone method[J]. Inorganic Chemicals Industry, 2013, 45(11): 37, 49. | |
| 26 | 王伟建, 潘智勇, 李文林, 等. 蒽醌法流化床与固定床的发展趋势[J]. 化工进展, 2016, 35(6): 1766-1773. |
| WANG Weijian, PAN Zhiyong, LI Wenlin, et al. Recent advances in development of the fluidized bed and fixed bed in the anthraquinone route[J]. Chemical Industry and Engineering Progress, 2016, 35(6): 1766-1773. | |
| 27 | LI Hongbo, ZHENG Bo, PAN Zhiyong, et al. Advances in the slurry reactor technology of the anthraquinone process for H2O2 production[J]. Frontiers of Chemical Science and Engineering, 2018, 12(1): 124-131. |
| 28 | WANG Li, ZHANG Yue, MA Qinging, et al. Hydrogenation of alkyl-anthraquinone over hydrophobically functionalized Pd/SBA-15 catalysts[J]. RSC Advances, 2019, 9(59): 34581-34588. |
| 29 | 张月琴, 高国华, 杨克勇, 等. 气相色谱-质谱法定性分析蒽醌系列工作液的组成[J]. 色谱, 2019, 37(4): 432-437. |
| ZHANG Yueqin, GAO Guohua, YANG Keyong, et al. Qualitative analysis of compositions of anthraquinone series working solution by gas chromatography-mass spectrometry[J]. Chinese Journal of Chromatography, 2019, 37(4): 432-437. | |
| 30 | 周集义. 过氧化氢生产装置氧化尾气处理研究进展[C]//中国无机盐协会过氧化物分会2013年会论文集. 洛阳: 中国无机盐协会过氧化物分会, 2013: 33-38. |
| ZHOU Jiyi. Research progress of oxidation tail gas treatment in hydrogen peroxide production plant[C]//The 2013 Annual Meeting proceedings of Peroxide Branch of China Inorganic Salt Association. Luoyang: Peroxide Branch of China Inorganic Salt Association, 2013: 33-38. | |
| 31 | SEBASTIAN J, ZHENG Mingyuan, JIANG Yu, et al. One-pot conversion of lysine to caprolactam over Ir/H-Beta catalysts[J]. Green Chemistry, 2019, 21(9):2462-2468. |
| 32 | 祁嘉昕. 环己酮生产工艺技术方案的对比分析[J]. 煤炭与化工, 2018, 41(4): 140-143. |
| QI Jiaxin. Comparison and analysis of technology scheme for cyclohexanone production[J]. Coal and Chemical Industry, 2018, 41(4): 140-143. | |
| 33 | 李惠友, 傅送保. 环己烷氧化废碱液化学处理工艺概述[J]. 精细石油化工, 2000(5): 5-8. |
| LI Huiyou, FU Songbao. The introduction of chemical treatment technology for waste salt solution of cyclohexane oxidation[J]. Speciality Petrochemicals, 2000(5): 5-8. | |
| 34 | SILVA A R, MOURÃO T, ROCHA J. Oxidation of cyclohexane by transition-metal complexes with biomimetic ligands[J]. Catalysis Today, 2013, 203(30): 81-86. |
| 35 | 佘远斌, 邓金辉, 张龙, 等. 氧气催化氧化环己烷[J]. 化学进展, 2018, 30(1): 124-136. |
| SHE Yuanbin, DENG Jinhui, ZHANG Long, et al. Catalytic oxidation of cyclohexane by O2 as an oxidant[J] Progress in Chemistry, 2018, 30(1): 124-136. | |
| 36 | STEYER F, SUNDMACHER K. VLE and LLE data for the system cyclohexane+cyclohexene+water+cyclohexanol[J]. Journal of Chemical & Engineering Data, 2004, 49(6): 1675-1681. |
| 37 | 邢亚峰, 赵培朝. 己内酰胺原料环己酮生产工艺的技术对比[J]. 化学工程与装备, 2015(3): 27-30. |
| XING Yafeng, ZHAO Peichao. Production process technical comparison of cyclohexanone, which is a raw material of caprolactam production[J]. Chemical Engineering & Equipment, 2015(3): 27-30. | |
| 38 | STEYER F, SUNDMACHER K. Cyclohexanol production via esterification of cyclohexene with formic acid and subsequent hydration of the ester—Reaction kinetics[J]. Industrial & Engineering Chemistry Research, 2007, 46(4): 1099-1104. |
| 39 | RAWESH K, SNEHA S, PRANGYA P D, et al. An overview of caprolactam synthesis[J]. Catalysis Reviews, 2019, 61(4):516-594. |
| 40 | MARCO T, GIOVANNI P, BRUNO N. Preparation of porous crystalline synthetic material comprised of silicon and titanium oxides: US4410501[P]. 1982-06-29. |
| 41 | 朱斌, 林民, 舒兴田, 等. 表面富钛TS-1分子筛的表征与评价[J]. 石油学报(石油加工), 2009, 25(S2): 112-115. |
| ZHU Bin, LIN Min, SHU Xingtian, et al. Characterization and evaluation of TS-1 with enriched Ti species on its surface[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2009, 25(S2): 112-115. | |
| 42 | 孙斌. 环己酮氨肟化反应过程中钛硅分子筛的溶解流失研究[J]. 石油炼制与化工, 2005, 36(11): 54-58. |
| SUN Bin. Study on dissolution erosion of titanium silicalite zeolite in cyclohexanone ammoximation[J]. Petroleum Processing and Petrochemicals, 2005, 36(11): 54-58. | |
| 43 | WU Wei, SUN Bin, LI Yongxiang, et al. Process for ammoximation of carbonyl compounds: US7408080[P]. 2003-05-30. |
| 44 | SUN Bin, WU Wei, WANG Enquan, et al. Process for regenerating titanium-containing catalysts: US7384882[P]. 2003-05-30. |
| 45 | 孙斌, 孟祥堃, 宗保宁. 己内酰胺绿色生产技术[J]. 化学通报, 2011, 74(11):999-1003. |
| SUN Bin, MENG Xiangkun, ZONG Baoning. Green caprolactam production technology[J]. Chemistry, 2011, 74(11):999-1003. | |
| 46 | ZONG Baoning, SUN Bin, CHENG Shibiao, et al. Green production technology of the monomer of nylon-6: caprolactam[J]. Engineering, 2017, 3(3):379-384. |
| 47 | 宗保宁, 潘智勇. 己内酰胺绿色生产技术的化学和工程基础[M]. 北京: 科学出版社, 2020: 85-94. |
| ZONG Baoning, PAN Zhiyong. The chemical and engineering basis of caprolactam green production technology[M]. Beijing: Science Press, 2020: 85-94. | |
| 48 | BELLUSSI G, PEREGO C. Industrial catalytic aspects of the synthesis of monomers for nylon production[J]. CATTECH, 2000, 4(1):4-16. |
| 49 | 宗保宁. “973”计划推动我国石油化学工业可持续发展[J]. 石油学报(石油加工), 2015, 31(2): 259-264. |
| ZONG Baoning. “973” Plans promoting the sustainable development of China petrochemical industry[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2015, 31(2): 259-264. | |
| 50 | 隆众资讯. 平衡格局下中国环氧丙烷市场现状及展望[EB/OL]. [2019-07-26].. |
| Information Longzhong. China's propylene oxide market status and prospect under balanced pattern[EB/OL]. [2019-07-26]. . | |
| 51 | NIJHUIS T A, HUIZINGA B J, MAKKEE M, et al. Direct epoxidation of propene using gold dispersed on TS-1 and other titanium-containing supports[J]. Industrial & Engineering Chemistry Research, 1999, 38(3): 884-891. |
| 52 | 刘波, 张晓莉, 赵丽, 等. 环氧丙烷生产工艺的发展现状[J]. 当代化工, 2016, 45(2):336-338, 341. |
| LIU Bo, ZHANG Xiaoli, ZHAO Li, et al. Development status of propylene oxide production process[J]. Contemporary Chemical Industry, 2016, 45(2): 336-338, 341. | |
| 53 | 钱伯章. 环氧丙烷的生产技术进展[J]. 化学推进剂与高分子材料, 2006, 4(2): 14-18. |
| QIAN Bozhang. Progress in production technology of propylene oxide[J]. Chemical Propellants & Polymeric Materials, 2006, 4(2):14-18. | |
| 54 | 肖峻. 国内环氧丙烷市场现状及发展趋势[J]. 石油化工技术与经济, 2015, 31(6): 1-5. |
| XIAO Jun. Current status and development trend of domestic propylene oxide market[J]. Technology & Economics in Petrochemicals, 2015, 31(6): 1-5. | |
| 55 | 于剑昆, 郭菊荣, 赵晓东, 等. 国内外HPPO工业化技术进展[J]. 化学推进剂与高分子材料, 2017, 15(1): 20-34. |
| YU Jiankun, GUO Jurong, ZHAO Xiaodong, et al. Progress of industrialized HPPO techniques at home and abroad[J]. Chemical Propellants & Polymeric Materials, 2017, 15(1): 20-34. | |
| 56 | RUSSO V, TESSER R, SANTACESARIA E, et al. Chemical and technical aspects of propene oxide production via hydrogen peroxide (HPPO process)[J]. Industrial & Engineering Chemistry Research, 2013, 52(3):1168-1178. |
| 57 | 朱斌. HPPO工艺催化剂活性组分研究[J]. 石油学报(石油加工), 2013, 29(2): 223-227. |
| ZHU Bin. Study on catalytic material used in HPPO process[J]. Acta Petrolei Sinica (Petroleum Processing Section), 2013, 29(2): 223-227. | |
| 58 | 林民, 李华, 王伟, 等. 1.0kt/a丙烯与双氧水环氧化制备环氧丙烷的中试研究[J]. 石油炼制与化工, 2013, 44(3): 1-5. |
| LIN Min, LI Hua, WANG Wei, et al. The preparation of propylene oxide by propylene epoxidation with hydrogen peroxide in 1.0 kt/a pilot plant[J]. Petroleum Processing and Petrochemicals, 2013, 44(3): 1-5. | |
| 59 | 郑结斌. 中国环氧氯丙烷行业现状及发展分析[J]. 中国氯碱, 2018(5): 24-25. |
| ZHENG Jiebin. Current situation and development analysis of epichlorohydrin industry in China[J]. China Chlor-Alkali, 2018(5): 24-25. | |
| 60 | 环氧氯丙烷2018年产能大幅增长[J]. 中国氯碱, 2018(5): 47. |
| Epichlorohydrin production capacity increased significantly in2018[J]. China Chlor-Alkali, 2018(5): 47. | |
| 61 | 朱桂生, 贾星原, 王爱丽, 等. 二氯丙醇粗产物皂化制备环氧氯丙烷研究[J]. 辽宁化工, 2018, 47(10): 983-985. |
| ZHU Guisheng, JIA Xingyuan, WANG Aili, et al. Research on preparation of epichlorohydrin from crude dichloropropanol by saponification[J]. Liaoning Chemical Industry, 2018, 47(10): 983-985. | |
| 62 | 刘声, 李军, 安庆大, 等. 烯丙基氯氧化制环氧氯丙烷的研究进展[J]. 化学通报, 2010, 73(7): 622-627. |
| LIU Sheng, LI Jun, AN Qingda, et al. Research progress of epoxidation of allyl chloride to epichlorohydrin[J]. Chemistry Bulletin, 2010, 73(7): 622-627. | |
| 63 | PANDEY R K, KUMAR R. Eco-friendly synthesis of epichlorohydrin catalyzed by titanium silicate (TS-1) molecular sieve and hydrogen peroxide[J]. Catalysis Communications, 2007, 8(3): 379-382. |
| 64 | XI Zuwei, ZHOU Ning, SUN Yu, et al. Reaction-controlled phase-transfer catalysis for propylene epoxidation to propylene oxide[J]. Science, 2001, 292(5519):1139-1141. |
| 65 | MARIO G C, UGO R. Process for the epoxidation of olefinic compounds and catalysis used therein: US4824976[P]. 1987-01-20. |
| 66 | WANG Linging, LIU Yueming, XIE Wei, et al. Highly efficient and selective production of epichlorohydrin through epoxidation of allyl chloride with hydrogen peroxide over Ti-MWW catalysts[J]. Journal of Catalysis, 2007, 246(1): 205-214. |
| 67 | CLERICI M G, BELLUSSI G, ROMANO U. Synthesis of propylene oxide from propylene and hydrogen peroxide catalyzed by titanium silicalite[J]. Journal of Catalysis, 1991, 129(1): 159-167. |
| 68 | KIM W, YUN C, KIM Y, et al. Modeling of a tubular reactor producing epichlorohydrin with consideration of reaction kinetics and deactivation of titanium silicate-1 catalyst[J]. Industrial Engineering Chemistry Research, 2011, 50(3): 1187-1195. |
| 69 | GAO Huanxin, LU Gongxuan, SUO Jishuan, et al. Epoxidation of allyl chloride with hydrogen peroxide catalyzed by titanium silicalite 1[J]. Applied Catalysis A:General, 1996, 138(1): 27-38. |
| 70 | 张生军, 郑化安, 朱敏莉. 环氧氯丙烷的技术进展和技术经济分析[J]. 应用化工, 2014(S1): 32-35. |
| ZHANG Shengjun, ZHENG Hua’an, ZHU Minli. Technology progress and tech-economic properties on production of epoxy chloropropane[J]. Applied Chemical Industry, 2014(S1): 32-35. | |
| 71 | 郭崇. 甘油法与氯丙烯直接环氧化法制环氧氯丙烷工艺的比较[J]. 氯碱工业, 2014, 50(10): 27-29. |
| GUO Chong. Comparison between glycerol process and direct epoxidation process of allyl chloride for epichlorohydrin synthesis[J]. Chlor-Alkali Industry, 2014, 50(10): 27-29. | |
| 72 | 张宇, 熊勇, 刘易, 等. TS-1催化氯丙烯直接环氧化反应的失活研究[J]. 石油炼制与化工, 2006, 37(7): 21-24. |
| ZHANG Yu, XIONG Yong, LIU Yi, et al. Study on the deactivation of TS 1 molecular sieve in catalyzing allyl chloride direct epoxidation[J]. Petroleum Processing and Petrochemicals, 2006, 37(7): 21-24. | |
| 73 | 屈叶青. 环氧氯丙烷的生产技术及国内外市场分析[J]. 石油化工技术与经济, 2015, 31(4): 26-30. |
| QU Yeqing. Production process of epoxy chloropropaneand analysis of its domestic and overseas market[J]. Technology & Economics in Petrochemicals, 2015, 31(4): 26-30. |
| [1] | 杨建平. 降低HPPO装置反应系统原料消耗的PSE[J]. 化工进展, 2023, 42(S1): 21-32. |
| [2] | 王福安. 300kt/a环氧丙烷工艺反应器降耗减排分析[J]. 化工进展, 2023, 42(S1): 213-218. |
| [3] | 张鹏, 潘原. 单原子催化剂在电催化氧还原直接合成过氧化氢中的研究进展[J]. 化工进展, 2023, 42(6): 2944-2953. |
| [4] | 张广宇, 赵健, 孙峰, 姜杰, 孙冰, 徐伟. CO2催化转化制碳酸丙烯酯研究进展:催化剂设计、性能与反应机理[J]. 化工进展, 2022, 41(S1): 177-189. |
| [5] | 廖兵, 胥雯, 叶秋月. 活化过碳酸盐及过氧碳酸氢盐在水处理领域中的研究进展[J]. 化工进展, 2022, 41(6): 3235-3248. |
| [6] | 宋英琪, 沈一丁, 刘勇兵, 党园园. 低有机氯含量PAE基纸张湿强剂的制备及性能评价[J]. 化工进展, 2022, 41(10): 5558-5566. |
| [7] | 肖博仁, 杨金星, 刘海鹏, 齐丽红, 左国民. 过氧化氢催化活化体系及其在危化品消毒中的应用[J]. 化工进展, 2021, 40(S1): 426-433. |
| [8] | 梁海瑞, 王涖, 刘国柱. 氢氧直接合成过氧化氢用催化剂的研究进展[J]. 化工进展, 2021, 40(4): 2060-2069. |
| [9] | 于鹏达, 张晓娴, 张金峰, 谈继淮, 王芳, 王芳, 付博, 朱新宝. 废弃芳纶粉末环氧化改性及其固化物性能[J]. 化工进展, 2021, 40(10): 5692-5698. |
| [10] | 马园园,寇伟,丁国生,徐联宾. 石墨烯负载纳米银的制备及其对H2O2的电化学检测[J]. 化工进展, 2019, 38(9): 4191-4196. |
| [11] | 李书香, 汪宁, 李娅, 付博, 朱新宝. 丙氧基化双酚A缩水甘油醚的合成与固化性能[J]. 化工进展, 2019, 38(06): 2961-2967. |
| [12] | 廖阳群, 张跃, 严生虎, 刘建武, 马晓明, 辜顺林, 沈介发, 陈代祥. 直接氧化法制备2,5-二氯苯酚[J]. 化工进展, 2019, 38(04): 1978-1983. |
| [13] | 杨瀚森, 李志会, 王晓曼, 徐元媛, 薛伟, 张东升, 赵新强, 王延吉. 离子液体在环己酮肟Beckmann重排反应中的研究进展[J]. 化工进展, 2018, 37(11): 4337-4342. |
| [14] | 徐言慧, 尹显洪, 邱江源, 黄在银, 谭春萍, 胡玉平. 多孔材料Cu3(BTB)2和H2O2处理偶氮染料废水及机理[J]. 化工进展, 2017, 36(12): 4700-4707. |
| [15] | 侯影飞, 李力军, 蒋驰, 郭宁, 牛青山. 活性炭负载磷钨酸催化剂的制备及其催化氧化脱硫性能[J]. 化工进展, 2017, 36(11): 4072-4079. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||