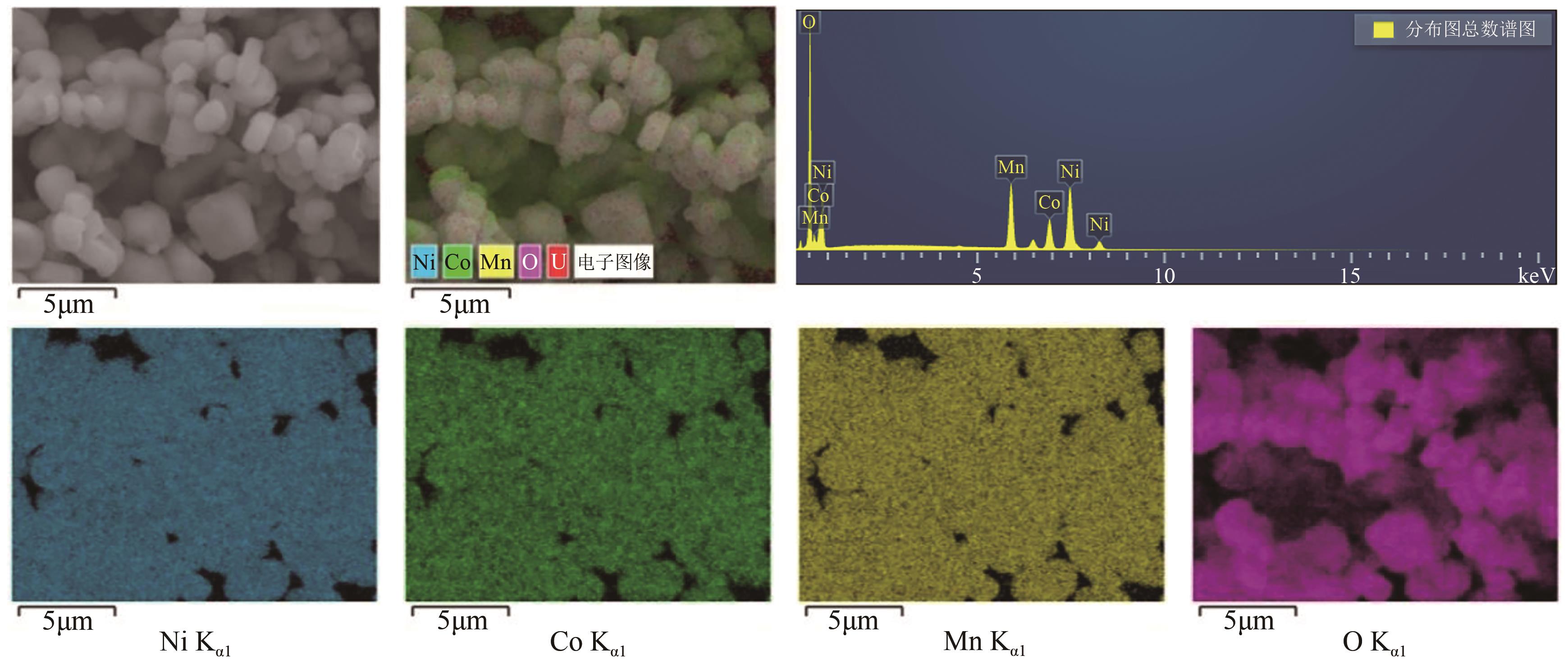
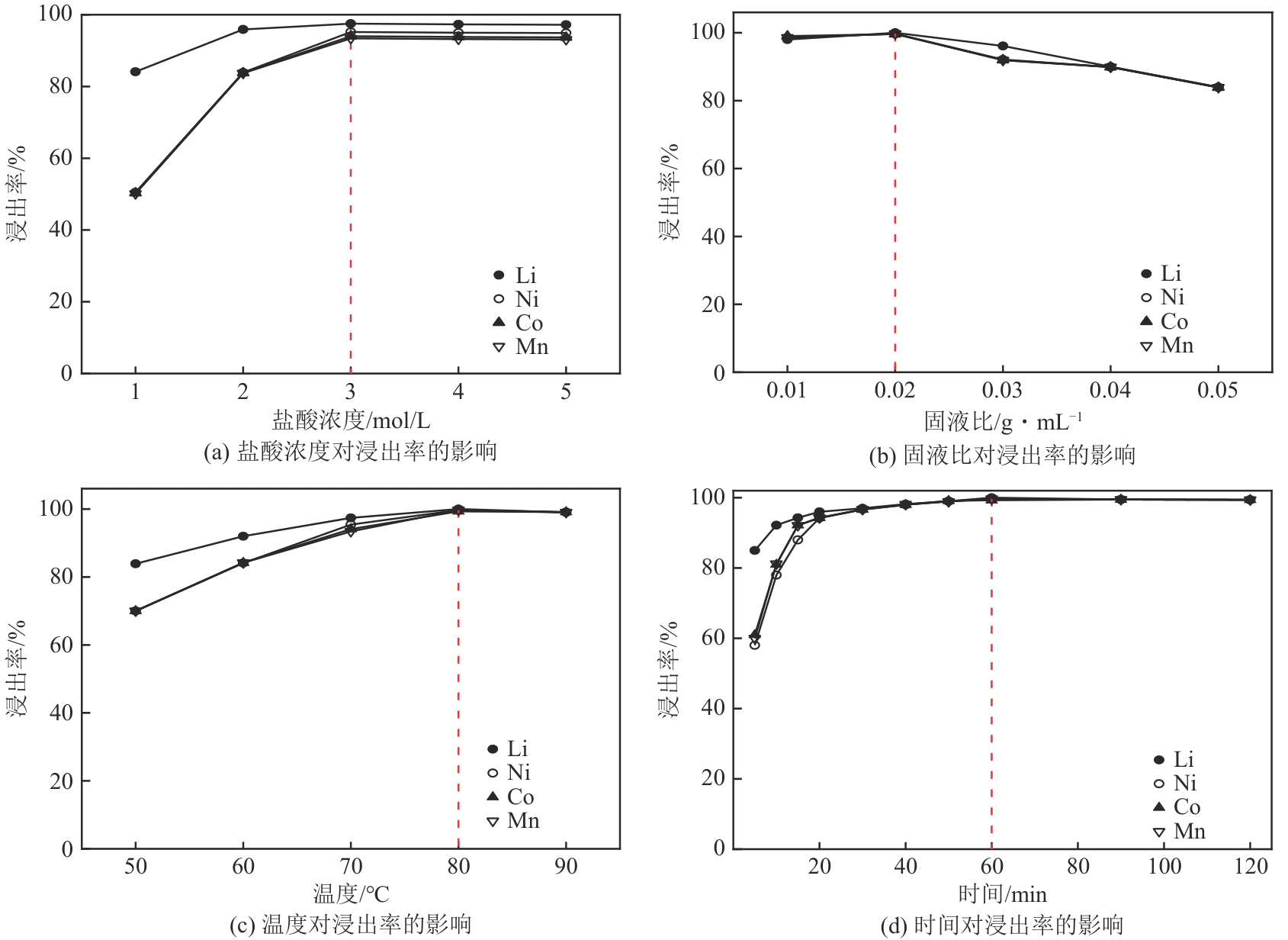
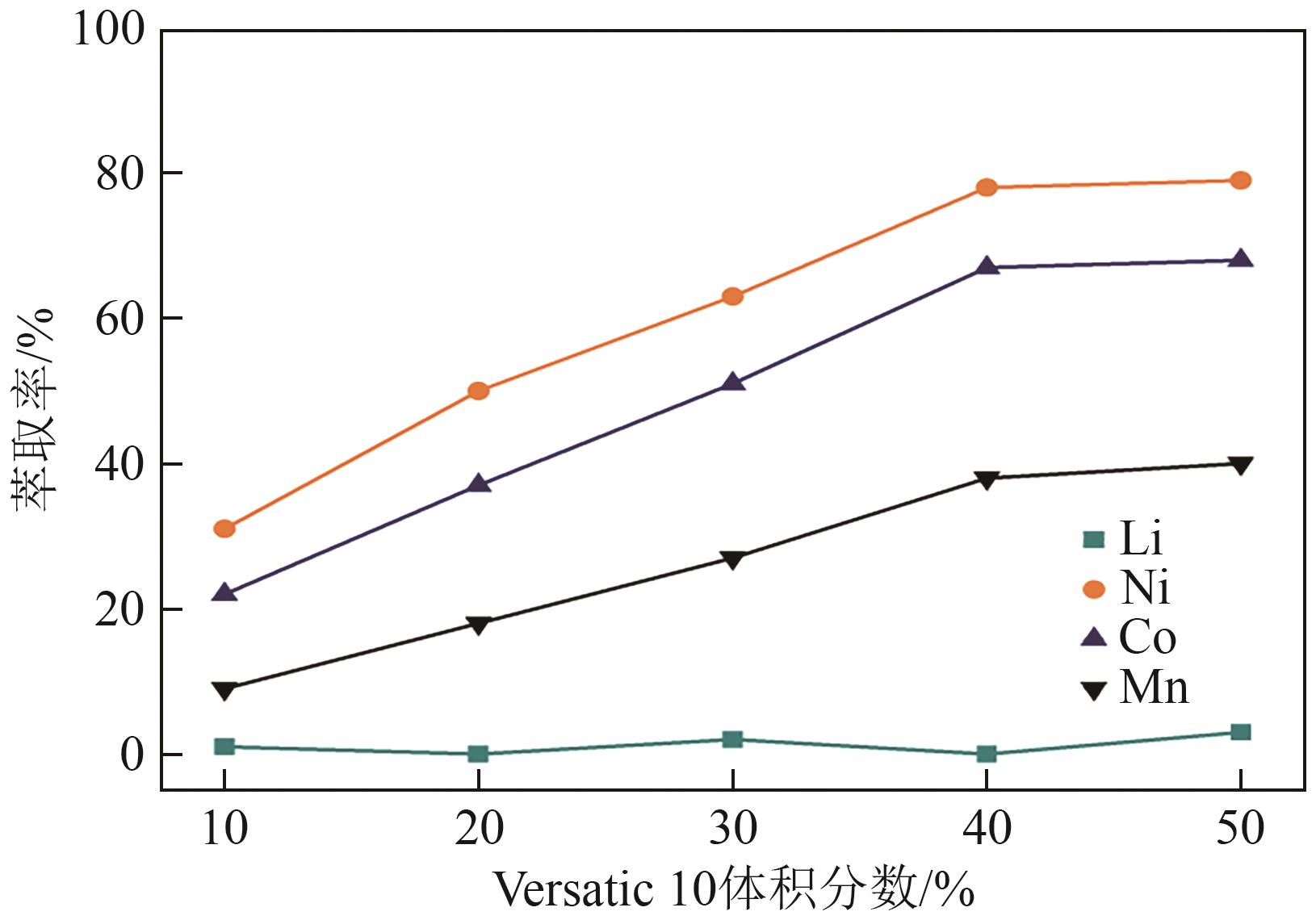
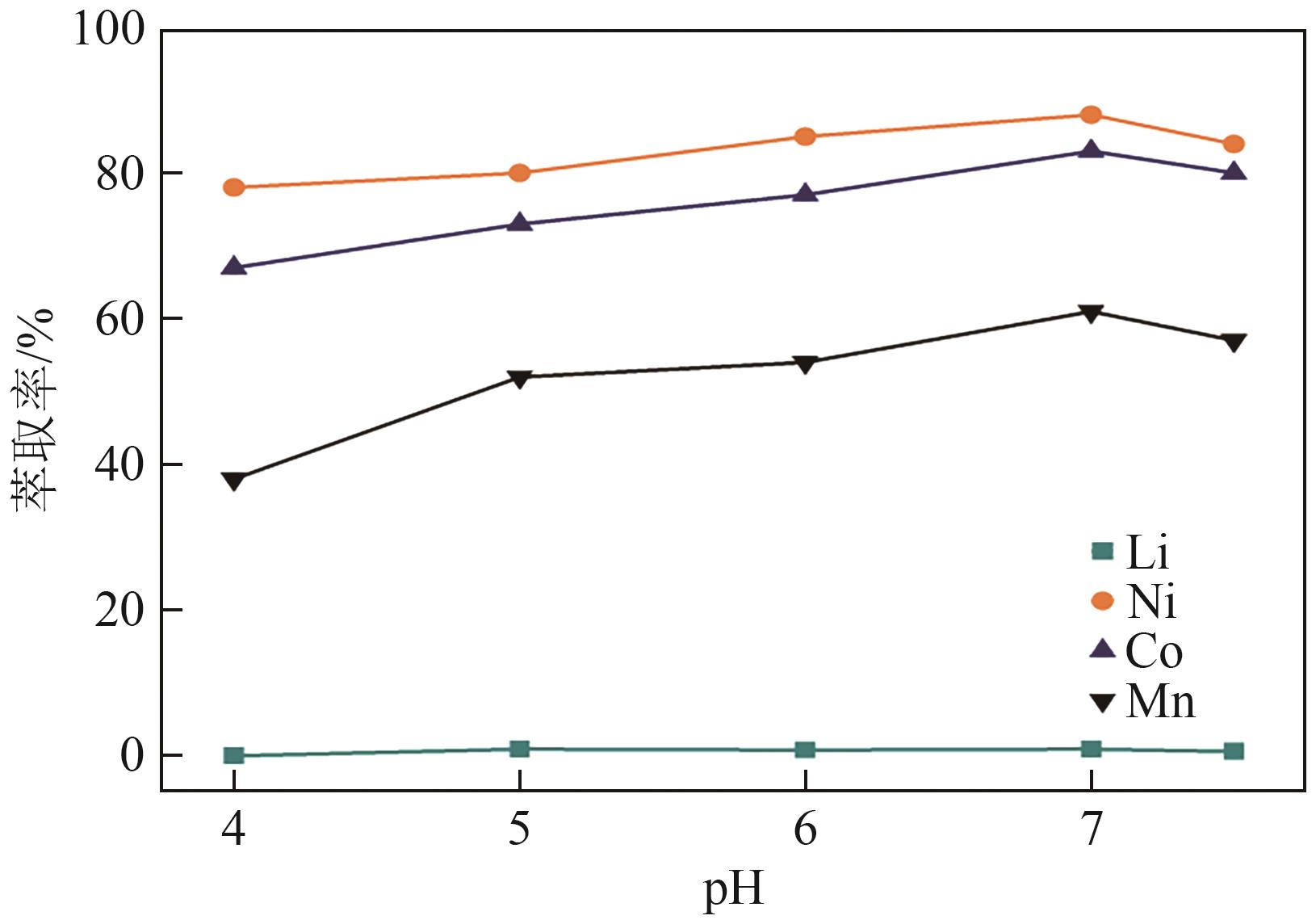
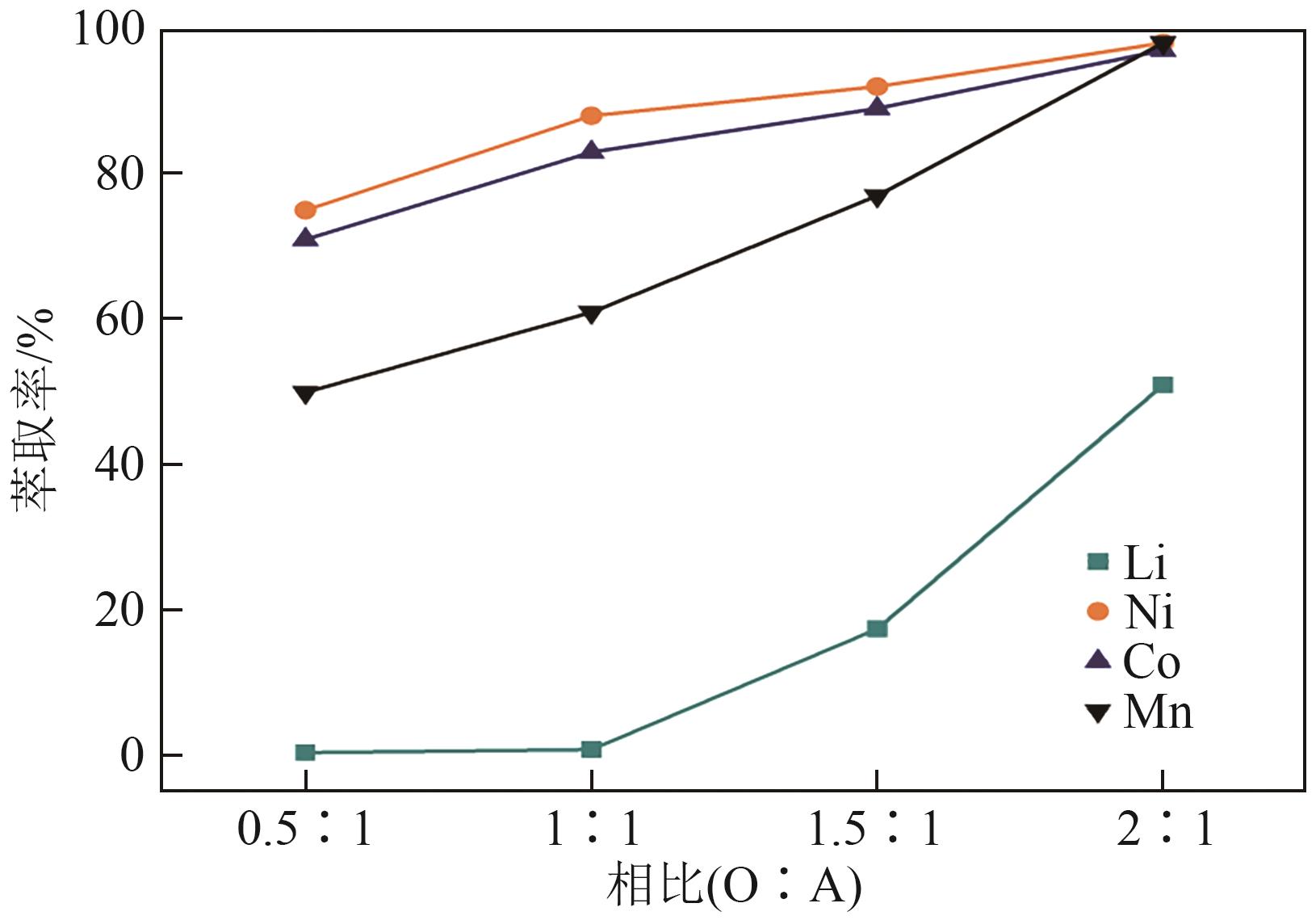
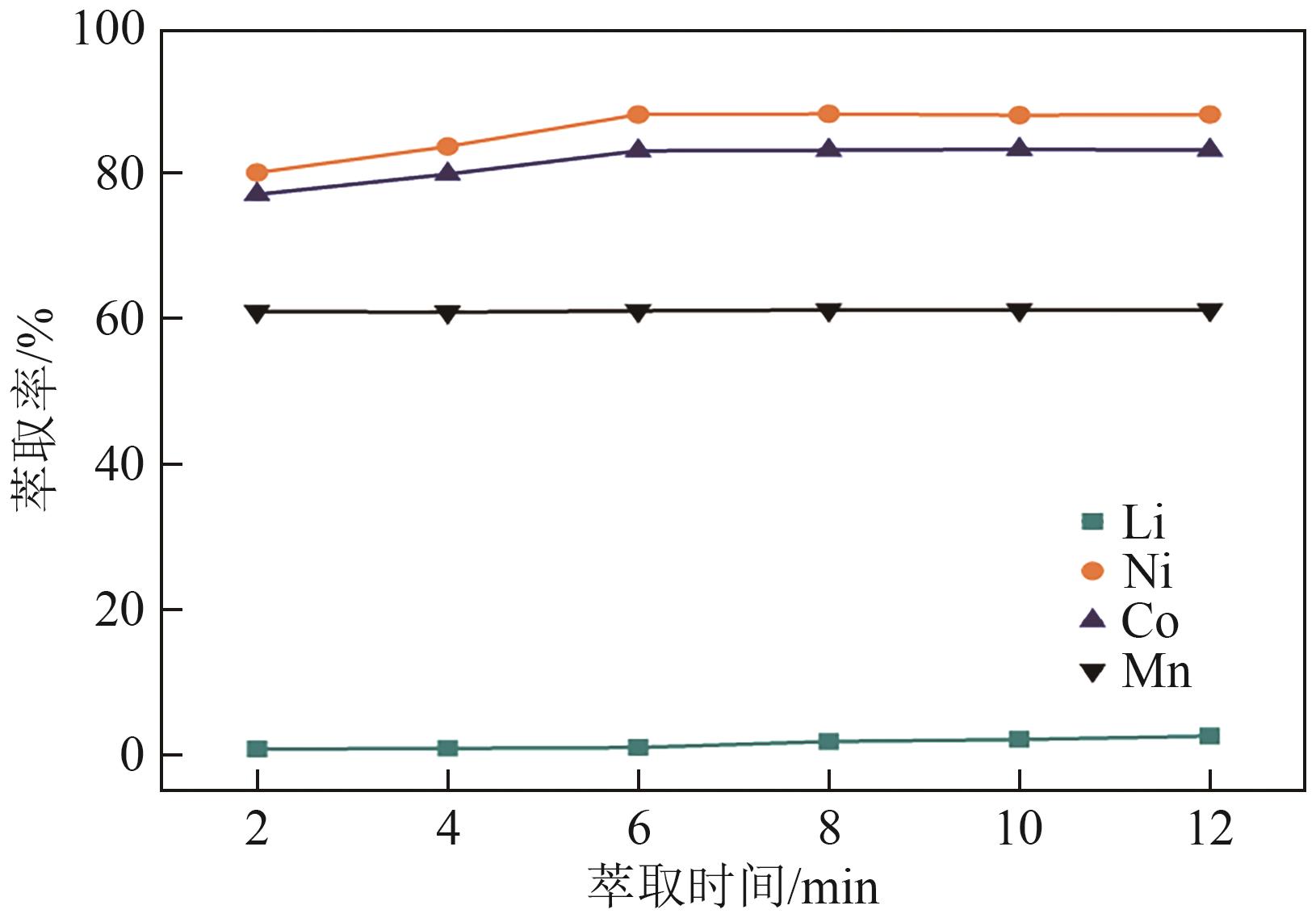
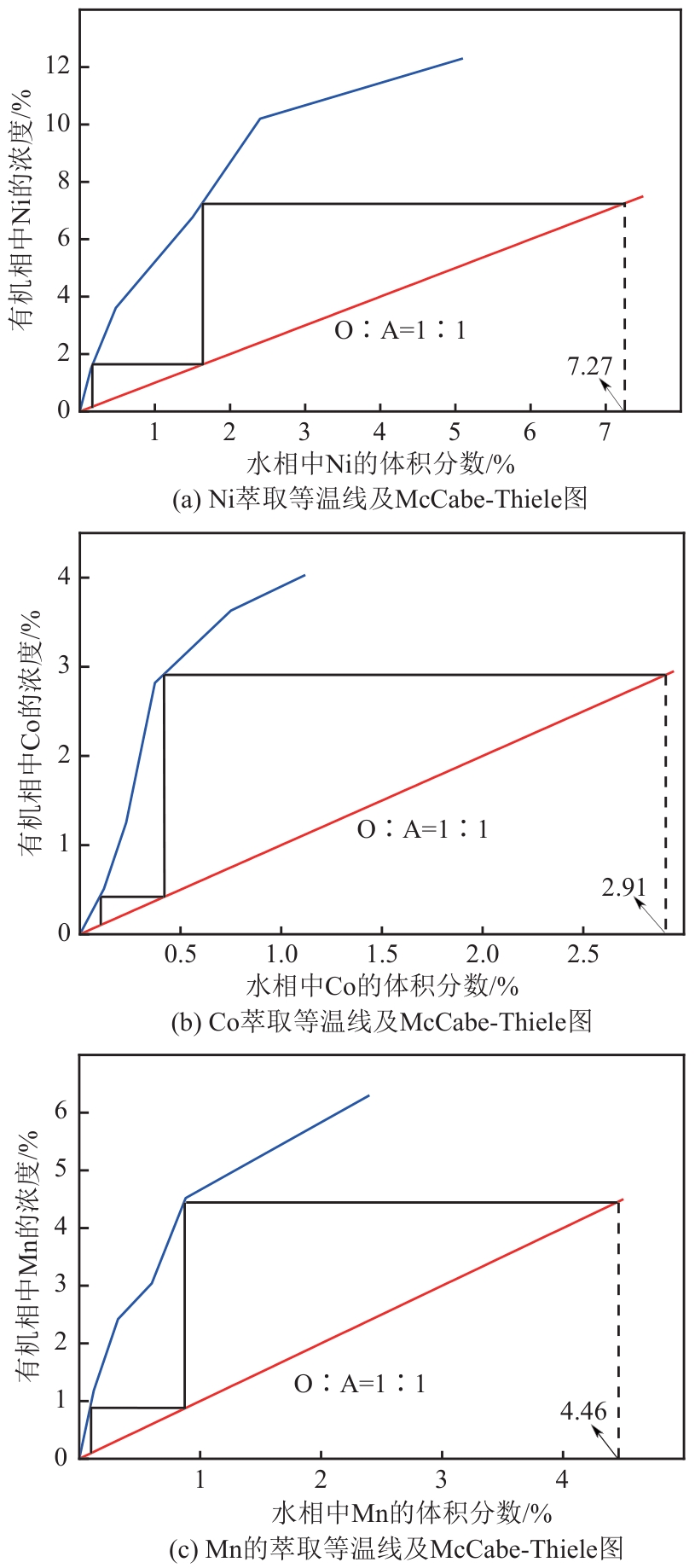
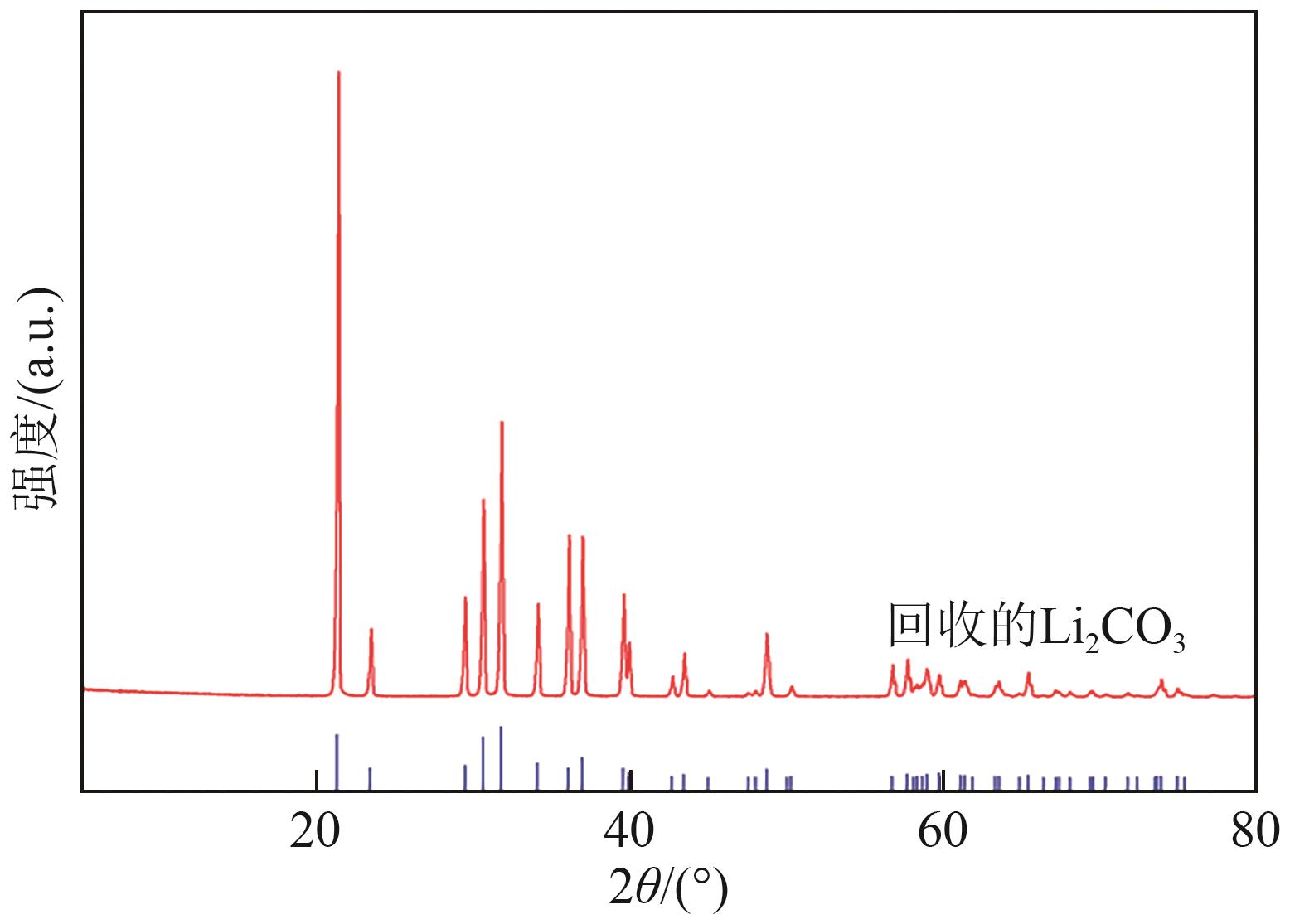
| [1] |
KIM Taehoon, SONG Wentao, Dae-Yong SON, et al. Lithium-ion batteries: Outlook on present, future, and hybridized technologies[J]. Journal of Materials Chemistry A, 2019, 7(7): 2942-2964.
|
| [2] |
ZHANG Ye, HU Yuehua, SUN Ning, et al. A novel precipitant for separating lithium from magnesium in high Mg/Li ratio brine[J]. Hydrometallurgy, 2019, 187: 125-133.
|
| [3] |
DUAN Xiaowei, ZHU Wenkun, RUAN Zhongkui, et al. Recycling of lithium batteries—A review[J]. Energies, 2022, 15(5): 1611.
|
| [4] |
MESHRAM Pratima, PANDEY B D, MANKHAND T R. Extraction of lithium from primary and secondary sources by pre-treatment, leaching and separation: A comprehensive review[J]. Hydrometallurgy, 2014, 150: 192-208.
|
| [5] |
Jelena SENĆANSKI, Danica BAJUK-BOGDANOVIĆ, Divna MAJSTOROVIĆ, et al. The synthesis of Li(CoMnNi)O2 cathode material from spent-Li ion batteries and the proof of its functionality in aqueous lithium and sodium electrolytic solutions[J]. Journal of Power Sources, 2017, 342: 690-703.
|
| [6] |
YANG Yue, SONG Shaole, LEI Shuya, et al. A process for combination of recycling lithium and regenerating graphite from spent lithium-ion battery[J]. Waste Management, 2019, 85: 529-537.
|
| [7] |
ZHANG Ye, HU Yuehua, SUN Ning, et al. Systematic review of feldspar beneficiation and its comprehensive application[J]. Minerals Engineering, 2018, 128: 141-152.
|
| [8] |
ZENG Xianlai, LI Jinhui. Innovative application of ionic liquid to separate Al and cathode materials from spent high-power lithium-ion batteries[J]. Journal of Hazardous Materials, 2014, 271: 50-56.
|
| [9] |
LIU Tianchi, CHEN Ji, SHEN Xing, et al. Regulating and regenerating the valuable metals from the cathode materials in lithium-ion batteries by nickel-cobalt-manganese co-extraction[J]. Separation and Purification Technology, 2021, 259: 118088.
|
| [10] |
Lijuan MEN, LIN Huaping, ZHU Likai, et al. Strategy for efficient recovery of NCM materials by the reagent-free method: Green recovery of lithium and high-value byproducts through capacitive deionization reverse applications[J]. ACS Sustainable Chemistry & Engineering, 2024, 12(23): 8780-8791.
|
| [11] |
NEGUSE Samuel Meles, YOON Songhak, Hyunjung LIM, et al. The pitfalls of deep eutectic solvents in the recycling of lithium-ion batteries[J]. Energy Technology, 2024, 12(4): 2301213.
|
| [12] |
YAN Qibin, DING Anting, LI Ming, et al. Green leaching of lithium-ion battery cathodes by ascorbic acid modified guanidine-based deep eutectic solvents[J]. Energy & Fuels, 2023, 37(2): 1216-1224.
|
| [13] |
ALAM Md Mofasserul, HUANG Haijian, YANG Zeheng, et al. Innovative green intense tetra-eutectic solvent (ITES) for recovery via occlusion co-precipitation at room temperature[J]. Sustainable Materials and Technologies, 2024, 40: e00971.
|
| [14] |
CARENA Eleonora, FERRARA Chiara. Exploiting deep eutectic solvents in LiFePO4 battery recycling: From cathode leaching to resynthesis[J]. ECS Meeting Abstracts, 2024(53): 2768.
|
| [15] |
王占昊, 丁威, 包申旭, 等. 采用Cyanex 301从废旧三元锂电浸出液中萃取分离镍和钴[J]. 中国有色金属学报,2024, 34(9): 3103-3113.
|
|
WANG Zhanhao, DING Wei, BAO Shenxu, et al. Extraction and separation of nickel and cobalt from spent ternary lithium batteries leachate using Cyanex 301[J]. The Chinese Journal of Nonferrous Metals, 2024, 34(9): 3103-3113.
|
| [16] |
GRANATA G, PAGNANELLI F, MOSCARDINI E, et al. Simultaneous recycling of nickel metal hydride, lithium ion and primary lithium batteries: Accomplishment of European guidelines by optimizing mechanical pre-treatment and solvent extraction operations[J]. Journal of Power Sources, 2012, 212: 205-211.
|
| [17] |
GAO Wenhao, NIE Chunchen, LI Lin, et al. Sustainable and efficient deep eutectic solvents in recycling of spent lithium-ion batteries: Recent advances and perspectives[J]. Journal of Cleaner Production, 2024, 464: 142735.
|
| [18] |
LIU Shuaiwei, HE Zhenjiang, KNAPP Michael. Sustainable electrochemical recycling of spent lithium-ion batteries: Selective extraction of lithium and direct regeneration of de-lithiated material[J]. ECS Meeting Abstracts, 2024, (55): 2957.
|
| [19] |
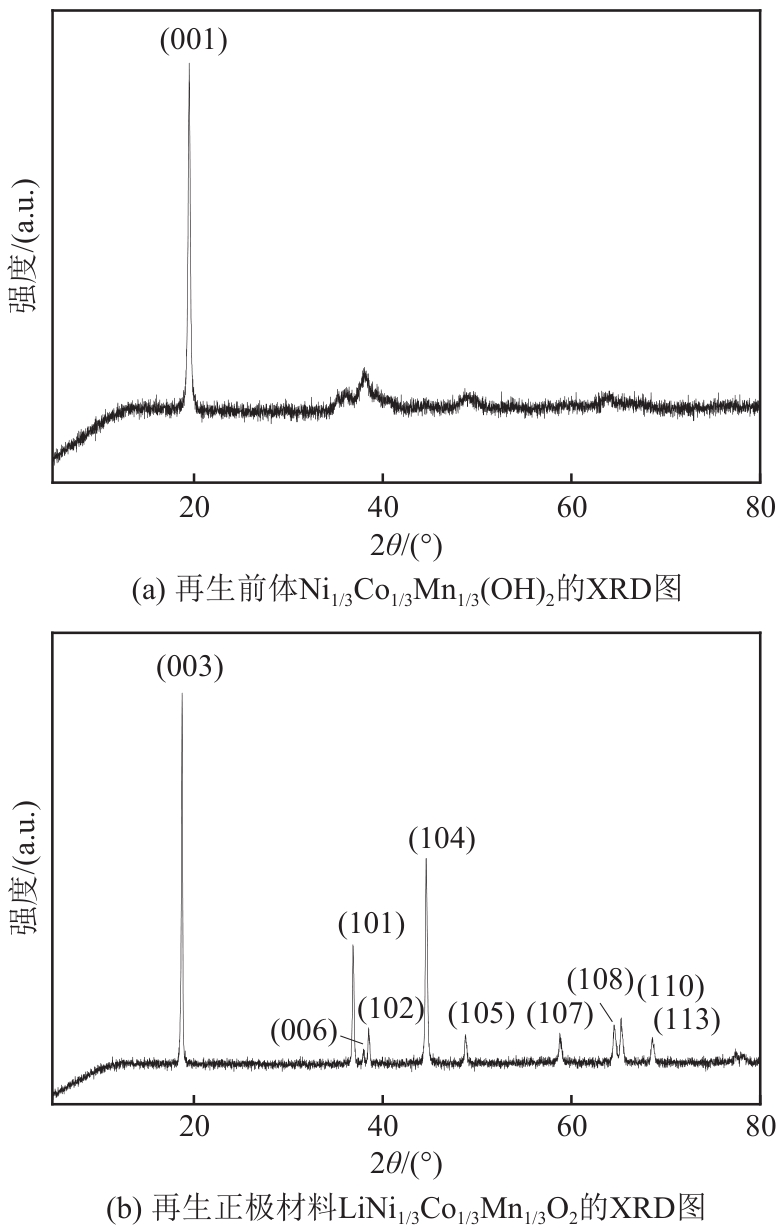
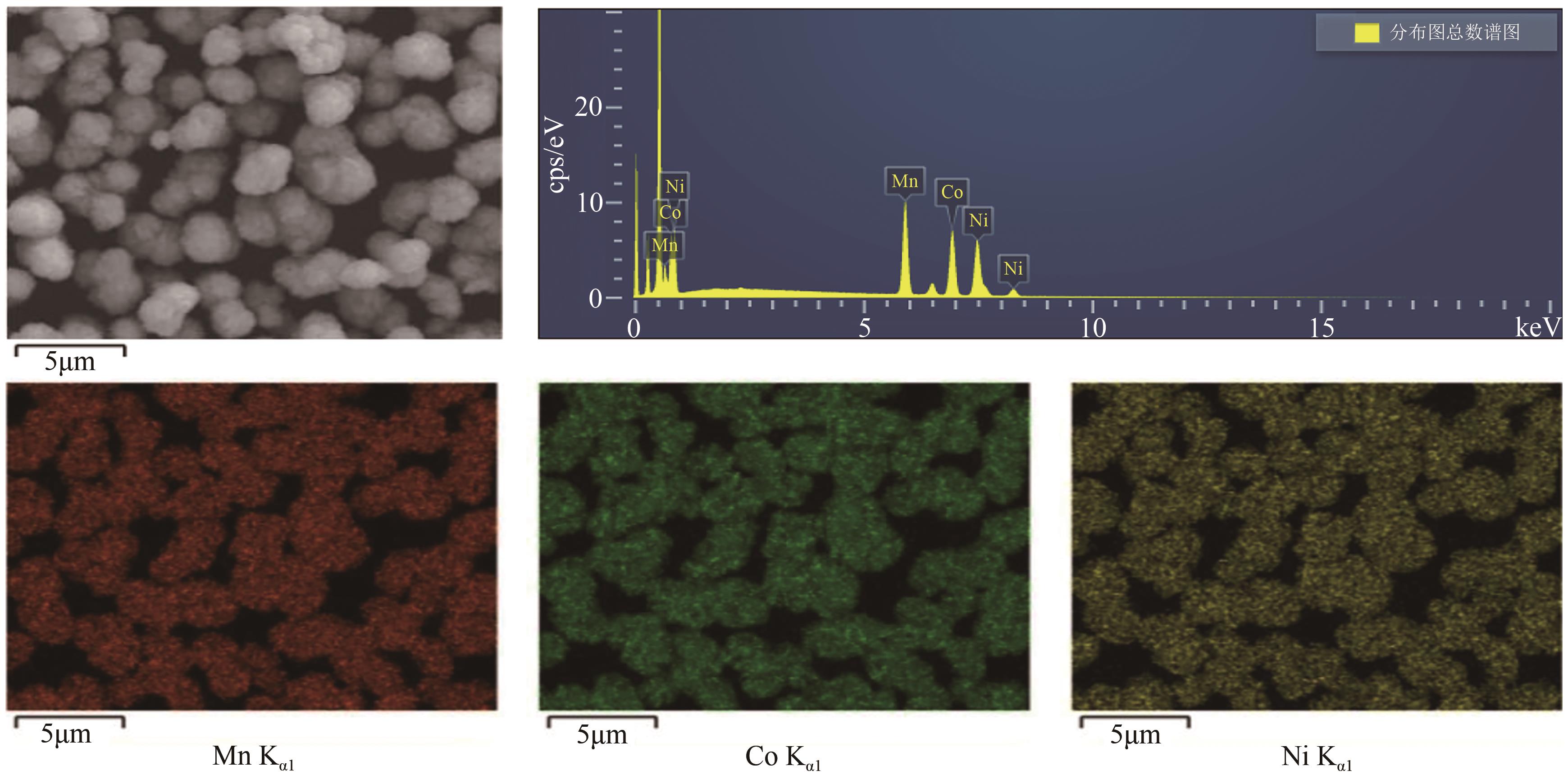
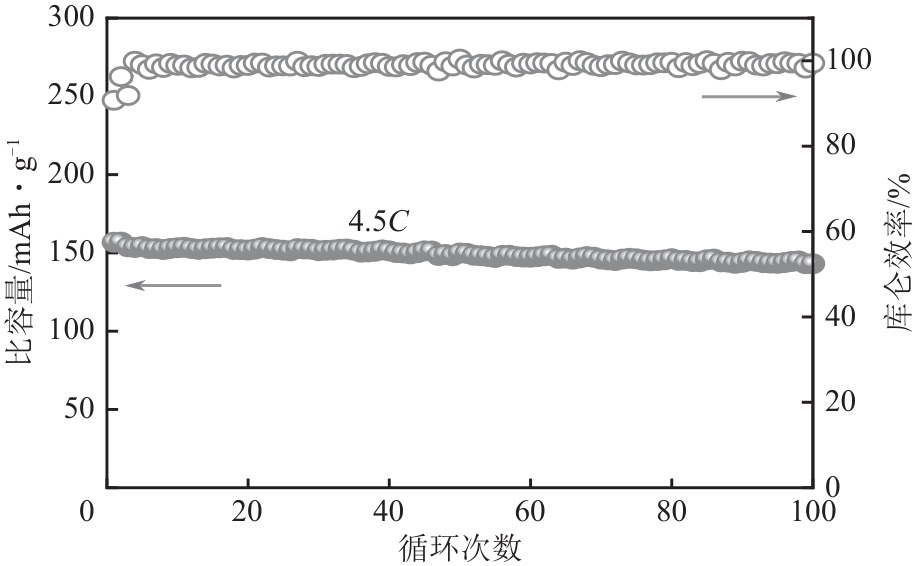
HE Lipo, SUN Shuying, SONG Xingfu, et al. Leaching process for recovering valuable metals from the LiNi1/3Co1/3Mn1/3O2 cathode of lithium-ion batteries[J]. Waste Management, 2017, 64: 171-181.
|
| [20] |
KOYAMA Yukinori, TANAKA Isao, ADACHI Hirohiko, et al. Crystal and electronic structures of superstructural Li1- x [Co1/3Ni1/3Mn1/3]O2 (0≤x≤1)[J]. Journal of Power Sources, 2003, 119/120/121: 644-648.
|
| [21] |
Sung-Ho JOO, SHIN Shun Myung, SHIN Dongju, et al. Extractive separation studies of manganese from spent lithium battery leachate using mixture of PC88A and Versatic 10 acid in kerosene[J]. Hydrometallurgy, 2015, 156: 136-141.
|
| [22] |
GAO Ruichuan, SUN Conghao, XU Lijun, et al. Recycling LiNi0.5Co0.2Mn0.3O2 material from spent lithium-ion batteries by oxalate co-precipitation[J]. Vacuum, 2020, 173: 109181.
|
| [23] |
HE Lipo, SUN Shuying, YU Jianguo. Performance of LiNi1/3Co1/3Mn1/3O2 prepared from spent lithium-ion batteries by a carbonate co-precipitation method[J]. Ceramics International, 2018, 44(1): 351-357.
|
| [24] |
YANG Yue, XU Shengming, HE Yinghe. Lithium recycling and cathode material regeneration from acid leach liquor of spent lithium-ion battery via facile co-extraction and co-precipitation processes[J]. Waste Management, 2017, 64: 219-227.
|
 ), 刘翔2, 胡庆喜1(
), 刘翔2, 胡庆喜1( ), 李金蓉1, 陈伟1
), 李金蓉1, 陈伟1
 ), LIU Xiang2, HU Qingxi1(
), LIU Xiang2, HU Qingxi1( ), LI Jinrong1, CHEN Wei1
), LI Jinrong1, CHEN Wei1