化工进展 ›› 2024, Vol. 43 ›› Issue (7): 3872-3890.DOI: 10.16085/j.issn.1000-6613.2023-0993
有机电极材料在水系电池中的应用研究进展
邵威1( ), 马壮1, 郑宏玮1, 刘光举1, 高翔1, 谢健2, 和庆钢1(
), 马壮1, 郑宏玮1, 刘光举1, 高翔1, 谢健2, 和庆钢1( )
)
- 1.浙江大学化学工程与生物工程学院,浙江 杭州 310027
2.浙江大学材料科学与工程学院,浙江 杭州 310027
-
收稿日期:2023-06-16修回日期:2023-11-02出版日期:2024-07-25发布日期:2024-08-14 -
通讯作者:和庆钢 -
作者简介:邵威(1989—),男,硕士,研究方向为水系有机电池。E-mail:jusswei@zju.edu.cn。 -
基金资助:中国南方电网公司储能研究专项(0470002022030103HX00002-01);国家自然科学基金(21978260)
Recent advances of organic materials for aqueous rechargeable batteries
SHAO Wei1( ), MA Zhuang1, ZHENG Hongwei1, LIU Guangju1, GAO Xiang1, XIE Jian2, HE Qinggang1(
), MA Zhuang1, ZHENG Hongwei1, LIU Guangju1, GAO Xiang1, XIE Jian2, HE Qinggang1( )
)
- 1.College of Chemical and Biological Engineering, Zhejiang University, Hangzhou 310027, Zhejiang, China
2.School of Material Science and Engineering, Zhejiang University, Hangzhou 310027, Zhejiang, China
-
Received:2023-06-16Revised:2023-11-02Online:2024-07-25Published:2024-08-14 -
Contact:HE Qinggang
摘要:
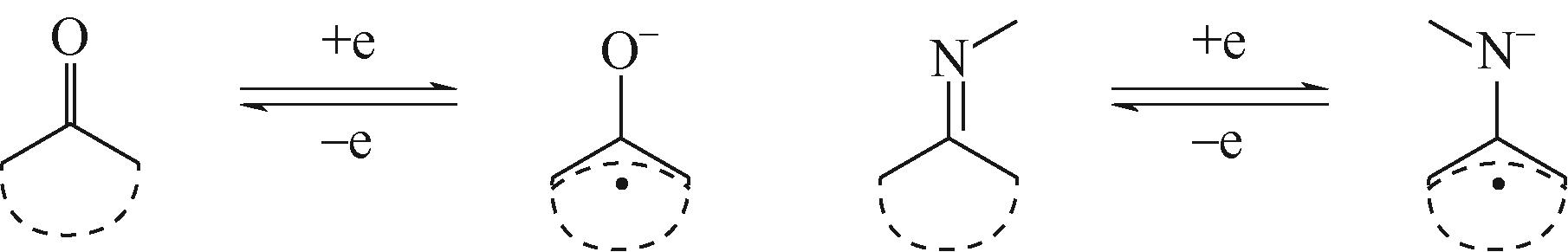
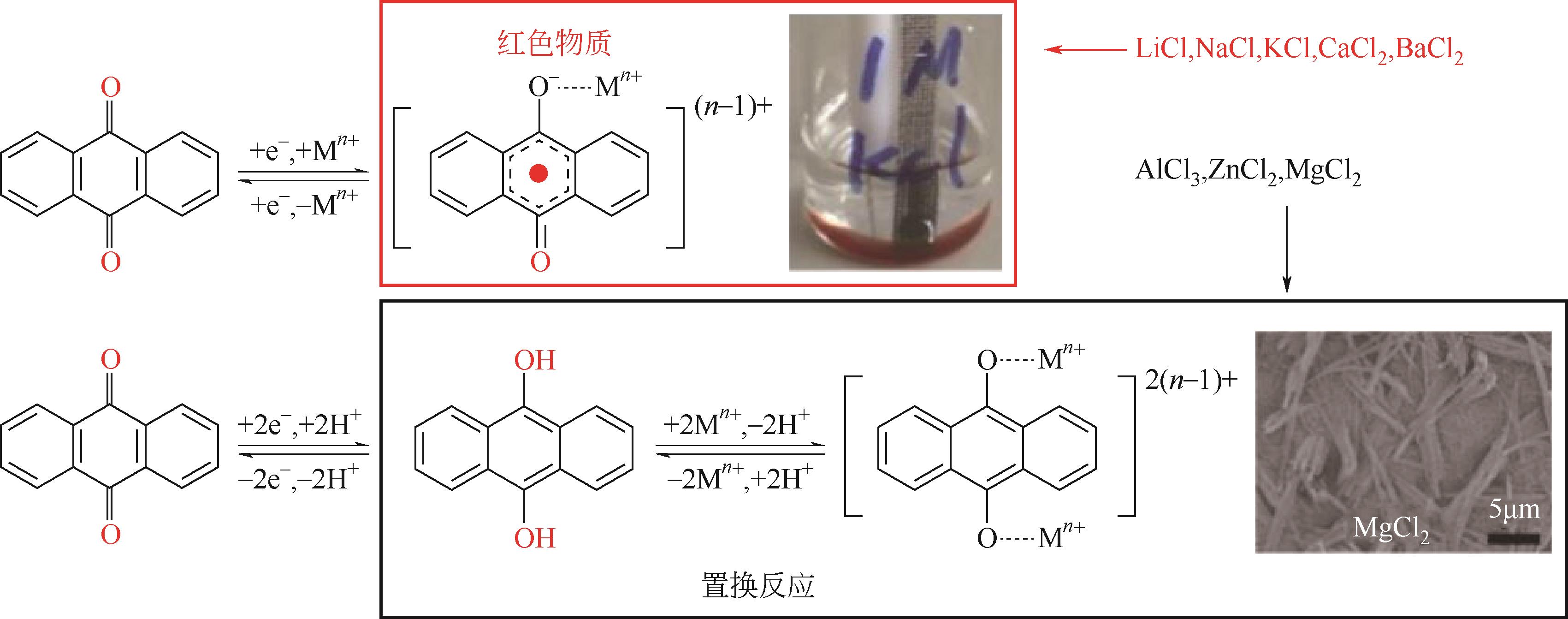
与商业化电池相比,水系有机电池(AOBs)具有低成本、高安全性、环保等特点,使其更适用于便携电子设备和电网储能应用。电极材料作为水系有机电池重要组成部分,在实现电池的高容量和长循环寿命方面起到至关重要的作用。基于绿色发展的需求,有机电极材料应具备理论比容量高、资源丰富、设计灵活性强等优点。本文综述了不同储能机理的有机电极材料的最新研究进展,包括羰基化合物、亚胺化合物、导电聚合物、COFs材料、MOFs材料以及复合材料等。同时总结了有机电极材料在水系电池中的导电性和溶解性问题,并提出了不同的解决策略。最后,讨论了水系有机电池的关键挑战以及未来努力方向,未来仍需要更多具有更好电子导电性和快速动力学的电极材料应用于水系有机电池。
中图分类号:
引用本文
邵威, 马壮, 郑宏玮, 刘光举, 高翔, 谢健, 和庆钢. 有机电极材料在水系电池中的应用研究进展[J]. 化工进展, 2024, 43(7): 3872-3890.
SHAO Wei, MA Zhuang, ZHENG Hongwei, LIU Guangju, GAO Xiang, XIE Jian, HE Qinggang. Recent advances of organic materials for aqueous rechargeable batteries[J]. Chemical Industry and Engineering Progress, 2024, 43(7): 3872-3890.
| 材料结构 | 电解液 | 对电极 | 电极组成 | 放电容量 /mAh·g-1, 电流密度 /A·g-1 | 倍率性能 /mAh·g-1, 电流密度 /A·g-1 | 循环稳定性/%, 循环次数 | 电池体系 | 参考文献 |
|---|---|---|---|---|---|---|---|---|
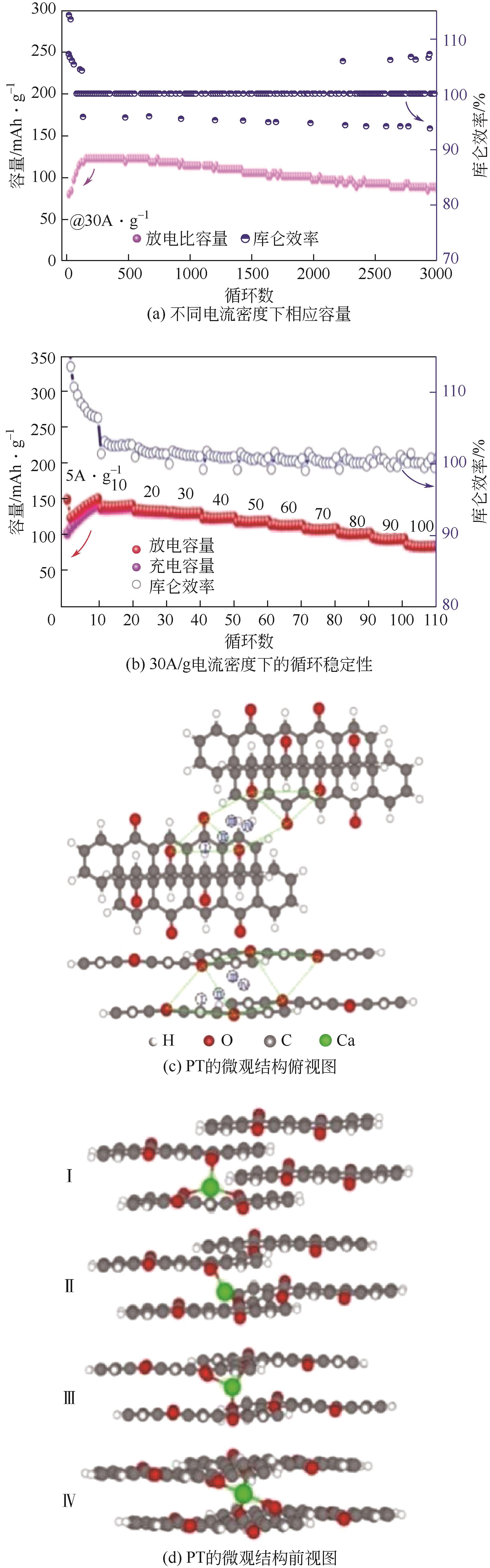
PT | 1mol/L CaCl2 | — | PT∶AC 6∶3 | 150,5 | 86.1,100 | 66.6,3000 | 水系钙离子电池 | [ |
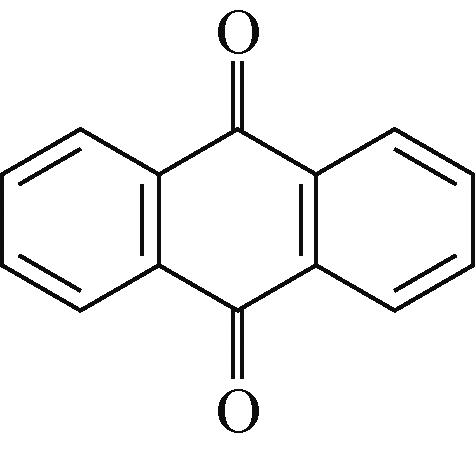
PTO | 4.4mol/L H2SO4 | PbO2 | PTO∶羧基碳管 1∶1 | 395,0.04 | 331.8,8 | 96,1500 | 铅酸电池 | [ |
PPTO | 2.5mol/L Li2SO4 | LiMn2O4 | PPTO∶SP∶ PTFE | 229 | 137.4,20C | 80,3000 | 水系锂离子电池 | [ |
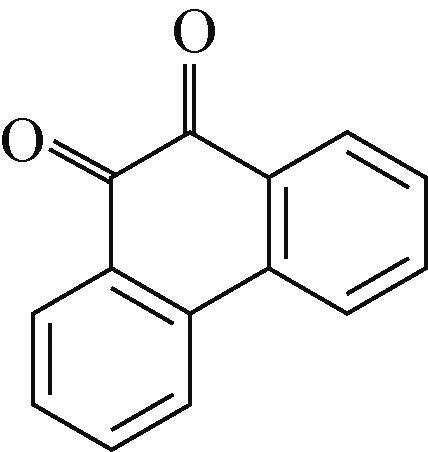
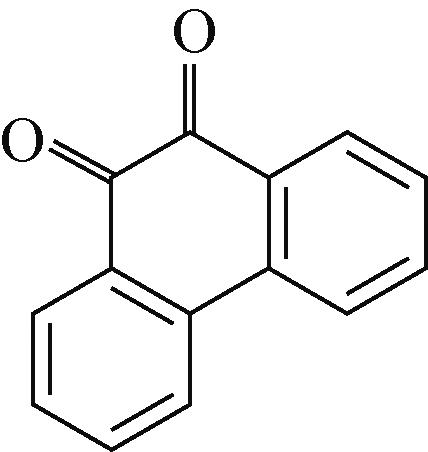
PAQS | 10mol/L KOH | AC | PAQS∶SP∶PTFE 7∶2∶1 | 200 | — | 176,1350 | 碱性水系 电池 | [ |
PQ | 3mol/L Zn(CF3SO3)2 | Zn | PQ∶Super P∶PTFE 6∶3.5∶0.5 | — | — | — | 水系锌离子电池 | [ |
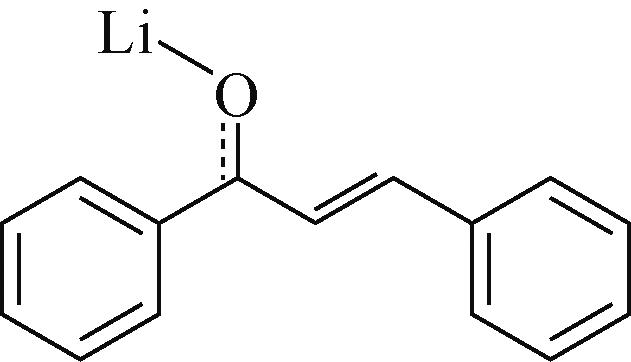
C4Q | 3mol/L Zn(CF3SO3)2 | 锌箔 | C4Q∶SP∶PVDF 6∶3.5∶0.5 | 335,0.02 | — | 87,1000 | 水系锌离子电池 | [ |
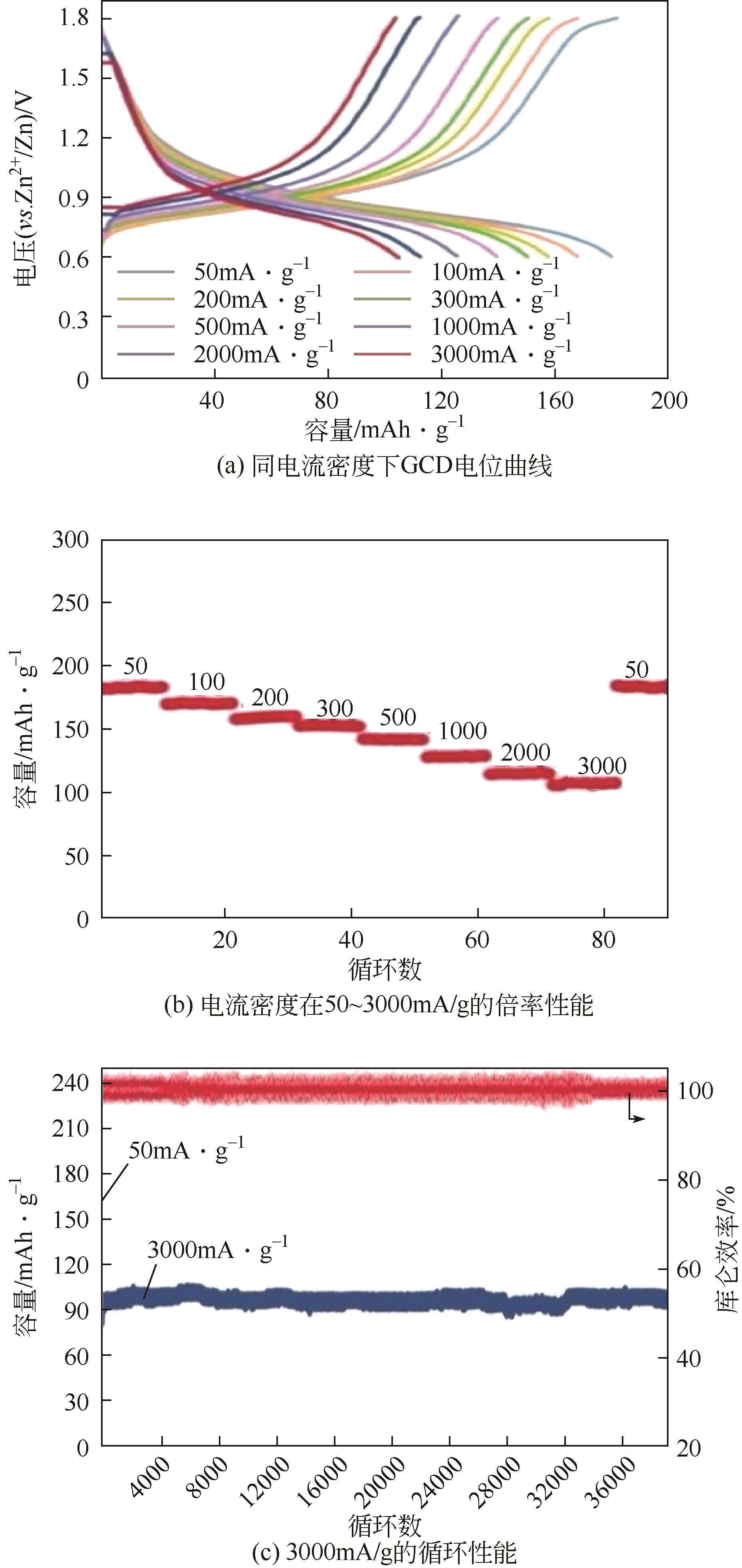
PQ | 2mol/L Zn(SO4)2 | Zn | PQ:AC 6∶4 | 150,0.1 | — | 96.3,36000 | 水系锌离子电池 | [ |
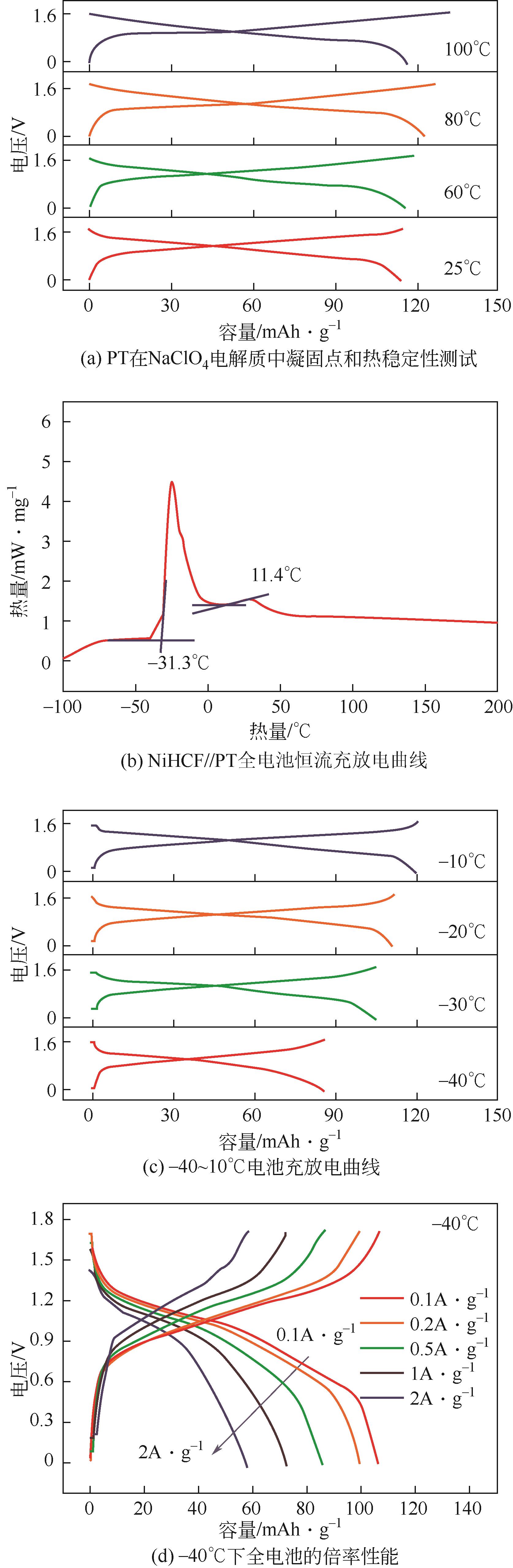
PT | 17mol/L NaClO4 | NiHCF | PT∶KB∶PTFE 6∶3∶1 | 126,0.2 | 87.9,50 | — | 水系钠离子电池 | [ |
锂化查尔酮 | 饱和Li2SO4 | LiFePO4 | — | 111.23,0.125C | 58.9,1C | 91,1000 | 水系锂离子电池 | [ |
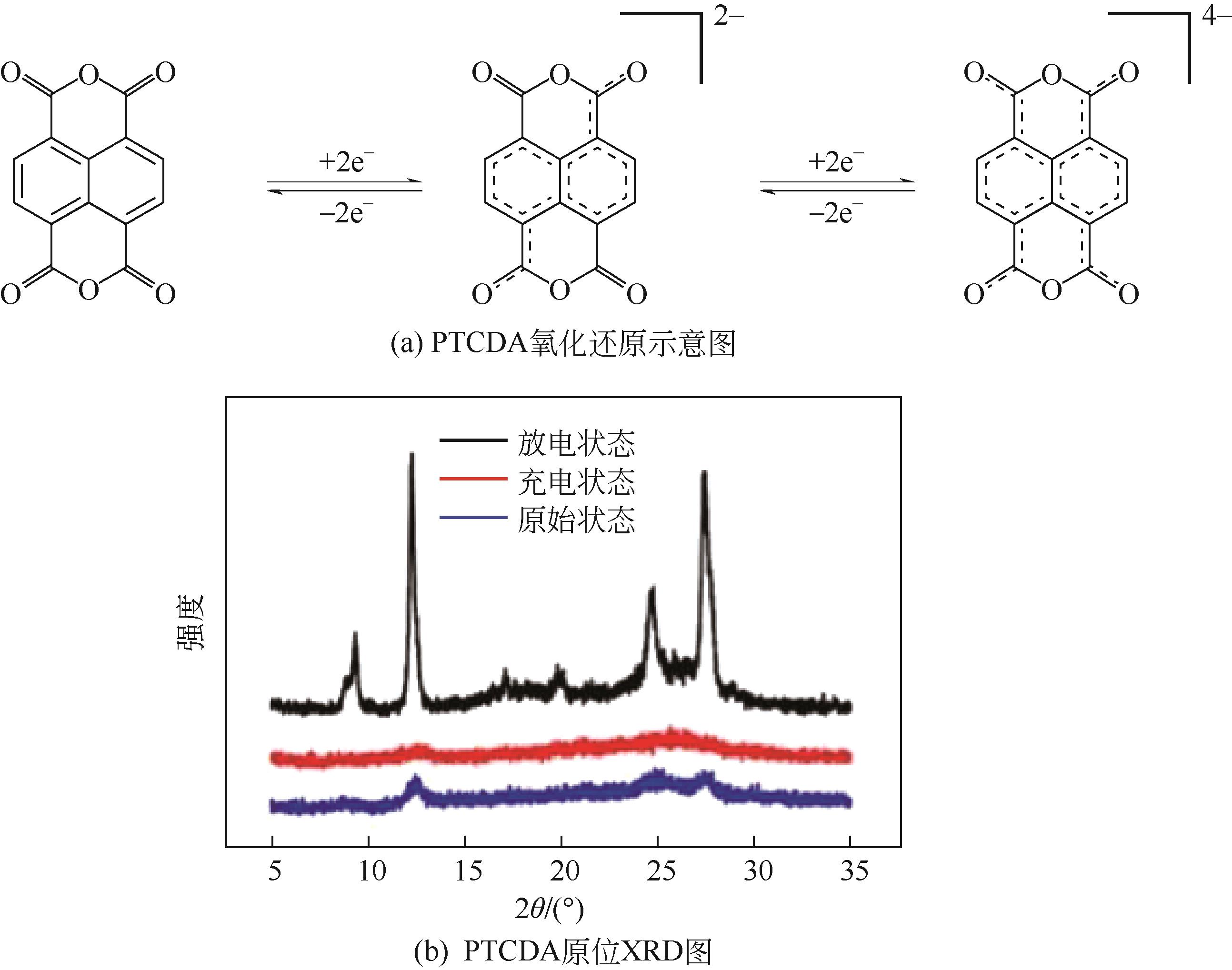
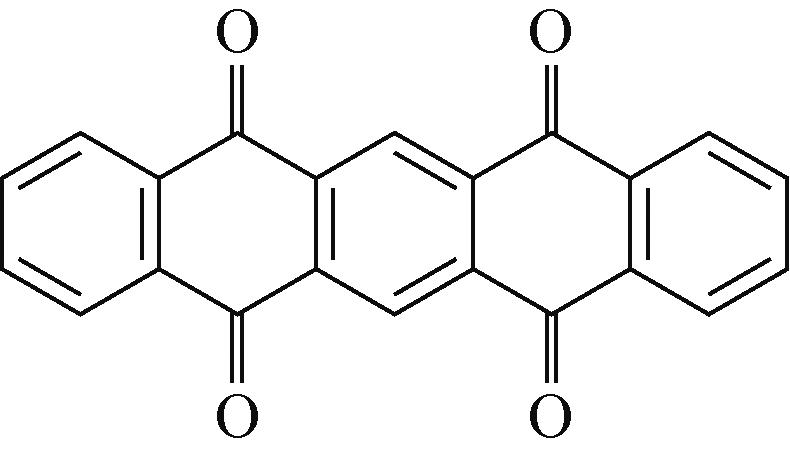
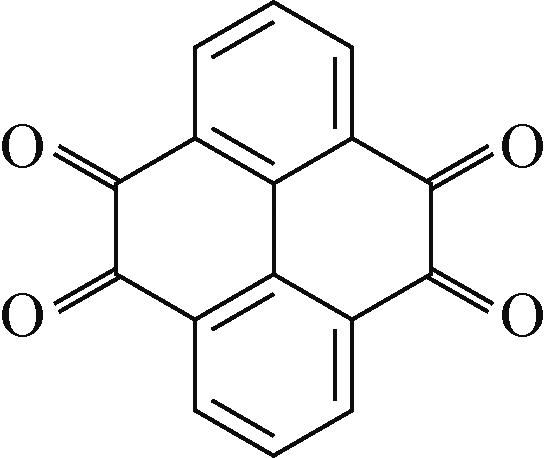
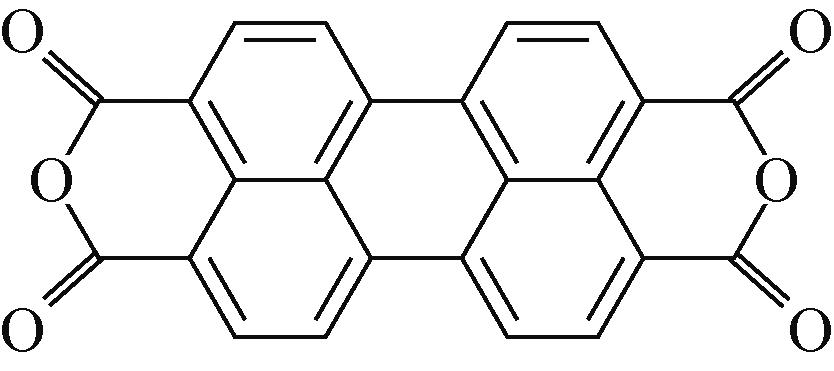
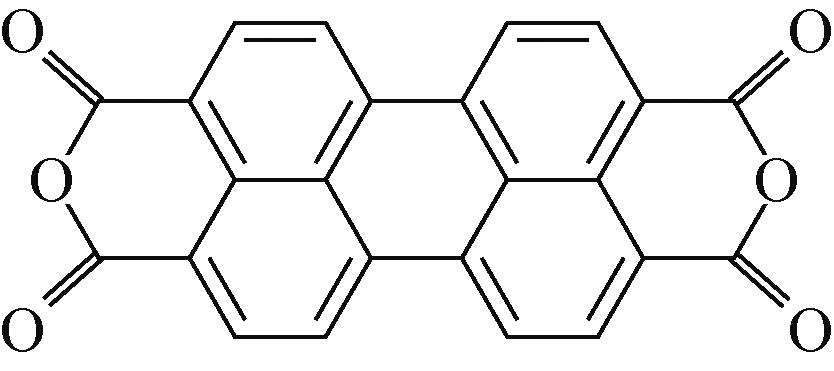
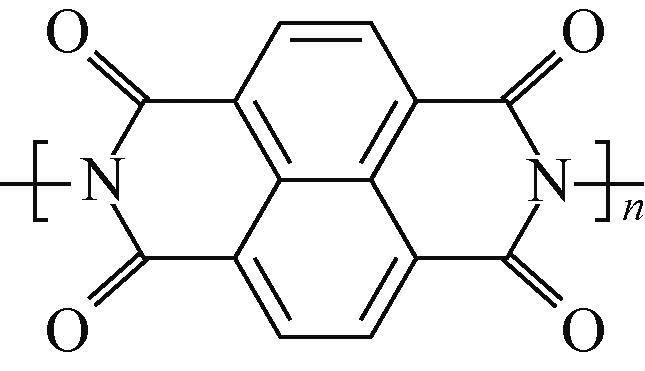
PTCDA | 2mol/L ZnSO4 | Zn | PTCDA∶SP∶PVDF 6∶3∶1 | 136,0.01 | 77,1 | 80,2000 | 水系锌离子电池 | [ |
PTCDA | 0.8mol/L Mg(NO3)2 | 活性炭 | PTCDA∶SP∶PVDF 7∶2∶1 | 125,0.02 | 70,0.5 | — | 水系镁离子电池 | [ |
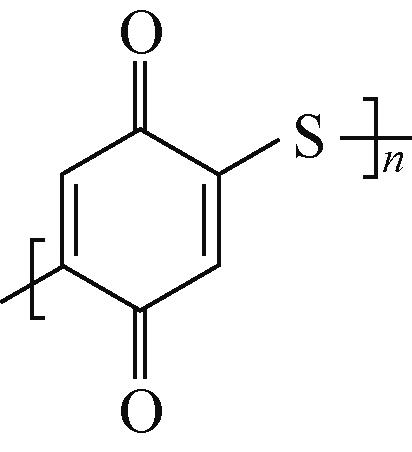
AQ | 1mol/L Al2(SO4)3 | 活性炭 | AQ∶SP 7∶3 | 211,0.8 | — | 94.5,500 | 水系铝离子电池 | [ |
PBQS | 3mol/L Zn(CF3SO3)2 | Zn | PBQS∶KB∶PTFE 6∶3∶1 | 203,0.1C | 126,5C | 83,50 | 水系锌离子电池 | [ |
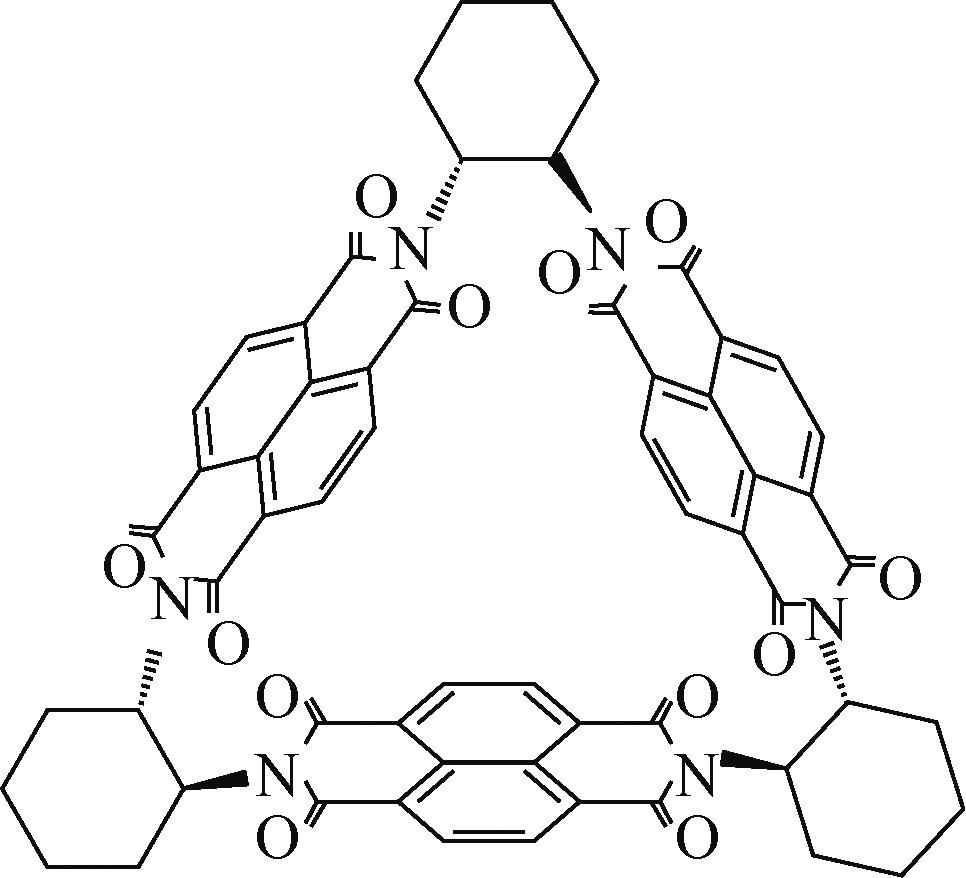
NDA-△ | — | 锂箔 | NDA-△:SP∶PVDF 5∶4∶1 | 146.4,0.1C | 58.1,100C | — | 锂离子 电池 | [ |
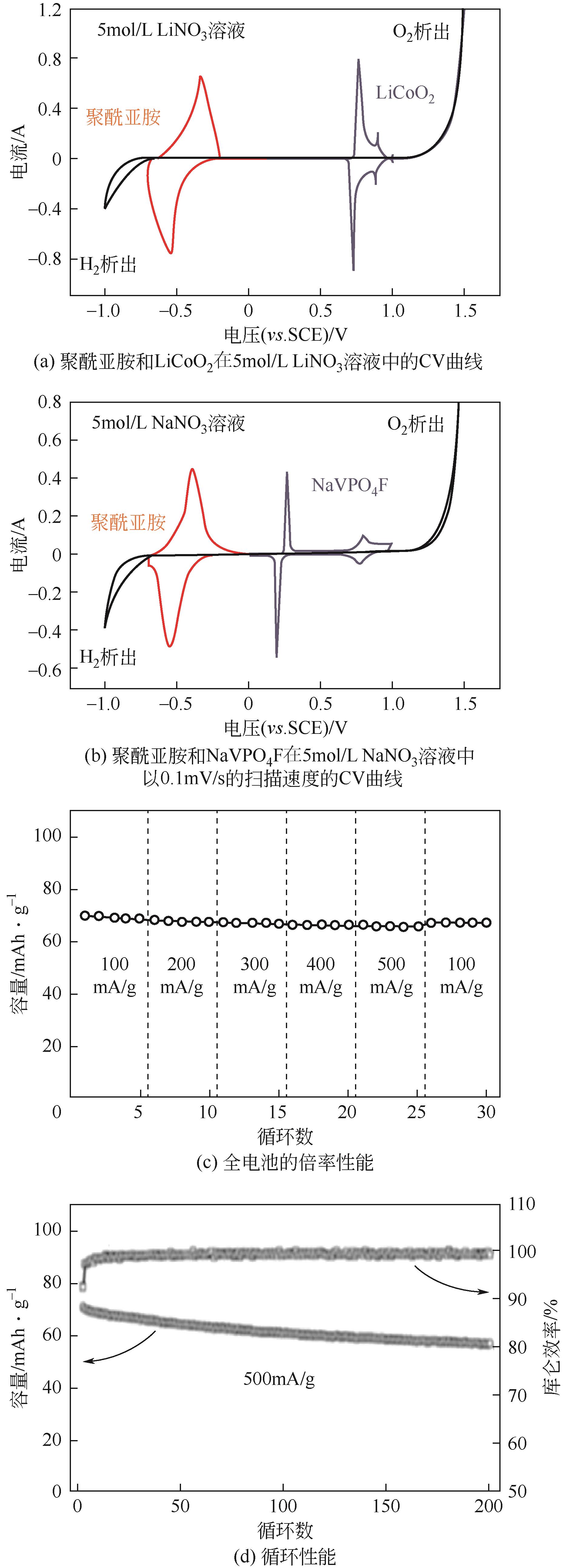
NTCDA | 5mol/L LiNO3 | LiCoO2 | NTCDA∶ XE2 carbon∶PTFE 6∶3∶1 | 71,0.1 | 65,0.5 | 80,200 | 水系锂离子电池 | [ |
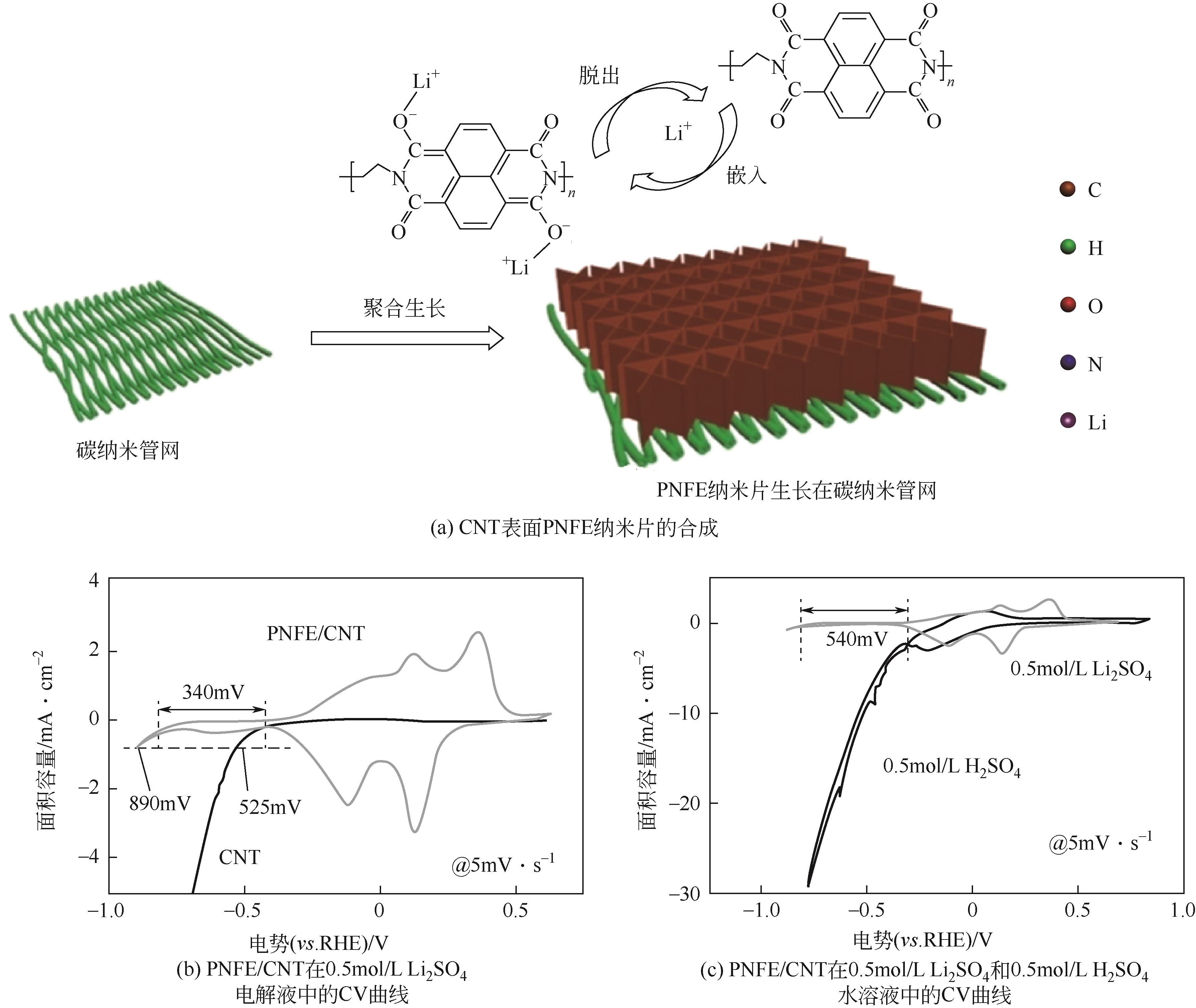
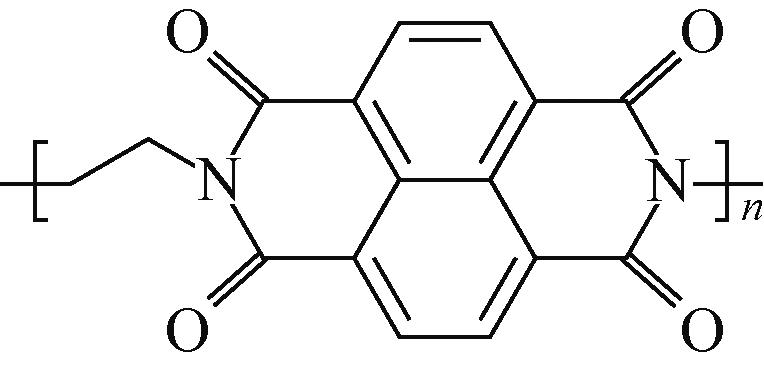
PNFE | 0.5mol/L Li2SO4 | MnO2 | — | 153.7,4C | 86.2,128C | 77.5,500 | 水系锂离子电池 | [ |
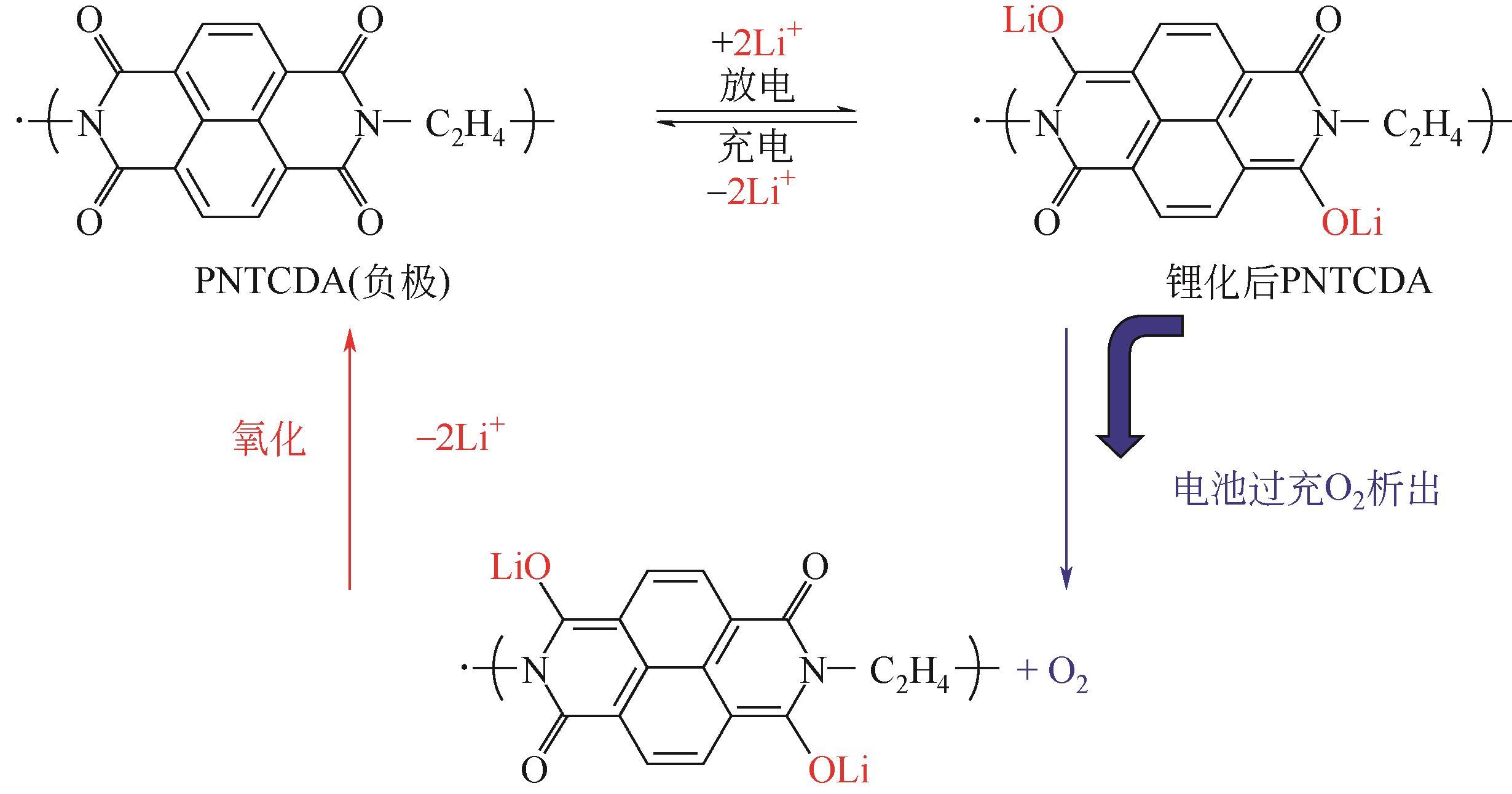
表1 羰基化合物电极材料在不同电池体系的电化学性能概况
| 材料结构 | 电解液 | 对电极 | 电极组成 | 放电容量 /mAh·g-1, 电流密度 /A·g-1 | 倍率性能 /mAh·g-1, 电流密度 /A·g-1 | 循环稳定性/%, 循环次数 | 电池体系 | 参考文献 |
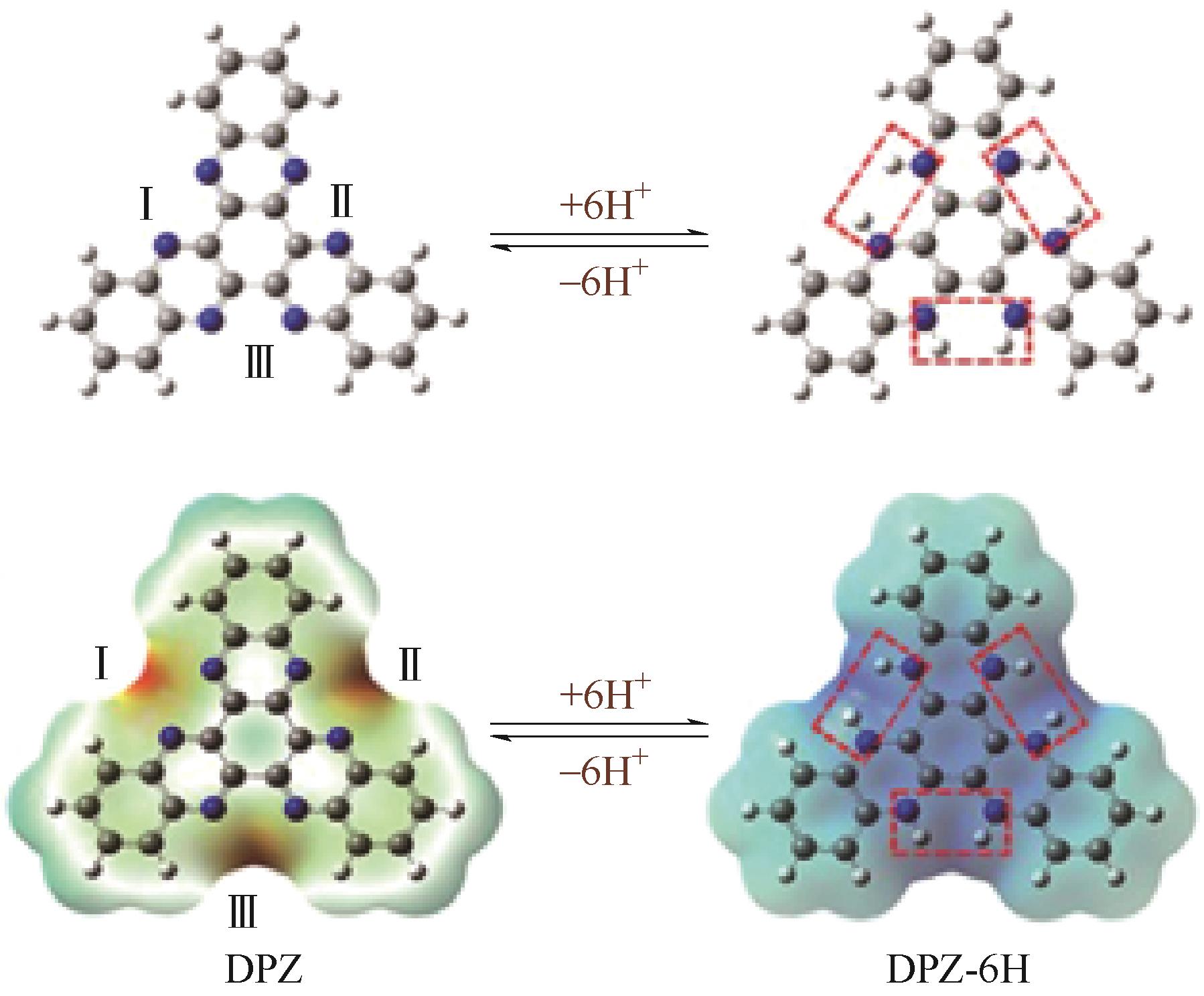
|---|---|---|---|---|---|---|---|---|
PT | 1mol/L CaCl2 | — | PT∶AC 6∶3 | 150,5 | 86.1,100 | 66.6,3000 | 水系钙离子电池 | [ |
PTO | 4.4mol/L H2SO4 | PbO2 | PTO∶羧基碳管 1∶1 | 395,0.04 | 331.8,8 | 96,1500 | 铅酸电池 | [ |
PPTO | 2.5mol/L Li2SO4 | LiMn2O4 | PPTO∶SP∶ PTFE | 229 | 137.4,20C | 80,3000 | 水系锂离子电池 | [ |
PAQS | 10mol/L KOH | AC | PAQS∶SP∶PTFE 7∶2∶1 | 200 | — | 176,1350 | 碱性水系 电池 | [ |
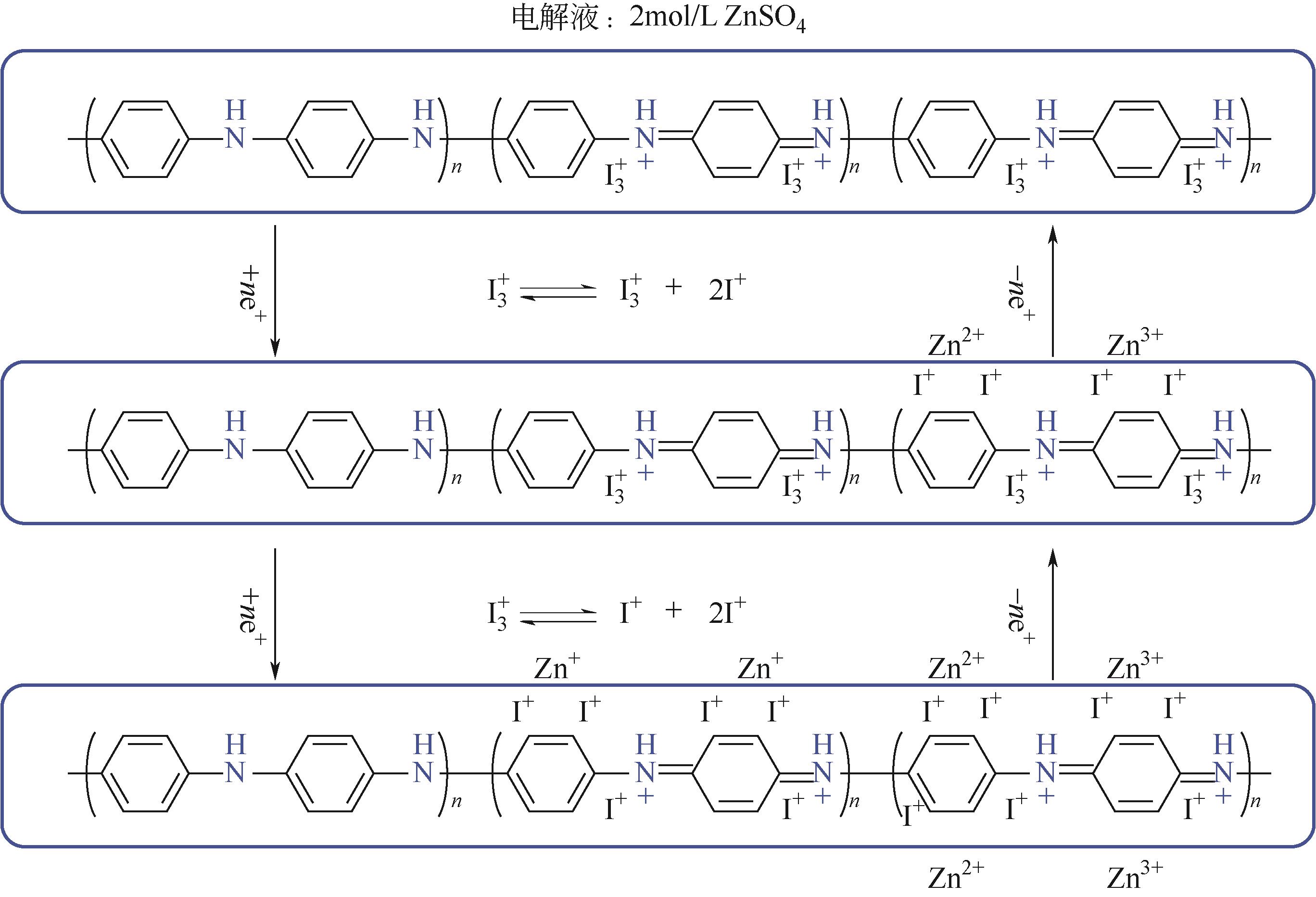
PQ | 3mol/L Zn(CF3SO3)2 | Zn | PQ∶Super P∶PTFE 6∶3.5∶0.5 | — | — | — | 水系锌离子电池 | [ |
C4Q | 3mol/L Zn(CF3SO3)2 | 锌箔 | C4Q∶SP∶PVDF 6∶3.5∶0.5 | 335,0.02 | — | 87,1000 | 水系锌离子电池 | [ |
PQ | 2mol/L Zn(SO4)2 | Zn | PQ:AC 6∶4 | 150,0.1 | — | 96.3,36000 | 水系锌离子电池 | [ |
PT | 17mol/L NaClO4 | NiHCF | PT∶KB∶PTFE 6∶3∶1 | 126,0.2 | 87.9,50 | — | 水系钠离子电池 | [ |
锂化查尔酮 | 饱和Li2SO4 | LiFePO4 | — | 111.23,0.125C | 58.9,1C | 91,1000 | 水系锂离子电池 | [ |
PTCDA | 2mol/L ZnSO4 | Zn | PTCDA∶SP∶PVDF 6∶3∶1 | 136,0.01 | 77,1 | 80,2000 | 水系锌离子电池 | [ |
PTCDA | 0.8mol/L Mg(NO3)2 | 活性炭 | PTCDA∶SP∶PVDF 7∶2∶1 | 125,0.02 | 70,0.5 | — | 水系镁离子电池 | [ |
AQ | 1mol/L Al2(SO4)3 | 活性炭 | AQ∶SP 7∶3 | 211,0.8 | — | 94.5,500 | 水系铝离子电池 | [ |
PBQS | 3mol/L Zn(CF3SO3)2 | Zn | PBQS∶KB∶PTFE 6∶3∶1 | 203,0.1C | 126,5C | 83,50 | 水系锌离子电池 | [ |
NDA-△ | — | 锂箔 | NDA-△:SP∶PVDF 5∶4∶1 | 146.4,0.1C | 58.1,100C | — | 锂离子 电池 | [ |
NTCDA | 5mol/L LiNO3 | LiCoO2 | NTCDA∶ XE2 carbon∶PTFE 6∶3∶1 | 71,0.1 | 65,0.5 | 80,200 | 水系锂离子电池 | [ |
PNFE | 0.5mol/L Li2SO4 | MnO2 | — | 153.7,4C | 86.2,128C | 77.5,500 | 水系锂离子电池 | [ |
| 种类 | 电解液 | 对电极 | 电极组成 | 放电容量/mAh·g-1, 电流密度/A·g-1 | 倍率性能/mAh·g-1, 电流密度/A·g-1 | 循环稳定性/%, 循环次数 | 电池体系 | 参考 文献 |
|---|---|---|---|---|---|---|---|---|
| PANI | 2mol/L ZnSO4 | Zn | — | 160,1.5 | — | 79,700 | 水系锌离子电池 | [ |
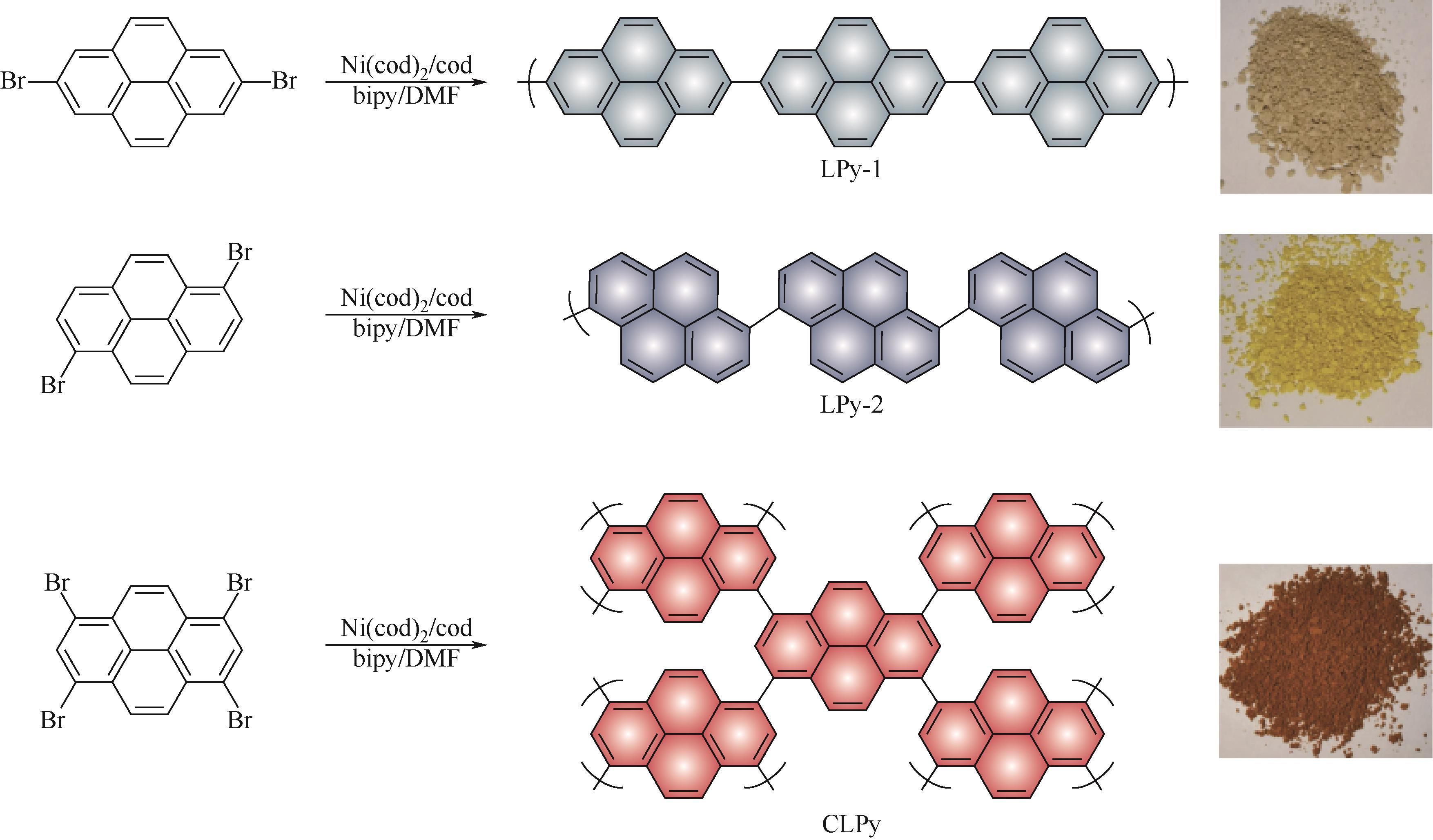
| CLPy | 30mol/L ZnCl2 | Zn | — | 105,3 | — | 96.4,38000 | 水系锌离子电池 | [ |
| PPy | LiSO4 | LiCoO2 | PPy∶AB∶ PVDF 8∶1∶1 | — | — | — | 水系锂离子电池 | [ |
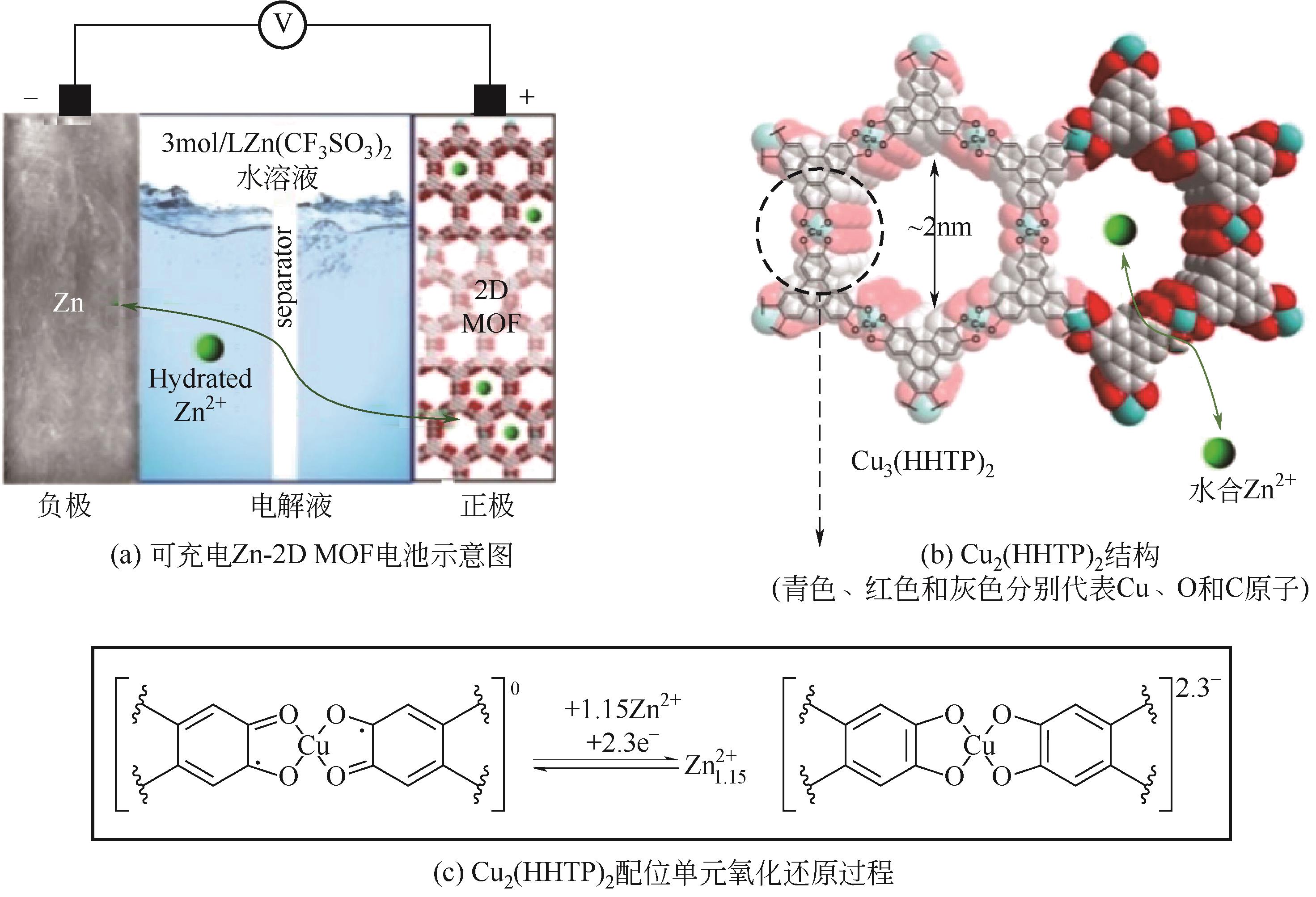
| Cu3(HHTP)2 | 3mol/L Zn(CF3SO3)2 | Zn | Cu3(HHTP)2∶AB∶PVDF 6∶2∶2 | 228,0.05 | — | 75,500 | 水系锌离子电池 | [ |
| PBA | 0.5mol/L K2SO4 | — | PBA∶AC∶PTFE 8∶1∶1 | — | — | 85,500 | 水系钾离子电池 | [ |
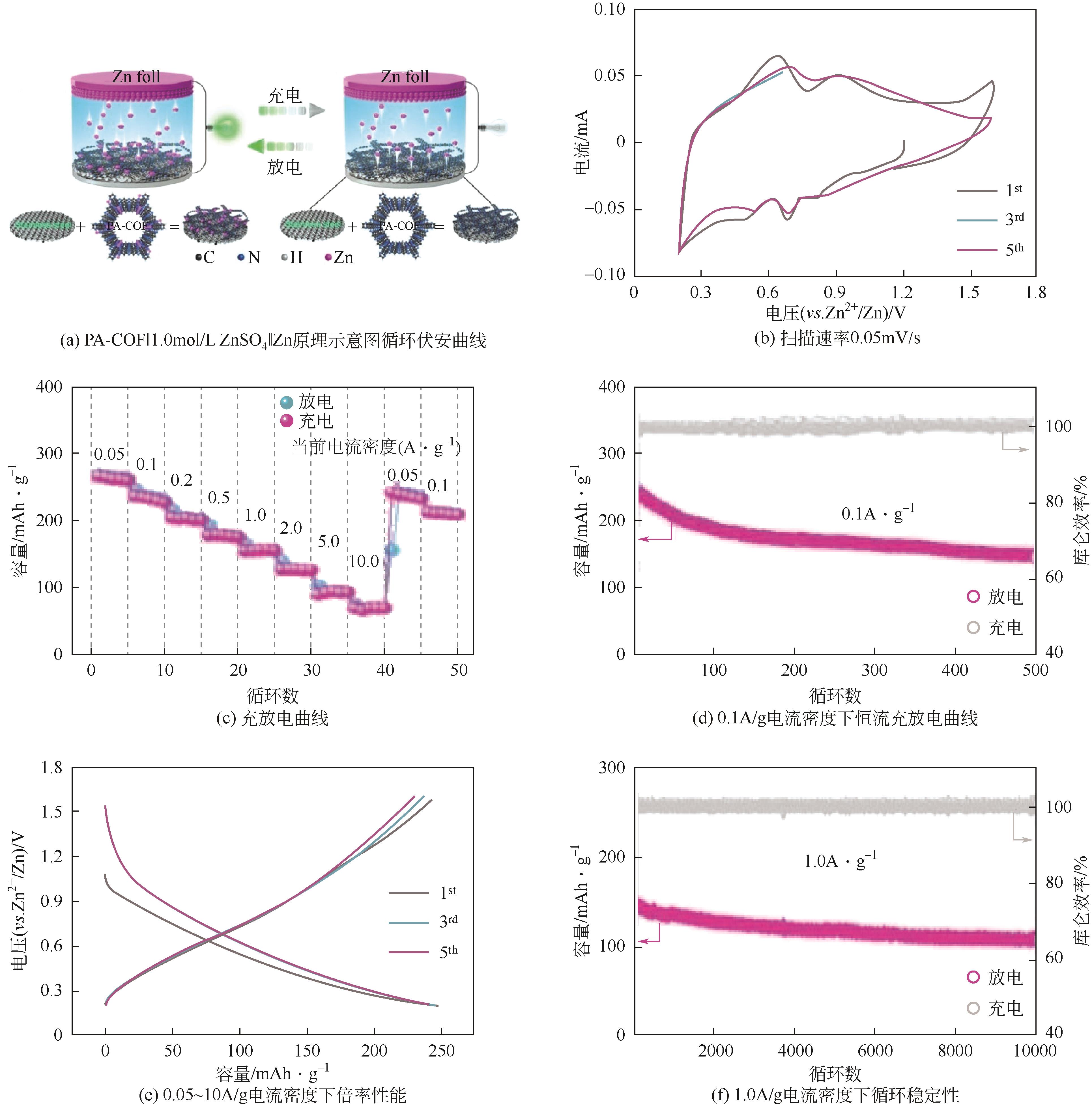
| PA-COF | 1mol/L ZnSO4 | Zn | PA-COF∶AB∶PTFE 6∶3∶1 | 265,0.05 | 68,10 | — | 水系锌离子电池 | [ |
| HqTp | 1mol/L CaCl2 | AC | PBA∶Super P∶PTFE 4∶4∶2 | 119.5,1 | 78.8,50 | 73.7,1600 | 水系钾离子电池 | [ |
表2 其他导电有机化合物在不同电池体系的电化学性能概况
| 种类 | 电解液 | 对电极 | 电极组成 | 放电容量/mAh·g-1, 电流密度/A·g-1 | 倍率性能/mAh·g-1, 电流密度/A·g-1 | 循环稳定性/%, 循环次数 | 电池体系 | 参考 文献 |
|---|---|---|---|---|---|---|---|---|
| PANI | 2mol/L ZnSO4 | Zn | — | 160,1.5 | — | 79,700 | 水系锌离子电池 | [ |
| CLPy | 30mol/L ZnCl2 | Zn | — | 105,3 | — | 96.4,38000 | 水系锌离子电池 | [ |
| PPy | LiSO4 | LiCoO2 | PPy∶AB∶ PVDF 8∶1∶1 | — | — | — | 水系锂离子电池 | [ |
| Cu3(HHTP)2 | 3mol/L Zn(CF3SO3)2 | Zn | Cu3(HHTP)2∶AB∶PVDF 6∶2∶2 | 228,0.05 | — | 75,500 | 水系锌离子电池 | [ |
| PBA | 0.5mol/L K2SO4 | — | PBA∶AC∶PTFE 8∶1∶1 | — | — | 85,500 | 水系钾离子电池 | [ |
| PA-COF | 1mol/L ZnSO4 | Zn | PA-COF∶AB∶PTFE 6∶3∶1 | 265,0.05 | 68,10 | — | 水系锌离子电池 | [ |
| HqTp | 1mol/L CaCl2 | AC | PBA∶Super P∶PTFE 4∶4∶2 | 119.5,1 | 78.8,50 | 73.7,1600 | 水系钾离子电池 | [ |
| 材料分类 | 代表性材料 | 优点 | 缺点 |
|---|---|---|---|
| 羰基化合物 | 酮、醌、酰亚胺、酸酐 | 高容量、快速反应动力学 | 高溶解度、低电导率 |
| 导电聚合物 | 聚吡咯、聚苯胺 | 高电导率 | 低容量、倾斜放电平台 |
| 亚胺类化合物 | 吩嗪类、异咯嗪 | 高容量、快速反应动力学 | 高溶解度、低电导率 |
| COFs | PA-COF | 高容量 | 低电导率 |
| MOFs | Cu3(HHTP)2 | 高电导率 | — |
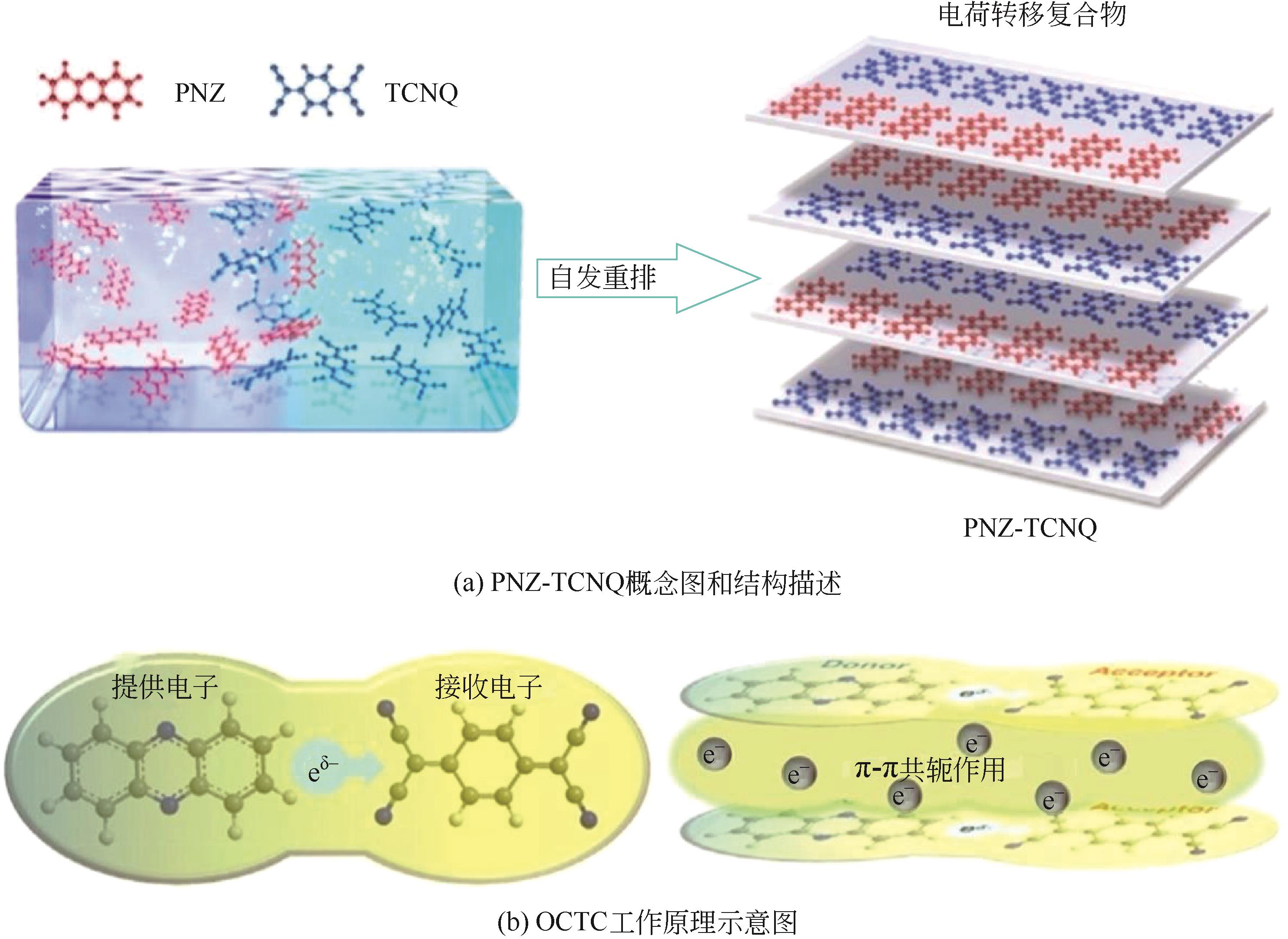
| 复合材料 | TTF-TCNQ | 高电导率 | — |
表3 水系电池中不同类型有机材料特性概述
| 材料分类 | 代表性材料 | 优点 | 缺点 |
|---|---|---|---|
| 羰基化合物 | 酮、醌、酰亚胺、酸酐 | 高容量、快速反应动力学 | 高溶解度、低电导率 |
| 导电聚合物 | 聚吡咯、聚苯胺 | 高电导率 | 低容量、倾斜放电平台 |
| 亚胺类化合物 | 吩嗪类、异咯嗪 | 高容量、快速反应动力学 | 高溶解度、低电导率 |
| COFs | PA-COF | 高容量 | 低电导率 |
| MOFs | Cu3(HHTP)2 | 高电导率 | — |
| 复合材料 | TTF-TCNQ | 高电导率 | — |
| 1 | 孙伟卿, 王思成, 刘宇宸. 支撑新型电力系统的储能技术综述与政策解读[J]. 自动化仪表, 2022, 43(12): 1-6, 18. |
| SUN Weiqing, WANG Sicheng, LIU Yuchen. Review and policy interpretation of energy storage technology supporting new power system[J]. Process Automation Instrumentation, 2022, 43(12): 1-6, 18. | |
| 2 | KOOHI-FAYEGH S, ROSEN M A. A review of energy storage types, applications and recent developments[J]. Journal of Energy Storage, 2020, 27: 101047. |
| 3 | DEANE J P, GALLACHÓIR B P Ó, MCKEOGH E J. Techno-economic review of existing and new pumped hydro energy storage plant[J]. Renewable and Sustainable Energy Reviews, 2010, 14(4): 1293-1302. |
| 4 | MOHIT Murarka, RANI Purohit Pranati, DIBAKAR Rakshit, et al. Progression of battery storage technology considering safe and sustainable stationary application[J]. Journal of Cleaner Production, 2022, 377: 134279. |
| 5 | CHAYAMBUKA Kudakwashe, MULDER Grietus, DANILOV Dmitri L, et al. From Li-ion batteries toward Na-ion chemistries: Challenges and opportunities[J]. Advanced Energy Materials, 2020, 10(38): 2001310. |
| 6 | YANG Jun, HU Chenyan, WANG Hao, et al. Review on the research of failure modes and mechanism for lead-acid batteries[J]. International Journal of Energy Research, 2017, 41(3): 336-352. |
| 7 | 朱晟, 彭怡婷, 闵宇霖, 等. 电化学储能材料及储能技术研究进展[J]. 化工进展, 2021, 40(9): 4837-4852. |
| ZHU Sheng, PENG Yiting, MIN Yulin, et al. Research progress on materials and technologies for electrochemical energy storage[J]. Chemical Industry and Engineering Progress, 2021, 40(9): 4837-4852. | |
| 8 | LI Lei, ZHANG Qichong, HE Bing, et al. Advanced multifunctional aqueous rechargeable batteries design: From materials and devices to systems[J]. Advanced Materials, 2022, 34(5): 2104327. |
| 9 | CAI Shengying, CHU Xingyuan, LIU Chang, et al. Water-salt oligomers enable supersoluble electrolytes for high-performance aqueous batteries[J]. Advanced Materials, 2021, 33(13): e2007470. |
| 10 | STRIETZEL Christian, STERBY Mia, HUANG Hao, et al. An aqueous conducting redox-polymer-based proton battery that can withstand rapid constant-voltage charging and sub-zero temperatures[J]. Angewandte Chemie International Edition, 2020, 59(24): 9631-9638. |
| 11 | Kouki OKA, STRIETZEL Christian, EMANUELSSON Rikard, et al. Cover feature: Conducting redox polymer as a robust organic electrode-active material in acidic aqueous electrolyte towards polymer-air secondary batteries[J]. ChemSusChem, 2020, 13(9): 2280-2285. |
| 12 | LI Leilei, CHEN Long, WEN Yuehua, et al. Phenazine anodes for ultralongcycle-life aqueous rechargeable batteries[J]. Journal of Materials Chemistry A, 2020, 8(48): 26013-26022. |
| 13 | GRIECO Rebecca, MOLINA Antonio, SANCHEZ Jaime S, et al. A significantly improved polymer||Ni(OH)2 alkaline rechargeable battery using anthraquinone-based conjugated microporous polymer anode[J]. Materials Today Energy, 2022, 27: 101014. |
| 14 | CLAUSEN Casper, Emil DRAŽEVIĆ, ANDERSEN Anders Søndergaard, et al. Anthraquinone oligomers as anode-active material in rechargeable nickel/oligomer batteries with aqueous electrolyte[J]. ACS Applied Energy Materials, 2018, 1(2): 243-248. |
| 15 | HAN Cuiping, LI Hongfei, LI Yu, et al. Proton-assisted calcium-ion storage in aromatic organic molecular crystal with coplanar stacked structure[J]. Nature Communications, 2021, 12(1): 2400. |
| 16 | HANNA Ortal, MALKA David, LUSKI Shalom, et al. Aqueous energy storage device based on LiMn2O4 (spinel) positive electrode and anthraquinone-modified carbon-negative electrode[J]. Energy Technology, 2019, 7(10): 1900589. |
| 17 | GU Tiantian, ZHOU Min, LIU Mengyun, et al. A polyimide-MWCNTs composite as high performance anode for aqueous Na-ion batteries[J]. RSC Advances, 2016, 6(58): 53319-53323. |
| 18 | LIU Yu, HUANG Meng, XIONG Fangyu, et al. Improved zinc-ion storage performance of the metal-free organic anode by the effect of binder[J]. Chemical Engineering Journal, 2022, 428: 131092. |
| 19 | HAN Cuiping, ZHU Jiaxiong, ZHI Chunyi, et al. The rise of aqueous rechargeable batteries with organic electrode materials[J]. Journal of Materials Chemistry A, 2020, 8(31): 15479-15512. |
| 20 | MATHY Sandrine, MENANTEAU Philippe, CRIQUI Patrick. After the Paris agreement: Measuring the global decarbonization wedges from national energy scenarios[J]. Ecological Economics, 2018, 150: 273-289. |
| 21 | TANG Mi, LI Hongyang, WANG Erjing, et al. Carbonyl polymeric electrode materials for metal-ion batteries[J]. Chinese Chemical Letters, 2018, 29(2): 232-244. |
| 22 | LEE Sechan, KWON Giyun, KU Kyojin, et al. Recent progress in organic electrodes for Li and Na rechargeable batteries[J]. Advanced Materials, 2018, 30(42): e1704682. |
| 23 | SONG Zhiping, ZHOU Haoshen. Towards sustainable and versatile energy storage devices: An overview of organic electrode materials[J]. Energy & Environmental Science, 2013, 6(8): 2280-2301. |
| 24 | KIM Haegyeom, HONG Jihyun, PARK Kyu-Young, et al. Aqueous rechargeable Li and Na ion batteries[J]. Chemical Reviews, 2014, 114(23): 11788-11827. |
| 25 | ZHANG Yugen, WANG Jinquan, RIDUAN Siti Nurhanna. Strategies toward improving the performance of organic electrodes in rechargeable lithium (sodium) batteries[J]. Journal of Materials Chemistry A, 2016, 4(39): 14902-14914. |
| 26 | Guiomar HERNÁNDEZ, CASADO Nerea, ZAMARAYEVA Alla M, et al. Perylene polyimide-polyether anodes for aqueous all-organic polymer batteries[J]. ACS Applied Energy Materials, 2018, 1(12): 7199-7205. |
| 27 | PENG Huiling, YU Qianchuan, WANG Shengping, et al. Molecular design strategies for electrochemical behavior of aromatic carbonyl compounds in organic and aqueous electrolytes[J]. Advanced Science, 2019, 6(17): 1900431. |
| 28 | LIANG Yanliang, JING Yan, GHEYTANI Saman, et al. Universal quinone electrodes for long cycle life aqueous rechargeable batteries[J]. Nature Materials, 2017, 16(8): 841-848. |
| 29 | Bernhard HÄUPLER, WILD Andreas, SCHUBERT Ulrich S. Carbonyls: Powerful organic materials for secondary batteries[J]. Advanced Energy Materials, 2015, 5(11): 1402034. |
| 30 | GENG Joaquin, BONNET Jean-Pierre, RENAULT Stéven, et al. Evaluation of polyketones with N-cyclic structure as electrode material for electrochemical energy storage: Case of tetraketopiperazine unit[J]. Energy & Environmental Science, 2010, 3(12): 1929-1933. |
| 31 | ZHAO Qing, HUANG Weiwei, LUO Zhiqiang, et al. High-capacity aqueous zinc batteries using sustainable quinone electrodes[J]. Science Advances, 2018, 4(3): eaao1761. |
| 32 | YANG Beibei, MA Yuanyuan, Duan BIN, et al. Ultralong-life cathode for aqueous zinc-organic batteries via pouring 9,10 - phenanthraquinone into active carbon[J]. ACS Applied Materials & Interfaces, 2021, 13(49): 58818-58826. |
| 33 | ZHANG Yu, XU Jie, LI Zhi, et al. All-climate aqueous Na-ion batteries using “water-in-salt” electrolyte[J]. Science Bulletin, 2022, 67(2): 161-170. |
| 34 | MUNIVENKATAPPA Chaithra, SHETTY Vijeth Rajshekar, SHIVAPPA Suresh Gurukar. Chalcone as anode material for aqueous rechargeable lithium-ion batteries[J]. Russian Journal of Electrochemistry, 2021, 57(4): 419-433. |
| 35 | YU Feng, WANG Yi, LIU Yu, et al. An aqueous rechargeable zinc-ion battery on basis of an organic pigment[J]. Rare Metals, 2022, 41(7): 2230-2236. |
| 36 | RODRÍGUEZ-PÉREZ Ismael A, YUAN Yifei, BOMMIER Clement, et al. Mg-ion battery electrode: An organic solid’s herringbone structure squeezed upon Mg-ion insertion[J]. Journal of the American Chemical Society, 2017, 139(37): 13031-13037. |
| 37 | ALT H, BINDER H, KÖHLING A, et al. Investigation into the use of quinone compounds-for battery cathodes[J]. Electrochimica Acta, 1972, 17(5): 873-887. |
| 38 | YAN Lijing, ZHAO Chenxuan, SHA Ying, et al. Electrochemical redox behavior of organic quinone compounds in aqueous metal ion electrolytes[J]. Nano Energy, 2020, 73: 104766. |
| 39 | HUANG Weiwei, LIU Shuai, LI Chaobo, et al. Calix[8]quinone: A new promising macrocyclic molecule as an efficient organic cathode in lithium ion batteries with a highly-concentrated electrolyte[J]. EcoMat, 2022, 4(5): e12214. |
| 40 | SONG Zhiping, QIAN Yumin, ZHANG Tao, et al. Poly(benzoquinonyl sulfide) as a high-energy organic cathode for rechargeable Li and Na batteries[J]. Advanced Science, 2015, 2(9): 1500124. |
| 41 | DAWUT Gulbahar, LU Yong, MIAO Licheng, et al. High-performance rechargeable aqueous Zn-ion batteries with a poly(benzoquinonyl sulfide) cathode[J]. Inorganic Chemistry Frontiers, 2018, 5(6): 1391-1396. |
| 42 | CHEN Dongyang, AVESTRO Alyssa-Jennifer, CHEN Zonghai, et al. A rigid naphthalenediimide triangle for organic rechargeable lithium-ion batteries[J]. Advanced Materials, 2015, 27(18): 2907-2912. |
| 43 | LI Yixin, LU Yong, NI Youxuan, et al. Quinone electrodes for alkali–acid hybrid batteries[J]. Journal of the American Chemical Society, 2022, 144(18): 8066-8072. |
| 44 | QIN H, SONG Z P, ZHAN H, et al. Aqueous rechargeable alkali-ion batteries with polyimide anode[J]. Journal of Power Sources, 2014, 249: 367-372. |
| 45 | CHEN Long, LI Wangyu, GUO Zhaowei, et al. Aqueous lithium-ion batteries using O2 self-elimination polyimides electrodes[J]. Journal of the Electrochemical Society, 2015, 162(10): A1972-A1977. |
| 46 | WANG Yuhang, CUI Xiaoqi, ZHANG Yueyu, et al. Achieving high aqueous energy storage via hydrogen-generation passivation[J]. Advanced Materials, 2016, 28(35): 7626-7632. |
| 47 | LI Jiahao, HUANG Lulu, Heng LYU, et al. Investigations on the electrochemical behaviors of hexaazatriphenylene derivative as high-performance electrode for batteries[J]. Electrochimica Acta, 2022, 432: 141206. |
| 48 | WANG Quan, LIU Yu, CHEN Pu. Phenazine-based organic cathode for aqueous zinc secondary batteries[J]. Journal of Power Sources, 2020, 468: 228401. |
| 49 | SUN Tianjiang, LIU Chang, WANG Jiayue, et al. A phenazine anode for high-performance aqueous rechargeable batteries in a wide temperature range[J]. Nano Research, 2020, 13(3): 676-683. |
| 50 | SHI Minjie, HE Jing, ZHAO Yue, et al. In-situ Raman investigation and application of phenazine-based organic electrode in aqueous proton batteries[J]. Materials & Design, 2022, 222: 111043. |
| 51 | HE Jing, WANG Renyuan, LI Lingyun, et al. Unraveling the superior aqueous Na-ion storage in a multicyano-substituted phenazine-based electrode material[J]. Chemical Communications, 2022, 58(85): 11925-11928. |
| 52 | ZENG Xiaomin, MENG Xiangjuan, JIANG Wei, et al. Anchoring polyiodide to conductive polymers as cathode for high-performance aqueous zinc–iodine batteries[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(38): 14280-14285. |
| 53 | ZHANG Chong, MA Wenyan, HAN Changzhi, et al. Tailoring the linking patterns of polypyrene cathodes for high-performance aqueous Zn dual-ion batteries[J]. Energy & Environmental Science, 2021, 14(1): 462-472. |
| 54 | WANG G J, YANG L C, QU Q T, et al. An aqueous rechargeable lithium battery based on doping and intercalation mechanisms[J]. Journal of Solid State Electrochemistry, 2010, 14(5): 865-869. |
| 55 | 蔡诗怡, 李津瑜, 吴丽霞, 等. 金属有机框架材料在锂硫电池的应用前沿进展[J]. 化工进展, 2021, 40(6): 3046-3057. |
| CAI Shiyi, LI Jinyu, WU Lixia, et al. Progress of MOF materials applied in Li-S batteries[J]. Chemical Industry and Engineering Progress, 2021, 40(6): 3046-3057. | |
| 56 | Kwan Woo NAM, PARK Sarah S, DOS REIS Roberto, et al. Conductive 2D metal-organic framework for high-performance cathodes in aqueous rechargeable zinc batteries[J]. Nature Communications, 2019, 10(1): 4948. |
| 57 | SU Dawei, MCDONAGH Andrew, QIAO Shizhang, et al. High-capacity aqueous potassium-ion batteries for large-scale energy storage[J]. Advanced Materials, 2017, 29(1): 1604007. |
| 58 | 徐喜连. MOF衍生分级结构材料的可控制备及其水系储能应用研究[D]. 杭州: 浙江工业大学, 2019. |
| XU Xilian. Controllable preparation of MOF-derived hierarchical structure materials and their application in aqueous energy storage[D]. Hangzhou: Zhejiang University of Technology, 2020. | |
| 59 | 马文杰, 姚卫棠. 共价有机框架(COFs)在锂离子电池中的应用[J]. 化工进展, 2023, 42 (10): 5339-5352. |
| MA Wenjie, YAO Weitang. Application of covalent organic frameworks (COFs) in lithium-ion batteries[J]. Chemical Industry and Engineering Progress, 2023, 42(10): 5339-5352. | |
| 60 | WANG Wenxi, KALE Vinayak S, CAO Zhen, et al. Phenanthroline covalent organic framework electrodes for high-performance zinc-ion supercapattery[J]. ACS Energy Letters, 2020, 5(7): 2256-2264. |
| 61 | LI Linyuan, ZHANG Guobin, DENG Xianming, et al. A covalent organic framework for high-rate aqueous calcium-ion batteries[J]. Journal of Materials Chemistry A, 2022, 10(39): 20827-20836. |
| 62 | LI Gaopeng, WANG Xinlu, Shuhui LYU, et al. Long-life and low-polarization Zn metal anodes enabled by a covalent triazine framework coating[J]. Chemical Engineering Journal, 2022, 450: 138116. |
| 63 | ZHANG Guangfeng, XU Zhixiao, LIU Ping, et al. A facile in situ polymerization strategy towards polyimide/carbon black composites as high performance lithium ion battery cathodes[J]. Electrochimica Acta, 2018, 260: 598-605. |
| 64 | SONG Zhiping, XU Terrence, GORDIN Mikhail L, et al. Polymer–graphene nanocomposites as ultrafast-charge and-discharge cathodes for rechargeable lithium batteries[J]. Nano Letters, 2012, 12(5): 2205-2211. |
| 65 | MENG Yuena, WU Haiping, ZHANG Yajie, et al. A flexible electrode based on a three-dimensional graphene network-supported polyimide for lithium-ion batteries[J]. Journal of Materials Chemistry A, 2014, 2(28): 10842-10846. |
| 66 | LEE Sechan, HONG Jihyun, JUNG Sung-Kyun, et al. Charge-transfer complexes for high-power organic rechargeable batteries[J]. Energy Storage Materials, 2019, 20: 462-469. |
| 67 | WANG Shuchan, HU Naiqi, HUANG Yongxin, et al. Charge-transfer complex promotes energy storage performance of single-moiety organic electrode materials in aqueous zinc-ion battery at low temperatures[J]. Applied Surface Science, 2023, 619: 156725. |
| [1] | 王文君, 刘瑞鑫, 王军, 张庆磊, 侯立安. 浅析二氧化钛材料可见光降解室内VOCs的研究进展[J]. 化工进展, 2025, 44(9): 5351-5362. |
| [2] | 杜磊, 曹志涛, 许浪, 张英杰, 孙宝昌, 邹海魁, 初广文, 陈建峰. 己二腈制备方法的研究进展[J]. 化工进展, 2025, 44(7): 3683-3696. |
| [3] | 任鹏锟, 仲兆平, 张小霓, 杨宇轩, 冉真真. 污泥-木屑基活性炭的制备及其对苯系VOCs的吸附性能[J]. 化工进展, 2025, 44(6): 3031-3040. |
| [4] | 张磊, 张新儒, 王永洪, 李晋平, 刘春波. 二维纳米材料混合基质膜在渗透汽化有机物分离的研究进展[J]. 化工进展, 2025, 44(6): 3324-3335. |
| [5] | 陈奥, 胡广, 盛全康, 陈龙, 张禹, 陈韶云, 胡成龙. 导电玻璃上原位电化学沉积有序聚吡咯纳米阵列及电化学性能[J]. 化工进展, 2025, 44(11): 6534-6541. |
| [6] | 代化, 吴军, 周章华, 张斌. 高能量密度及高功率特性锂一次电池研究进展[J]. 化工进展, 2024, 43(12): 6680-6691. |
| [7] | 张婷婷, 左旭乾, 田玲娣, 王世猛. 化工园区挥发性有机物排放清单及因子库构建方法[J]. 化工进展, 2023, 42(S1): 549-557. |
| [8] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [9] | 王鹏, 史会兵, 赵德明, 冯保林, 陈倩, 杨妲. 过渡金属催化氯代物的羰基化反应研究进展[J]. 化工进展, 2023, 42(9): 4649-4666. |
| [10] | 王帅晴, 杨思文, 李娜, 孙占英, 安浩然. 元素掺杂生物质炭材料在电化学储能中的研究进展[J]. 化工进展, 2023, 42(8): 4296-4306. |
| [11] | 陈飞, 刘成宝, 陈丰, 钱君超, 邱永斌, 孟宪荣, 陈志刚. g-C3N4基超级电容器用电极材料的研究进展[J]. 化工进展, 2023, 42(5): 2566-2576. |
| [12] | 王科菊, 赵成, 胡晓玫, 云军阁, 魏凝涵, 姜雪迎, 邹昀, 陈志航. 金属氧化物低温催化氧化VOCs的研究进展[J]. 化工进展, 2023, 42(5): 2402-2412. |
| [13] | 刘静, 林琳, 张健, 赵峰. 生物质基炭材料孔径调控及电化学性能研究进展[J]. 化工进展, 2023, 42(4): 1907-1916. |
| [14] | 蔡江涛, 候刘华, 兰雨金, 张晨陈, 刘国阳, 朱由余, 张建兰, 赵世永, 张亚婷. 沥青基多孔炭材料的制备及在超级电容器中的应用进展[J]. 化工进展, 2023, 42(4): 1895-1906. |
| [15] | 卓祖优, 宋生南, 黄明堦, 杨旋, 卢贝丽, 陈燕丹. 草酸钾-尿素协同活化法制备超大比表面积面粉基多级孔炭及其电化学储能应用[J]. 化工进展, 2023, 42(2): 925-933. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||