化工进展 ›› 2023, Vol. 42 ›› Issue (11): 6042-6052.DOI: 10.16085/j.issn.1000-6613.2022-2333
• 资源与环境化工 • 上一篇
FeS/壳聚糖基碳气凝胶复合材料的制备及对Cr(Ⅵ)的吸附
- 西安科技大学地质与环境学院,陕西 西安 710054
-
收稿日期:2022-12-19修回日期:2023-02-19出版日期:2023-11-20发布日期:2023-12-15 -
通讯作者:程爱华 -
作者简介:常娟(1997—),女,硕士研究生,研究方向为水处理技术。E-mail:876474652@qq.com。 -
基金资助:国家自然科学基金青年项目(52206277)
Preparation and Cr(Ⅵ) adsorption properties of FeS/chitosan-based carbon aerogel composites
- College of Geology and Environment, Xi’an University of Science and Technology, Xi’an 710054, Shaanxi, China
-
Received:2022-12-19Revised:2023-02-19Online:2023-11-20Published:2023-12-15 -
Contact:CHENG Aihua
摘要:
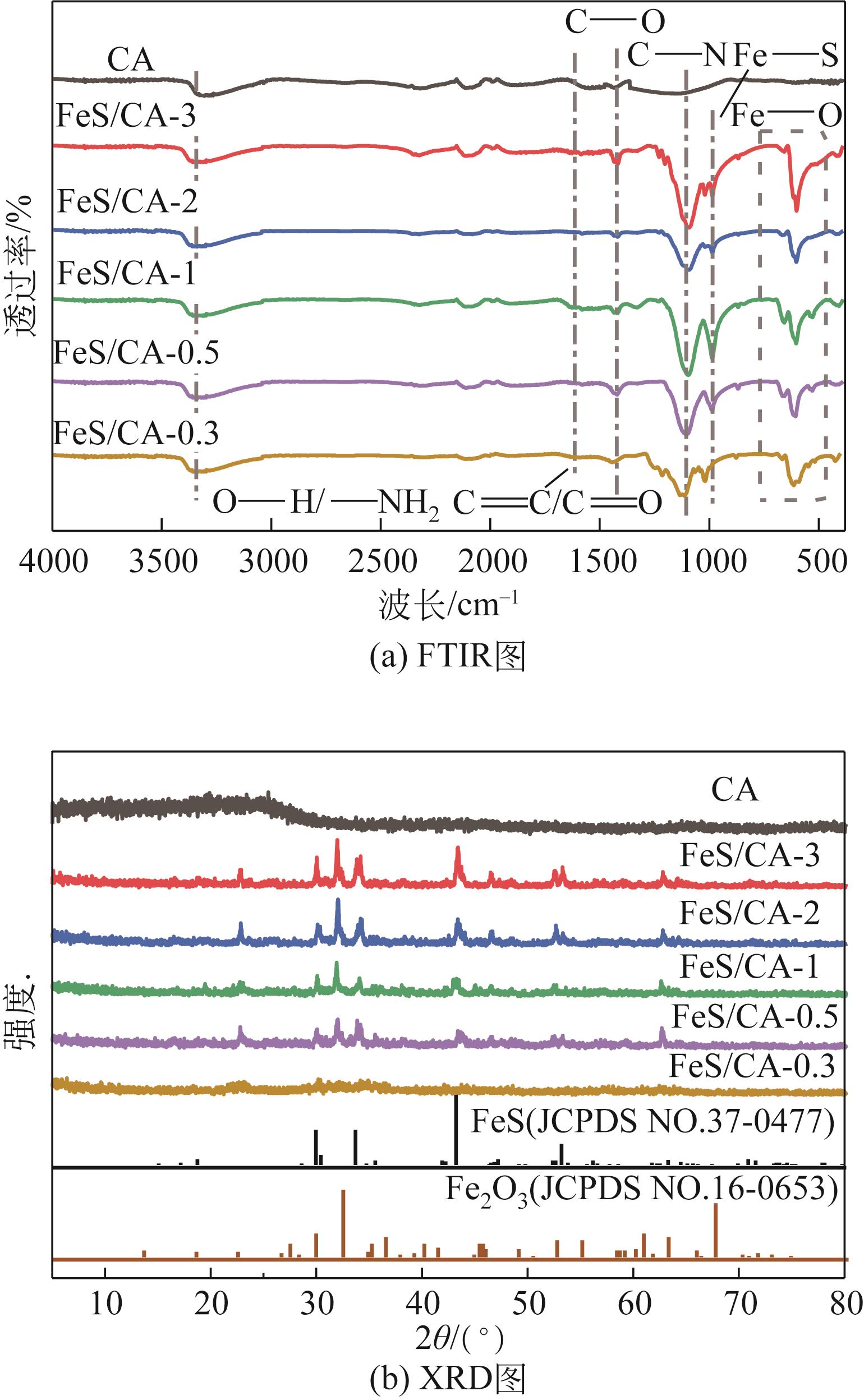
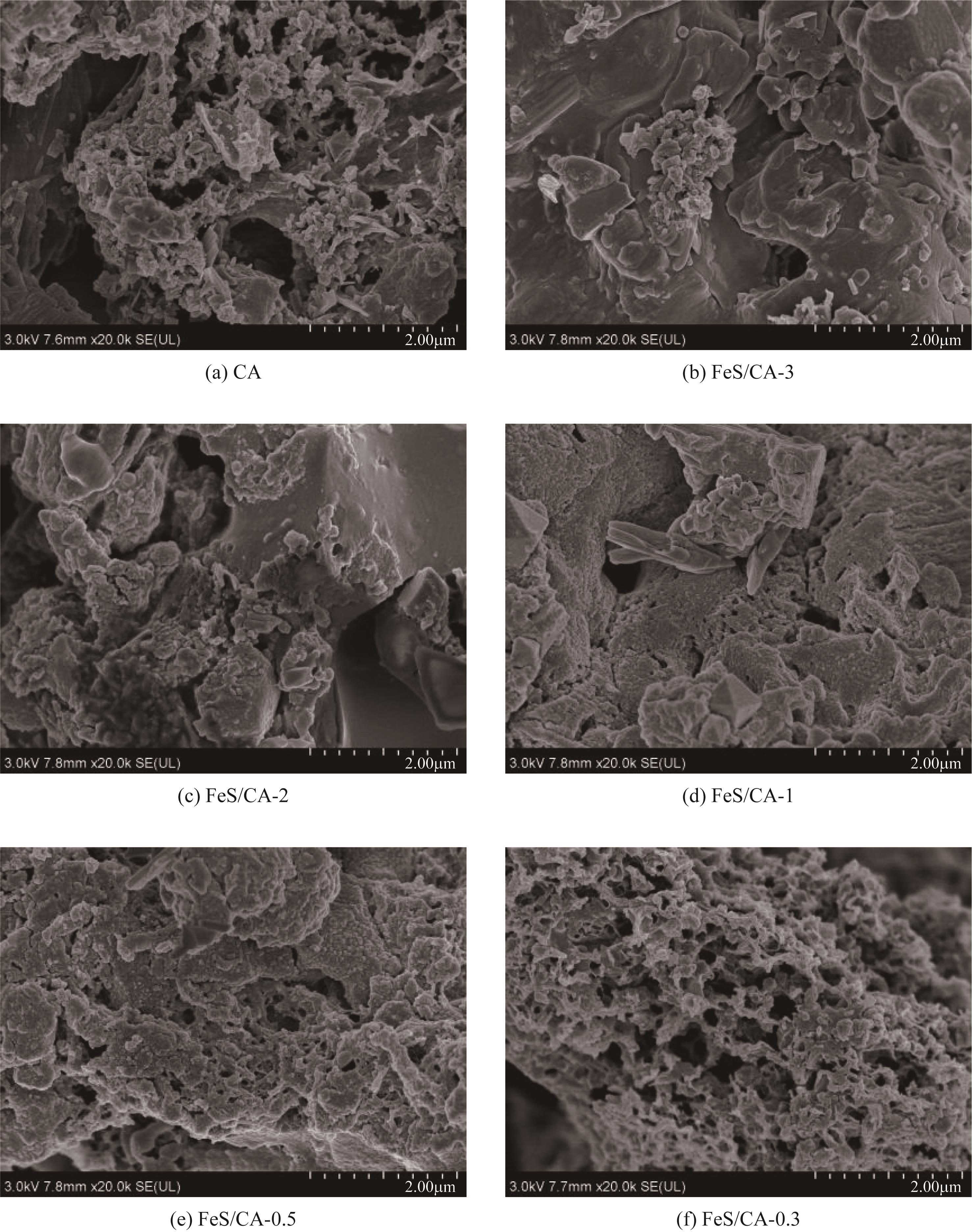
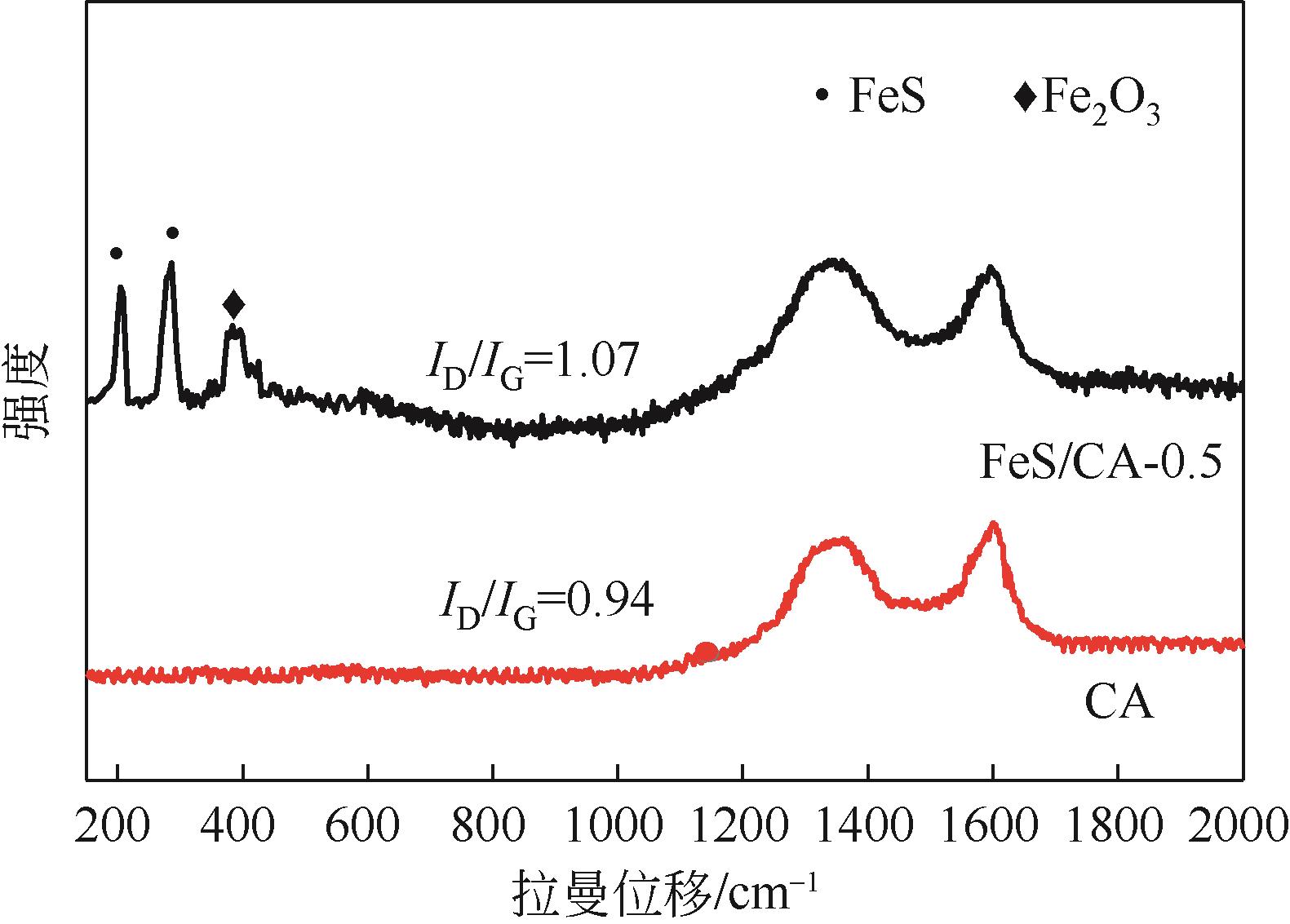
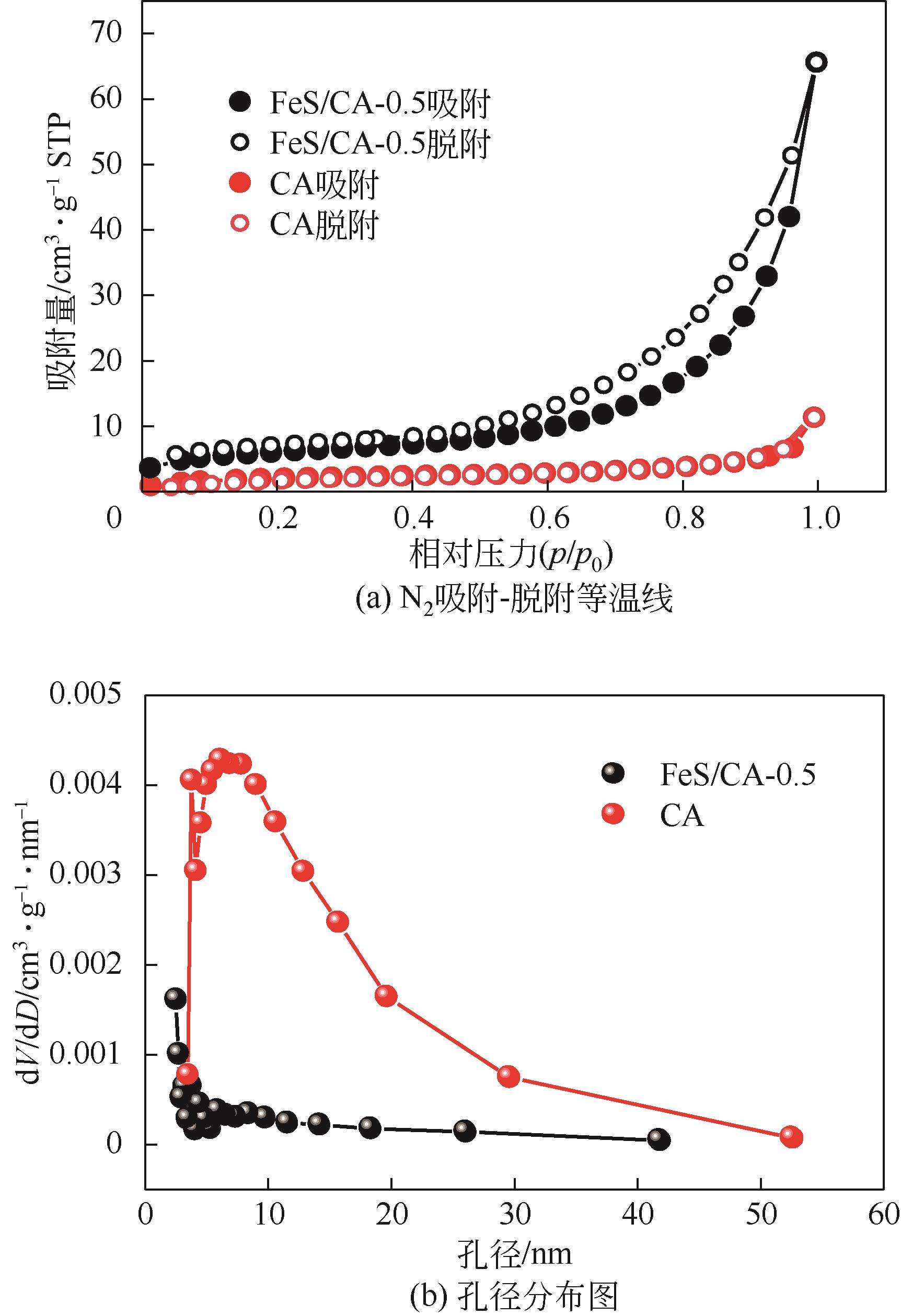
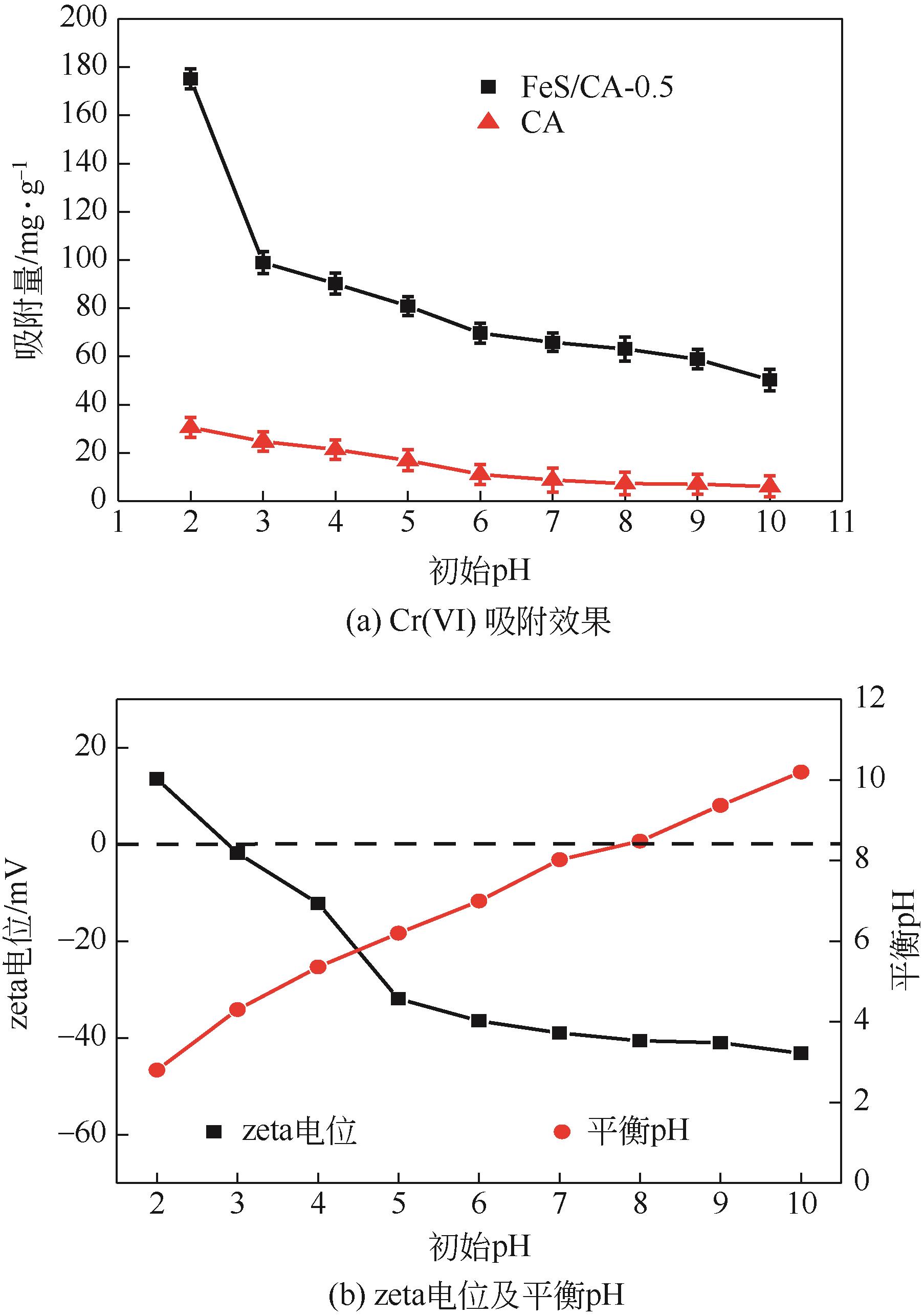
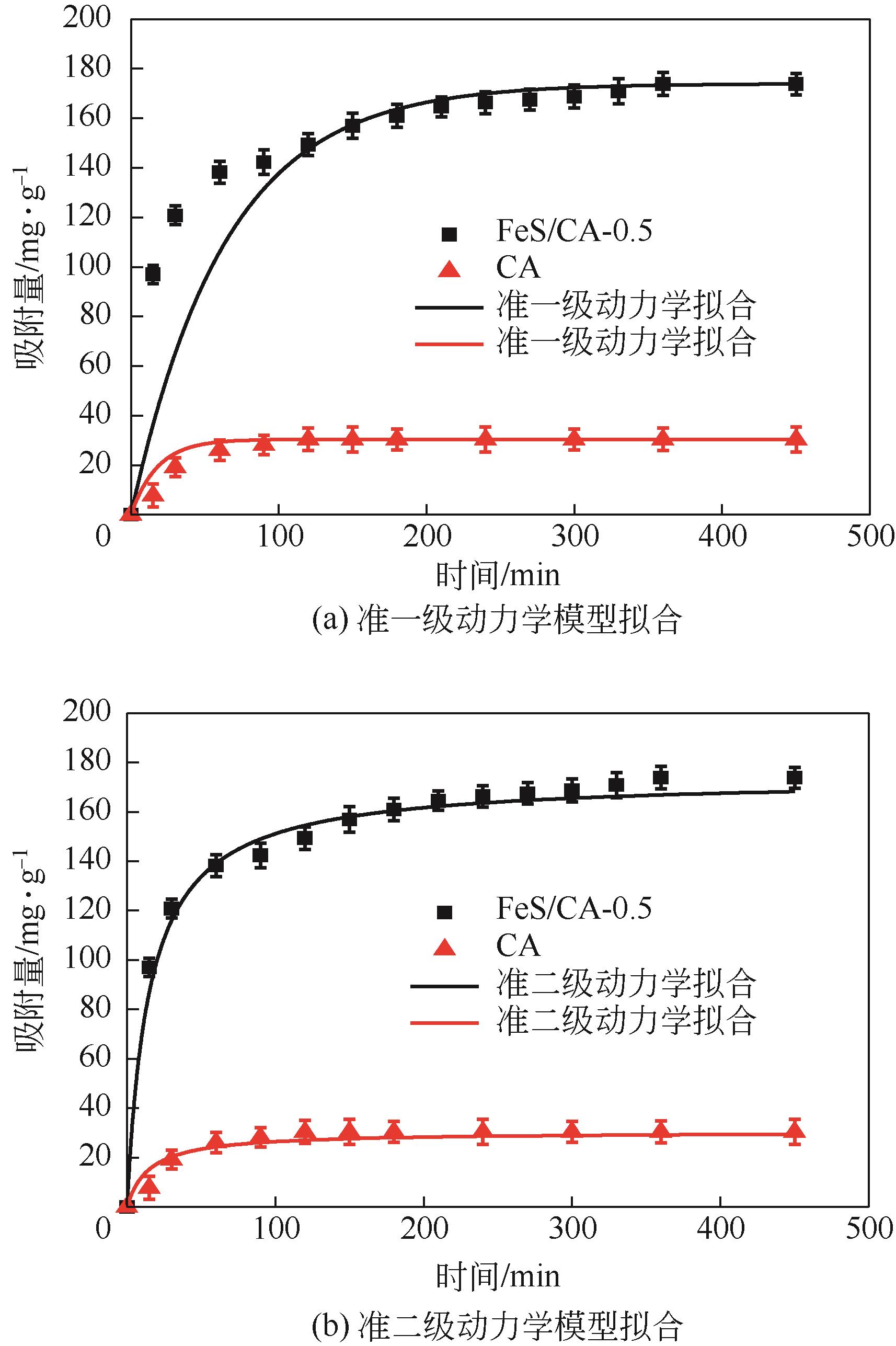
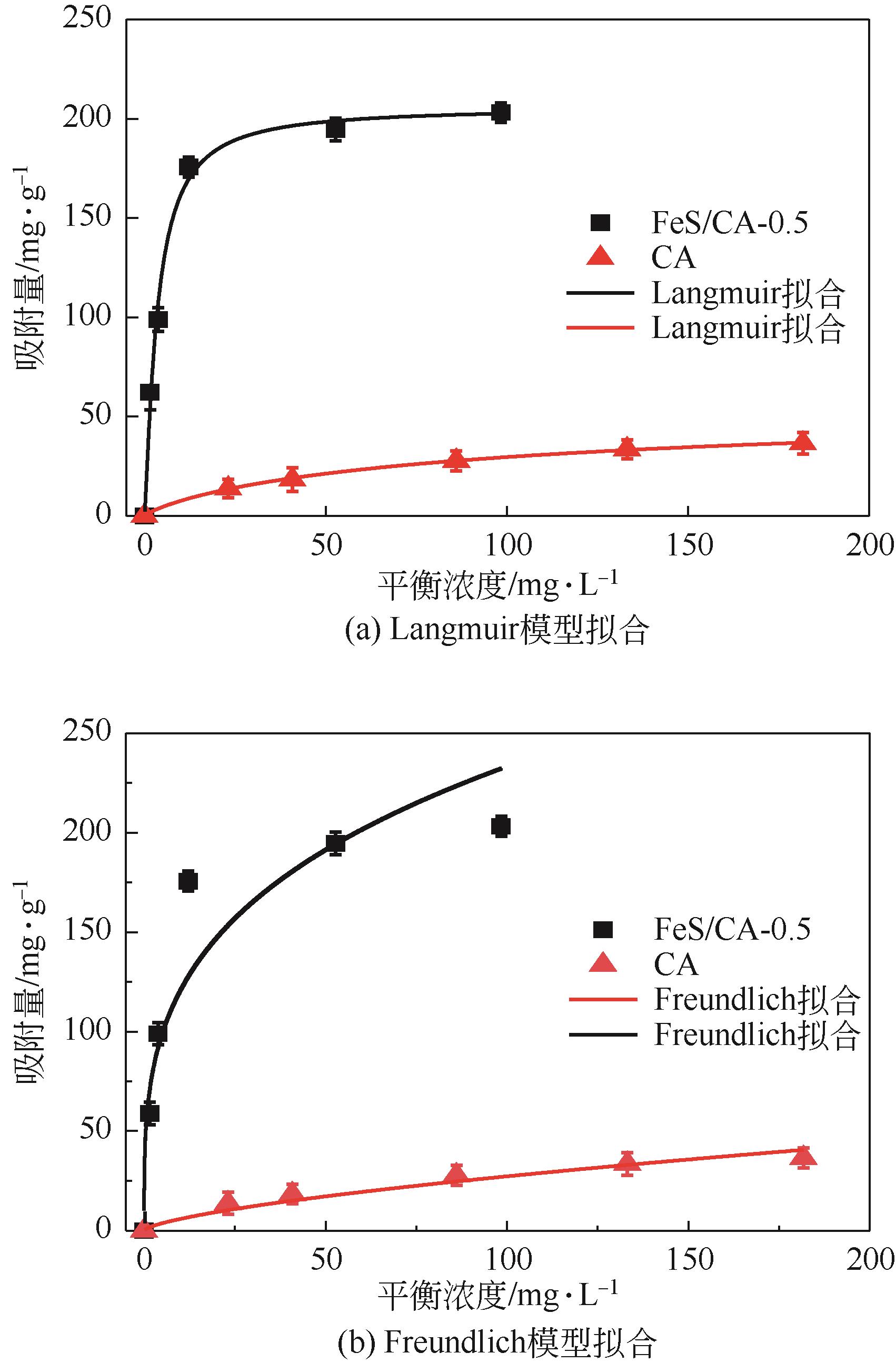
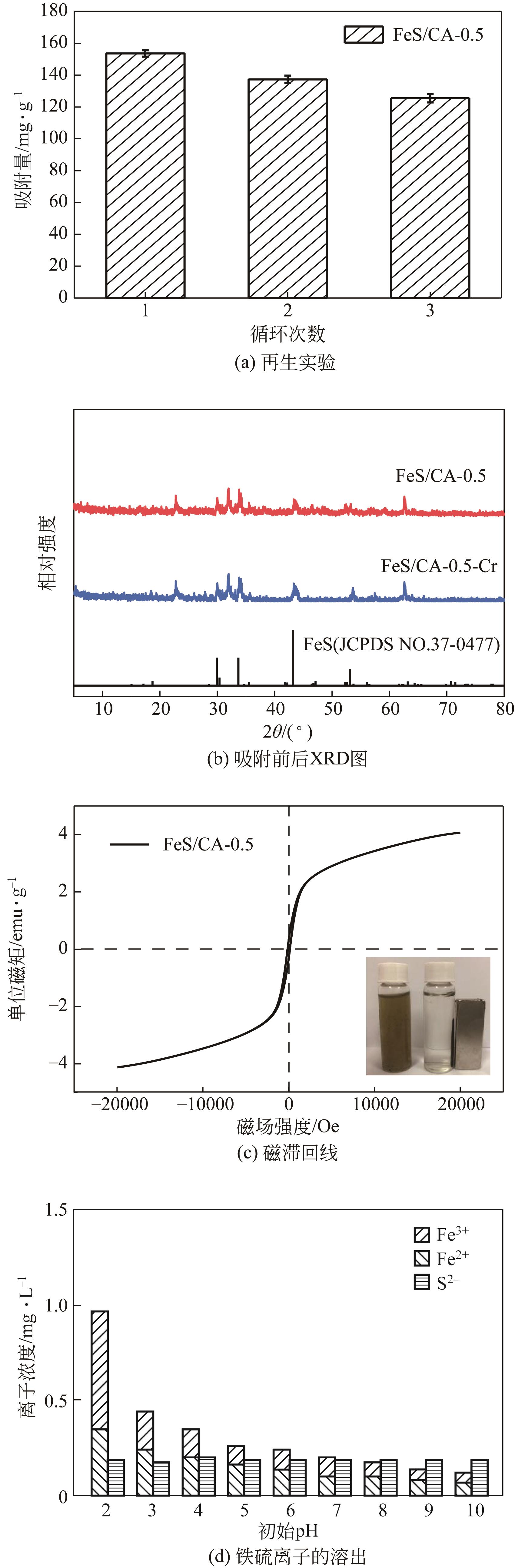
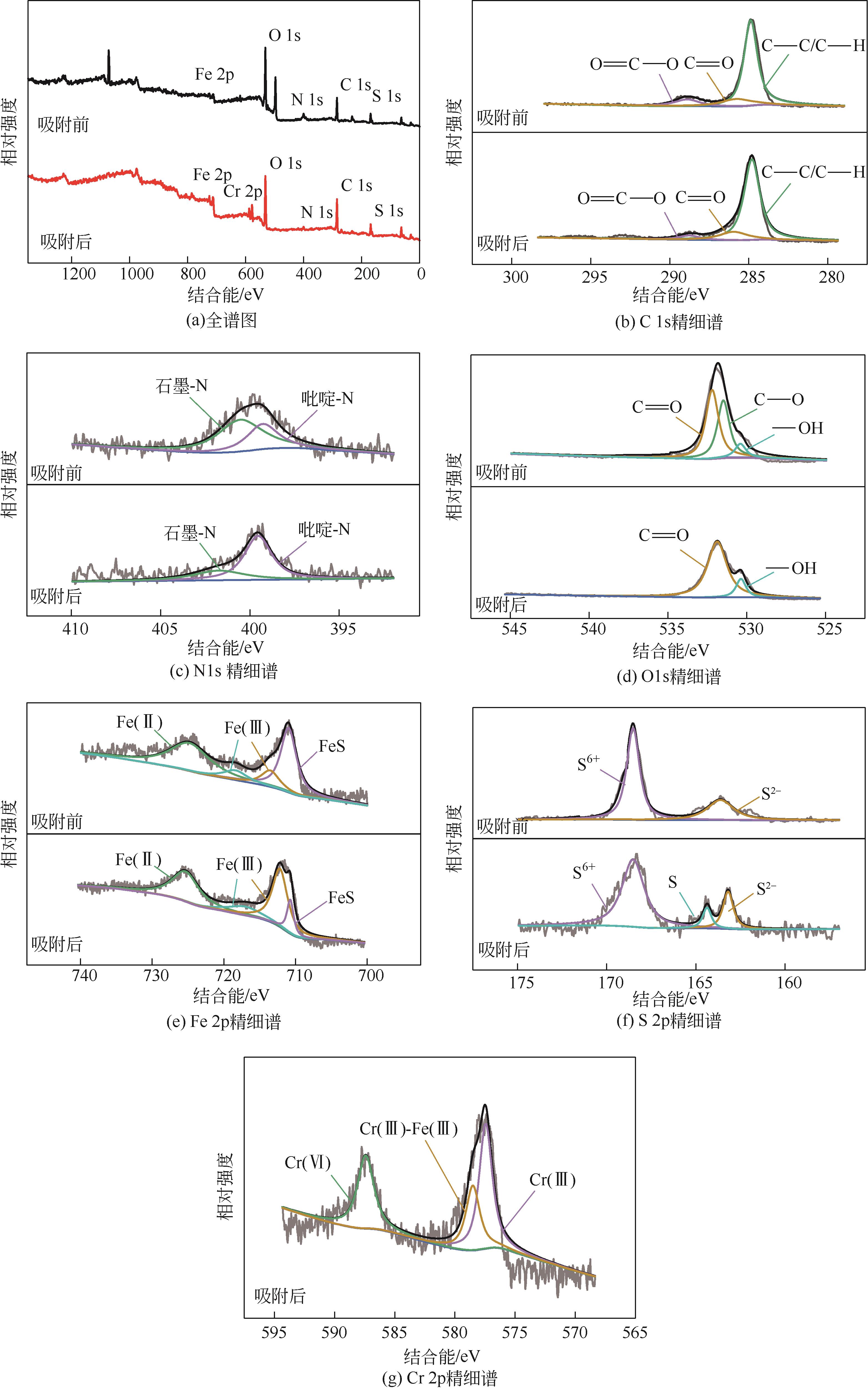
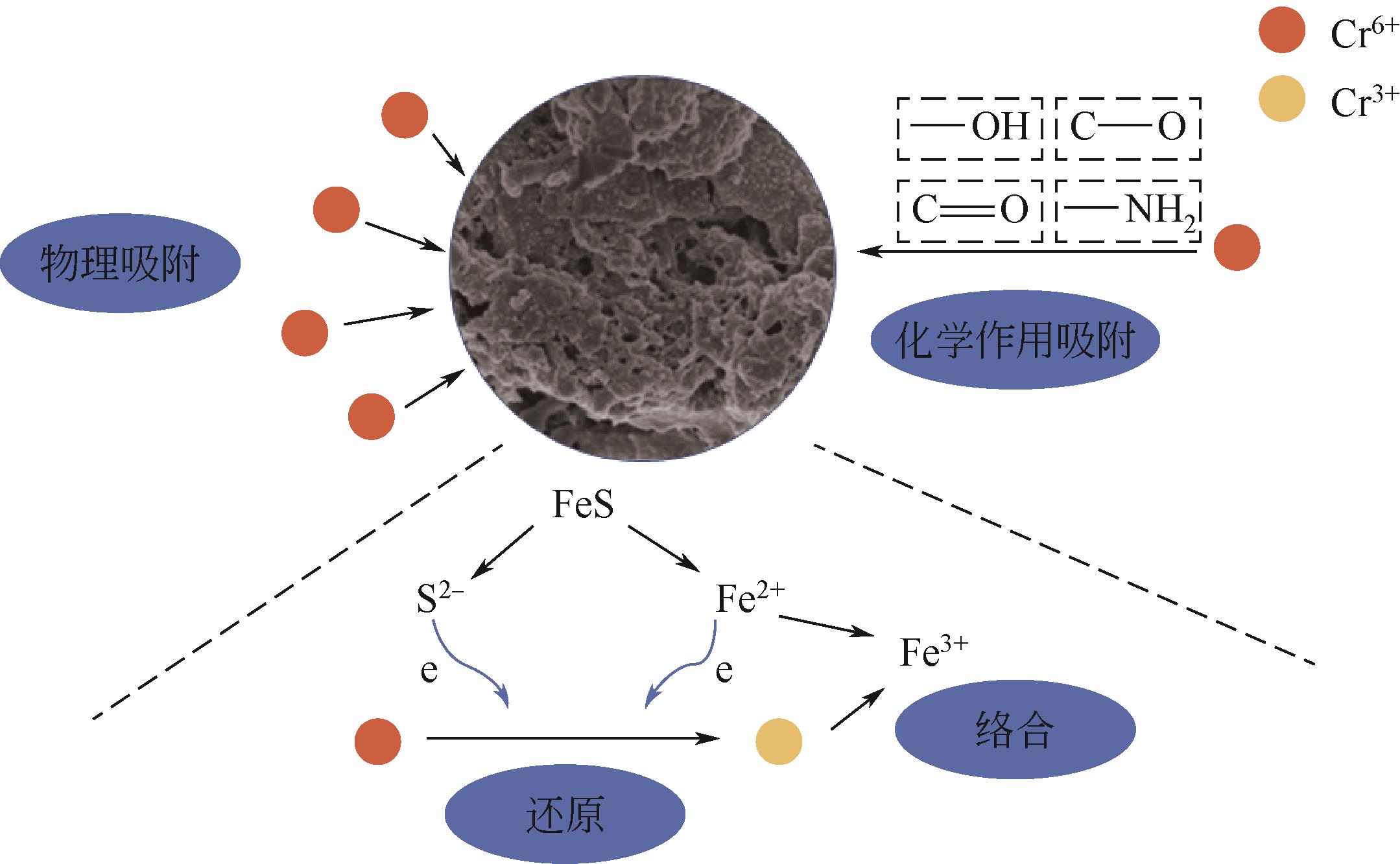
以壳聚糖为前体材料,通过溶胶-凝胶、冷冻干燥、碳化等方法制备FeS/壳聚糖基碳气凝胶复合材料(FeS/CA-0.5)。利用SEM、FTIR、XRD及XPS等方法对FeS/CA-0.5的微观形态和结构进行了表征,考察了FeS/CA-0.5对Cr(Ⅵ)的吸附性能,探究了反应机制。结果表明:FeS/CA-0.5保留壳聚糖基碳气凝胶的结构且成功分散FeS,比表面积大,具有超顺磁性;在100mL 100mg/L的Cr(Ⅵ)溶液中投加0.05gFeS/CA-0.5,pH为2,吸附360min可达到平衡,FeS/CA-0.5对Cr(Ⅵ)的平衡吸附量可达173.84mg/g;吸附过程符合准二级动力学和Langmuir等温吸附模型,颗粒内扩散模型表明FeS/CA-0.5对Cr(Ⅵ)的吸附是多步骤过程,整个吸附过程是自发吸热的;FeS/CA-0.5除铬机制主要是物理吸附、化学吸附及化学还原作用。FeS/CA-0.5在处理含铬废水方面有潜在应用前景。
中图分类号:
引用本文
常娟, 程爱华. FeS/壳聚糖基碳气凝胶复合材料的制备及对Cr(Ⅵ)的吸附[J]. 化工进展, 2023, 42(11): 6042-6052.
CHANG Juan, CHENG Aihua. Preparation and Cr(Ⅵ) adsorption properties of FeS/chitosan-based carbon aerogel composites[J]. Chemical Industry and Engineering Progress, 2023, 42(11): 6042-6052.
| 样品 | 比表面积/m2·g-1 | 孔体积/cm3·g-1 | 孔径/nm |
|---|---|---|---|
| CA | 45.89 | 0.008 | 19.15 |
| FeS/CA-3 | 83.10 | 0.024 | 8.60 |
| FeS/CA-2 | 87.95 | 0.044 | 9.05 |
| FeS/CA-1 | 189.97 | 0.063 | 8.30 |
| FeS/CA-0.5 | 210.32 | 0.100 | 7.40 |
| FeS/CA-0.3 | 220.07 | 0.097 | 6.60 |
表1 CA和不同比例FeS/CA复合材料的比表面积和孔隙性质
| 样品 | 比表面积/m2·g-1 | 孔体积/cm3·g-1 | 孔径/nm |
|---|---|---|---|
| CA | 45.89 | 0.008 | 19.15 |
| FeS/CA-3 | 83.10 | 0.024 | 8.60 |
| FeS/CA-2 | 87.95 | 0.044 | 9.05 |
| FeS/CA-1 | 189.97 | 0.063 | 8.30 |
| FeS/CA-0.5 | 210.32 | 0.100 | 7.40 |
| FeS/CA-0.3 | 220.07 | 0.097 | 6.60 |
| 吸附剂 | 准一级动力学模型 | 准二级动力学模型 | ||||
|---|---|---|---|---|---|---|
| k1/min-1 | R2 | qe/mg·g-1 | k2/g·mg-1·min-1 | R2 | qe/mg·g-1 | |
| CA | 0.022 | 0.972 | 31.222 | 0.029 | 0.889 | 46.211 |
| FeS/CA-0.5 | 0.071 | 0.989 | 73.484 | 0.038 | 0.998 | 180.180 |
表2 CA和FeS/CA-0.5吸附Cr(Ⅵ)的动力学参数
| 吸附剂 | 准一级动力学模型 | 准二级动力学模型 | ||||
|---|---|---|---|---|---|---|
| k1/min-1 | R2 | qe/mg·g-1 | k2/g·mg-1·min-1 | R2 | qe/mg·g-1 | |
| CA | 0.022 | 0.972 | 31.222 | 0.029 | 0.889 | 46.211 |
| FeS/CA-0.5 | 0.071 | 0.989 | 73.484 | 0.038 | 0.998 | 180.180 |
| 吸附剂 | 颗粒内扩散模型 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| kd1 | C1 | R2 | kd2 | C2 | R2 | kd3 | C3 | R2 | |||
| CA | 4.48 | -7.59 | 0.859 | 0.18 | 27.35 | 0.348 | — | — | — | ||
| FeS/CA-0.5 | 10.65 | 58.13 | 0.924 | 4.47 | 100.64 | 0.986 | 2.09 | 133.35 | 0.927 | ||
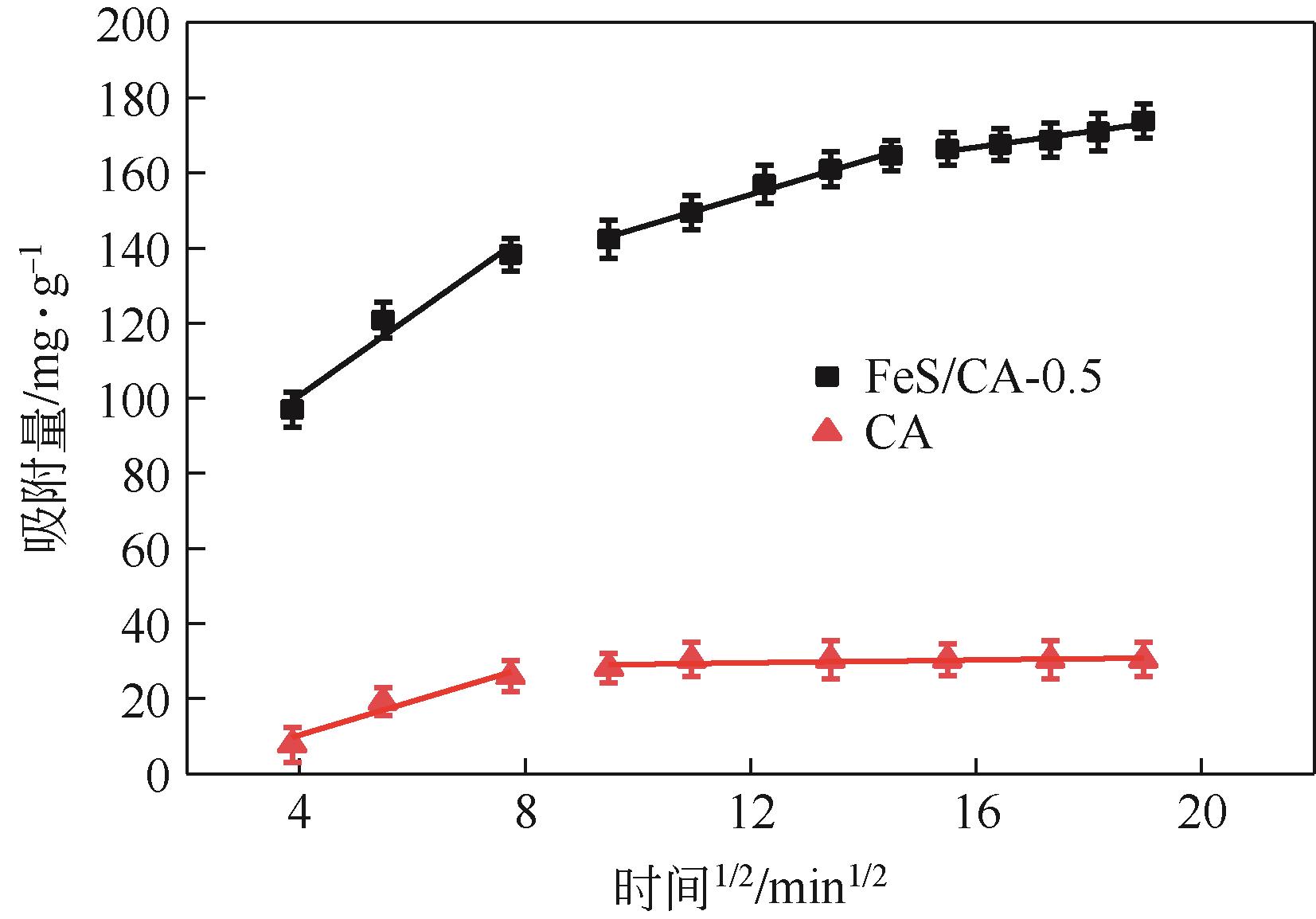
表3 CA和FeS/CA-0.5吸附Cr(Ⅵ)颗粒内扩散模型拟合参数
| 吸附剂 | 颗粒内扩散模型 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| kd1 | C1 | R2 | kd2 | C2 | R2 | kd3 | C3 | R2 | |||
| CA | 4.48 | -7.59 | 0.859 | 0.18 | 27.35 | 0.348 | — | — | — | ||
| FeS/CA-0.5 | 10.65 | 58.13 | 0.924 | 4.47 | 100.64 | 0.986 | 2.09 | 133.35 | 0.927 | ||
| 吸附剂 | Langmuir模型 | Freundlich模型 | ||||
|---|---|---|---|---|---|---|
| b | R2 | Q0/mg·g-1 | KF | R2 | n | |
| CA | 0.018 | 0.990 | 46.253 | 3.004 | 0.990 | 2.053 |
| FeS/CA-0.5 | 0.245 | 0.993 | 184.592 | 62.434 | 0.864 | 3.493 |
表4 CA和FeS/CA-0.5吸附Cr(Ⅵ)的Langmuir和Freundlich吸附等温线参数
| 吸附剂 | Langmuir模型 | Freundlich模型 | ||||
|---|---|---|---|---|---|---|
| b | R2 | Q0/mg·g-1 | KF | R2 | n | |
| CA | 0.018 | 0.990 | 46.253 | 3.004 | 0.990 | 2.053 |
| FeS/CA-0.5 | 0.245 | 0.993 | 184.592 | 62.434 | 0.864 | 3.493 |
| C0/mg·L-1 | ΔG/kJ·mol-1 | ΔH/kJ·mol-1 | ΔS/kJ·mol-1·K-1 | ||
|---|---|---|---|---|---|
| 298 | 308 | 318 | |||
| 100 | -23.476 | -26.086 | -28.696 | 54.302 | 0.261 |
| 150 | -20.131 | -21.461 | -22.791 | 19.503 | 0.133 |
| 200 | -19.017 | -20.157 | -21.297 | 14.955 | 0.114 |
表5 FeS/CA-0.5吸附Cr(Ⅵ)的热力学参数
| C0/mg·L-1 | ΔG/kJ·mol-1 | ΔH/kJ·mol-1 | ΔS/kJ·mol-1·K-1 | ||
|---|---|---|---|---|---|
| 298 | 308 | 318 | |||
| 100 | -23.476 | -26.086 | -28.696 | 54.302 | 0.261 |
| 150 | -20.131 | -21.461 | -22.791 | 19.503 | 0.133 |
| 200 | -19.017 | -20.157 | -21.297 | 14.955 | 0.114 |
| 1 | YU Shujun, TANG Hao, ZHANG Di, et al. MXenes as emerging nanomaterials in water purification and environmental remediation[J]. Science of the Total Environment, 2022, 811: 152280. |
| 2 | YU Shujun, PANG Hongwei, HUANG Shuyi, et al. Recent advances in metal-organic framework membranes for water treatment: A review [J]. Science of the Total Environment, 2021, 800: 149662. |
| 3 | WU Zhibin, YUAN Xingzhong, ZENG Guangming, et al. Highly efficient photocatalytic activity and mechanism of Yb3+/Tm3+ codoped In2S3 from ultraviolet to near infrared light towards chromium(VI) reduction and rhodamine B oxydative degradation[J]. Applied Catalysis B: Environmental, 2018, 225: 8-21. |
| 4 | YU Peijing, FU Fenglian, SUN Guangzhao, et al. Effects of oxalate and citrate on the behavior and redistribution of Cr(Ⅵ) during ferrihydrite-Cr( Ⅵ ) co-precipitates transformation[J]. Chemosphere, 2021, 266: 128977. |
| 5 | LU Wanli, DUAN Chao, ZHANG Yanling, et al. Cellulose-based electrospun nanofiber membrane with core-sheath structure and robust photocatalytic activity for simultaneous and efficient oil emulsions separation, dye degradation and Cr(Ⅵ) reduction[J]. Carbohydrate Polymers, 2021, 258: 117676-117686. |
| 6 | SENGUTTUVAN S, SENTHILKUMAR P, JANAKI V, et al. Significance of conducting polyaniline based composites for the removal of dyes and heavy metals from aqueous solution and wastewaters—A review [J]. Chemosphere, 2021, 267: 129201. |
| 7 | 周春地, 阳婷, 闵熙泽, 等. 零价铁、铜改性生物炭及其对Cr(Ⅵ)吸附性能的影响[J]. 化工进展, 2020, 39(10): 4275-4282. |
| ZHOU Chundi, YANG Ting, MIN Xize, et al. Influence of zero valent iron and copper modified biochar on Cr(Ⅵ) adsorption[J]. Chemical Industry and Engineering Progress, 2020, 39(10): 4275-4282. | |
| 8 | LEE Jong-hoon, PARK Soo-jin. Recent advances in preparations and applications of carbon aerogels: A review[J]. Carbon, 2020, 163: 1-18. |
| 9 | YANG Chen, LIU Dan, HUANG Shaoming, et al. Pressure-induced monolithic carbon aerogel from metal-organic framework[J]. Energy Storage Materials, 2020, 28: 393-400. |
| 10 | WANG Jingyu, LIU Wenting, LI Xiaopeng, et al. Strong hydrophilicity NiS2/Fe7S8 heterojunctions encapsulated in N-doped carbon nanotubes for enhanced oxygen evolution reaction[J]. Chemical Communications, 2020, 56(10): 1489-1492. |
| 11 | YU Miao, HAN Yingying, LI Jian, et al. Magnetic N-doped carbon aerogel from sodium carboxymethyl cellulose/collagen composite aerogel for dye adsorption and electrochemical supercapacitor[J].International Journal of Biological Macromolecules, 2018, 115: 185-193. |
| 12 | JING Zefeng, DING Jichao, ZHANG Tao, et al. Flexible, versatility and superhydrophobic biomass carbon aerogels derived from corn bracts for efficient oil/water separation[J]. Food and Bioproducts Processing, 2019, 115: 134-142. |
| 13 | SONG Junying, MESSELE Selamawit Ashagre, MENG Lingjun, et al. Adsorption of metals from oil sands process water (OSPW) under natural pH by sludge-based Biochar/Chitosan composite[J]. Water Research, 2021, 194: 116930. |
| 14 | WU Qiong, HU Jinqiu, CAO Shuangshuang, et al. Heteroatom-doped hierarchical porous carbon aerogels from chitosan for high performance supercapacitors[J]. International Journal of Biological Macromolecules, 2020, 155: 131-141. |
| 15 | WANG Yifan, VAN ZWIETEN Lukas, WANG Hailong, et al. Sorption of Pb(Ⅱ) onto biochar is enhanced through co-sorption of dissolved organic matter[J]. Science of The Total Environment, 2022, 825: 153686. |
| 16 | WANG Zhiwei, WANG Yahuan, CAO Shuai, et al. Fabrication of core@shell structural Fe-Fe2O3@PHCP nanochains with high saturation magnetization and abundant amino groups for hexavalent chromium adsorption and reduction[J]. Journal of Hazardous Materials, 2020, 384: 121483. |
| 17 | JIANG Xiancai, XIANG Xiaotong, PENG Shuijiao, et al. Facile preparation of nitrogen-doped activated mesoporous carbon aerogel from chitosan for methyl orange adsorption from aqueous solution[J]. Cellulose, 2019, 26(7): 4515-4527. |
| 18 | LI Jiahao, CHENG Rong, CHEN Jiaao, et al. Microscopic mechanism about the selective adsorption of Cr(Ⅵ) from salt solution on nitrogen-doped carbon aerogel microsphere pyrolysis products[J]. Science of the Total Environment, 2021, 798: 149331. |
| 19 | YUAN Yanmei, TAO Hong, FAN Jinhong, et al. Degradation of p-chloroaniline by persulfate activated with ferrous sulfide ore particles[J]. Chemical Engineering Journal, 2015, 268: 38-46. |
| 20 | HE Xingyu, MIN Xiaobo, PENG Tianyu, et al. Mechanochemically activated microsized zero-valent iron/pyrite composite for effective hexavalent chromium sequestration in aqueous solution[J]. Journal of Chemical & Engineering Data, 2020, 65(4): 1936-1945. |
| 21 | 张浩. 壳聚糖稳定的硫化亚铁磁性复合材料的制备及其对铬的去除研究[D]. 长沙: 湖南农业大学, 2019. |
| ZHANG Hao. The preparation of chitosan-stabilized FeS magnetic composites and its application for chromium removal[D]. Changsha: Hunan Agricultural University, 2019. | |
| 22 | 郭秦铭, 崔金萍, 陈明星, 等. CA/MoS2复合物的制备及其去除六价铬的性能[J]. 浙江理工大学学报(自然科学版), 2021, 45(2): 212-220. |
| GUO Qinming, CUI Jinping, CHEN Mingxing, et al. Preparation of CA/MoS2 composite and its removal of hexavalent chromium[J]. Journal of Zhejiang Sci-Tech University (Natural Sciences Edition), 2021, 45(2): 212-220. | |
| 23 | 孙悦. 硫化亚铁复合材料的制备及其对水中汞离子的去除性能研究[D]. 杭州: 浙江大学, 2018. |
| SUN Yue. Preparation of iron sulfide composites for the removal of mercury from aqueous system[D]. Hangzhou: Zhejiang University, 2018. | |
| 24 | ZHANG Hao, PENG Liang, CHEN Anwei, et al. Chitosan-stabilized FeS magnetic composites for chromium removal: characterization, performance, mechanism, and stability[J]. Carbohydrate Polymers, 2019, 214: 276-285. |
| 25 | 郭成, 郝军杰, 李明阳, 等. 海藻酸钠/聚乙烯亚胺凝胶球的合成及对Cr(Ⅵ)的吸附性能和机制[J]. 复合材料学报, 2021, 38(7): 2140-2151. |
| GUO Cheng, HAO Junjie, LI Mingyang, et al. Adsorption of Cr(Ⅵ) on porous sodium alginate/polyethyleneimine hydrogel beads and its mechanistic study[J]. Acta Materiae Compositae Sinica, 2021, 38(7): 2140-2151. | |
| 26 | CHEN Yongjun, MA Rui, PU Xunchi, et al. The characterization of a novel magnetic biochar derived from sulfate-reducing sludge and its application for aqueous Cr(Ⅵ) removal through synergistic effects of adsorption and chemical reduction[J]. Chemosphere, 2022, 308: 136258. |
| 27 | SUN Zhichao, LIU Yunguo, HUANG Yuanqing, et al. Fast adsorption of Cd2+ and Pb2+ by EGTA dianhydride (EGTAD) modified ramie fiber [J]. Journal of Colloid and Interface Science, 2014, 434: 152-158. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 胡喜, 王明珊, 李恩智, 黄思鸣, 陈俊臣, 郭秉淑, 于博, 马志远, 李星. 二硫化钨复合材料制备与储钠性能研究进展[J]. 化工进展, 2023, 42(S1): 344-355. |
| [3] | 崔守成, 徐洪波, 彭楠. 两种MOFs材料用于O2/He吸附分离的模拟分析[J]. 化工进展, 2023, 42(S1): 382-390. |
| [4] | 陈崇明, 陈秋, 宫云茜, 车凯, 郁金星, 孙楠楠. 分子筛基CO2吸附剂研究进展[J]. 化工进展, 2023, 42(S1): 411-419. |
| [5] | 许春树, 姚庆达, 梁永贤, 周华龙. 共价有机框架材料功能化策略及其对Hg(Ⅱ)和Cr(Ⅵ)的吸附性能研究进展[J]. 化工进展, 2023, 42(S1): 461-478. |
| [6] | 顾永正, 张永生. HBr改性飞灰对Hg0的动态吸附及动力学模型[J]. 化工进展, 2023, 42(S1): 498-509. |
| [7] | 郭强, 赵文凯, 肖永厚. 增强流体扰动强化变压吸附甲硫醚/氮气分离的数值模拟[J]. 化工进展, 2023, 42(S1): 64-72. |
| [8] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [9] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [10] | 林晓鹏, 肖友华, 管奕琛, 鲁晓东, 宗文杰, 傅深渊. 离子聚合物-金属复合材料(IPMC)柔性电极的研究进展[J]. 化工进展, 2023, 42(9): 4770-4782. |
| [11] | 杨莹, 侯豪杰, 黄瑞, 崔煜, 王兵, 刘健, 鲍卫仁, 常丽萍, 王建成, 韩丽娜. 利用煤焦油中酚类物质Stöber法制备碳纳米球用于CO2吸附[J]. 化工进展, 2023, 42(9): 5011-5018. |
| [12] | 张振, 李丹, 陈辰, 吴菁岚, 应汉杰, 乔浩. 吸附树脂对唾液酸的分离纯化[J]. 化工进展, 2023, 42(8): 4153-4158. |
| [13] | 姜晶, 陈霄宇, 张瑞妍, 盛光遥. 载锰生物炭制备及其在环境修复中应用研究进展[J]. 化工进展, 2023, 42(8): 4385-4397. |
| [14] | 于静文, 宋璐娜, 刘砚超, 吕瑞东, 武蒙蒙, 冯宇, 李忠, 米杰. 一种吲哚基超交联聚合物In-HCP对水中碘的吸附作用[J]. 化工进展, 2023, 42(7): 3674-3683. |
| [15] | 李艳玲, 卓振, 池亮, 陈曦, 孙堂磊, 刘鹏, 雷廷宙. 氮掺杂生物炭的制备与应用研究进展[J]. 化工进展, 2023, 42(7): 3720-3735. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||