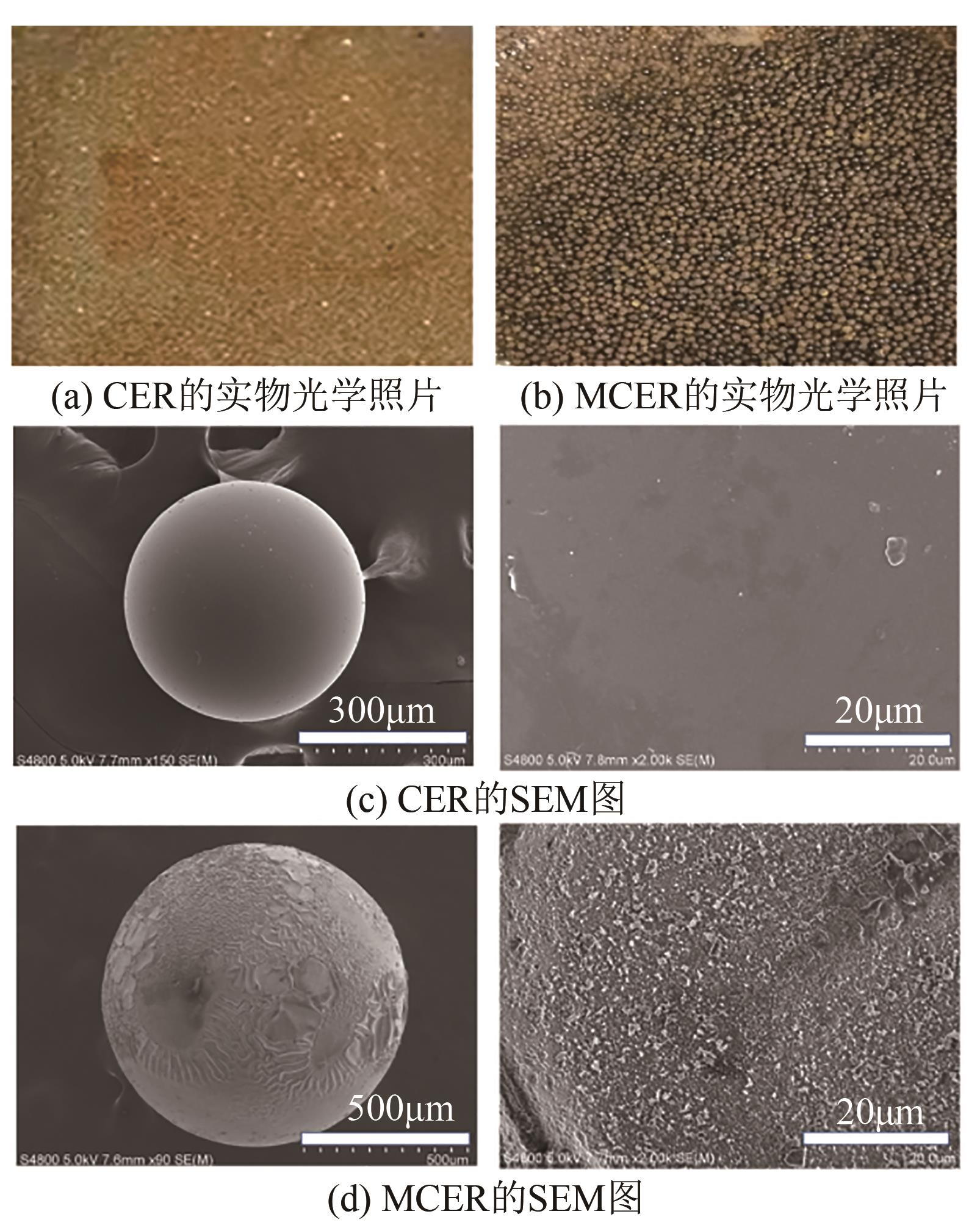
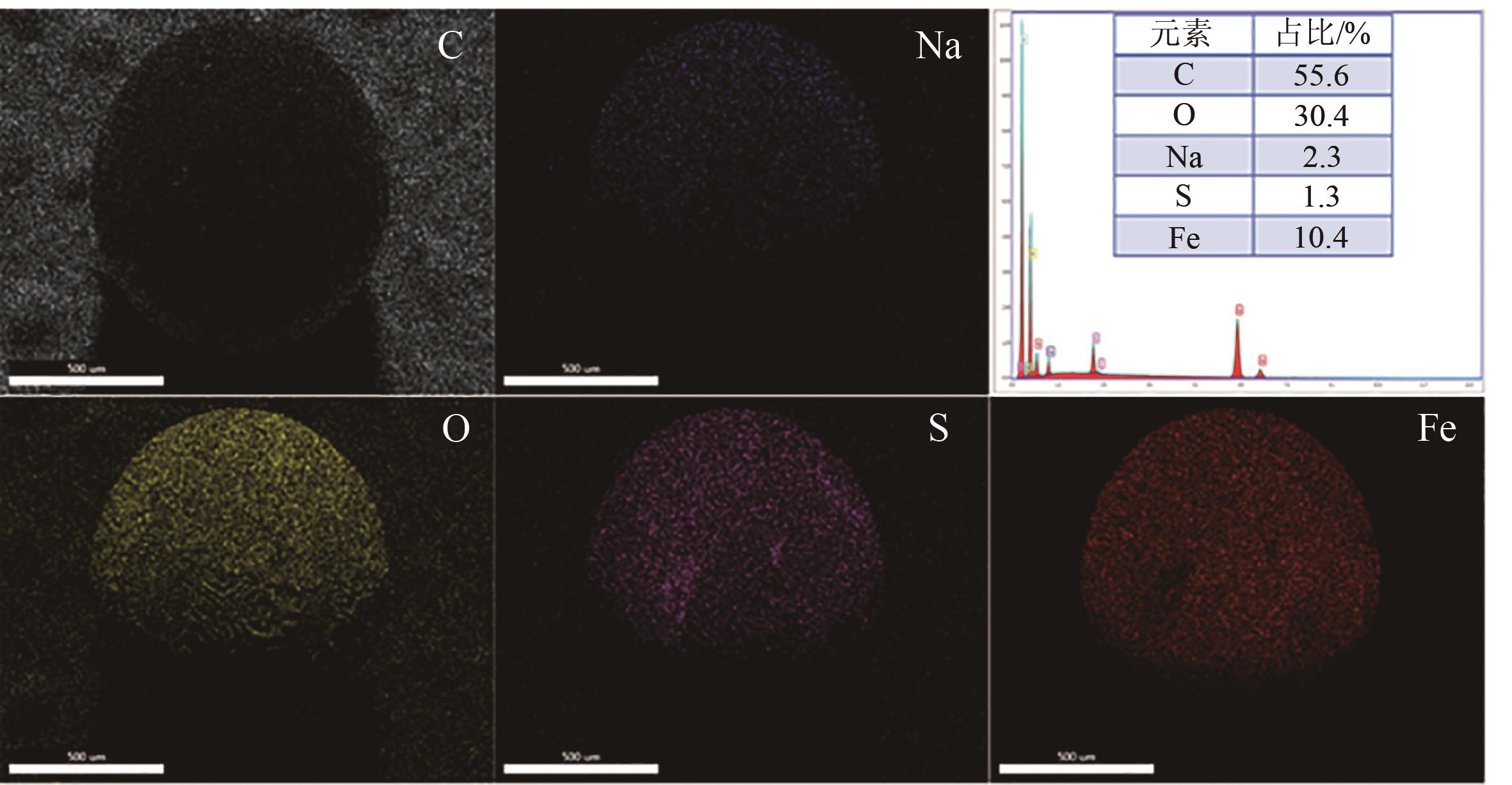
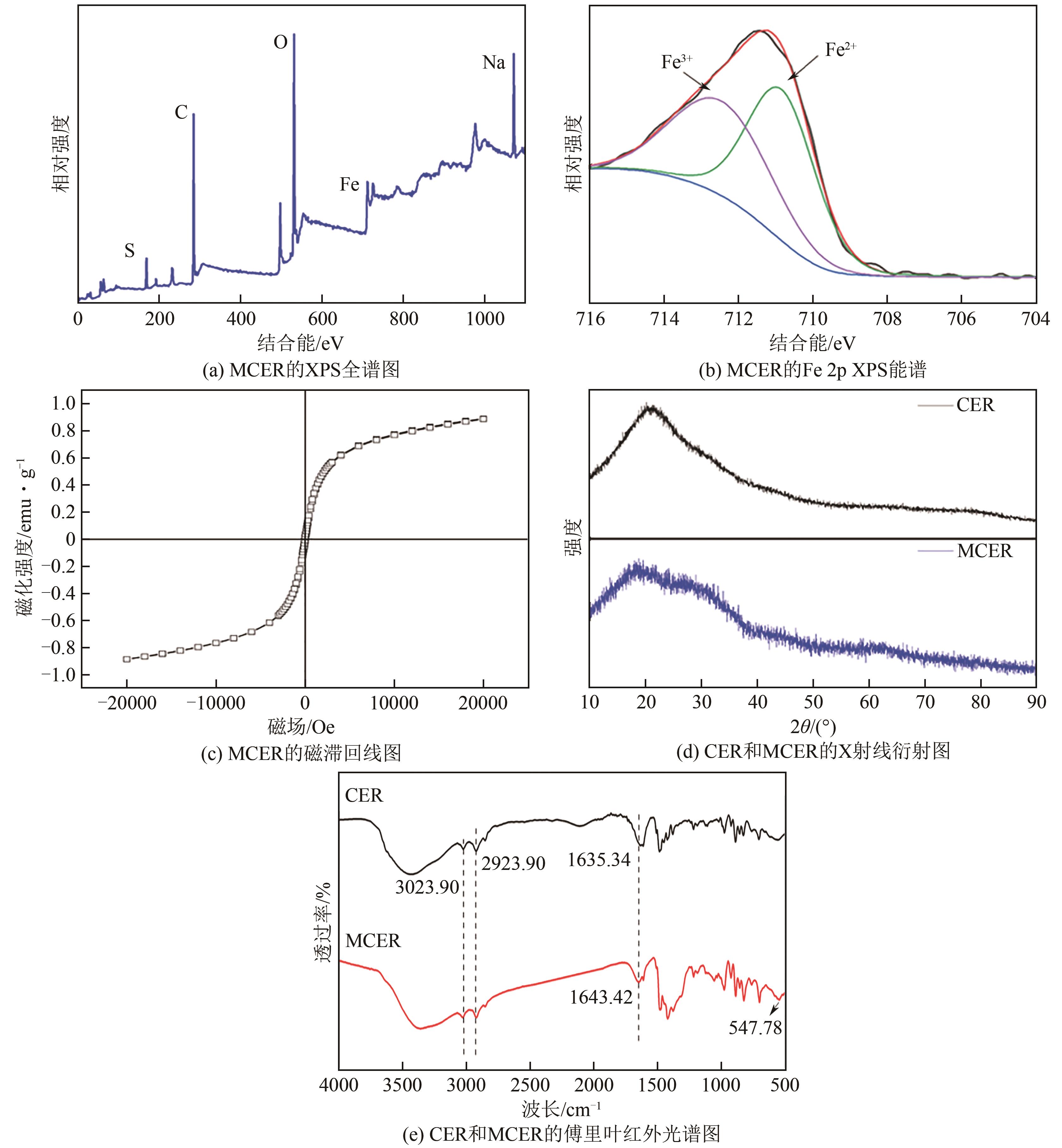
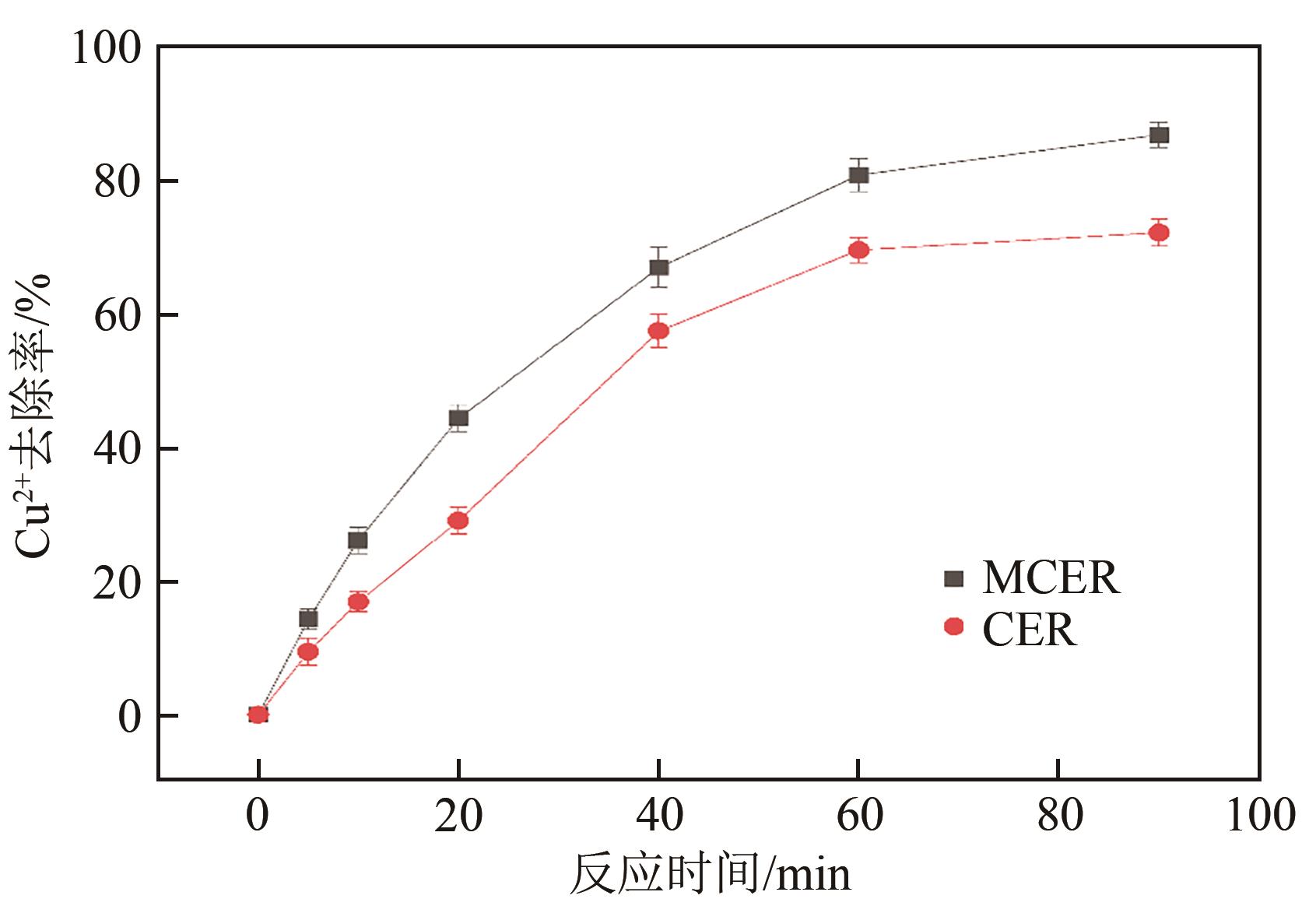
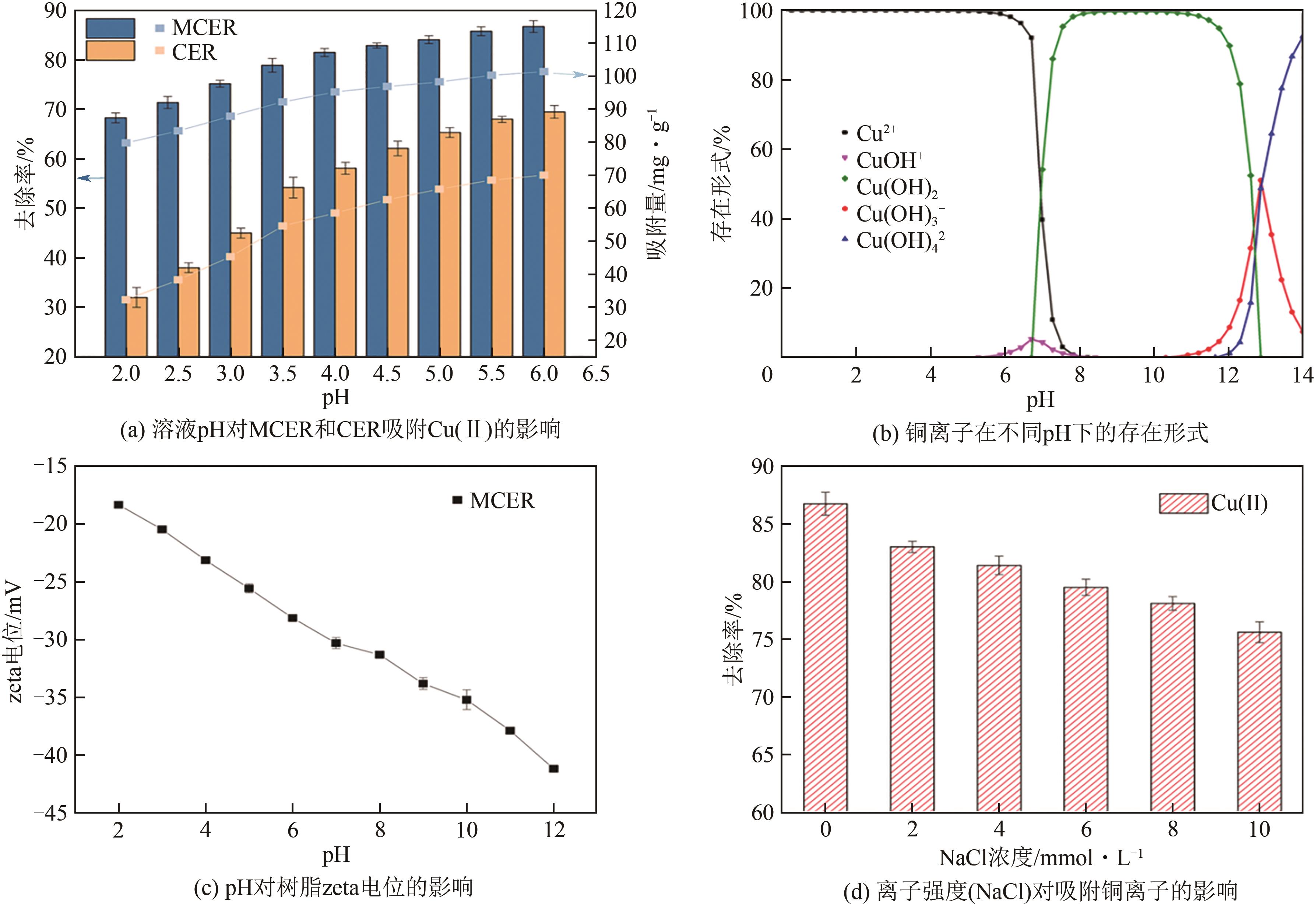
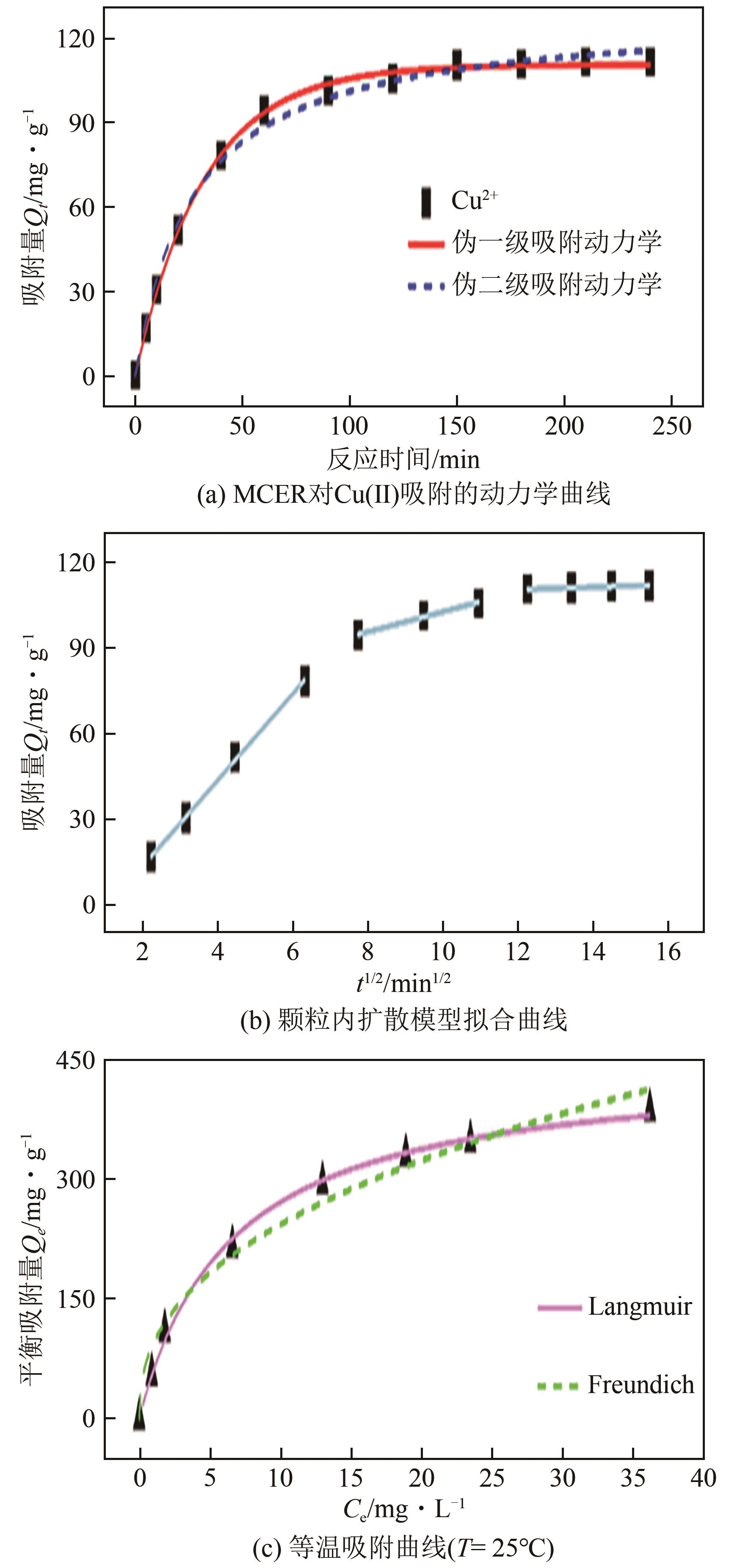
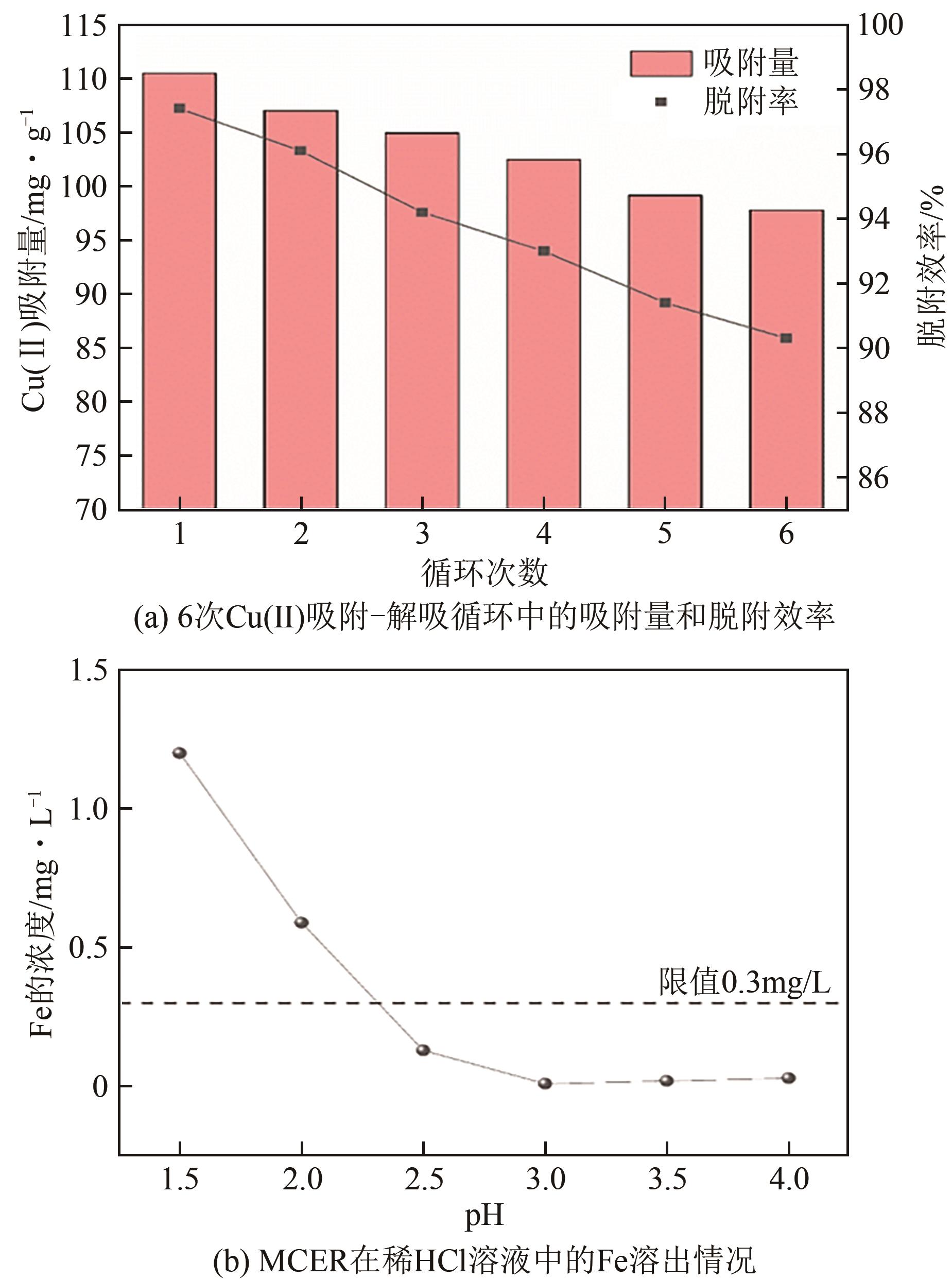
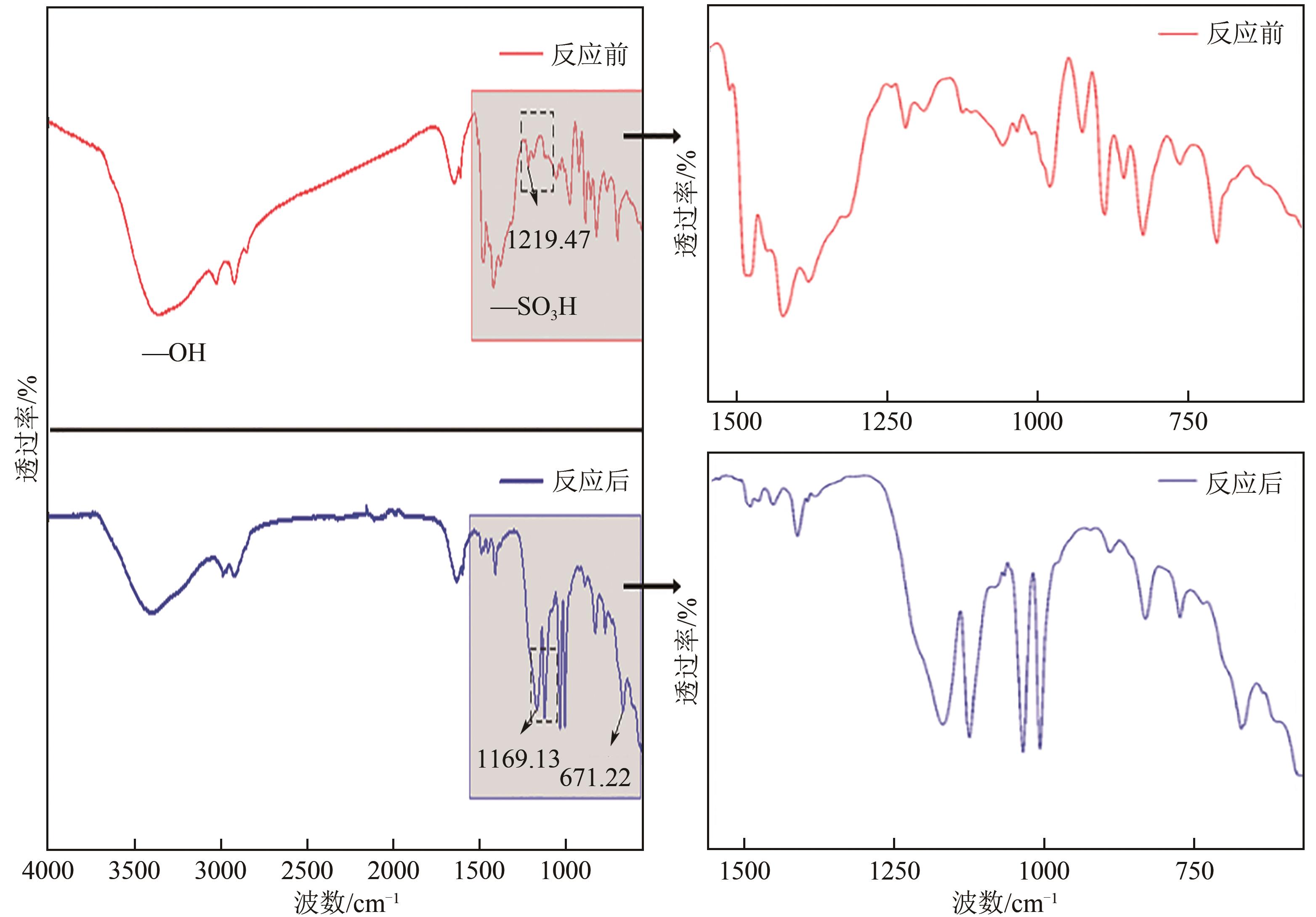
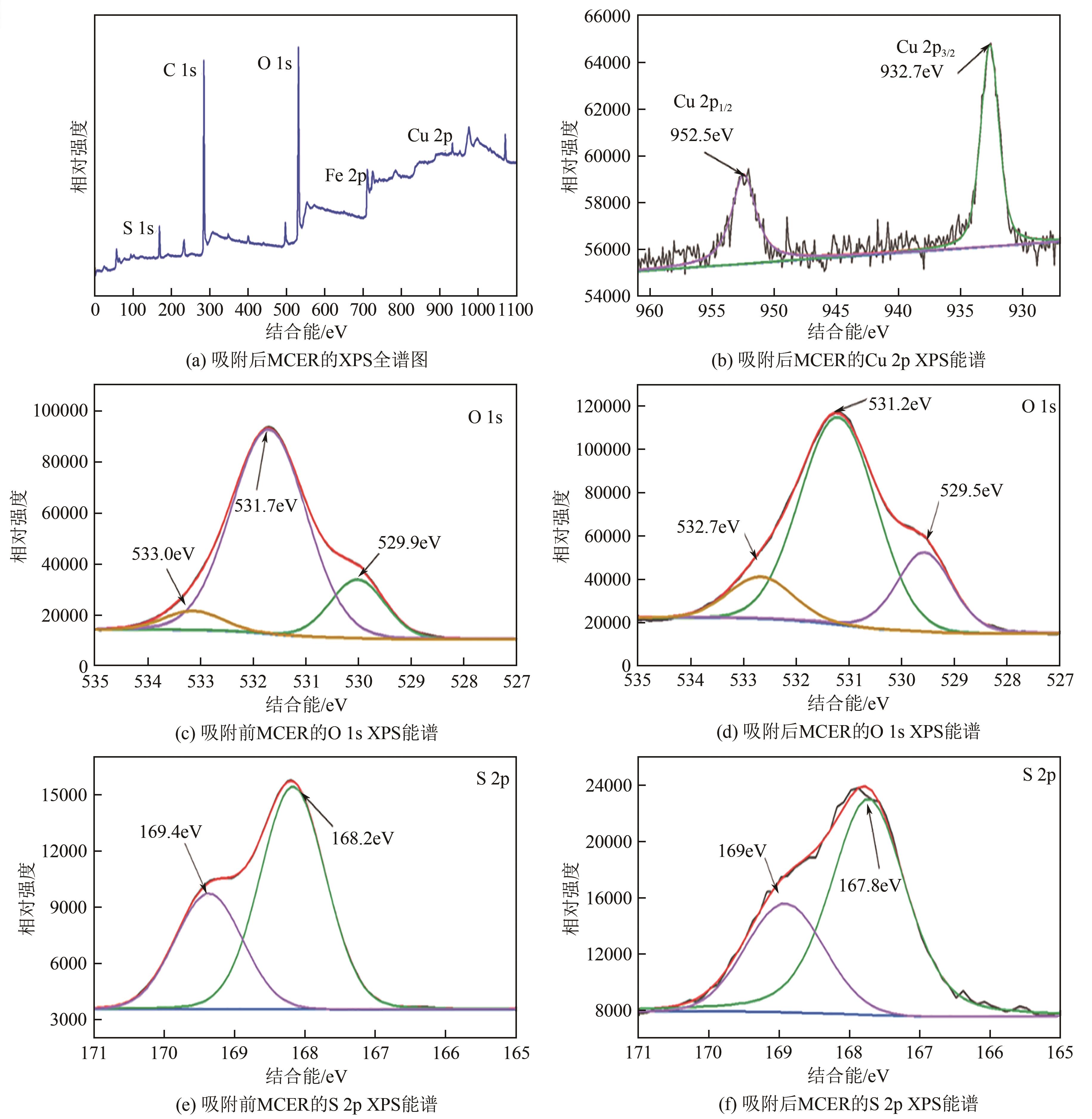
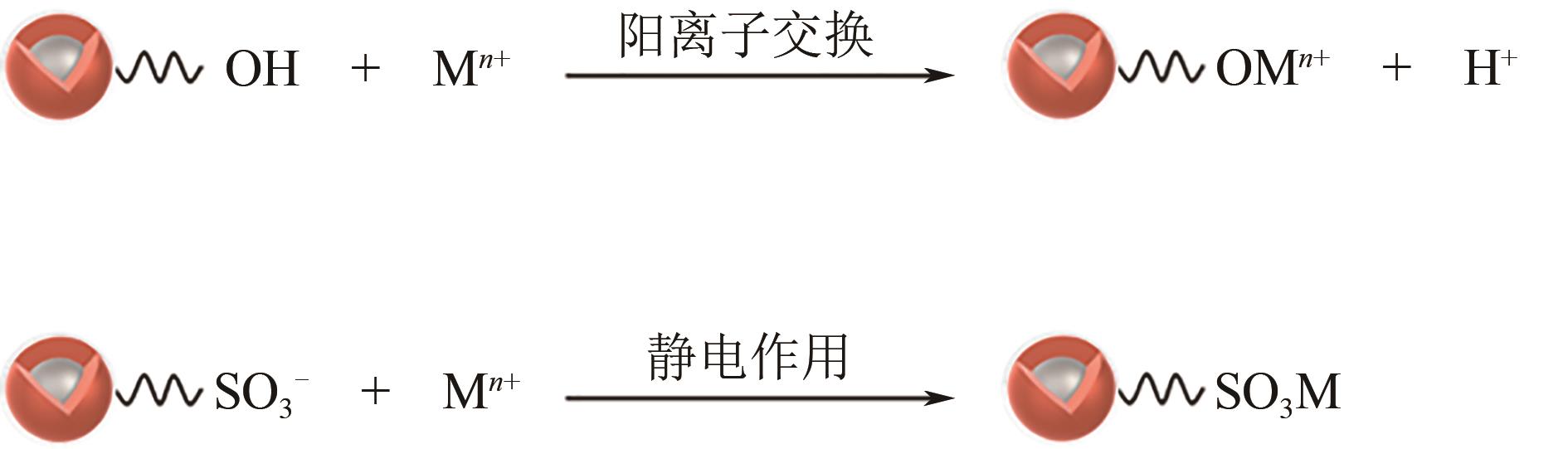
| 1 |
李征. 重金属污染水体的环境保护处理技术研究[J]. 环境与发展, 2018, 30(11): 110-111.
|
|
LI Zheng. Research on environmental protection treatment technology based on heavy metal polluted water body[J]. Environment and Development, 2018, 30(11): 110-111.
|
| 2 |
雷兆武, 孙颖. 离子交换技术在重金属废水处理中的应用[J]. 环境科学与管理, 2008, 33(10): 82-84.
|
|
LEI Zhaowu, SUN Ying. Application of lon exchange technology in heavy metals wastewater treatment[J]. Environmental Science and Management, 2008, 33(10): 82-84.
|
| 3 |
BERGQUIST Allison M, CHOE Jong Kwon, STRATHMANN Timothy J, et al. Evaluation of a hybrid ion exchange-catalyst treatment technology for nitrate removal from drinking water[J]. Water Research,2016, 96: 177-187.
|
| 4 |
CHANTHAPON Nopphorn, SARKAR Sudipta, KIDKHUNTHOD Pinit, et al. Lead removal by a reusable gel cation exchange resin containing nano-scale zero valent iron[J]. Chemical Engineering Journal, 2018, 331: 545-555.
|
| 5 |
李海斌, 张克华, 陈庆典, 等. 氨基磷酸螯合树脂(D418)高效去除Cu(Ⅱ)的性能与机制[J]. 复合材料学报, 2021, 38(4): 1128-1138.
|
|
LI Haibin, ZHANG Kehua, CHEN Qingdian, et al. Performance and mechanism of the amino methylene phosphonic acid chelating resin(D418) for the efficient removal of Cu(Ⅱ)[J]. Acta Materiae Compositae Sinica, 2021, 38(4): 1128-1138.
|
| 6 |
GE Yuru, LI Yushu, ZU Baiyi, et al. AM-DMC-AMPS multi-functionalized magnetic nanoparticles for efficient purification of complex multiphase water system[J]. Nanoscale Research Letters, 2016, 11(1): 217.
|
| 7 |
路翠萍. 磁性聚苯乙烯基大孔树脂的制备及对水溶液中金属离子的吸附性能研究[D]. 兰州: 兰州理工大学, 2014.
|
|
LU Cuiping. Study on the synthesis of magnetic polystyrene based macroporous resins and their adsorption performance to metal ions in aqueous solution[D]. Lanzhou: Lanzhou University of Technology, 2014.
|
| 8 |
LI Qimeng, FU Lichun, Wang Zheng, et al. Synthesis and characterization of a novel magnetic cation exchange resin and its application for efficient removal of Cu2+ and Ni2+ from aqueous solutions[J]. Journal of Cleaner Production, 2017, 165: 801-810.
|
| 9 |
王彤彤, 马江波, 曲东, 等. 两种木材生物炭对铜离子的吸附特性及其机制[J]. 环境科学, 2017, 38(5): 2161-2171.
|
|
WANG Tongtong, MA Jiangbo, QU Dong, et al. Characteristics and mechanism of copper adsorption from aqueous solutio ns on biochar produced from sawdust and apple branch[J]. Environmental Science, 2017, 38(5): 2161-2171.
|
| 10 |
谢超然, 王兆炜, 朱俊民, 等. 核桃青皮生物炭对重金属铅、铜的吸附特性研究[J]. 环境科学学报, 2016, 36(4): 1190-1198.
|
|
XIE Chaoran, WANG Zhaowei, ZHU Junmin, et al. Adsorption of lead and copper from aqueous solutions on biochar produced from walnut green husk[J]. Acta Scientiae Circumstantiae, 2016, 36(4): 1190-1198.
|
| 11 |
ZHENG Xinyu, ZHENG Huaili, XIONG Zikang, et al. Novel anionic polyacrylamide-modify-chitosan magnetic composite nanoparticles with excellent adsorption capacity for cationic dyes and pH-independent adsorption capability for metal ions[J]. Chemical Engineering Journal, 2020, 392: 123706.
|
| 12 |
LIU Biming, LIU Zhenxue, WU Haixia, et al. Effective and simultaneous removal of organic/inorganic arsenic using polymer-based hydrated iron oxide adsorbent: Capacity evaluation and mechanism[J]. The Science of the Total Environment, 2020, 742: 140508.
|
| 13 |
HUANG Feng, LI Wenzhi, LIU Qingchuan, et al. Sulfonated tobacco stem carbon as efficient catalyst for dehydration of C6 carbohydrate to 5-hydroxymethylfurfural in γ-valerolactone/water[J]. Fuel Processing Technology, 2018, 181: 294-303.
|
 ), LU Jiayue1,2, WANG Fanggang1,2
), LU Jiayue1,2, WANG Fanggang1,2