化工进展 ›› 2023, Vol. 42 ›› Issue (2): 765-773.DOI: 10.16085/j.issn.1000-6613.2022-0703
Cu改性对稀土尾矿催化剂NH3-SCR脱硝的影响
侯丽敏1,2,3( ), 许杰1, 付善聪1, 武文斐1,2,3(
), 许杰1, 付善聪1, 武文斐1,2,3( )
)
- 1.内蒙古科技大学能源与环境学院,内蒙古自治区高效洁净燃烧重点实验室,内蒙古 包头 014010
2.内蒙古科技大学白云鄂博矿多金属资源综合利用重点实验室,内蒙古 包头 014010
3.内蒙古科技大学白云鄂博共伴生矿资源高效综合利用省部共建协同创新中心,内蒙古 包头 014010
-
收稿日期:2022-04-19修回日期:2022-09-21出版日期:2023-02-25发布日期:2023-03-13 -
通讯作者:武文斐 -
作者简介:侯丽敏(1988—),女,博士,讲师,研究方向为稀土尾矿改性制备催化剂、矿产资源综合利用。E-mail:neuhlm@163.com。 -
基金资助:国家重点研发计划(2020YFC1909102);内蒙古自然科学基金(2019ZD13);国家自然科学基金(51866013);内蒙古自治区本级事业单位引进人才科研启动支持经费;内蒙古自治区直属高校基本科研业务费
Effect of Cu modification on NH3-SCR denitration of rare earth tailings catalyst
HOU Limin1,2,3( ), XU Jie1, FU Shancong1, WU Wenfei1,2,3(
), XU Jie1, FU Shancong1, WU Wenfei1,2,3( )
)
- 1.Key Laboratory of Efficient and Clean Combustion, School of Energy and Environment, Inner Mongolia University of Science & Technology, Baotou 014010, Inner Mongolia, China
2.Key Laboratory of Integrated Exploitation of Bayan Obo Multi-Metal Resources, Inner Mongolia University of Science & Technology, Baotou 014010, Inner Mongolia, China
3.Collaborative Innovation Center of Integrated Exploitation of Bayan Obo Multi-Metal Resources, Inner Mongolia University of Science and Technology, Baotou 014010, Inner Mongolia, China
-
Received:2022-04-19Revised:2022-09-21Online:2023-02-25Published:2023-03-13 -
Contact:WU Wenfei
摘要:
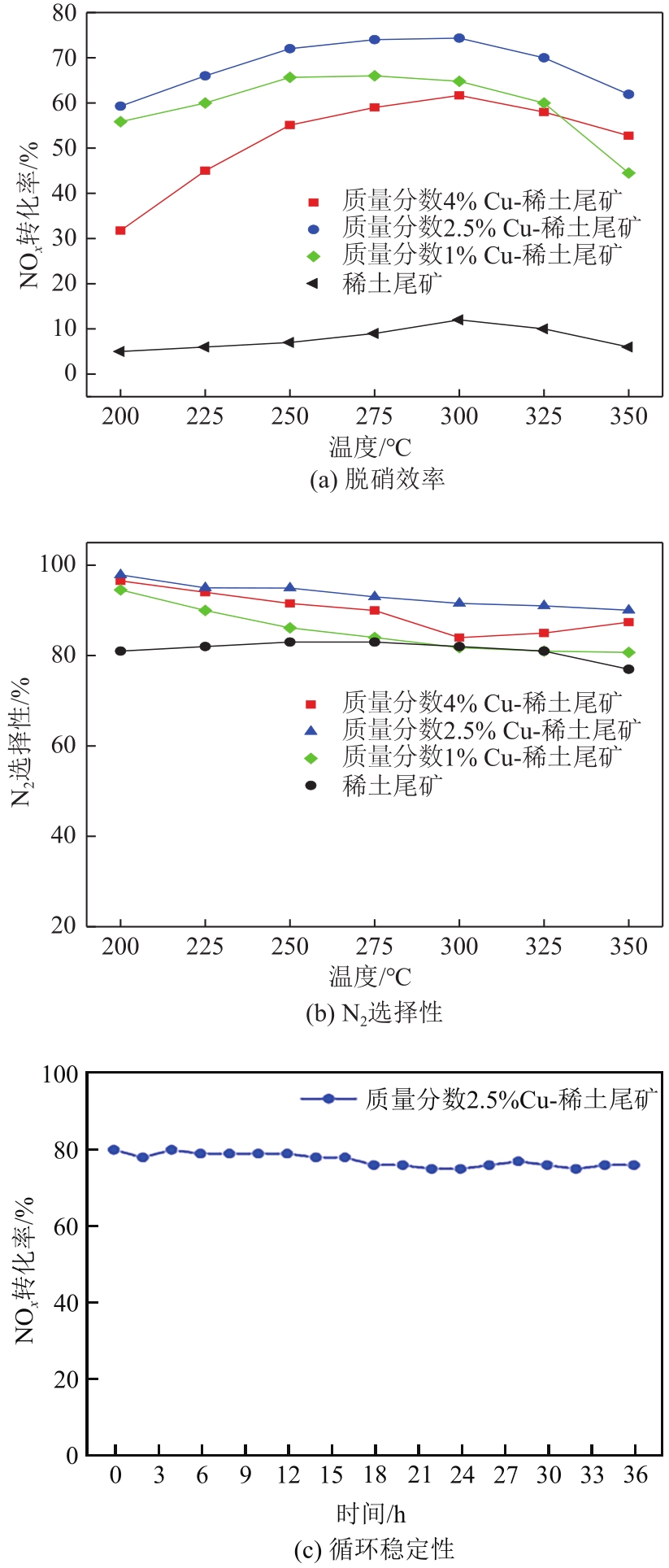
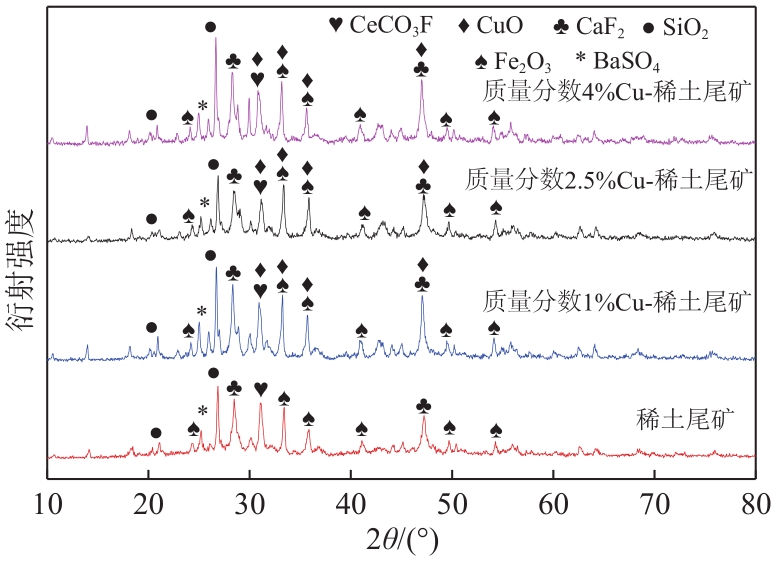
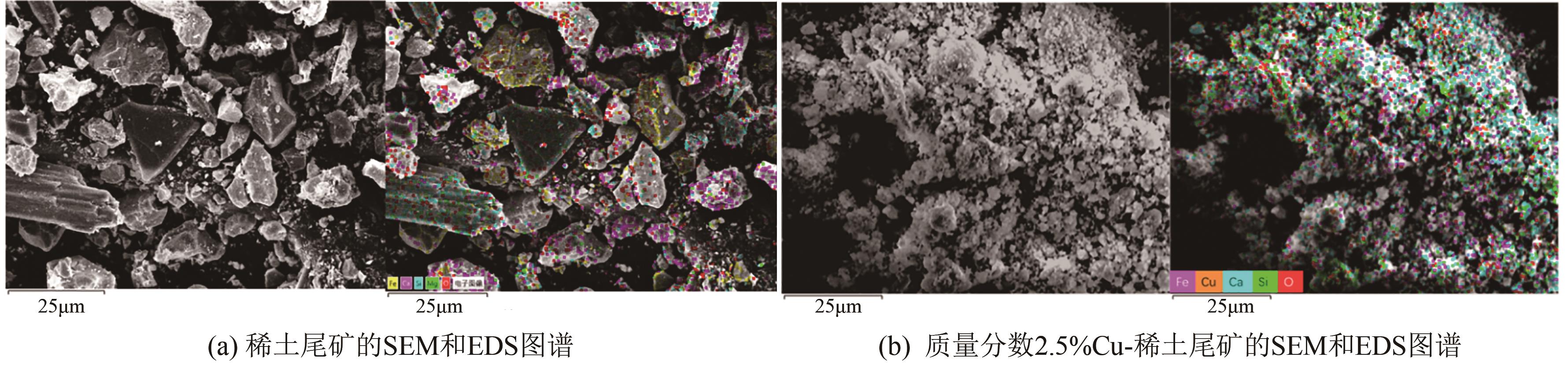
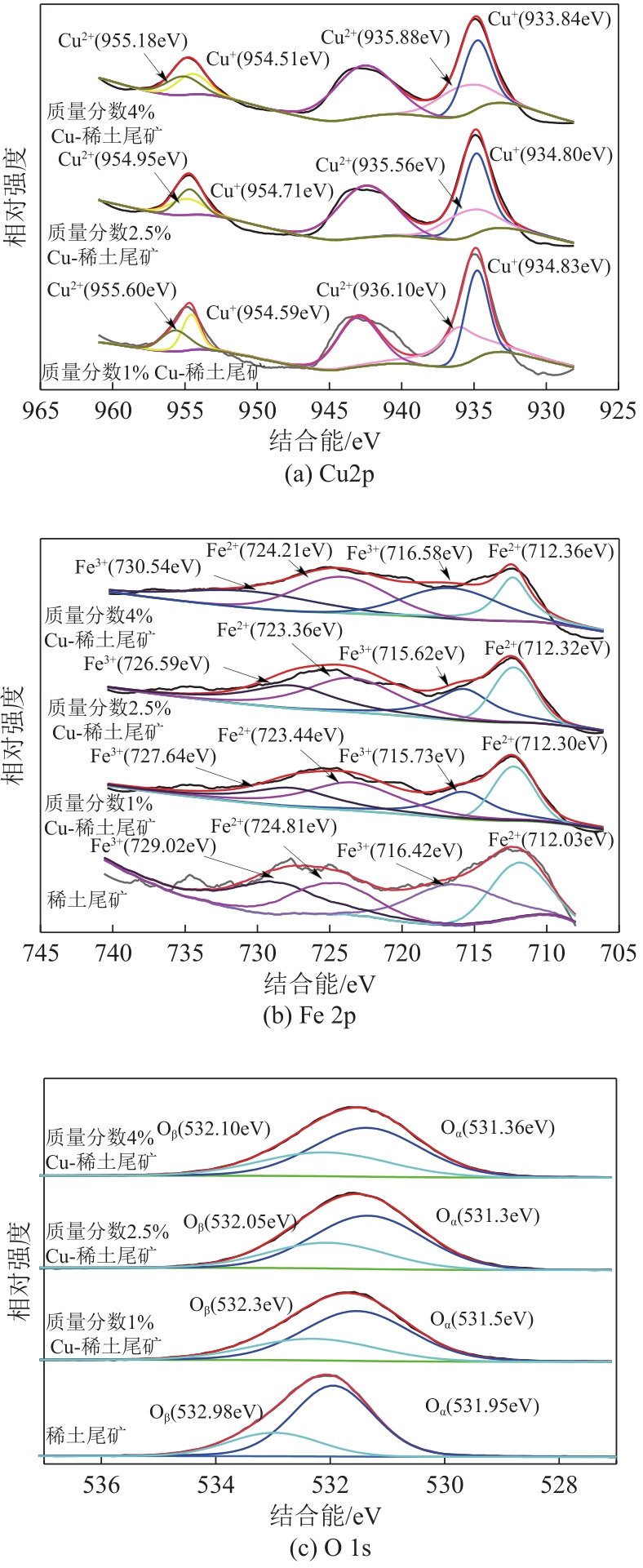
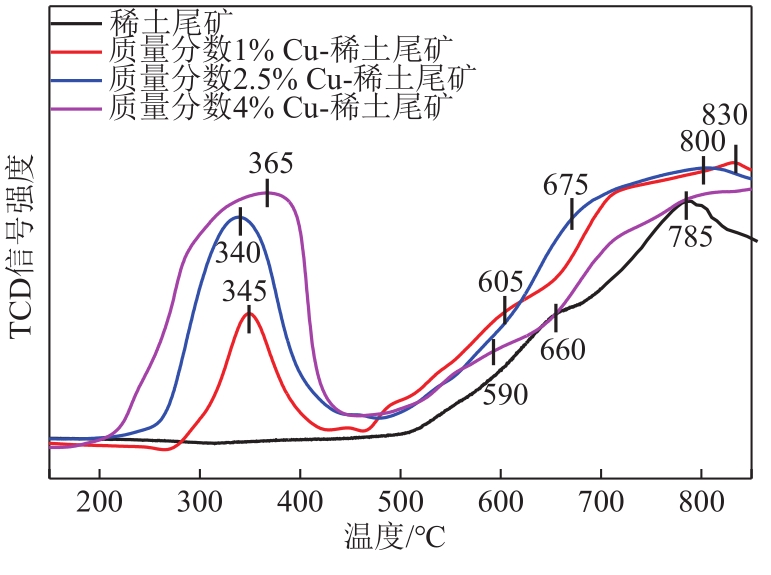
近年来,天然矿物由于储量大、活性组分丰富、价格低廉、绿色环保等优点成为脱硝催化剂研究的热点。本文以稀土尾矿为研究对象,利用浸渍-水热法对稀土尾矿进行Cu改性,通过XRD、SEM-EDS、XPS、H2-TPR、NH3-TPD、BET等表征探究Cu改性对稀土尾矿催化剂性能的影响。结果表明,Cu改性后,稀土尾矿催化剂的脱硝性能有大幅提升,且活性反应温度窗口拓宽至200~350℃。随着Cu改性量的增加,催化剂的脱硝效率先升高后降低,改性量为2.5%(质量分数)时,性能提升效果最明显。Cu改性提高了稀土尾矿催化剂的比表面积,Cu元素主要以CuO形式存在于催化剂表面,且与Fe2O3存在相互作用,催化剂中Cu2+、Fe2+和Fe3+的结合能发生明显的偏移,诱导Fe离子周围电子云密度降低,且质量分数2.5%Cu-稀土尾矿催化剂中两者相互作用最强烈,提升了催化剂的氧化还原能力和酸活性中心,CuO的还原温度对脱硝活性的影响大于氧化能力的影响,Fe2O3的氧化能力对脱硝活性的影响大于还原温度的影响。催化剂表面流动性较强的吸附氧更有利于脱硝反应的进行。
中图分类号:
引用本文
侯丽敏, 许杰, 付善聪, 武文斐. Cu改性对稀土尾矿催化剂NH3-SCR脱硝的影响[J]. 化工进展, 2023, 42(2): 765-773.
HOU Limin, XU Jie, FU Shancong, WU Wenfei. Effect of Cu modification on NH3-SCR denitration of rare earth tailings catalyst[J]. Chemical Industry and Engineering Progress, 2023, 42(2): 765-773.
| 元素 | 含量/% | 元素 | 含量/% | 元素 | 含量/% | 元素 | 含量/% |
|---|---|---|---|---|---|---|---|
| Fe | 17.40 | S | 0.72 | Th | 0.03 | Zr | 0.004 |
| Ca | 17.80 | Ba | 0.99 | Zn | 0.04 | Ni | 0.002 |
| F | 11.84 | Nd | 0.68 | Pb | 0.02 | Sn | 0.005 |
| Si | 8.80 | Mn | 0.93 | Sc | 0.01 | Cr | 0.001 |
| Mg | 3.10 | K | 0.57 | Cl | 0.08 | Rb | 0.002 |
| Ce | 1.78 | Ti | 0.31 | V | 0.008 | Te | 0.002 |
| Al | 1.49 | Nb | 0.11 | Co | 0.005 | As | 0.0004 |
| Na | 1.34 | Pr | 0.10 | Pd | 0.003 | W | 0.0005 |
| P | 1.16 | Sr | 0.10 | I | 0.006 | Cu | 0.0025 |
表1 稀土尾矿主要元素及含量(质量分数)
| 元素 | 含量/% | 元素 | 含量/% | 元素 | 含量/% | 元素 | 含量/% |
|---|---|---|---|---|---|---|---|
| Fe | 17.40 | S | 0.72 | Th | 0.03 | Zr | 0.004 |
| Ca | 17.80 | Ba | 0.99 | Zn | 0.04 | Ni | 0.002 |
| F | 11.84 | Nd | 0.68 | Pb | 0.02 | Sn | 0.005 |
| Si | 8.80 | Mn | 0.93 | Sc | 0.01 | Cr | 0.001 |
| Mg | 3.10 | K | 0.57 | Cl | 0.08 | Rb | 0.002 |
| Ce | 1.78 | Ti | 0.31 | V | 0.008 | Te | 0.002 |
| Al | 1.49 | Nb | 0.11 | Co | 0.005 | As | 0.0004 |
| Na | 1.34 | Pr | 0.10 | Pd | 0.003 | W | 0.0005 |
| P | 1.16 | Sr | 0.10 | I | 0.006 | Cu | 0.0025 |
| 项目 | 稀土尾矿 | 质量分数1%Cu-稀土尾矿 | 质量分数2.5%Cu-稀土尾矿 | 质量分数4%Cu-稀土尾矿 |
|---|---|---|---|---|
| Cu2+原子分数/% | — | 64.0 | 61.0 | 66.0 |
| Cu+原子分数/% | — | 36.0 | 39.0 | 44.0 |
| Cu2+/Cu+ | — | 1.7 | 1.56 | 1.5 |
| Fe2+原子分数/% | 44.6 | 55.0 | 52.0 | 51.0 |
| Fe3+原子分数/% | 55.4 | 45.0 | 48.0 | 49.0 |
| Fe2+/Fe3+ | 0.8 | 1.2 | 1.1 | 1.1 |
| Oα原子分数/% | 74.0 | 62.4 | 69.4 | 64.2 |
| Oβ原子分数/% | 26.0 | 37.6 | 30.6 | 35.8 |
| Oα/Oβ | 2.8 | 1.7 | 2.3 | 1.8 |
表2 稀土尾矿及Cu改性稀土尾矿催化剂表面元素及其价态占比
| 项目 | 稀土尾矿 | 质量分数1%Cu-稀土尾矿 | 质量分数2.5%Cu-稀土尾矿 | 质量分数4%Cu-稀土尾矿 |
|---|---|---|---|---|
| Cu2+原子分数/% | — | 64.0 | 61.0 | 66.0 |
| Cu+原子分数/% | — | 36.0 | 39.0 | 44.0 |
| Cu2+/Cu+ | — | 1.7 | 1.56 | 1.5 |
| Fe2+原子分数/% | 44.6 | 55.0 | 52.0 | 51.0 |
| Fe3+原子分数/% | 55.4 | 45.0 | 48.0 | 49.0 |
| Fe2+/Fe3+ | 0.8 | 1.2 | 1.1 | 1.1 |
| Oα原子分数/% | 74.0 | 62.4 | 69.4 | 64.2 |
| Oβ原子分数/% | 26.0 | 37.6 | 30.6 | 35.8 |
| Oα/Oβ | 2.8 | 1.7 | 2.3 | 1.8 |
| 催化剂 | 温度/℃ | 脱附峰面积 | ||||
|---|---|---|---|---|---|---|
| T1 | T2 | T3 | S1 | S2 | S3 | |
| 稀土尾矿 | 141 | 339 | 487 | 172 | 407 | 138 |
| 质量分数1%Cu-稀土尾矿 | 209 | 370 | — | 553 | 615 | — |
| 质量分数2.5%Cu-稀土尾矿 | 232 | 433 | 533 | 603 | 537 | 45 |
| 质量分数4%Cu-稀土尾矿 | 209 | 445 | — | 558 | 563 | — |
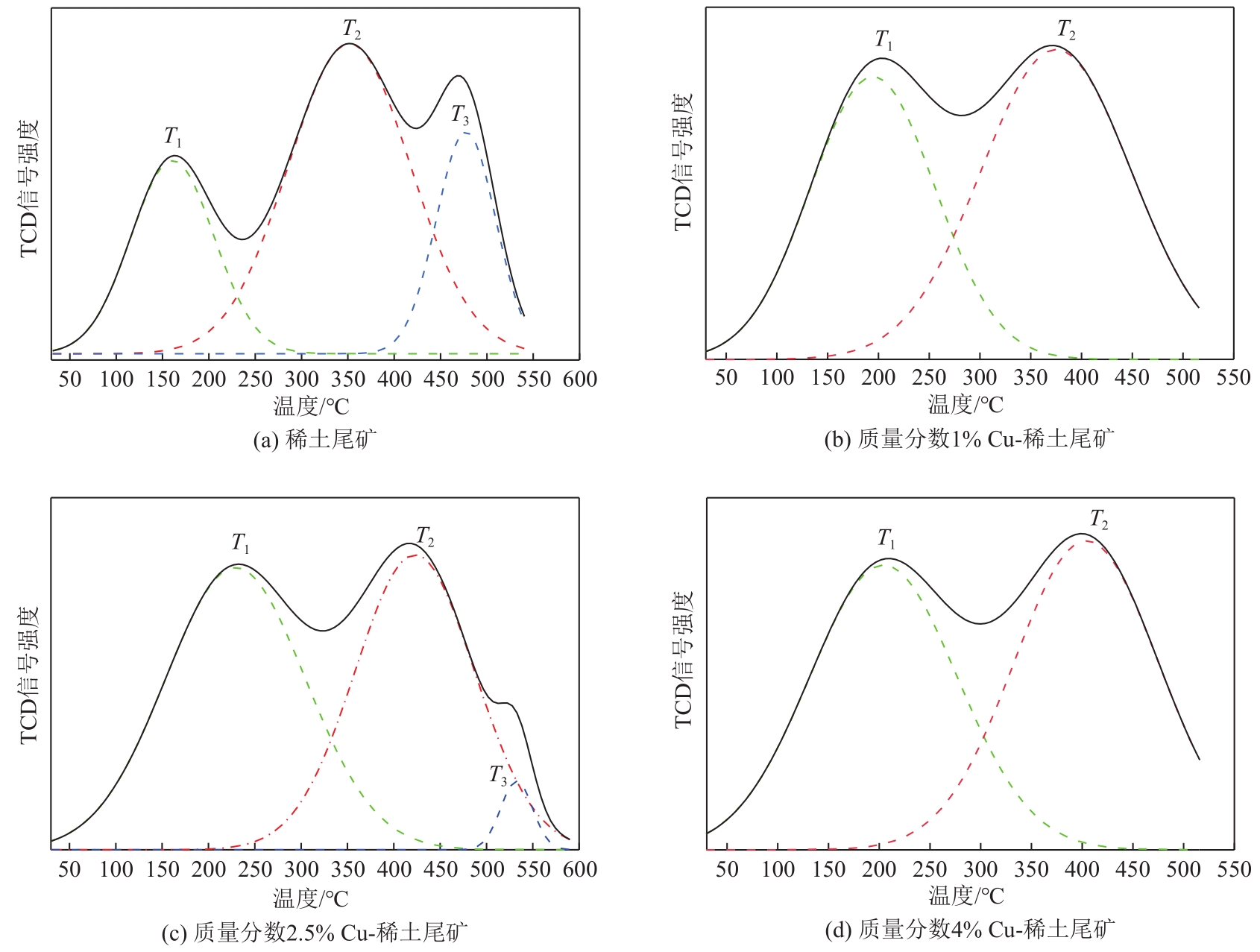
表3 稀土尾矿及Cu改性稀土尾矿催化剂表面酸活性中心种类及其分布
| 催化剂 | 温度/℃ | 脱附峰面积 | ||||
|---|---|---|---|---|---|---|
| T1 | T2 | T3 | S1 | S2 | S3 | |
| 稀土尾矿 | 141 | 339 | 487 | 172 | 407 | 138 |
| 质量分数1%Cu-稀土尾矿 | 209 | 370 | — | 553 | 615 | — |
| 质量分数2.5%Cu-稀土尾矿 | 232 | 433 | 533 | 603 | 537 | 45 |
| 质量分数4%Cu-稀土尾矿 | 209 | 445 | — | 558 | 563 | — |
| 催化剂 | 比表面积 /m2·g-1 | 孔径 /nm | 孔容 /cm3·g-1 |
|---|---|---|---|
| 稀土尾矿 | 20.10 | 22.21 | 0.11 |
| 质量分数1%Cu-稀土尾矿 | 38.10 | 8.88 | 0.08 |
| 质量分数2.5%Cu-稀土尾矿 | 59.67 | 8.69 | 0.13 |
| 质量分数4%Cu-稀土尾矿 | 42.02 | 9.17 | 0.09 |
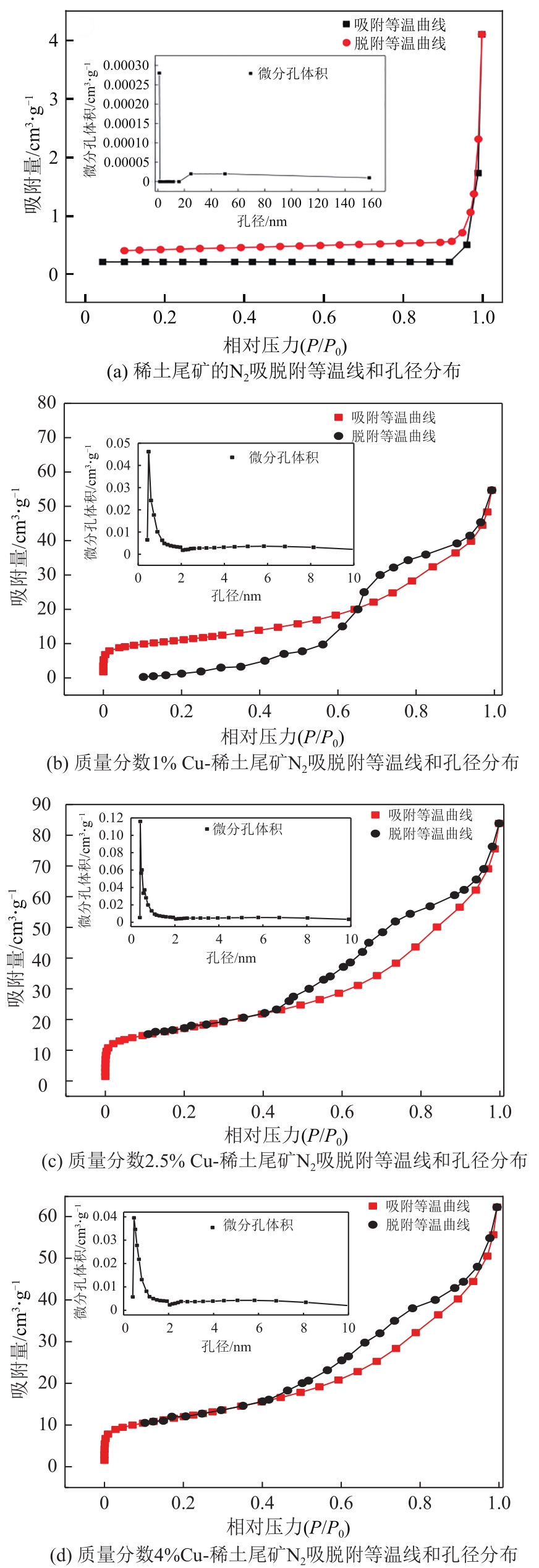
表4 稀土尾矿及Cu改性稀土尾矿催化剂的比表面积、孔容、孔径分析
| 催化剂 | 比表面积 /m2·g-1 | 孔径 /nm | 孔容 /cm3·g-1 |
|---|---|---|---|
| 稀土尾矿 | 20.10 | 22.21 | 0.11 |
| 质量分数1%Cu-稀土尾矿 | 38.10 | 8.88 | 0.08 |
| 质量分数2.5%Cu-稀土尾矿 | 59.67 | 8.69 | 0.13 |
| 质量分数4%Cu-稀土尾矿 | 42.02 | 9.17 | 0.09 |
| 1 | 赵乾. SCR烟气脱硝系统模拟优化及喷氨量最优控制[D]. 重庆: 重庆大学, 2012. |
| ZHAO Qian. Simulation optimization and optimal control on spraying ammonia of SCR flue gas denitrification[D]. Chongqing: Chongqing University, 2012. | |
| 2 | LIU Kaijie, YU Qingbo, WANG Baolan, et al. Activated carbon-supported catalyst loading of CH4N2O for selective reduction of NO from flue gas at low temperatures[J]. International Journal of Hydrogen Energy, 2019, 44(26): 13523-13537. |
| 3 | 赵毅, 孙中豪, 曾韵洁. 低温SCR脱硝催化剂的研究进展[J]. 化工环保, 2019, 39(1): 1-5. |
| ZHAO Yi, SUN Zhonghao, ZENG Yunjie. Research progresses of low temperature SCR denitration catalyst[J]. Environmental Protection of Chemical Industry, 2019, 39(1): 1-5. | |
| 4 | CHAE Ho Jeong, In-Sik NAM, Sung-Won HAM, et al. Characteristics of vanadia on the surface of V2O5/Ti-PILC catalyst for the reduction of NO x by NH3 [J]. Applied Catalysis B: Environmental, 2004, 53(2): 117-126. |
| 5 | NOVA Isabella, LIETTI Luca, TRONCONI Enrico, et al. Dynamics of SCR reaction over a TiO2-supported vanadia-tungsta commercial catalyst[J]. Catalysis Today, 2000, 60(1/2): 73-82. |
| 6 | CHEN Liang, LI Junhua, GE Maofa. The poisoning effect of alkali metals doping over nano V2O5-WO3/TiO2 catalysts on selective catalytic reduction of NO x by NH3 [J]. Chemical Engineering Journal, 2011, 170(2/3): 531-537. |
| 7 | MARBERGER Adrian, ELSENER Martin, NUGUID Rob Jeremiah G, et al. Thermal activation and aging of a V2O5/WO3-TiO2 catalyst for the selective catalytic reduction of NO with NH3 [J]. Applied Catalysis A: General, 2019, 573: 64-72. |
| 8 | WANG Xiaoxiang, CONG Qiliang, CHEN Liang, et al. The alkali resistance of CuNbTi catalyst for selective reduction of NO by NH3: A comparative investigation with VWTi catalyst[J]. Applied Catalysis B: Environmental, 2019, 246: 166-179. |
| 9 | LIANG Zengying, MA Xiaoqian, LIN Hai, et al. The energy consumption and environmental impacts of SCR technology in China[J]. Applied Energy, 2011, 88(4): 1120-1129. |
| 10 | BERA Parthasarathi, PATIL K C, JAYARAM V, et al. Ionic dispersion of Pt and Pd on CeO2 by combustion method: Effect of metal-ceria interaction on catalytic activities for NO reduction and CO and hydrocarbon oxidation[J]. Journal of Catalysis, 2000, 196(2): 293-301. |
| 11 | 边雪, 肖坤宇, 王书豪, 等. Fe、Ce改性锰钛催化剂的制备及其低温脱硝性能研究[J]. 功能材料, 2019, 50(7): 7079-7084, 7089. |
| BIAN Xue, XIAO Kunyu, WANG Shuhao, et al. Preparation and the study on low-temperature denitrification of Fe, Ce modified manganese titanium catalyst[J]. Journal of Functional Materials, 2019, 50(7): 7079-7084, 7089. | |
| 12 | LIU Zhiming, ZHU Junzhi, ZHANG Shaoxuan, et al. Selective catalytic reduction of NO x by NH3 over MoO3-promoted CeO2/TiO2 catalyst[J]. Catalysis Communications, 2014, 46: 90-93. |
| 13 | LI Zhe, SHEN Lintao, HUANG Wei, et al. Kinetics of selective catalytic reduction of NO by NH3 on Fe-Mo/ZSM-5 catalyst[J]. Journal of Environmental Sciences, 2007, 19(12): 1516-1519. |
| 14 | 刘向辉, 张娜, 白宏科, 等. MnO x /DPC催化剂SCR脱硝活性研究[J]. 煤炭加工与综合利用, 2020(3): 75-78. |
| LIU Xianghui, ZHANG Na, BAI Hongke, et al. Study on SCR denitrification activity of MnO x /DPC catalyst[J]. Coal Processing & Comprehensive Utilization, 2020(3): 75-78. | |
| 15 | WANG Xiaobo, WU Shiguo, ZOU Weixin, et al. Fe-Mn/Al2O3 catalysts for low temperature selective catalytic reduction of NO with NH3 [J]. Chinese Journal of Catalysis, 2016, 37(8): 1314-1323. |
| 16 | SINGOREDJO Lydia, KORVER Ruben, KAPTEIJN Freek, et al. Alumina supported manganese oxides for the low-temperature selective catalytic reduction of nitric oxide with ammonia[J]. Applied Catalysis B: Environmental, 1992, 1(4): 297-316. |
| 17 | CHANG Huazhen, CHEN Xiaoyin, LI Junhua, et al. Improvement of activity and SO2 tolerance of Sn-modified MnO x -CeO2 catalysts for NH3-SCR at low temperatures[J]. Environmental Science & Technology, 2013, 47(10): 5294-5301. |
| 18 | CHANG Huazhen, LI Junhua, CHEN Xiaoyin, et al. Effect of Sn on MnO x -CeO2 catalyst for SCR of NO x by ammonia: Enhancement of activity and remarkable resistance to SO2 [J]. Catalysis Communications, 2012, 27: 54-57. |
| 19 | XING Mengmeng, SUN Qian, ZENG Chun, et al. Modulating Cu+ distribution on the surface of Ce-doped CuO composite oxides for SO2-resistant NH3-selective catalytic reduction of NO[J]. RSC Advances, 2017, 7(31): 18830-18837. |
| 20 | 郭娜. 复合型锰基催化剂的制备和脱硝性能研究[D]. 西安: 西安建筑科技大学, 2013. |
| GUO Na. Study on the preparation and properties of manganese base SCR catalyst[D]. Xi’an: Xi’an University of Architecture and Technology, 2013. | |
| 21 | 卢慧霞. 菱/锰铁矿石低温SCR脱硝催化剂的制备及改性研究[D]. 南京: 东南大学, 2017. |
| LU Huixia. Preparation and modification of siderite and pyrolusite catalysts for low-temperature SCR of NO x with NH3 [D]. Nanjing: Southeast University, 2017. | |
| 22 | KAMASAMUDRAM Krishna, CURRIER Neal W, CHEN Xu, et al. Overview of the practically important behaviors of zeolite-based urea-SCR catalysts, using compact experimental protocol[J]. Catalysis Today, 2010, 151(3/4): 212-222. |
| 23 | SONG Zhongxian, ZHANG Qiulin, NING Ping, et al. Effect of copper precursors on the catalytic activity of Cu/ZSM-5 catalysts for selective catalytic reduction of NO by NH3 [J]. Research on Chemical Intermediates, 2016, 42(10): 7429-7445. |
| 24 | 郑强. 综合回收白云鄂博弱磁尾矿中铁、稀土、氟和磷的研究[D]. 沈阳: 东北大学, 2017. |
| ZHENG Qiang. Studies on comprehensive recovery of iron, rare earth, fluorine and phosphorus from Bayan obo weakly magnetic tailings[D]. Shenyang: Northeastern University, 2017. | |
| 25 | 侯丽敏, 闫笑, 乔超越, 等. 从工艺矿物学角度分析稀土尾矿作为NH3-SCR催化剂的可行性[J]. 中国稀土学报, 2022, 40(2): 216-224. |
| HOU Limin, YAN Xiao, QIAO Chaoyue, et al. Feasibility of rare earth tailings as NH3-SCR catalyst from perspective of process mineralogy[J]. Journal of the Chinese Society of Rare Earths, 2022, 40(2): 216-224. | |
| 26 | 付金艳. γ-Al2O3球磨修饰稀土尾矿NH3-SCR脱硝特性研究[D]. 包头: 内蒙古科技大学, 2020. |
| FU Jinyan. Study on NH3-SCR denitration characteristics of rare earth tailings modified by γ-Al2O3 [D]. Baotou: Inner Mongolia University of Science & Technology, 2020. | |
| 27 | 彭罡. Cu和Fe改性MCM-22催化剂的NH3-SCR性能研究[D]. 武汉: 武汉科技大学, 2020. |
| PENG Gang. Investigation on catalytic performance of MCM-22 modified by Cu and Fe in NH3-SCR[D]. Wuhan: Wuhan University of Science and Technology, 2020. | |
| 28 | 王建. 稀土尾矿催化CO还原脱硝特性实验研究[D]. 包头: 内蒙古科技大学, 2020. |
| WANG Jian. Experimental study on catalytic performance of rare earth tailings for CO reduction and denitrification[D]. Baotou: Inner Mongolia University of Science & Technology, 2020. | |
| 29 | 侯丽敏, 闫笑, 乔超越, 等. 机械力-微波活化对稀土尾矿NH3-SCR脱硝性能的影响[J]. 化工进展, 2021, 40(10): 5818-5828. |
| HOU Limin, YAN Xiao, QIAO Chaoyue, et al. Effect of mechanical force and microwave on the NH3-SCR denitration of rare earth tailings[J]. Chemical Industry and Engineering Progress, 2021, 40(10): 5818-5828. | |
| 30 | 李秀凤. 固体超强酸SO4 2-/ZrO2-CeO2的制备及在生物柴油中的应用[D]. 昆明: 昆明理工大学, 2010. |
| LI Xiufeng. Preparation of solid superacid SO4 2-/ZrO2-CeO2 and its application in biodiesel[D]. Kunming: Kunming University of Science and Technology, 2010. | |
| 31 | Feng BIN, SONG Chonglin, Gang LYU, et al. Selective catalytic reduction of nitric oxide with ammonia over zirconium-doped copper/ZSM-5 catalysts[J]. Applied Catalysis B: Environmental, 2014, 150/151: 532-543. |
| 32 | GONG Jinlong, YUE Hairong, ZHAO Yujun, et al. Synthesis of ethanol via syngas on Cu/SiO2 catalysts with balanced Cu0-Cu+ sites[J]. Journal of the American Chemical Society, 2012, 134(34): 13922-13925. |
| 33 | CAO Fan, SU Sheng, XIANG Jun, et al. The activity and mechanism study of Fe-Mn-Ce/γ-Al2O3 catalyst for low temperature selective catalytic reduction of NO with NH3 [J]. Fuel, 2015, 139: 232-239. |
| 34 | BONINGARI Thirupathi, PAPPAS Dimitrios K, ETTIREDDY Padmanabha R, et al. Influence of SiO2 on M/TiO2 (M = Cu, Mn, and Ce) formulations for low-temperature selective catalytic reduction of NO x with NH3: Surface properties and key components in relation to the activity of NO x reduction[J]. Industrial & Engineering Chemistry Research, 2015, 54(8): 2261-2273. |
| 35 | KANG Min, PARK Eun Duck, KIM Ji Man, et al. Manganese oxide catalysts for NO x reduction with NH3 at low temperatures[J]. Applied Catalysis A: General, 2007, 327(2): 261-269. |
| 36 | 杨勇, 陶智超, 张成华, 等. 焙烧温度对Fe-Mn催化剂结构和F-T合成性能影响[J]. 燃料化学学报, 2004, 32(6): 717-722. |
| YANG Yong, TAO Zhichao, ZHANG Chenghua, et al. Effect of calcination temperature on the structure and Fischer-Tropsch performance of Fe-Mn catalyst[J]. Journal of Fuel Chemistry and Technology, 2004, 32(6): 717-722. | |
| 37 | Ismail BOZ, SAHIBZADA Mortaza, METCALFE Ian S. Kinetics of the higher alcohol synthesis over a K-promoted CuO/ZnO/Al2O3 catalyst[J]. Industrial & Engineering Chemistry Research, 1994, 33(9): 2021-2028. |
| 38 | 刘建国, 定明月, 王铁军, 等. Cu-Fe基双孔载体催化剂结构和低碳醇合成反应性能[J]. 物理化学学报, 2012, 28(8): 1964-1970. |
| LIU Jianguo, DING Mingyue, WANG Tiejun, et al. Structure and performance of Cu-Fe bimodal support for higher alcohol syntheses[J]. Acta Physico-Chimica Sinica, 2012, 28(8): 1964-1970. | |
| 39 | ZHU Zhenping, LIU Zhenyu, LIU Shoujun, et al. Catalytic NO reduction with ammonia at low temperatures on V2O5/AC catalysts: Effect of metal oxides addition and SO2 [J]. Applied Catalysis B: Environmental, 2001, 30(3/4): 267-276. |
| 40 | XIA Yan, ZHAN Wangcheng, GUO Yun, et al. Fe-Beta zeolite for selective catalytic reduction of NO x with NH3: Influence of Fe content[J]. Chinese Journal of Catalysis, 2016, 37(12): 2069-2078. |
| 41 | 胡海鹏, 王学涛, 张兴宇, 等. Fe-Cu/ZSM-5催化剂的NH3-SCR脱硝性能[J]. 燃料化学学报, 2018, 46(2): 225-232. |
| HU Haipeng, WANG Xuetao, ZHANG Xingyu, et al. Performance of Fe-Cu/ZSM-5 catalyst in the deNO x process via NH3-SCR[J]. Journal of Fuel Chemistry and Technology, 2018, 46(2): 225-232. | |
| 42 | SING K S W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity[J]. Pure and Applied Chemistry, 1985, 57(4): 603-619. |
| 43 | 李娜, 陈泽东, 李华, 等. 氟碳铈精矿矿物学分析及负载锰的NH3-SCR脱硝性能的研究[J]. 中国稀土学报, 2021, 39(6): 890-902. |
| LI Na, CHEN Zedong, LI Hua, et al. Mineralogical analysis of bastnaesite concentrate and denitration performance of NH3-SCR loaded with manganese[J]. Journal of the Chinese Society of Rare Earths, 2021, 39(6): 890-902. | |
| 44 | WEI Mengqi, YU Qingbo, MU Tongtong, et al. Preparation and characterization of waste ion-exchange resin-based activated carbon for CO2 capture[J]. Adsorption, 2016, 22(3): 385-396. |
| 45 | WANG Xiaoxiang, SHI Yun, LI Sujing, et al. Promotional synergistic effect of Cu and Nb doping on a novel Cu/Ti-Nb ternary oxide catalyst for the selective catalytic reduction of NO x with NH3 [J]. Applied Catalysis B: Environmental, 2018, 220: 234-250. |
| 46 | MA Ziran, WU Xiaodong, SI Zhichun, et al. Impacts of niobia loading on active sites and surface acidity in NbO x /CeO2-ZrO2 NH3-SCR catalysts[J]. Applied Catalysis B: Environmental, 2015, 179: 380-394. |
| 47 | ARFAOUI Jihene, GHORBEL Abdelhamid, PETITTO Carolina, et al. WITHDRAWN: Novel vanadium supported onto mixed molybdenum-titanium pillared clay catalysts for the low temperature SCR-NO by NH3 [J]. Chemical Engineering Journal, 2017 |
| 48 | WAN Yaping, ZHAO Wenru, TANG Yu, et al. Ni-Mn bi-metal oxide catalysts for the low temperature SCR removal of NO with NH3 [J]. |
| [1] | 张明焱, 刘燕, 张雪婷, 刘亚科, 李从举, 张秀玲. 非贵金属双功能催化剂在锌空气电池研究进展[J]. 化工进展, 2023, 42(S1): 276-286. |
| [2] | 时永兴, 林刚, 孙晓航, 蒋韦庚, 乔大伟, 颜彬航. 二氧化碳加氢制甲醇过程中铜基催化剂活性位点研究进展[J]. 化工进展, 2023, 42(S1): 287-298. |
| [3] | 谢璐垚, 陈崧哲, 王来军, 张平. 用于SO2去极化电解制氢的铂基催化剂[J]. 化工进展, 2023, 42(S1): 299-309. |
| [4] | 杨霞珍, 彭伊凡, 刘化章, 霍超. 熔铁催化剂活性相的调控及其费托反应性能[J]. 化工进展, 2023, 42(S1): 310-318. |
| [5] | 王家庆, 宋广伟, 李强, 郭帅成, DAI Qingli. 橡胶混凝土界面改性方法及性能提升路径[J]. 化工进展, 2023, 42(S1): 328-343. |
| [6] | 陈崇明, 陈秋, 宫云茜, 车凯, 郁金星, 孙楠楠. 分子筛基CO2吸附剂研究进展[J]. 化工进展, 2023, 42(S1): 411-419. |
| [7] | 高雨飞, 鲁金凤. 非均相催化臭氧氧化作用机理研究进展[J]. 化工进展, 2023, 42(S1): 430-438. |
| [8] | 王乐乐, 杨万荣, 姚燕, 刘涛, 何川, 刘逍, 苏胜, 孔凡海, 朱仓海, 向军. SCR脱硝催化剂掺废特性及性能影响[J]. 化工进展, 2023, 42(S1): 489-497. |
| [9] | 顾永正, 张永生. HBr改性飞灰对Hg0的动态吸附及动力学模型[J]. 化工进展, 2023, 42(S1): 498-509. |
| [10] | 赵景超, 谭明. 表面活性剂对电渗析减量化工业含盐废水的影响[J]. 化工进展, 2023, 42(S1): 529-535. |
| [11] | 邓丽萍, 时好雨, 刘霄龙, 陈瑶姬, 严晶颖. 非贵金属改性钒钛基催化剂NH3-SCR脱硝协同控制VOCs[J]. 化工进展, 2023, 42(S1): 542-548. |
| [12] | 王谨航, 何勇, 史伶俐, 龙臻, 梁德青. 气体水合物阻聚剂研究进展[J]. 化工进展, 2023, 42(9): 4587-4602. |
| [13] | 程涛, 崔瑞利, 宋俊男, 张天琪, 张耘赫, 梁世杰, 朴实. 渣油加氢装置杂质沉积规律与压降升高机理分析[J]. 化工进展, 2023, 42(9): 4616-4627. |
| [14] | 朱杰, 金晶, 丁正浩, 杨会盼, 侯封校. 化学链气化中准东煤灰对CaSO4载氧体改性及其作用机理[J]. 化工进展, 2023, 42(9): 4628-4635. |
| [15] | 王晋刚, 张剑波, 唐雪娇, 刘金鹏, 鞠美庭. 机动车尾气脱硝催化剂Cu-SSZ-13的改性研究进展[J]. 化工进展, 2023, 42(9): 4636-4648. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||