化工进展 ›› 2022, Vol. 41 ›› Issue (2): 901-910.DOI: 10.16085/j.issn.1000-6613.2021-0637
TOCNF与磁性羧甲基壳聚糖纳米粒子复合物的制备及吸附Pb2+的特性
- 上海海洋大学食品学院,上海 201306
-
收稿日期:2021-03-29修回日期:2021-04-29出版日期:2022-02-05发布日期:2022-02-23 -
通讯作者:陈舜胜 -
作者简介:周丽莎(1995—),女,硕士研究生,研究方向为重金属吸附及环境治理。E-mail:1663530608@qq.com 。 -
基金资助:国家重点研发计划(2017YFD0400105-02)
Preparation of TOCNF and magnetic carboxymethyl chitosan nanoparticles composite and adsorption properties of Pb2+
ZHOU Lisha( ), LI Ruonan, BIAN Yujie, CHEN Shunsheng(
), LI Ruonan, BIAN Yujie, CHEN Shunsheng( )
)
- College of Food Science and Technology, Shanghai Ocean University, Shanghai 201306, China
-
Received:2021-03-29Revised:2021-04-29Online:2022-02-05Published:2022-02-23 -
Contact:CHEN Shunsheng
摘要:
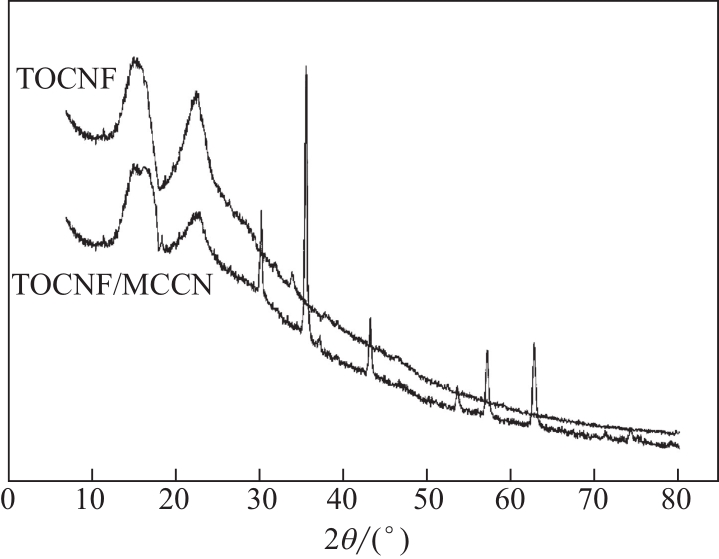
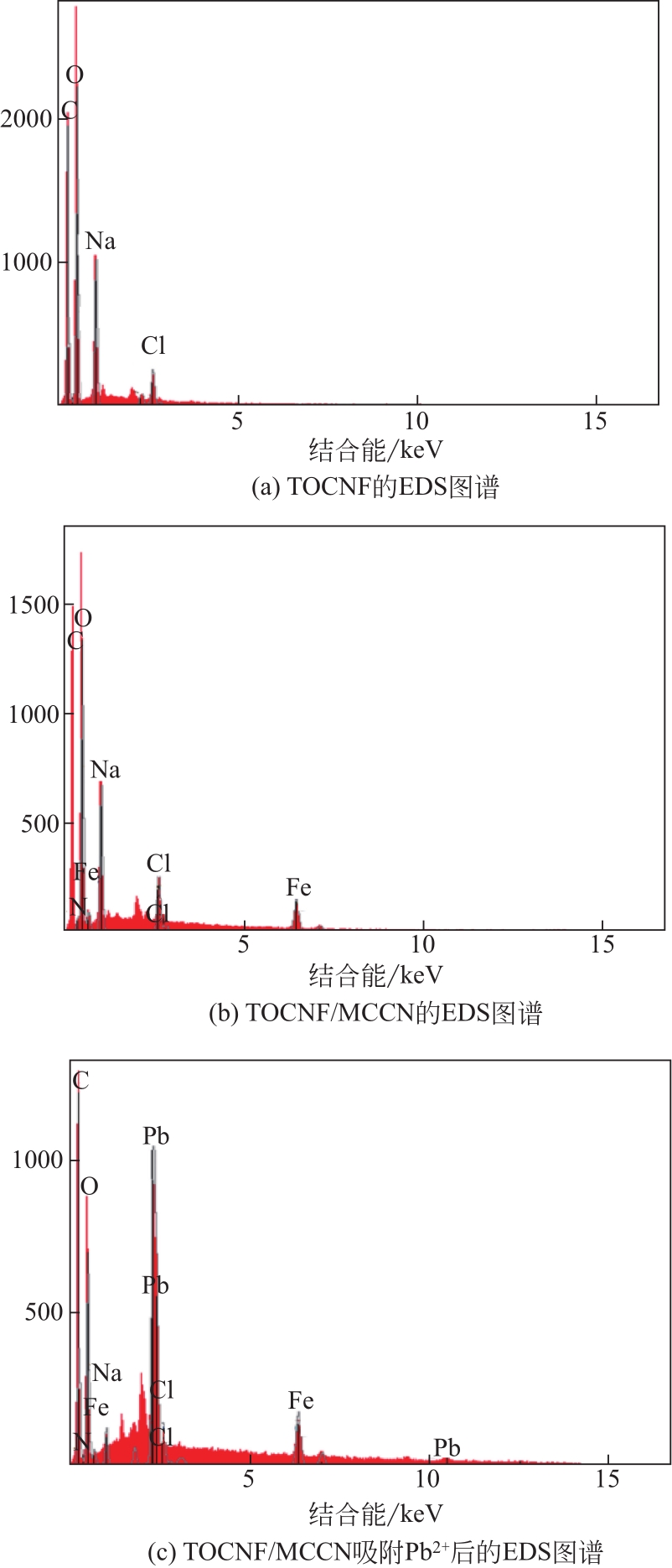
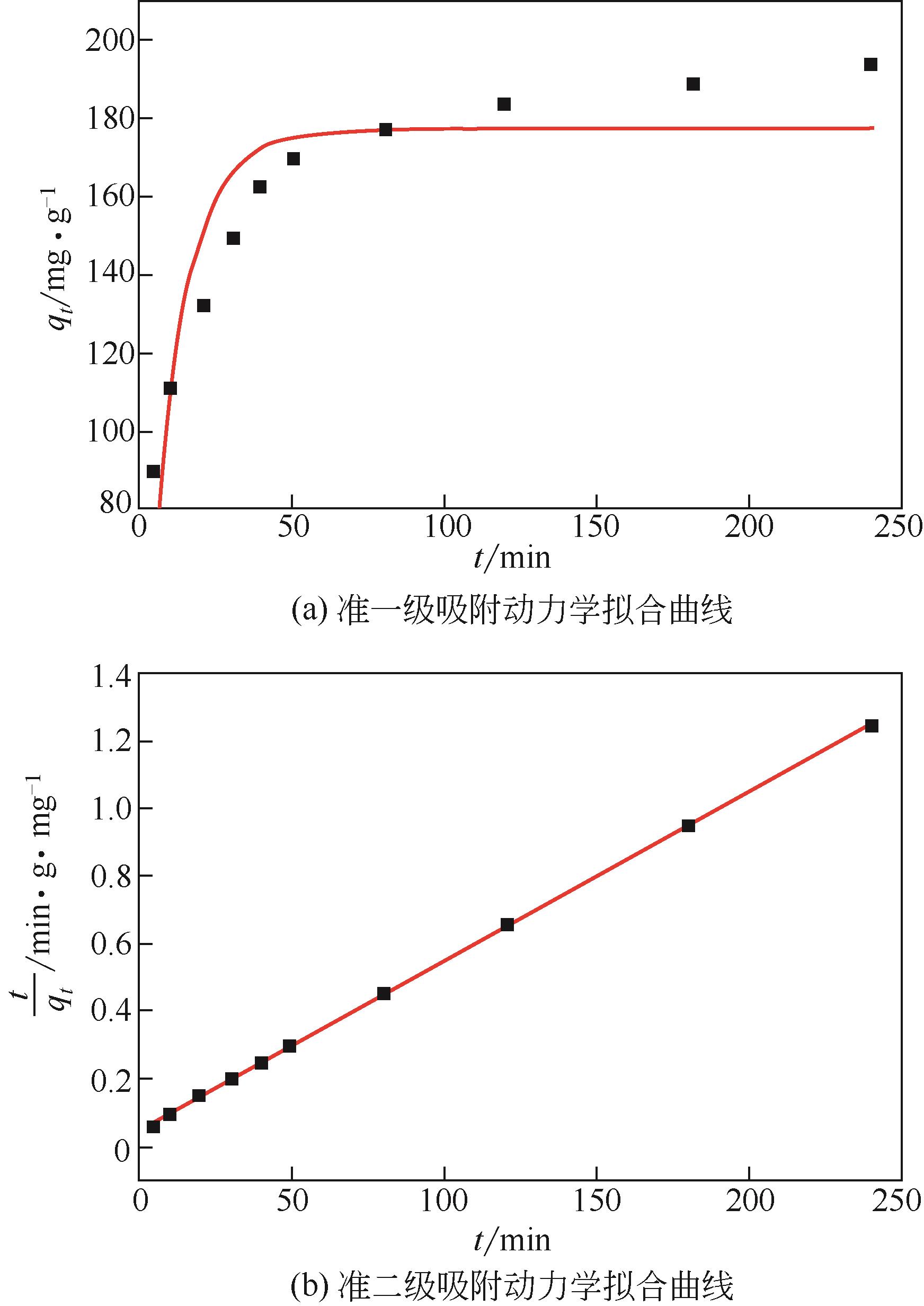
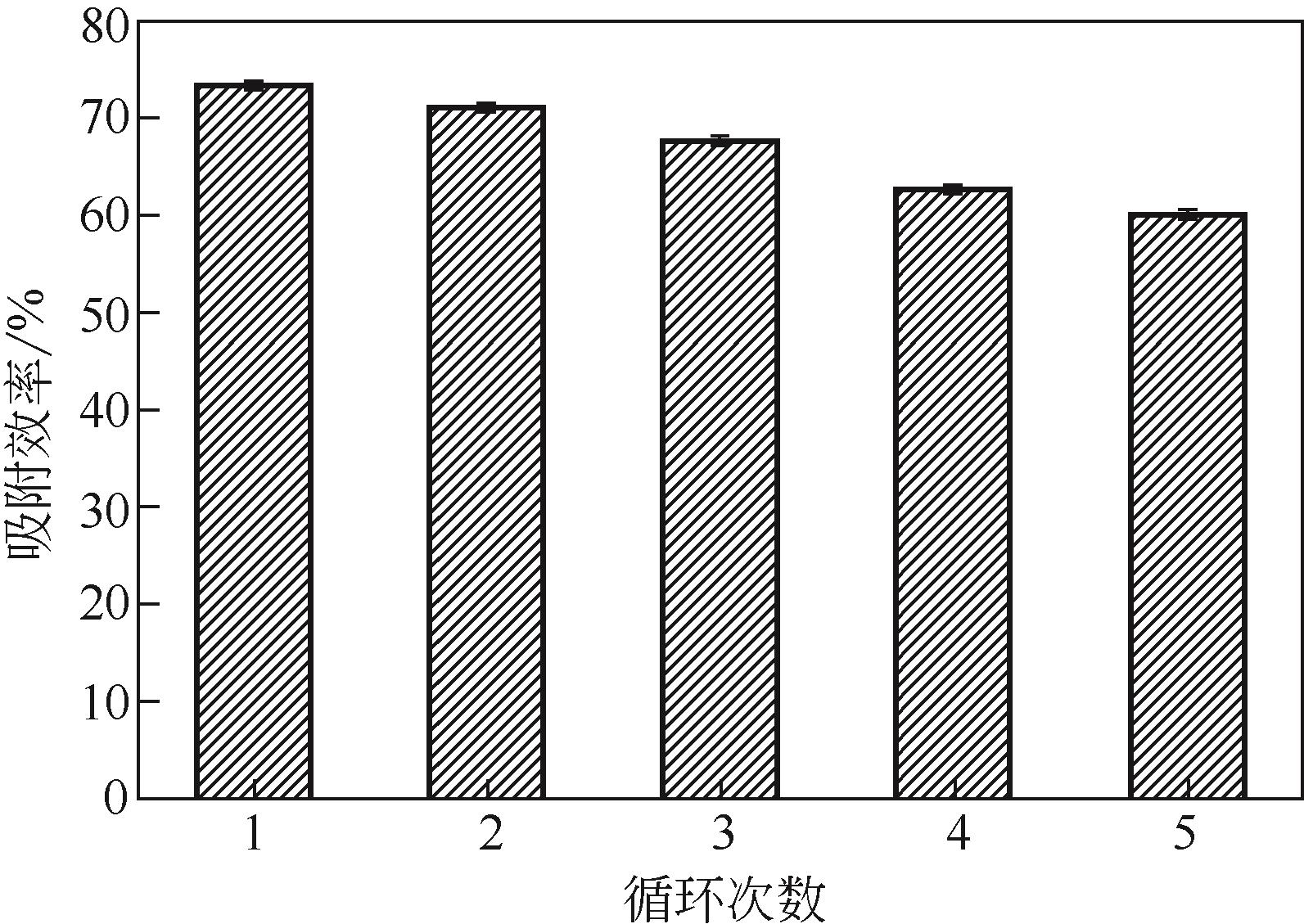
水体中铅污染对环境及人的健康安全造成了极大的危害。本文将四甲基呱啶(TEMPO)氧化的纤维素纳米纤维(TEMPO-oxidized cellulose nanofibers,TOCNF)与磁性羧甲基壳聚糖纳米粒子(magnetic carboxymethyl chitosan nanoparticles,MCCN)交联复合制备一种经济高效且对环境无毒害的Pb2+吸附剂,对复合前后的材料进行结构表征。通过单因素实验研究pH、Pb2+初始浓度、吸附时间及温度对Pb2+吸附效率的影响,确定最优吸附条件后比较TOCNF/MCCN及TOCNF对Pb2+的吸附效果,研究复合材料对Pb2+的吸附特性。研究结果表明,Fe3O4成功被羧甲基壳聚糖纳米粒子包裹并与TOCNF交联复合,在最优吸附条件(pH=5,Pb2+初始浓度为100mg/L,吸附时间为240min,常温下进行实验)下,TOCNF/MCCN吸附Pb2+的饱和容量为193.5mg/g,比TOCNF高了近一倍。复合吸附剂吸附Pb2+的过程更符合准二级动力学方程,说明决定吸附速率的主要是化学吸附。等温吸附方程的相关系数R2表明,Langmuir方程能更好地拟合吸附过程,说明复合吸附剂对Pb2+的吸附主要是表面基团的单分子层吸附。通过线性方程的斜率计算得到的理论饱和吸附量为201.1mg/g,与实际值差3.8%,经过5次解吸再吸附的过程,吸附剂的吸附效率仅下降了13%,表明该吸附剂有良好的可再生性,具有很好的应用前景。
中图分类号:
引用本文
周丽莎, 李若男, 卞雨洁, 陈舜胜. TOCNF与磁性羧甲基壳聚糖纳米粒子复合物的制备及吸附Pb2+的特性[J]. 化工进展, 2022, 41(2): 901-910.
ZHOU Lisha, LI Ruonan, BIAN Yujie, CHEN Shunsheng. Preparation of TOCNF and magnetic carboxymethyl chitosan nanoparticles composite and adsorption properties of Pb2+[J]. Chemical Industry and Engineering Progress, 2022, 41(2): 901-910.
| 吸附剂吸附Pb2+ | qe/mg·g-1 | k/min-1 | R2 |
|---|---|---|---|
| 准一级动力学方程 | 177.6 | 0.0909 | 0.8179 |
| 准二级动力学方程 | 199.3 | 0.0005628 | 0.9996 |
表1 TOCNF/MCCN复合材料吸附Pb2+动力学方程参数
| 吸附剂吸附Pb2+ | qe/mg·g-1 | k/min-1 | R2 |
|---|---|---|---|
| 准一级动力学方程 | 177.6 | 0.0909 | 0.8179 |
| 准二级动力学方程 | 199.3 | 0.0005628 | 0.9996 |
| Langmuir等温线模型 | Freundlich等温线模型 | |||||
|---|---|---|---|---|---|---|
| qm/mg·g-1 | b/mg·L-1 | R2 | n | K/mg·L-1 | R2 | |
| 201.1 | 0.04235 | 0.9980 | 2.663 | 3.265 | 0.8246 | |
表2 TOCNF/MCCN复合材料吸附Pb2+等温模型拟合参数
| Langmuir等温线模型 | Freundlich等温线模型 | |||||
|---|---|---|---|---|---|---|
| qm/mg·g-1 | b/mg·L-1 | R2 | n | K/mg·L-1 | R2 | |
| 201.1 | 0.04235 | 0.9980 | 2.663 | 3.265 | 0.8246 | |
| 吸附剂 | 饱和吸附容量/mg·g-1 | pH | 吸附模型 | 循环次数 | 参考文献 |
|---|---|---|---|---|---|
| TOCNF/MCCN复合材料 | 201.1 | 5 | Langmuir和Freundlich | 5 | 本研究 |
| 硫醇改性CNF复合膜 | 137.7 | 5 | Langmuir | 3 | [ |
| 氧化石墨烯/羧甲基纤维素纳米纤维复合材料 | 99 | 5 | Langmuir和Freundlich | — | [ |
| 丙烯酸接枝TOCNF | 100 | 5.3 | Langmuir和Freundlich | — | [ |
| 纤维素/壳聚糖复合气凝胶 | 137.8 | 3~6 | Langmuir | 5 | [ |
| 羟基磷灰石静电纺乙酸纤维素纳米纤维 | 49.75 | 6 | Langmuir | — | [ |
| 细菌纤维素/羟基磷灰石复合材料 | 192 | 3~5 | Langmuir和Freundlich | — | [ |
表3 TOCNF/MCCN复合材料与其他纤维素基吸附剂吸附Pb2+效果
| 吸附剂 | 饱和吸附容量/mg·g-1 | pH | 吸附模型 | 循环次数 | 参考文献 |
|---|---|---|---|---|---|
| TOCNF/MCCN复合材料 | 201.1 | 5 | Langmuir和Freundlich | 5 | 本研究 |
| 硫醇改性CNF复合膜 | 137.7 | 5 | Langmuir | 3 | [ |
| 氧化石墨烯/羧甲基纤维素纳米纤维复合材料 | 99 | 5 | Langmuir和Freundlich | — | [ |
| 丙烯酸接枝TOCNF | 100 | 5.3 | Langmuir和Freundlich | — | [ |
| 纤维素/壳聚糖复合气凝胶 | 137.8 | 3~6 | Langmuir | 5 | [ |
| 羟基磷灰石静电纺乙酸纤维素纳米纤维 | 49.75 | 6 | Langmuir | — | [ |
| 细菌纤维素/羟基磷灰石复合材料 | 192 | 3~5 | Langmuir和Freundlich | — | [ |
| 1 | LI J, WANG X, ZHAO G, et al. Metal-organic framework-based materials: superior adsorbents for the capture of toxic and radioactive metal ions[J]. Chemical Society Reviews, 2018, 47(7): 2322-2356. |
| 2 | NARANJO V I, HENDRICKS M, JONES K S. Lead toxicity in children: an unremitting public health problem[J]. Pediatric Neurology, 2020, 113: 51-55. |
| 3 | ZHANG J L, LI T, LI X Y, et al. A key role of inner-cation-π interaction in adsorption of Pb(Ⅱ) on carbon nanotubes: experimental and DFT studies[J]. Journal of Hazardous Materials, 2021, 412: 125187. |
| 4 | TU Y J, CHANG C K, YOU C F, et al. Treatment of complex heavy metal wastewater using a multi-staged ferrite process[J]. Journal of Hazardous Materials, 2012, 209/210: 379-384. |
| 5 | ABOU-SHADY A, PENG C S, ALMERIA O J, et al. Effect of pH on separation of Pb(Ⅱ) and NO3- from aqueous solutions using electrodialysis[J]. Desalination, 2012, 285: 46-53. |
| 6 | DONG L H, HOU L A, WANG Z S, et al. A new function of spent activated carbon in BAC process: removing heavy metals by ion exchange mechanism[J]. Journal of Hazardous Materials, 2018, 359: 76-84. |
| 7 | LONG J Y, YUVARAJA G, ZHOU S Y, et al. Inactive Fusarium Fungal strains (ZSY and MJY) isolation and application for the removal of Pb(II) ions from aqueous environment[J]. Journal of Industrial and Engineering Chemistry, 2019, 72: 442-452. |
| 8 | TAN P, SUN J, HU Y Y, et al. Adsorption of Cu2+, Cd2+ and Ni2+ from aqueous single metal solutions on graphene oxide membranes[J]. Journal of Hazardous Materials, 2015, 297: 251-260. |
| 9 | CHEN T, ZHOU Z Y, HAN R, et al. Adsorption of cadmium by biochar derived from municipal sewage sludge: impact factors and adsorption mechanism[J]. Chemosphere, 2015, 134: 286-293. |
| 10 | ERDEM E, KARAPINAR N, DONAT R. The removal of heavy metal cations by natural zeolites[J]. Journal of Colloid and Interface Science, 2004, 280(2): 309-314. |
| 11 | 肖瑶, 吴中杰, 崔美, 等. 生物炭-膨润土共改性及其铅离子吸附与稳定化研究[J]. 无机材料学报, 2021: 1-9. |
| XIAO Yao, WU Zhongjie, CUI Mei, et al. Co-modification of biochar and bentonite for adsorption and stabilization of Pb2+ ions[J]. Journal of Inorganic Materials, 2021: 1-9. | |
| 12 | SINGH K, ARORA J K, SINHA T J M, et al. Functionalization of nanocrystalline cellulose for decontamination of Cr(Ⅲ) and Cr(Ⅵ) from aqueous system: computational modeling approach[J]. Clean Technologies and Environmental Policy, 2014, 16(6): 1179-1191. |
| 13 | SHEIKHI A, SAFARI S, YANG H, et al. Copper removal using electrosterically stabilized nanocrystalline cellulose[J]. ACS Applied Materials & Interfaces, 2015, 7(21): 11301-11308. |
| 14 | GUPTA A D, RAWAT K P, BHADAURIA V, et al. Recent trends in the application of modified starch in the adsorption of heavy metals from water: a review[J]. Carbohydrate Polymers, 2021, 269: 117763. |
| 15 | LIU Q, LI F, LU H, et al. Enhanced dispersion stability and heavy metal ion adsorption capability of oxidized starch nanoparticles[J]. Food Chemistry, 2018, 242: 256-263. |
| 16 | ZIA Q, TABASSUM M, MENG J M, et al. Polydopamine-assisted grafting of chitosan on porous poly (L-lactic acid) electrospun membranes for adsorption of heavy metal ions[J]. International Journal of Biological Macromolecules, 2021, 167: 1479-1490. |
| 17 | ELWAHED M S ABD, EL-AZIZ M E ABD, SHAABAN E A, et al. New trend to use biochar as foliar application for wheat plants (Triticum Aestivum)[J]. Journal of Plant Nutrition, 2019, 42(10): 1180-1191. |
| 18 | YOUSSEF A M, HASANIN M S, EL-AZIZ M E ABD, et al. Green, economic, and partially biodegradable wood plastic composites via enzymatic surface modification of lignocellulosic fibers[J]. Heliyon, 2019, 5(3): e01332. |
| 19 | ZIMMERMANN T, BORDEANU N, STRUB E. Properties of nanofibrillated cellulose from different raw materials and its reinforcement potential[J]. Carbohydrate Polymers, 2010, 79(4): 1086-1093. |
| 20 | EKSILER K, ANDOU Y, YILMAZ F, et al. Dynamically controlled fibrillation under combination of ionic liquid with mechanical grinding[J]. Journal of Applied Polymer Science, 2017, 134(7): 44469. |
| 21 | NINOMIYA K, ABE M, TSUKEGI T, et al. Lignocellulose nanofibers prepared by ionic liquid pretreatment and subsequent mechanical nanofibrillation of bagasse powder: application to esterified bagasse/polypropylene composites[J]. Carbohydrate Polymers, 2018, 182: 8-14. |
| 22 | CHEN W S, ABE K, UETANI K, et al. Individual cotton cellulose nanofibers: pretreatment and fibrillation technique[J]. Cellulose, 2014, 21(3): 1517-1528. |
| 23 | ZHU F, ZHENG Y M, ZHANG B G, et al. A critical review on the electrospun nanofibrous membranes for the adsorption of heavy metals in water treatment[J]. Journal of Hazardous Materials, 2021, 401: 123608. |
| 24 | XU Y L, SONG Y J, XU F. TEMPO oxidized cellulose nanofibers-based heterogenous membrane employed for concentration-gradient-driven energy harvesting[J]. Nano Energy, 2021, 79: 105468. |
| 25 | BEAUMONT M, WINKLEHNER S, VEIGEL S, et al. Wet esterification of never-dried cellulose: a simple process to surface-acetylated cellulose nanofibers[J]. Green Chemistry, 2020, 22(17): 5605-5609. |
| 26 | YULIASMI S, GINTING N, WAHYUNI H S, et al. The effect of alkalization on carboxymethil cellulose synthesis from stem and peel cellulose of banana[J]. Open Access Macedonian Journal of Medical Sciences, 2019, 7(22): 3874-3877. |
| 27 | AMALY N, MA Y, EL-MOGHAZY A Y, et al. Copper complex formed with pyridine rings grafted on cellulose nanofibrous membranes for highly efficient lysozyme adsorption[J]. Separation and Purification Technology, 2020, 250: 117086. |
| 28 | ABOU-ZEID R E, KAMAL K H, EL-AZIZ M E ABD, et al. Grafted TEMPO-oxidized cellulose nanofiber embedded with modified magnetite for effective adsorption of lead ions[J]. International Journal of Biological Macromolecules, 2021, 167: 1091-1101. |
| 29 | ZHANG H, OMER A M, HU Z H, et al. Fabrication of magnetic bentonite/carboxymethyl chitosan/sodium alginate hydrogel beads for Cu(Ⅱ) adsorption[J]. International Journal of Biological Macromolecules, 2019, 135: 490-500. |
| 30 | ABOU-ZEID R E, DACRORY S, ALI K A, et al. Novel method of preparation of tricarboxylic cellulose nanofiber for efficient removal of heavy metal ions from aqueous solution[J]. International Journal of Biological Macromolecules, 2018, 119: 207-214. |
| 31 | HONG R Y, ZHANG S Z, DI G Q, et al. Preparation, characterization and application of Fe3O4/ZnO core/shell magnetic nanoparticles[J]. Materials Research Bulletin, 2008, 43(8/9): 2457-2468. |
| 32 | ZHANG H C, TAN X, QIU T T, et al. A novel and biocompatible Fe3O4 loaded chitosan polyelectrolyte nanoparticles for the removal of Cd2+ ion[J]. International Journal of Biological Macromolecules, 2019, 141: 1165-1174. |
| 33 | HONG H J, BAN G, KIM H S, et al. Fabrication of cylindrical 3D cellulose nanofibril(CNF) aerogel for continuous removal of copper(Cu2+) from wastewater[J]. Chemosphere, 2021, 278: 130288. |
| 34 | QURESHI A A, JAVED S, JAVED H M A, et al. Facile formation of SnO2-TiO2 based photoanode and Fe3O4@rGO based counter electrode for efficient dye-sensitized solar cells[J]. Materials Science in Semiconductor Processing, 2021, 123: 105545. |
| 35 | BAKKARI M E, BINDIGANAVILE V, GONCALVES J, et al. Preparation of cellulose nanofibers by TEMPO-oxidation of bleached chemi-thermomechanical pulp for cement applications[J]. Carbohydrate Polymers, 2019, 203: 238-245. |
| 36 | 许时婴, 沈欣, 蒋雄图. 羧甲基壳聚糖结构与性质研究[J]. 无锡轻工业学院学报, 1993, 12(2): 92-100. |
| XU Shiying, SHEN Xin, JIANG Xiongtu. Studies on the structure and the properties of carboxymethylchitosans[J]. Journal of Wuxi University of Light Industry, 1993, 12(2): 92-100. | |
| 37 | YANG R, AUBRECHT K B, MA H Y, et al. Thiol-modified cellulose nanofibrous composite membranes for chromium (Ⅵ) and lead (Ⅱ) adsorption[J]. Polymer, 2014, 55(5): 1167-1176. |
| 38 | GARG U, KAUR M P, JAWA G K, et al. Removal of cadmium (Ⅱ) from aqueous solutions by adsorption on agricultural waste biomass[J]. Journal of Hazardous Materials, 2008, 154(1/2/3): 1149-1157. |
| 39 | 施华珍, 刘坤, 汤睿, 等. 有机改性磁性碱性钙基膨润土的制备及对Cu(Ⅱ)和Mn(Ⅱ)的吸附[J]. 化工进展, 2018, 37(11): 4509-4521. |
| SHI Huazhen, LIU Kun, TANG Rui, et al. Organically modification of magnetic alkaline Ca-bentonite and its adsorption for Cu(Ⅱ) and Mn(Ⅱ)[J]. Chemical Industry and Engineering Progress, 2018, 37(11): 4509-4521. | |
| 40 | 叶代勇, 伊双莉. 纤维素纳米晶须表面紫外光接枝丙烯酸及其吸附Cu2+[J]. 功能材料, 2014, 45(5): 5097-5101. |
| YE Daiyong, YI Shuangli. Cellulose nanowhiskers’ UV-grafted with acrylic acid and its adsorption performance for Cu2+[J]. Journal of Functional Materials, 2014, 45(5): 5097-5101. | |
| 41 | YU H, HONG H J, KIM S M, et al. Mechanically enhanced graphene oxide/carboxymethyl cellulose nanofibril composite fiber as a scalable adsorbent for heavy metal removal[J]. Carbohydrate Polymers, 2020, 240: 116348. |
| 42 | NAJAFLOU S, RAD M F, BAGHDADI M, et al. Removal of Pb(Ⅱ) from contaminated waters using cellulose sulfate/chitosan aerogel: equilibrium, kinetics, and thermodynamic studies[J]. Journal of Environmental Management, 2021, 286: 112167. |
| 43 | HAMAD A A, HASSOUNA M S, SHALABY T I, et al. Electrospun cellulose acetate nanofiber incorporated with hydroxyapatite for removal of heavy metals[J]. International Journal of Biological Macromolecules, 2020, 151: 1299-1313. |
| 44 | NÚÑEZ D, CÁCERES R, IDE W, et al. An ecofriendly nanocomposite of bacterial cellulose and hydroxyapatite efficiently removes lead from water[J]. International Journal of Biological Macromolecules, 2020, 165: 2711-2720. |
| [1] | 崔守成, 徐洪波, 彭楠. 两种MOFs材料用于O2/He吸附分离的模拟分析[J]. 化工进展, 2023, 42(S1): 382-390. |
| [2] | 陈崇明, 陈秋, 宫云茜, 车凯, 郁金星, 孙楠楠. 分子筛基CO2吸附剂研究进展[J]. 化工进展, 2023, 42(S1): 411-419. |
| [3] | 许春树, 姚庆达, 梁永贤, 周华龙. 共价有机框架材料功能化策略及其对Hg(Ⅱ)和Cr(Ⅵ)的吸附性能研究进展[J]. 化工进展, 2023, 42(S1): 461-478. |
| [4] | 顾永正, 张永生. HBr改性飞灰对Hg0的动态吸附及动力学模型[J]. 化工进展, 2023, 42(S1): 498-509. |
| [5] | 郭强, 赵文凯, 肖永厚. 增强流体扰动强化变压吸附甲硫醚/氮气分离的数值模拟[J]. 化工进展, 2023, 42(S1): 64-72. |
| [6] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [7] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [8] | 杨莹, 侯豪杰, 黄瑞, 崔煜, 王兵, 刘健, 鲍卫仁, 常丽萍, 王建成, 韩丽娜. 利用煤焦油中酚类物质Stöber法制备碳纳米球用于CO2吸附[J]. 化工进展, 2023, 42(9): 5011-5018. |
| [9] | 张振, 李丹, 陈辰, 吴菁岚, 应汉杰, 乔浩. 吸附树脂对唾液酸的分离纯化[J]. 化工进展, 2023, 42(8): 4153-4158. |
| [10] | 姜晶, 陈霄宇, 张瑞妍, 盛光遥. 载锰生物炭制备及其在环境修复中应用研究进展[J]. 化工进展, 2023, 42(8): 4385-4397. |
| [11] | 于静文, 宋璐娜, 刘砚超, 吕瑞东, 武蒙蒙, 冯宇, 李忠, 米杰. 一种吲哚基超交联聚合物In-HCP对水中碘的吸附作用[J]. 化工进展, 2023, 42(7): 3674-3683. |
| [12] | 李艳玲, 卓振, 池亮, 陈曦, 孙堂磊, 刘鹏, 雷廷宙. 氮掺杂生物炭的制备与应用研究进展[J]. 化工进展, 2023, 42(7): 3720-3735. |
| [13] | 白亚迪, 邓帅, 赵睿恺, 赵力, 杨英霞. 变温吸附碳捕集机组标准化测试方案探讨及性能实验[J]. 化工进展, 2023, 42(7): 3834-3846. |
| [14] | 张雪伟, 黄亚继, 许月阳, 程好强, 朱志成, 李金壘, 丁雪宇, 王圣, 张荣初. 碱性吸附剂对燃煤烟气中SO3的吸附特性[J]. 化工进展, 2023, 42(7): 3855-3864. |
| [15] | 陆洋, 周劲松, 周启昕, 王瑭, 刘壮, 李博昊, 周灵涛. CeO2/TiO2吸附剂煤气脱汞产物的浸出规律[J]. 化工进展, 2023, 42(7): 3875-3883. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||