化工进展 ›› 2022, Vol. 41 ›› Issue (2): 881-891.DOI: 10.16085/j.issn.1000-6613.2021-0109
Fe3O4@PGMA-Arg磁性吸附剂的制备及对亚铁离子的吸附性能
- 华东理工大学化工学院, 绿色能源化工国际联合研究中心,上海 200237
-
收稿日期:2021-01-16修回日期:2021-04-07出版日期:2022-02-05发布日期:2022-02-23 -
通讯作者:杨敬一 -
作者简介:田忠宇(1992—),男,硕士研究生,研究方向为石油化学。E-mail:tianzysl@163.com 。
Preparation of Fe3O4@PGMA-Arg magnetic adsorbents and its adsorption performance for ferrous ion
TIAN Zhongyu( ), YANG Jingyi(
), YANG Jingyi( ), WANG Yixing, XU Xinru
), WANG Yixing, XU Xinru
- International Joint Research Center of Green Energy Chemical Engineering, School of Chemical Engineering, East China University of Science and Technology, Shanghai 200237, China
-
Received:2021-01-16Revised:2021-04-07Online:2022-02-05Published:2022-02-23 -
Contact:YANG Jingyi
摘要:
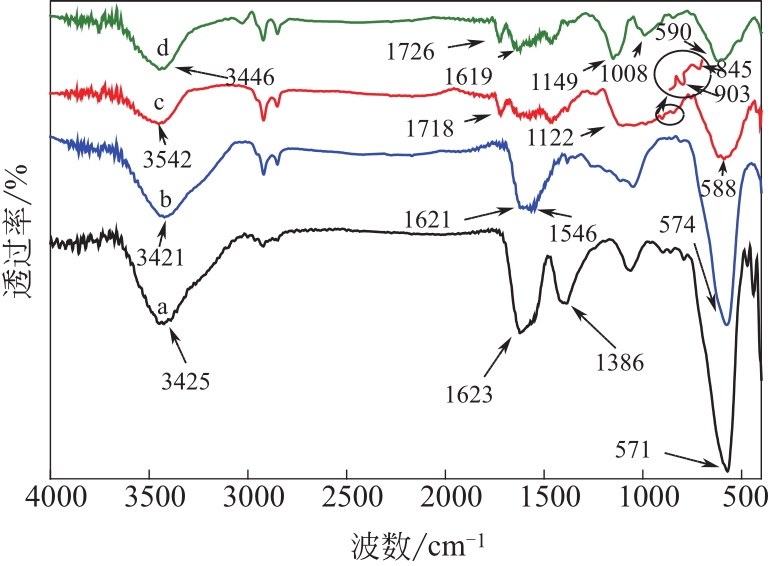
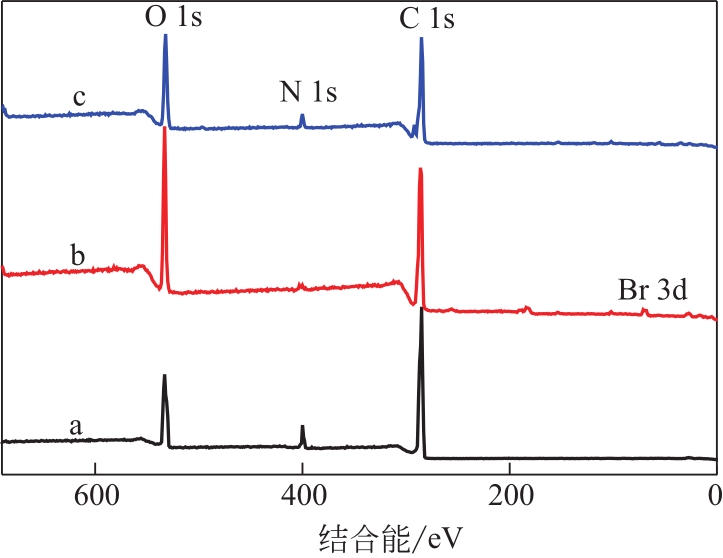
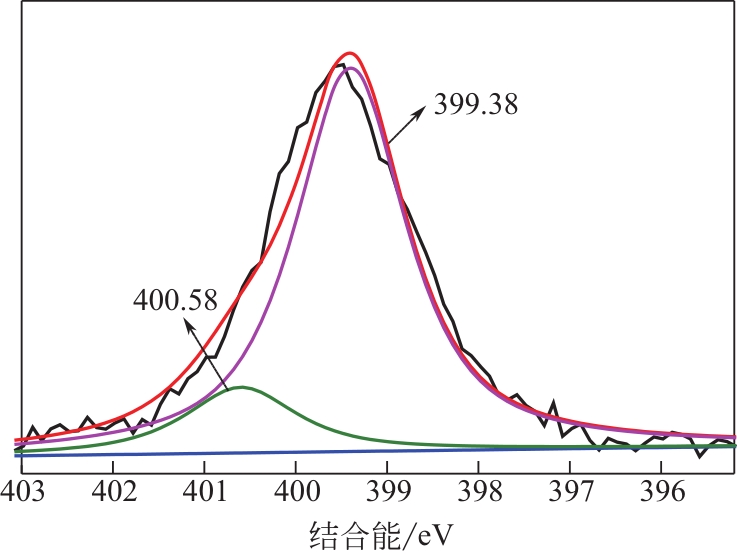
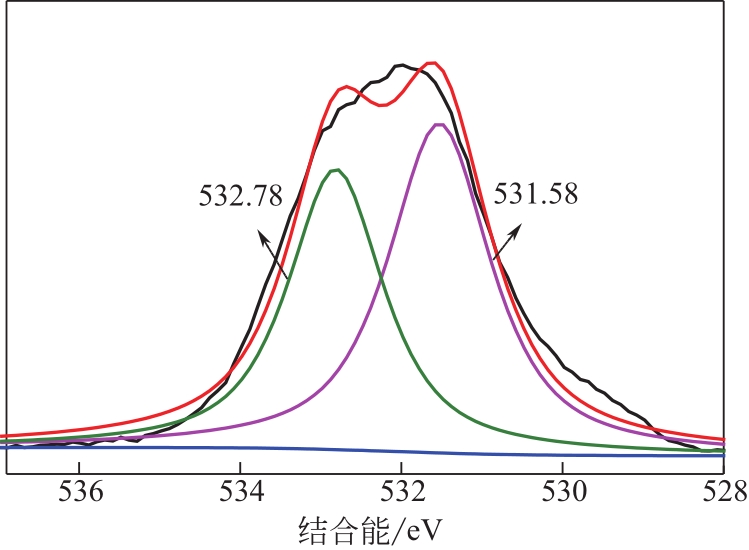
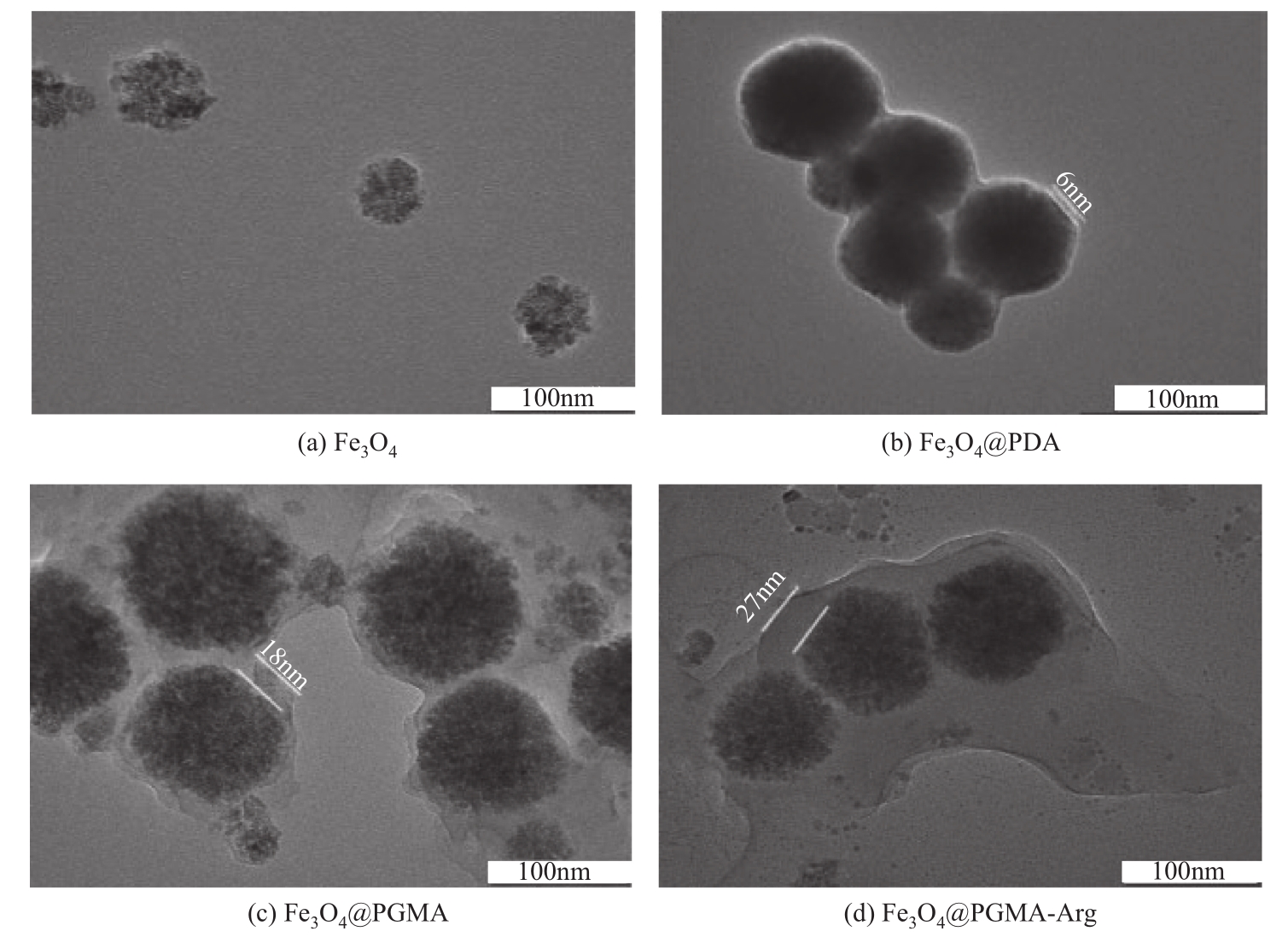
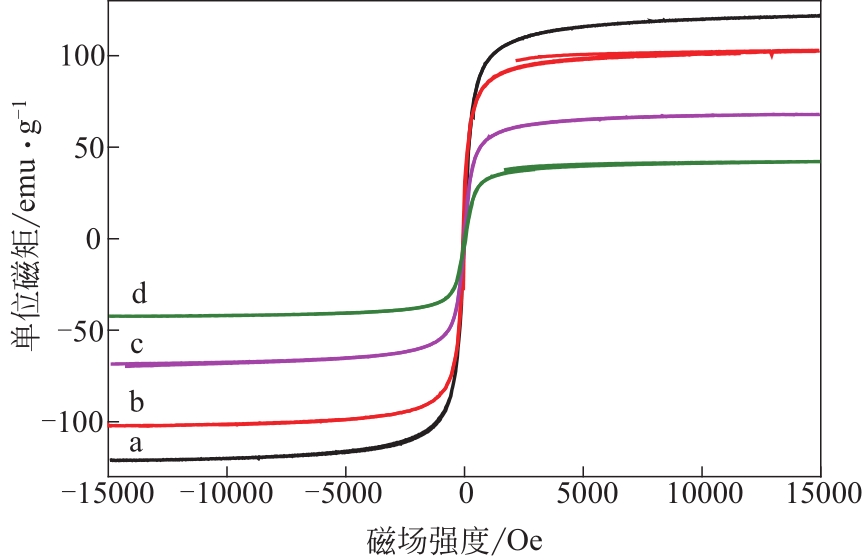
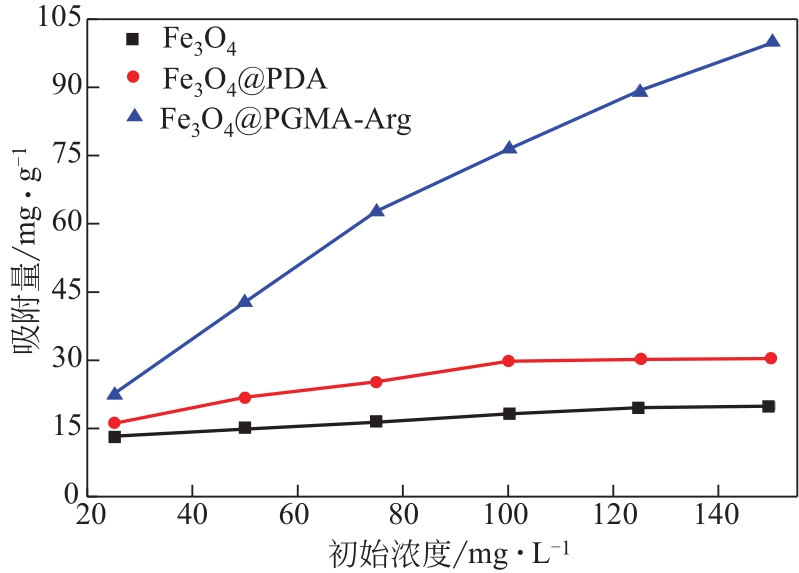
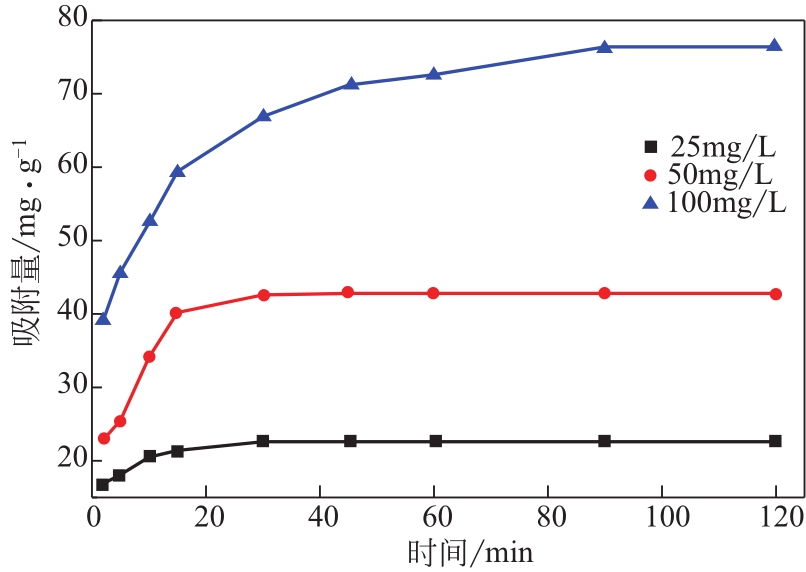
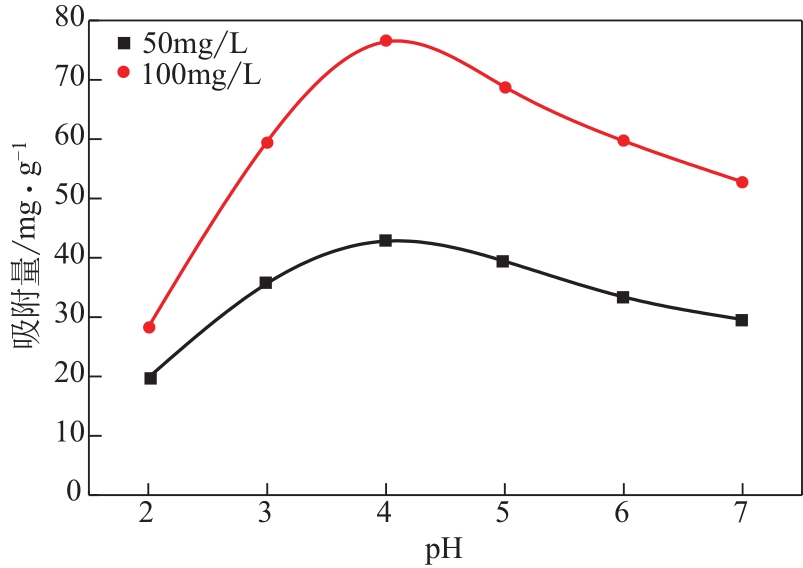
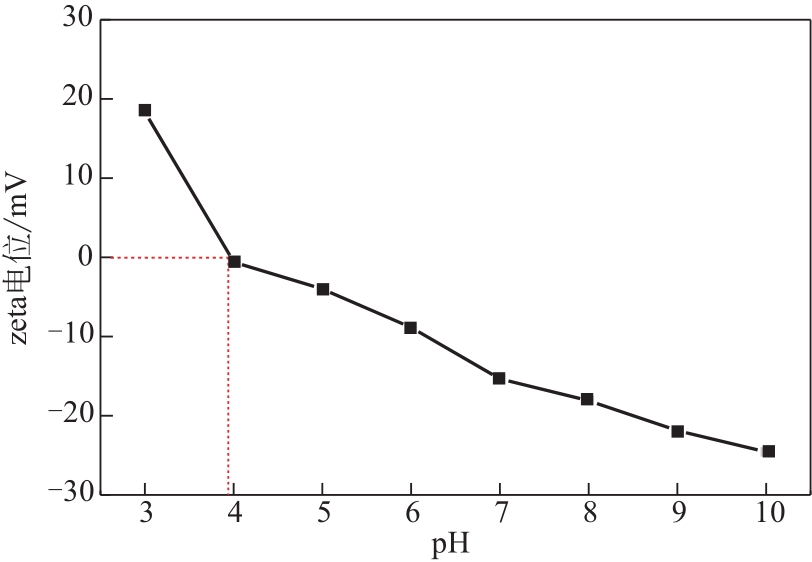
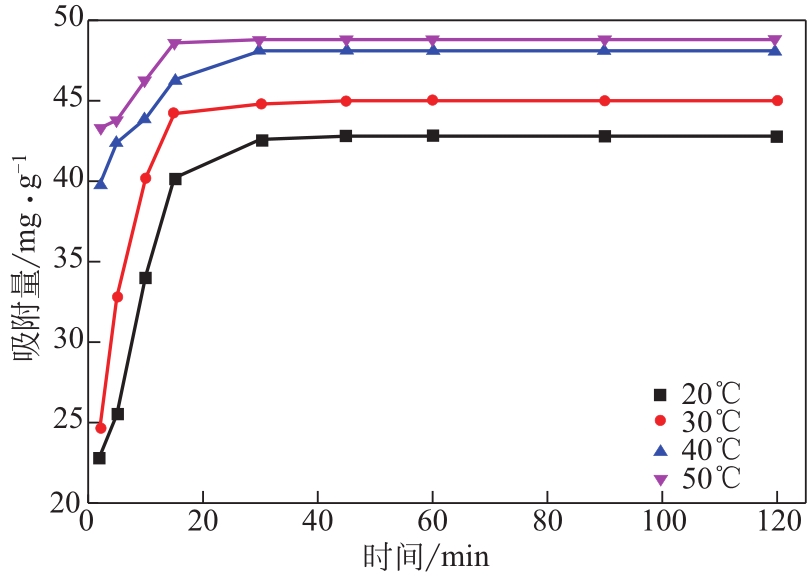
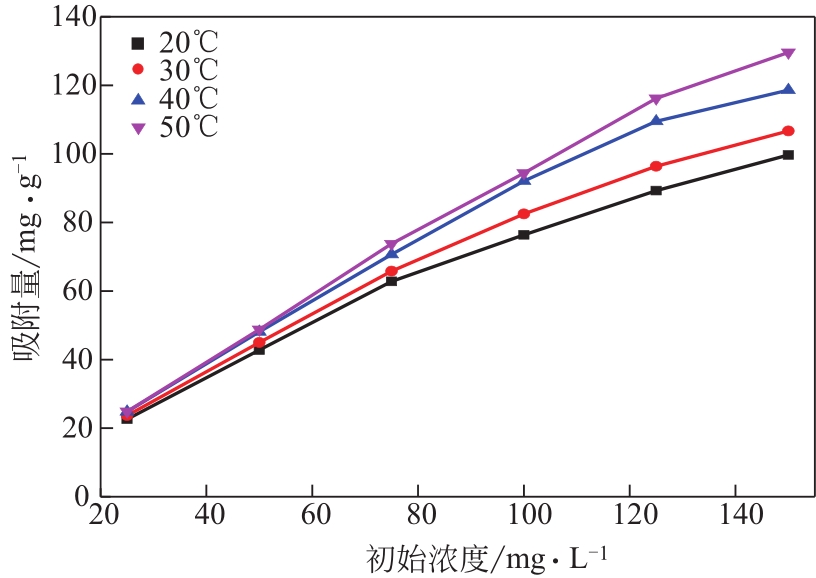
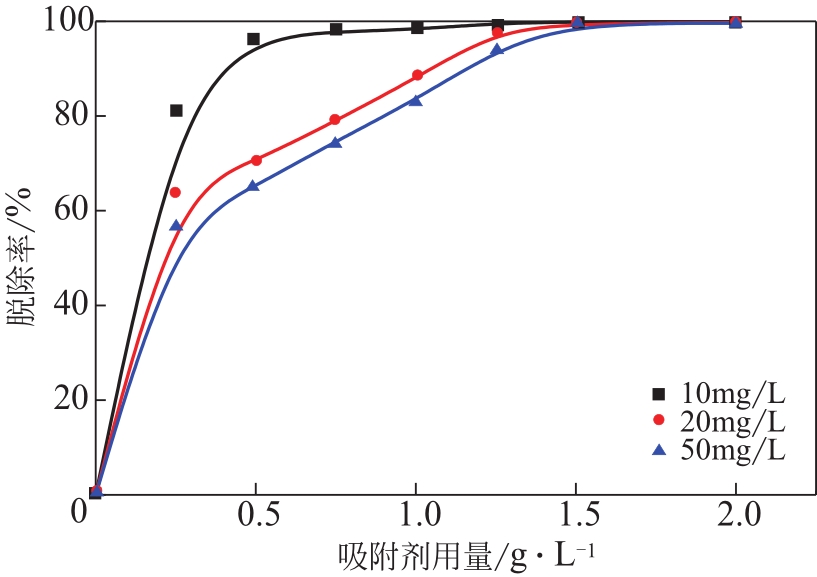
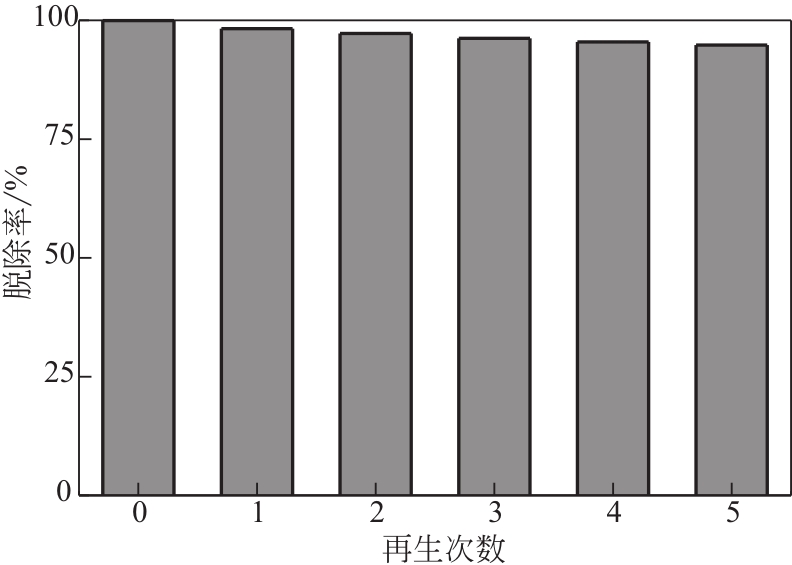
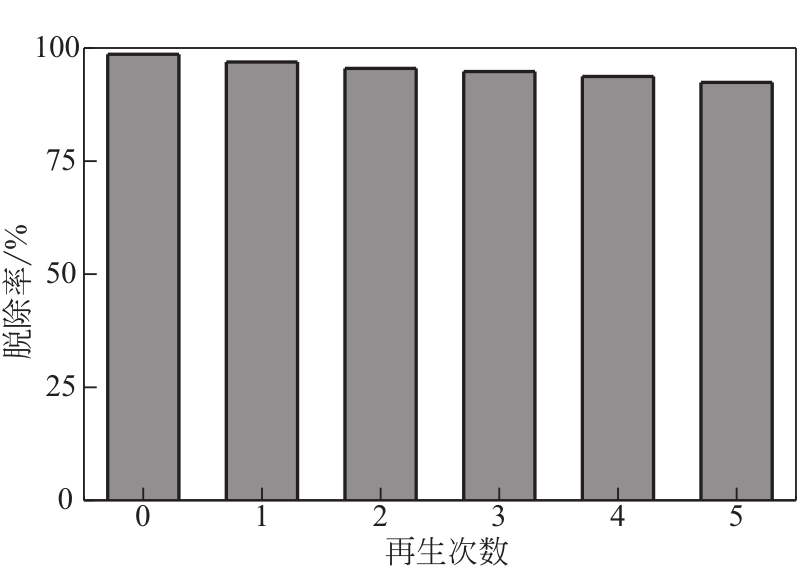
为脱除油田采出水中的Fe(Ⅱ)合成磁性吸附剂,本文以溶剂热法制得亲水性Fe3O4纳米颗粒,使用盐酸多巴胺(DA)进行包覆得到Fe3O4@PDA,再以甲基丙烯酸缩水甘油酯(GMA)在其表面聚合接枝得到Fe3O4@PGMA,后经精氨酸(Arg)修饰后得到功能化的Fe3O4@PGMA-Arg。通过红外光谱、X射线光电子能谱、X射线衍射和磁化强度对制备的纳米颗粒进行表征,结果表明Fe3O4@PGMA-Arg中具有伯胺、亚胺双键、羟基和羧基官能团,其中伯胺基团和亚胺双键上的N可与Fe(Ⅱ)形成配位键,羟基和羧基的O可与Fe(Ⅱ)形成配位键,从而达到吸附Fe(Ⅱ)的目的。合成产物仍保持了Fe3O4的反尖晶石结构,具有好的磁响应性能。通过静态吸附实验探究吸附条件对Fe3O4@PGMA-Arg吸附Fe(Ⅱ)的影响因素,结果表明,Fe(Ⅱ)的吸附量随着温度和初始浓度的增加而增加,适宜的pH为4。动力学和热力学研究表明,吸附Fe(Ⅱ)过程符合准二级反应动力学模型,吸附等温线符合Langmuir模型,吸附过程活化能为45.60kJ/mol,为化学吸附。Fe3O4@PGMA-Arg经5次再生后,对亚铁离子仍保持较高的脱除率。
中图分类号:
引用本文
田忠宇, 杨敬一, 王怿兴, 徐心茹. Fe3O4@PGMA-Arg磁性吸附剂的制备及对亚铁离子的吸附性能[J]. 化工进展, 2022, 41(2): 881-891.
TIAN Zhongyu, YANG Jingyi, WANG Yixing, XU Xinru. Preparation of Fe3O4@PGMA-Arg magnetic adsorbents and its adsorption performance for ferrous ion[J]. Chemical Industry and Engineering Progress, 2022, 41(2): 881-891.
| 样品 | C 1s(摩尔分数)/% | N 1s(摩尔分数)/% | O 1s(摩尔分数)/% | Br 3d(摩尔分数)/% |
|---|---|---|---|---|
| Fe3O4@PDA | 73.97 | 7.73 | 18.66 | — |
| Fe3O4@PGMA | 72.04 | 0.42 | 24.48 | 1.06 |
| Fe3O4@PGMA-Arg | 70.99 | 4.10 | 24.73 | 0.18 |
表1 Fe3O4@PDA、Fe3O4@PGMA和Fe3O4@PGMA-Arg表面元素含量
| 样品 | C 1s(摩尔分数)/% | N 1s(摩尔分数)/% | O 1s(摩尔分数)/% | Br 3d(摩尔分数)/% |
|---|---|---|---|---|
| Fe3O4@PDA | 73.97 | 7.73 | 18.66 | — |
| Fe3O4@PGMA | 72.04 | 0.42 | 24.48 | 1.06 |
| Fe3O4@PGMA-Arg | 70.99 | 4.10 | 24.73 | 0.18 |
浓度 /mg·L-1 | 准一级动力学模型 | 准二级动力学模型 | |||||
|---|---|---|---|---|---|---|---|
| k1/min-1 | Qe/mg·g-1 | R2 | k2/g·mg-1·min-1 | Qe/mg·g-1 | R2 | ||
| 25 | 0.7465 | 22.5 | 0.8833 | 0.05338 | 22.8 | 0.9999 | |
| 50 | 1.9614 | 42.8 | 0.8601 | 0.01167 | 43.8 | 0.9994 | |
| 100 | 1.8076 | 70.5 | 0.8520 | 0.00030 | 79.0 | 0.9990 | |
表2 Fe3O4@PGMA-Arg不同起始浓度下吸附动力学模型拟合参数
浓度 /mg·L-1 | 准一级动力学模型 | 准二级动力学模型 | |||||
|---|---|---|---|---|---|---|---|
| k1/min-1 | Qe/mg·g-1 | R2 | k2/g·mg-1·min-1 | Qe/mg·g-1 | R2 | ||
| 25 | 0.7465 | 22.5 | 0.8833 | 0.05338 | 22.8 | 0.9999 | |
| 50 | 1.9614 | 42.8 | 0.8601 | 0.01167 | 43.8 | 0.9994 | |
| 100 | 1.8076 | 70.5 | 0.8520 | 0.00030 | 79.0 | 0.9990 | |
温度 /℃ | Langmuir模型 | Freundlich模型 | ||||
|---|---|---|---|---|---|---|
| KL/L·mg-1 | Qm/mg·g-1 | R2 | KF/mg1-1/n·L1/n·g-1 | n | R2 | |
| 20 | 0.08276 | 121.2 | 0.9931 | 16.05 | 2.05 | 0.9704 |
| 30 | 0.13181 | 123.3 | 0.9924 | 22.37 | 2.28 | 0.9803 |
| 40 | 0.39558 | 127.2 | 0.9921 | 42.24 | 3.04 | 0.9783 |
| 50 | 0.67623 | 136.8 | 0.9877 | 54.51 | 3.15 | 0.9325 |
表3 Fe3O4@PGMA-Arg吸附等温线模型拟合参数
温度 /℃ | Langmuir模型 | Freundlich模型 | ||||
|---|---|---|---|---|---|---|
| KL/L·mg-1 | Qm/mg·g-1 | R2 | KF/mg1-1/n·L1/n·g-1 | n | R2 | |
| 20 | 0.08276 | 121.2 | 0.9931 | 16.05 | 2.05 | 0.9704 |
| 30 | 0.13181 | 123.3 | 0.9924 | 22.37 | 2.28 | 0.9803 |
| 40 | 0.39558 | 127.2 | 0.9921 | 42.24 | 3.04 | 0.9783 |
| 50 | 0.67623 | 136.8 | 0.9877 | 54.51 | 3.15 | 0.9325 |
| 温度/℃ | ΔG?/kJ·mol-1 | ΔH?/kJ·mol-1 | ΔS?/J·mol-1·K-1 |
|---|---|---|---|
| 20 | -13.55 | 67.34 | 298.39 |
| 30 | -22.44 | ||
| 40 | -26.04 | ||
| 50 | -28.32 |
表4 Fe3O4@PGMA-Arg热力学曲线拟合参数
| 温度/℃ | ΔG?/kJ·mol-1 | ΔH?/kJ·mol-1 | ΔS?/J·mol-1·K-1 |
|---|---|---|---|
| 20 | -13.55 | 67.34 | 298.39 |
| 30 | -22.44 | ||
| 40 | -26.04 | ||
| 50 | -28.32 |
| 1 | XIONG Boya, MILLER Z, ROMAN-WHITE S, et al. Chemical degradation of polyacrylamide during hydraulic fracturing[J]. Environmental Science & Technology, 2018, 52(1): 327-336. |
| 2 | ZHANG Ping, ZHANG Zhang, LIU Yuan, et al. Investigation of the impact of ferrous species on the performance of common oilfield scale inhibitors for mineral scale control[J]. Journal of Petroleum Science and Engineering, 2019, 172: 288-296. |
| 3 | KAVEESHWAR A R, SANDERS M, PONNUSAMY S K, et al. Chitosan as a biosorbent for adsorption of iron (II) from fracking wastewater[J]. Polymers for Advanced Technologies, 2018, 29(2): 961-969. |
| 4 | 黄漫, 李美蓉, 田兰兰, 等. 亚铁离子对驱油聚合物溶液黏度的影响及其降黏机理[J]. 应用化学, 2013, 30(12): 1399-1403. |
| HUANG Man, LI Meirong, TIAN Lanlan, et al. Effect of ferrous ion on reducing the viscosity of oil flooding polymer solution and its mechanism[J]. Chinese Journal of Applied Chemistry, 2013, 30(12): 1399-1403. | |
| 5 | GREGORY K B, VIDIC R D, DZOMBAK D A. Water management challenges associated with the production of shale gas by hydraulic fracturing[J]. Elements, 2011, 7(3): 181-186. |
| 6 | 刘金燕, 刘立华, 薛建荣, 等. 重金属废水吸附处理的研究进展[J]. 环境化学, 2018, 37(9): 2016-2024. |
| LIU Jinyan, LIU Lihua, XUE Jianrong, et al. Research progress on treatment of heavy metal wastewater by adsorption[J]. Environmental Chemistry, 2018, 37(9): 2016-2024. | |
| 7 | 汪彩虹, 陈硕然, 叶常青, 等. 单分散磁性Fe3O4纳米粒子的研究进展[J]. 化工进展, 2016, 35(S1): 242-247. |
| WANG Caihong, CHEN Shuoran, YE Changqing, et al. Research progress on monodisperse Fe3O4 magnetic nanoparticles[J]. Chemical Industry and Engineering Progress, 2016, 35(S1): 242-247. | |
| 8 | MASOUMI A, GHAEMY M, BAKHT A N. Removal of metal ions from water using poly(MMA-co-MA)/modified-Fe3O4 magnetic nanocomposite: isotherm and kinetic study[J]. Industrial & Engineering Chemistry Research, 2014, 53(19): 8188-8197. |
| 9 | 刘明强, 王会才, 孙强. 端氨基超支聚合物功能化磁性复合材料对Cu2+和甲基橙的吸附研究[J]. 化工新型材料, 2017, 45(2): 91-94. |
| LIU Mingqiang, WANG Huicai, SUN Qiang. Study on adsorption of amino terminated hyperbranched polymer functional magnetic composite materials for Cu2+ and methyl orange[J]. New Chemical Materials, 2017, 45(2): 91-94. | |
| 10 | ZHANG Sai, ZHOU Yifeng, NIE Wangyan, et al. Preparation of Fe3O4/chitosan/poly(acrylic acid) composite particles and its application in adsorbing copper ion (II)[J]. Cellulose, 2012, 19(6): 2081-2091. |
| 11 | MA Zhiyuan, JIA Xin, HU Jiamei, et al. Mussel-inspired thermosensitive polydopamine-graft-poly(N-isopropylacrylamide) coating for controlled-release fertilizer[J]. Journal of Agricultural and Food Chemistry, 2013, 61(50): 12232-12237. |
| 12 | 陈思远, 董旭, 查刘生. 表面引发原子转移自由基聚合法合成无机/有机核壳复合纳米粒子[J]. 化学进展, 2015, 27(7): 831-840. |
| CHEN Siyuan, DONG Xu, ZHA Liusheng. Inorganic/organic core-shell composite nanoparticles by surface-initiated atom transfer radical polymerization[J]. Progress in Chemistry, 2015, 27(7): 831-840. | |
| 13 | BUAMAH R, PETRUSEVSKI B, SCHIPPERS J C. Oxidation of adsorbed ferrous iron: kinetics and influence of process conditions[J]. Water Science and Technology, 2009, 60(9): 2353-2363. |
| 14 | SRIVASTAVA V, WENG C H, SINGH V K, et al. Adsorption of nickel ions from aqueous solutions by nano alumina: kinetic, mass transfer, and equilibrium studies[J]. Journal of Chemical & Engineering Data, 2011, 56(4): 1414-1422. |
| 15 | NEELI S T, RAMSURN H, NG C Y, et al. Removal of Cr (Ⅵ), As (Ⅴ), Cu(Ⅱ), and Pb(Ⅱ) using cellulose biochar supported iron nanoparticles: a kinetic and mechanistic study[J]. Journal of Environmental Chemical Engineering, 2020, 8(5): 103886. |
| 16 | ÖZACAR M, ŞENGIL İ A. A kinetic study of metal complex dye sorption onto pine sawdust[J]. Process Biochemistry, 2005, 40(2): 565-572. |
| 17 | PHOLOSI A, NAIDOO E B, OFOMAJA A E. Intraparticle diffusion of Cr(Ⅵ) through biomass and magnetite coated biomass: a comparative kinetic and diffusion study[J]. South African Journal of Chemical Engineering, 2020, 32: 39-55. |
| 18 | TSENG R L, WU F C. Inferring the favorable adsorption level and the concurrent multi-stage process with the Freundlich constant[J]. Journal of Hazardous Materials, 2008, 155(1/2): 277-287. |
| 19 | GUO Xuan, WANG Jianlong. Comparison of linearization methods for modeling the Langmuir adsorption isotherm[J]. Journal of Molecular Liquids, 2019, 296: 111850. |
| [1] | 崔守成, 徐洪波, 彭楠. 两种MOFs材料用于O2/He吸附分离的模拟分析[J]. 化工进展, 2023, 42(S1): 382-390. |
| [2] | 陈崇明, 陈秋, 宫云茜, 车凯, 郁金星, 孙楠楠. 分子筛基CO2吸附剂研究进展[J]. 化工进展, 2023, 42(S1): 411-419. |
| [3] | 许春树, 姚庆达, 梁永贤, 周华龙. 共价有机框架材料功能化策略及其对Hg(Ⅱ)和Cr(Ⅵ)的吸附性能研究进展[J]. 化工进展, 2023, 42(S1): 461-478. |
| [4] | 顾永正, 张永生. HBr改性飞灰对Hg0的动态吸附及动力学模型[J]. 化工进展, 2023, 42(S1): 498-509. |
| [5] | 郭强, 赵文凯, 肖永厚. 增强流体扰动强化变压吸附甲硫醚/氮气分离的数值模拟[J]. 化工进展, 2023, 42(S1): 64-72. |
| [6] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [7] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [8] | 杨莹, 侯豪杰, 黄瑞, 崔煜, 王兵, 刘健, 鲍卫仁, 常丽萍, 王建成, 韩丽娜. 利用煤焦油中酚类物质Stöber法制备碳纳米球用于CO2吸附[J]. 化工进展, 2023, 42(9): 5011-5018. |
| [9] | 姜晶, 陈霄宇, 张瑞妍, 盛光遥. 载锰生物炭制备及其在环境修复中应用研究进展[J]. 化工进展, 2023, 42(8): 4385-4397. |
| [10] | 张振, 李丹, 陈辰, 吴菁岚, 应汉杰, 乔浩. 吸附树脂对唾液酸的分离纯化[J]. 化工进展, 2023, 42(8): 4153-4158. |
| [11] | 王知彩, 刘伟伟, 周璁, 潘春秀, 闫洪雷, 李占库, 颜井冲, 任世彪, 雷智平, 水恒福. 基于煤基腐殖酸的高效减水剂合成与性能表征[J]. 化工进展, 2023, 42(7): 3634-3642. |
| [12] | 于静文, 宋璐娜, 刘砚超, 吕瑞东, 武蒙蒙, 冯宇, 李忠, 米杰. 一种吲哚基超交联聚合物In-HCP对水中碘的吸附作用[J]. 化工进展, 2023, 42(7): 3674-3683. |
| [13] | 李艳玲, 卓振, 池亮, 陈曦, 孙堂磊, 刘鹏, 雷廷宙. 氮掺杂生物炭的制备与应用研究进展[J]. 化工进展, 2023, 42(7): 3720-3735. |
| [14] | 白亚迪, 邓帅, 赵睿恺, 赵力, 杨英霞. 变温吸附碳捕集机组标准化测试方案探讨及性能实验[J]. 化工进展, 2023, 42(7): 3834-3846. |
| [15] | 张雪伟, 黄亚继, 许月阳, 程好强, 朱志成, 李金壘, 丁雪宇, 王圣, 张荣初. 碱性吸附剂对燃煤烟气中SO3的吸附特性[J]. 化工进展, 2023, 42(7): 3855-3864. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||