化工进展 ›› 2021, Vol. 40 ›› Issue (1): 515-525.DOI: 10.16085/j.issn.1000-6613.2020-0559
废旧轮胎热解及热解产物研究展望
蒋智慧1,2( ), 刘洋1,2, 宋永猛1,2, 邓泽宇1,2, 张天昊1,2, 付洁1,2, 敖文雅1,2, 代建军1,2(
), 刘洋1,2, 宋永猛1,2, 邓泽宇1,2, 张天昊1,2, 付洁1,2, 敖文雅1,2, 代建军1,2( )
)
- 1.北京化工大学化学工程学院,北京 100029
2.有机无机复合材料国家重点实验室,北京 100029
-
收稿日期:2020-04-09出版日期:2021-01-05发布日期:2021-01-12 -
通讯作者:代建军 -
作者简介:蒋智慧(1994—),女,硕士研究生,研究方向为固废处理与转化。E-mail:wisdomj0310@163.com 。 -
基金资助:国家重点研发计划(2017YFE0124800)
Review of pyrolysis for waste tires and research prospects of pyrolysis products
Zhihui JIANG1,2( ), Yang LIU1,2, Yongmeng SONG1,2, Zeyu DENG1,2, Tianhao ZHANG1,2, Jie FU1,2, Wenya AO1,2, Jianjun DAI1,2(
), Yang LIU1,2, Yongmeng SONG1,2, Zeyu DENG1,2, Tianhao ZHANG1,2, Jie FU1,2, Wenya AO1,2, Jianjun DAI1,2( )
)
- 1.College of Chemical Engineering, Beijing University of Chemical Technology, Beijing 100029, China
2.State Key Laboratory of Organic-Inorganic Composites, Beijing 100029, China
-
Received:2020-04-09Online:2021-01-05Published:2021-01-12 -
Contact:Jianjun DAI
摘要:
热解作为废旧轮胎处置的重要技术手段,可以有效实现其减量化、无害化和资源化利用。本文综述了废旧轮胎热解的影响因素以及热解产物的研究进展,对废旧轮胎热解的经济、环境和社会效益进行了说明,指出当前工业化热解废旧轮胎存在的问题,并展望了未来节能环保式热解工艺的应用前景。结合现有的工业化热解设备,优化工艺条件和反应器结构型式,进一步分析了热解产物即热解气、热解油及热解炭的成分结构与应用,通过对热解产物改性活化与提质处理,创造更大的经济效益。提出应基于环境法规要求和绿色发展理念,糅合多种处理技术,研制适合废旧轮胎热解的工艺装备,开发集收集/预处理/热解/产物回收与提质于一体的废旧轮胎处置技术,实现废旧轮胎高效清洁转化和高值利用。
中图分类号:
引用本文
蒋智慧, 刘洋, 宋永猛, 邓泽宇, 张天昊, 付洁, 敖文雅, 代建军. 废旧轮胎热解及热解产物研究展望[J]. 化工进展, 2021, 40(1): 515-525.
Zhihui JIANG, Yang LIU, Yongmeng SONG, Zeyu DENG, Tianhao ZHANG, Jie FU, Wenya AO, Jianjun DAI. Review of pyrolysis for waste tires and research prospects of pyrolysis products[J]. Chemical Industry and Engineering Progress, 2021, 40(1): 515-525.
| 技术 | 主要内容 | 特点 |
|---|---|---|
| 重建 | ||
| 原形利用 | 防护堤坝、游乐玩具等 | 处理量低,<总量1% |
| 翻新 | 翻新轮胎寿命等同新轮胎寿命60%~90% | 翻新率低;硫化过程中产生危害性有机物 |
| 材料回收 | ||
| 再生橡胶 | 加工制备成能重新使用的橡胶 | 二次污染严重、再生手段不够成熟,推广度低 |
| 生产胶粉 | 用于制备离子交换剂等 | 工艺不够成熟,造成二次污染 |
| 能量回收 | ||
| 焚烧 | 直接焚烧,部分能量得以回收 | 二次污染,产生二 英、VOC、颗粒物等 英、VOC、颗粒物等 |
| 气化 | 采用气化法处理废轮胎制取可燃气体 | 产品较少,与热解相比,经济性低 |
| 热解 | 无氧热分解,生产富含烃的热解气、油和热解炭 | 减量化、无害化,资源化综合利用好 |
表1 废旧轮胎处理技术[5-6]
| 技术 | 主要内容 | 特点 |
|---|---|---|
| 重建 | ||
| 原形利用 | 防护堤坝、游乐玩具等 | 处理量低,<总量1% |
| 翻新 | 翻新轮胎寿命等同新轮胎寿命60%~90% | 翻新率低;硫化过程中产生危害性有机物 |
| 材料回收 | ||
| 再生橡胶 | 加工制备成能重新使用的橡胶 | 二次污染严重、再生手段不够成熟,推广度低 |
| 生产胶粉 | 用于制备离子交换剂等 | 工艺不够成熟,造成二次污染 |
| 能量回收 | ||
| 焚烧 | 直接焚烧,部分能量得以回收 | 二次污染,产生二 英、VOC、颗粒物等 英、VOC、颗粒物等 |
| 气化 | 采用气化法处理废轮胎制取可燃气体 | 产品较少,与热解相比,经济性低 |
| 热解 | 无氧热分解,生产富含烃的热解气、油和热解炭 | 减量化、无害化,资源化综合利用好 |
| 过程 | 热解温度/℃ | 反应 |
|---|---|---|
| 初级反应 | <200 | 水分蒸发 |
| >200 | 油类助剂、增塑剂等有机助剂分解 | |
| 200~300 | 天然橡胶分解 | |
| 250~500 | 合成橡胶分解 | |
| 二次反应 | 500~900 | Diels-Alder聚合反应及二次炭化反应 |
表2 废旧轮胎热解过程[7-8]
| 过程 | 热解温度/℃ | 反应 |
|---|---|---|
| 初级反应 | <200 | 水分蒸发 |
| >200 | 油类助剂、增塑剂等有机助剂分解 | |
| 200~300 | 天然橡胶分解 | |
| 250~500 | 合成橡胶分解 | |
| 二次反应 | 500~900 | Diels-Alder聚合反应及二次炭化反应 |
| 催化剂 | 轮胎种类(粒径) | 热解温度/℃ | 实验结论 | 参考文献 |
|---|---|---|---|---|
| ZSM-5 | 汽车(1.0~1.4mm) | 430 | 苯和二甲苯等芳烃产率提高 | [ |
| Al2O3 | 混合轮胎粉末(2.0~3.5mm) | 450 | 热解油中芳香族化合物含量为84.6% | [ |
| Ca(OH)2 | 450 | 热解油产率最高,其中芳香族化合物降低至60.9% | [ | |
| 天然沸石 | 450 | 热解油中芳香族化合物含量为71.0% | [ | |
| Si∶Al=3∶7 | 汽车(0.01~0.09mm) | 500 | 热解油产率最高为55.7%,且芳香族化合物最高 | [ |
| Nano HZSM-5/γ-Al2O3 | 汽车(0.5~2.0mm) | 400 | 对轻质烯烃的选择性更高 | [ |
| Ru/MCM-41 | 汽车(8~18目) | 470 | 轻质烯烃产率提高 | [ |
| NaOH(35%) | 汽车(20~100目) | 550 | 热解油产率最高 | [ |
| Fe粉(35%) | 550 | 热解炭产物分布影响最大 | [ | |
| 高炉渣(35%) | 550 | 热解气产物产率最高 | [ | |
| TiO2 | 汽车(2.0mm) | 550 | 热解气产率提高 | [ |
| CaO(2.5%) | 汽车轮胎小块(100mm×120mm) | 500 | 液相产率提高到48.0% | [ |
| ZnO(2.5%) | 530 | 液相产率提高到48.0% | [ | |
| CaO,ZnO,TiO2 | 600 | 热解油和热解炭黑产率大幅度下降,热解气产率上升 | [ |
表3 一些催化剂对废旧轮胎热解的影响
| 催化剂 | 轮胎种类(粒径) | 热解温度/℃ | 实验结论 | 参考文献 |
|---|---|---|---|---|
| ZSM-5 | 汽车(1.0~1.4mm) | 430 | 苯和二甲苯等芳烃产率提高 | [ |
| Al2O3 | 混合轮胎粉末(2.0~3.5mm) | 450 | 热解油中芳香族化合物含量为84.6% | [ |
| Ca(OH)2 | 450 | 热解油产率最高,其中芳香族化合物降低至60.9% | [ | |
| 天然沸石 | 450 | 热解油中芳香族化合物含量为71.0% | [ | |
| Si∶Al=3∶7 | 汽车(0.01~0.09mm) | 500 | 热解油产率最高为55.7%,且芳香族化合物最高 | [ |
| Nano HZSM-5/γ-Al2O3 | 汽车(0.5~2.0mm) | 400 | 对轻质烯烃的选择性更高 | [ |
| Ru/MCM-41 | 汽车(8~18目) | 470 | 轻质烯烃产率提高 | [ |
| NaOH(35%) | 汽车(20~100目) | 550 | 热解油产率最高 | [ |
| Fe粉(35%) | 550 | 热解炭产物分布影响最大 | [ | |
| 高炉渣(35%) | 550 | 热解气产物产率最高 | [ | |
| TiO2 | 汽车(2.0mm) | 550 | 热解气产率提高 | [ |
| CaO(2.5%) | 汽车轮胎小块(100mm×120mm) | 500 | 液相产率提高到48.0% | [ |
| ZnO(2.5%) | 530 | 液相产率提高到48.0% | [ | |
| CaO,ZnO,TiO2 | 600 | 热解油和热解炭黑产率大幅度下降,热解气产率上升 | [ |
| 反应器 | 最佳反应条件 | 液相产量最高时三相分布 | 参考文献 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 热解温度/℃ | 轮胎粒径 | 升温速率/℃·min-1 | 气氛 | 停留时间/min | 液体/% | 固体/% | 气体/% | ||
| 固定床 | 400 | 1.0~4.0mm | 5 | — | 60 | 38.4 | 34.0 | 27.2 | [ |
| 固定床 | 500 | 粉末,80目 | 15 | 氮气 | 60 | 32.0 | 41.0 | 27.0 | [ |
| 回转窑 | 550 | 5.0mm | — | 氮气 | — | 43.7 | 34.5 | 21.7 | [ |
| 流化床 | 500 | 小块 | — | 氮气 | — | 40.3 | 47.9 | 11.8 | [ |
| 锥形喷射床 | 475 | 颗粒 | — | 氮气 | 30 | 58.2 | 35.9 | 5.9 | [ |
| 移动床 | 600 | 颗粒,5mm | — | 氮气 | 3.7 | 48.4 | 39.9 | 11.7 | [ |
| 间歇反应器 | 500 | 50~150cm | 5 | 氮气 | 90 | 38.3 | 56.2 | 5.5 | [ |
| 微波设备 | 592.1 | 3.6mm | — | 氮气 | 30 | 40.0 | 35 | 25 | [ |
表4 不同热解反应器和工艺实验研究
| 反应器 | 最佳反应条件 | 液相产量最高时三相分布 | 参考文献 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 热解温度/℃ | 轮胎粒径 | 升温速率/℃·min-1 | 气氛 | 停留时间/min | 液体/% | 固体/% | 气体/% | ||
| 固定床 | 400 | 1.0~4.0mm | 5 | — | 60 | 38.4 | 34.0 | 27.2 | [ |
| 固定床 | 500 | 粉末,80目 | 15 | 氮气 | 60 | 32.0 | 41.0 | 27.0 | [ |
| 回转窑 | 550 | 5.0mm | — | 氮气 | — | 43.7 | 34.5 | 21.7 | [ |
| 流化床 | 500 | 小块 | — | 氮气 | — | 40.3 | 47.9 | 11.8 | [ |
| 锥形喷射床 | 475 | 颗粒 | — | 氮气 | 30 | 58.2 | 35.9 | 5.9 | [ |
| 移动床 | 600 | 颗粒,5mm | — | 氮气 | 3.7 | 48.4 | 39.9 | 11.7 | [ |
| 间歇反应器 | 500 | 50~150cm | 5 | 氮气 | 90 | 38.3 | 56.2 | 5.5 | [ |
| 微波设备 | 592.1 | 3.6mm | — | 氮气 | 30 | 40.0 | 35 | 25 | [ |
| 炭黑来源(粒径) | 改性方法 | 应用途径 | 应用效果 | 参考文献 |
|---|---|---|---|---|
| 轮胎碎片550℃热解(8μm) | 酸/碱洗:将炭黑前后分别与HCl和NaOH机械搅拌,过滤-洗涤-干燥 | 橡胶补强剂 | 改性炭黑具有补强作用,增强了橡胶的力学性能、抗拉强度、硬度等机械性能 | [ |
| 两种尺寸炭黑(25和150μm) | 熔融混合:将沥青加热至150℃,加入两种炭黑,高速搅拌 | 改性沥青 | 改性沥青弹性增加,耐热和抗氧化老化能力提高,储存稳定性提高 | [ |
| 轮胎颗粒800℃热解(1~2mm) | 循环活化:用HNO3氧化20min,用H2O2和(NH4)2S2O8氧化2h | 活性炭 | 具有较高的中孔体积(0.47~0.68cm3/g),微孔(0.03~15cm3/g) | [ |
| 轮胎碎片500°C热解(250~400μm) | 酸洗:炭黑加入硝酸,在80℃下剧烈搅拌60min, 过滤-洗涤-干燥,加入钛酸酯偶联剂 | 油墨 | 改性炭黑的油墨细度、黑度、流动性和黏度指数可作为胶印油墨 | [ |
| 选用轮胎粉末,利用Hummers法制备洋葱状炭 | 电极材料 | 废轮胎洋葱状炭作为优质阳极材料,电导率高 | [ |
表5 改性炭黑应用途径
| 炭黑来源(粒径) | 改性方法 | 应用途径 | 应用效果 | 参考文献 |
|---|---|---|---|---|
| 轮胎碎片550℃热解(8μm) | 酸/碱洗:将炭黑前后分别与HCl和NaOH机械搅拌,过滤-洗涤-干燥 | 橡胶补强剂 | 改性炭黑具有补强作用,增强了橡胶的力学性能、抗拉强度、硬度等机械性能 | [ |
| 两种尺寸炭黑(25和150μm) | 熔融混合:将沥青加热至150℃,加入两种炭黑,高速搅拌 | 改性沥青 | 改性沥青弹性增加,耐热和抗氧化老化能力提高,储存稳定性提高 | [ |
| 轮胎颗粒800℃热解(1~2mm) | 循环活化:用HNO3氧化20min,用H2O2和(NH4)2S2O8氧化2h | 活性炭 | 具有较高的中孔体积(0.47~0.68cm3/g),微孔(0.03~15cm3/g) | [ |
| 轮胎碎片500°C热解(250~400μm) | 酸洗:炭黑加入硝酸,在80℃下剧烈搅拌60min, 过滤-洗涤-干燥,加入钛酸酯偶联剂 | 油墨 | 改性炭黑的油墨细度、黑度、流动性和黏度指数可作为胶印油墨 | [ |
| 选用轮胎粉末,利用Hummers法制备洋葱状炭 | 电极材料 | 废轮胎洋葱状炭作为优质阳极材料,电导率高 | [ |
| 支出与收入 | 名称 | 单价/CNY·t-1 | 年处理量或年产量/t | 总额/×104CNY·a-1 |
|---|---|---|---|---|
| 支出 | 设施建设 | — | 30000 | 9000 |
| 处理费用 | 3000 | 30000 | 9000 | |
| 原材料收购 | 400 | 30000 | 1200 | |
| 收入(热解产物) | 柠檬油精 | 20000 | 1620 | 3240 |
| 普通炭黑 | 1200 | 10500 | 1260 | |
| 轻质石脑油 | 6100 | 2700 | 1647 | |
| 重油 | 4100 | 9450 | 3874.5 | |
| 废钢丝 | 1100 | 1500 | 165 | |
| 直接为热裂解提供热能,不出售 | 热解气 | 2500 | 4230 | 1057.5 |
| 毛收益 | 合计 | — | — | 8986.5 |
表6 废旧轮胎微负压热解技术路线收支情况[67]
| 支出与收入 | 名称 | 单价/CNY·t-1 | 年处理量或年产量/t | 总额/×104CNY·a-1 |
|---|---|---|---|---|
| 支出 | 设施建设 | — | 30000 | 9000 |
| 处理费用 | 3000 | 30000 | 9000 | |
| 原材料收购 | 400 | 30000 | 1200 | |
| 收入(热解产物) | 柠檬油精 | 20000 | 1620 | 3240 |
| 普通炭黑 | 1200 | 10500 | 1260 | |
| 轻质石脑油 | 6100 | 2700 | 1647 | |
| 重油 | 4100 | 9450 | 3874.5 | |
| 废钢丝 | 1100 | 1500 | 165 | |
| 直接为热裂解提供热能,不出售 | 热解气 | 2500 | 4230 | 1057.5 |
| 毛收益 | 合计 | — | — | 8986.5 |
| 1 | 国家统计局. 中华人民共和国国家统计局月度数据报表[R]. 2018. |
| National Bureau of Statistics. Monthly data report of the National Bureau of Statistics of the People Republic of China[R]. 2018. | |
| 2 | 中华人民共和国国家商务部. 中国再生资源回收行业发展报告(2018)[R]. 2018. |
| Ministry of Commerce of the Peoples Republic of China. China Renewable Resources Recycling Industry Development Report (2018)[R]. 2018. | |
| 3 | 中华人民共和国工业和信息化部. 《废旧轮胎综合利用指导意见》[R]. 2010. |
| Ministry of Industry and Information Technology of the People's Republic of China. Guiding Opinions on Comprehensive Utilization of Waste Tires[R]. 2020. | |
| 4 | 中华人民共和国工业和信息化部. 对《废旧轮胎综合利用行业规范条件(修订征求意见稿)》《废旧轮胎综合利用行业规范公告管理暂行办法(修订征求意见稿)》公开征求意见[R]. 2019. |
| Ministry of Industry and Information Technology of the People's Republic of China. Public comment on the Regulations for the Comprehensive Utilization of Waste Tire Industry (Revised Draft) and Interim Measures for the Management of the Announcement of the Specification for Comprehensive Utilization of Waste Tire Industry (Revised Draft)[R]. 2019. | |
| 5 | 张峰, 郦亮, 饶绮麟. 废旧轮胎再利用技术[J]. 矿冶, 2011, 20(4): 105-108. |
| ZHANG F, LI L, RAO Q L. Waste tire recycling technology[J]. Mining and Metallurgy, 2011, 20(4): 105-108. | |
| 6 | 朱茂电, 王加龙, 赵吕明. 废旧轮胎回收利用技术及其应用前景[J]. 再生资源与循环经济, 2008(8): 32-35. |
| ZHU M D, WANG J L, ZHAO L M. Waste tire recycling technology and its application prospects[J]. Recyclable Resources and Circular Economy, 2008(8): 32-35. | |
| 7 | XU F F, WANG B, YANG D, et al. TG-FTIR and Py-GC/MS study on pyrolysis mechanism and products distribution of waste bicycle tire[J]. Energy Conversion and Management, 2018, 175: 288-297. |
| 8 | LI S Q, YAO Q, CHI Y, et al. Pilot-scale pyrolysis of scrap tires in a continuous rotary kiln reactor[J]. Ind. Eng. Chem. Res., 2004, 43: 5133-5145. |
| 9 | BOUVIER J M, CHARBEL F, GELUS M. Gas-solid pyrolysis of tire wastes—Kinetics and material balances of batch pyrolysis of used tires[J]. Resources and Conservation, 1987, 3(15): 205-214. |
| 10 | AYLÓN E, CALLÉN M S, LÓPEZ J M, et al. Assessment of tire devolatilization kinetics[J]. Journal of Analytical and Applied Pyrolysis, 2005,75(1/2): 259-264. |
| 11 | YANG J, TANGUY P A, ROY C. Heat transfer, mass transfer and kinetics study of the vacuum pyrolysis of a large used tire particle[J]. Chemical Engineering Science, 1995, 50(12):1909-1922. |
| 12 | AUGUSTINE Quek, RAJASHEKHAR Balasubramanian. An algorithm for the kinetics of tire pyrolysis under different heating rates[J]. Journal of Hazardous Materials, 2009, 166(1): 126-132. |
| 13 | TALEB Dzuhairy Ab, HAMID Hamidah Abd, DERIS Raja Razuan Raja, et al. Insights into pyrolysis of waste tire in fixed bed reactor: thermal behavior[J]. Materials Today: Proceedings, 2020, 31: 178-186. |
| 14 | Hüseyin AYDIN, Cumali İLKILIÇ. Optimization of fuel production from waste vehicle tires by pyrolysis and resembling to diesel fuel by various desulfurization methods[J]. Fuel, 2012(102): 605-612. |
| 15 | 崔洪, 杨建丽, 刘振宇. 废旧轮胎热解行为的TG/DTA研究[J]. 化工学报, 1999, 50(6): 826-833. |
| CUI H, YANG J L, LIU Z Y. TG/DTA study on pyrolysis behavior of waste tires[J]. CIESC Journal, 1999, 50(6): 826-833. | |
| 16 | BANAR V, AKYILDIZ A, OZKAN Z, et al. Characterization of pyrolytic oil obtained from pyrolysis of TDF (Tire Derived Fuel)[J]. Energy Conversion and Management, 2012 (62): 22-30. |
| 17 | MKHIZE N M, DANON B, GRYP P VAN DER, et al. Kinetic study of the effect of the heating rate on the waste tyre pyrolysis to maximise limonene production[J]. Chemical Engineering Research and Design, 2019, 152: 363-371. |
| 18 | WILLIAMS Paul T, BESLER Serpil, TAYLOR David T. The pyrolysis of scrap automotive tyres: the influence of temperature and heating rate on product composition[J]. Fuel, 1990, 69(12): 1474-1482. |
| 19 | AYLÓN E, FERNÁNDEZ-COLINO A, MURILLO R, et al. Valorisation of waste tyre by pyrolysis in a moving bed reactor[J]. Waste Management, 2010, 30(7): 1220-1224. |
| 20 | POLICELLA Matteo, WANG Zhiwei, BURRA Kiran G, et al. Characteristics of syngas from pyrolysis and CO2-assisted gasification of waste tires[J]. Applied Energy, 2019(254): 113678. |
| 21 | BETANCUR Mariluz, MARTÍNEZ Juan Daniel, MURILLO Ramón. Production of activated carbon by waste tire thermochemical degradation with CO2[J]. Journal of Hazardous Materials, 2009, 168(2/3): 882-887. |
| 22 | KAR Y. Catalytic pyrolysis of car tire waste using expanded perlite[J]. Waste Management, 2011, 31(8): 1772-1782. |
| 23 | 楚雅杰, 仪垂杰, 陈贺, 等. 催化裂解废旧轮胎的试验研究[J]. 科学技术与工程, 2017, 17(15): 213-217. |
| CHU Y J, YI C J, CHEN Y, et al. Experimental study on catalytic pyrolysis of waste tires[J]. Science Technology and Engineering, 2017, 17(15): 213-217. | |
| 24 | Nguy&#x DŨNG100166;n Anh, KLAEWKLA Raweewan, WONGKASEMJIT Sujitra,et al. Light olefins and light oil production from catalytic pyrolysis of waste tire[J]. Journal of Analytical and Applied Pyrolysis,2009, 86(2): 281-286. |
| 25 | SHEN B X, WU C F, LIANG C, et al. Pyrolysis of waste tires: the influence of USY catalyst/tire ratio on products[J]. Journal of Analytical and Applied Pyrolysis, 2007, 2(78): 243-249. |
| 26 | 王文选, 仲兆平, 陈晓平,等. 催化剂在轮胎裂解中作用的研究[J]. 东南大学学报, 1999(S1): 47-50. |
| WANG W X, ZHONG Z P, CHEN X P, et al. Research on catalysts in the pyrolysis of waste tires[J]. Journal of Southeast University, 1999(S1): 47-50. | |
| 27 | WILLIAMS P T, BRINDLE A J. Catalytic pyrolysis of tires: influence of catalyst temperature[J]. Fuel, 2002, 81: 2425-2434. |
| 28 | MIANDAD R, BARAKAT M A. Effect of advanced catalysts on tire waste pyrolysis oil[J]. Process Safety and Environmental Protection, 2018(116): 542-552. |
| 29 | LI W, HUANG C F, LI D P, et al. Derived oil production by catalytic pyrolysis of scrap tires[J]. Chinese Journal of Catalysis, 2016, 37(4): 526-532. |
| 30 | HE Z Z, JIAO Q Z. Light olefin production from catalytic pyrolysis of waste tires using nano-HZSM-5/γ-Al2O3 catalysts[J]. Journal of Analytical and Applied Pyrolysis, 2018(129): 66-71. |
| 31 | DUNG N A, KLAEWKLA R, WONGKASEMJIT S, et al. Light olefins and light oil production from catalytic pyrolysis of waste tire[J]. Journal of Analytical and Applied Pyrolysis, 2009,86: 281-286. |
| 32 | HIJAZI Ayman, BOYADJIAN Cassia. Solar pyrolysis of waste rubber tires using photoactive catalysts[J]. Waste Management, 2018, 77: 10-21. |
| 33 | 张兴华, 常杰, 王铁军, 等. 真空条件下金属氧化物催化废轮胎热解研究[J]. 能源工程, 2006(1): 41-45. |
| ZHANG X H, CHANG J, WANG T J, et al. A study on metal-oxide catalyzed pyrolysis of waste tires under vacuum conditions[J]. Energy Engineering, 2006(1): 41-45. | |
| 34 | DAI X W, YIN X L, WU C Z, et al. Pyrolysis of waste tires in a circulating fluidized-bed reactor[J]. Energy, 2001, 26(4): 385-399. |
| 35 | UCAR Suat, KARAGOZ Selhan, OZKAN Ahmet R, et al. Evaluation of two different scrap tires as hydrocarbon source by pyrolysis[J]. Fuel, 2005 84(14/15): 1884-1892. |
| 36 | SINGH R K, MONDAL S, RUJ B, et al. Interaction of three categories of tyre waste during co-pyrolysis: effect on product yield and quality[J]. Journal of Analytical and Applied Pyrolysis, 2019, 141: 104618. |
| 37 | WANG M Y, ZHANG L, LI A M, et al. Comparative pyrolysis behaviors of tire tread and side wall from waste tire and characterization of the resulting chars[J]. Journal of Environmental Management, 2019, 232: 364-371. |
| 38 | YAZDANI Esmaeil, HASHEMABADI Seyed Hassan, TAGHIZADEH Afshin. Study of waste tire pyrolysis in a rotary kiln reactor in a wide range of pyrolysis temperature[J]. Waste Management, 2019, 85(15): 195-201. |
| 39 | RYMS M, JANUSZEWICZ K, LEWANDOWSKI W M, et al. Pyrolysis process of whole waste tires as a biomass energy recycling[J]. Ecol. Chem. Eng., 2013(20): 93-107. |
| 40 | LOPEZ G, ALVAREZ J. Waste truck-tire processing by flash pyrolysis in a conical spouted bed reactor[J]. Energy Conversion and Management, 2017, 142: 523-532. |
| 41 | CUNLIFFE Adrian M, WILLIAMS Paul T. Composition of oils derived from the batch pyrolysis of tires[J]. Journal of Analytical and Applied Pyrolysis, 1998, 2(44): 131-152. |
| 42 | ZHANG Y Z, BIAN T T, ZHANG Y, et al. High-efficiency green microwave pyrolysis waste tire method[J]. Journal of Zhejiang University-Science A(Applied Physics & Engineering), 2018,19(12): 951-960. |
| 43 | MKHIZE N M, DANON B, GRYP P VAN DER, et al. Condensation of the hot volatiles from waste tyre pyrolysis by quenching[J]. Journal of Analytical and Applied Pyrolysis, 2017, 124: 180-185. |
| 44 | ELBABA Ibrahim F, WILLIAMS Paul T. Two stage pyrolysis-catalytic gasification of waste tyres: influence of process parameters[J]. Applied Catalysis B: Environmental, 2012, 125: 136-143. |
| 45 | SONG Z L, YANG Y Q, SUN J, et al. Effect of power level on the microwave pyrolysis of tire powder[J]. Energy, 2017, 127: 571-580. |
| 46 | WILLIAMS P T, BOTTRILL R P, CUNLIFFE A M. Combustion of tyre pyrolysis oil[J]. Process Safety and Environmental Protection, 1998, 76(4): 291-301. |
| 47 | BUTLER Eoin, DEVLIN Ger, MEIER Dietrich, et al. A review of recent laboratory research and commercial developments in fast pyrolysis and upgrading[J]. Renewable and Sustainable Energy Reviews, 2011, 15(8): 4171-4186. |
| 48 | ALVAREZ J, LOPEZ G, AMUTIO M, et al. Evaluation of the properties of tyre pyrolysis oils obtained in a conical spouted bed reactor[J]. Energy, 2017, 128: 463-474. |
| 49 | KAMINSKY W, MENNERICH C, ZHANG Z. Feedstock recycling of synthetic and natural rubber by pyrolysis in a fluidized bed[J]. Journal of Analytical and Applied Pyrolysis, 2009, 85: 334-337. |
| 50 | MARTÍNEZ Juan Daniel, MURILLO Ramón, Tomás GARCÍA, et al. Demonstration of the waste tire pyrolysis process on pilot scale in a continuous auger reactor[J]. Journal of Hazardous Materials, 2013, 261: 637-645. |
| 51 | BLACK J W, RROWN D B. Development of a continuous ablative reactor for fast pyrolysis[C]//Advances in Thermochemical Biomass Conversion, 1992. |
| 52 | MARTÍNEZ Juan D, VESES Alberto, MASTRAL Ana M, et al. Co-pyrolysis of biomass with waste tires: upgrading of liquid bio-fuel[J]. Fuel Processing Technology, 2014, 119: 263-271. |
| 53 | 唐兰, 黄海涛, 吴创之,等. 等离子体热解处理废轮胎实验研究[J]. 环境科学与技术, 2004, 27(6): 82-83, 99. |
| TANG L, HUANG H T, WU C Z,et al. Experimental research on plasma pyrolysis treatment of waste tires[J]. Environmental Science and Technology, 2004, 27(6): 82-83, 99. | |
| 54 | ZHANG X, WANG T, MA L. et al. Vacuum pyrolysis of waste tires with basic additives[J]. Waste Management, 2005, 28(11): 2301-2310. |
| 55 | 梁著文. 烟气余热回收装置的利用[J]. 沿海企业与科技, 2010(10): 111-113. |
| LIANG Z W. Utilization of flue gas waste heat recovery device[J]. Coastal Enterprises and Technology, 2010(10): 111-113. | |
| 56 | 徐宗平, 郭庆民. 废轮胎热解回收中的废气综合利用[J]. 中国轮胎资源综合利用, 2018(11):36-39. |
| XU Z P, GUO Q M. The comprehensive utilization of waste gas in waste tire pyrolysis recycling[J]. China Tire Resources Recycling, 2018(11): 36-39. | |
| 57 | Mustafa KARAGÖZ, AĞBULUTÜmit, SARIDEMIR Suat. Waste to energy: production of waste tire pyrolysis oil and comprehensive analysis of its usability in diesel engines[J]. Fuel, 2020, 275: 117844. |
| 58 | ZHANG Y L, JI G Z, LI C J, et al. Templating synthesis of hierarchical porous carbon from heavy residue of tire pyrolysis oil for methylene blue removal[J]. Chemical Engineering Journal, 2020, 390: 124398. |
| 59 | 郭豪, 梁鹏, 郭庆民. 废轮胎热解回收的产业现状与创新技术[J]. 再生资源与循环经济, 2013, 6(8): 22-26. |
| GUO H, LIANG P, GUO Q M. Industry status and innovative technology of waste tire pyrolysis and recycling[J]. Renewable Resources and Circular Economy, 2013, 6(8): 22-26. | |
| 60 | ZHOU J, WAN G G J D, REN X H, et al. Surface modification of pyrolytic carbon black from waste tires and its use as pigment for offset printing ink[J]. Chinese Journal of Chemical Engineering, 2006, 14(5): 654-659. |
| 61 | Rubén GÓMEZ-HERNÁNDEZ, Yesmin PANECATL-BERNAL, MÉNDEZ-ROJAS Miguel Ángel. High yield and simple one-step production of carbon black nanoparticles from waste tires[J]. Heliyon, 2019, 5(7): 102139. |
| 62 | 肖国良, 彭小芹, 盖国胜. 废轮胎裂解炭黑的超细粉碎和表面改性及在NR中的应用[J]. 橡胶工业, 2004(2): 78-82. |
| XIAO G L, PENG X Q, GAI G S. Superfine crushing and surface modification of waste tire pyrolysis carbon black and its application in NR[J]. Rubber Industry, 2004(2): 78-82. | |
| 63 | SAGAR M, NIBEDITA K, MANOHAR N, et al. A potential utilization of end-of-life tyres as recycled carbon black in EPDM rubber[J]. Waste Management, 2018, 74: 110-122. |
| 64 | FENG Z G, RAO W Y, CHEN C, et al. Performance evaluation of bitumen modified with pyrolysis carbon black made from waste tyres[J]. Construction and Building Materials, 2016, 111: 495-501. |
| 65 | HERAS F, JIMENEZ-CORDERO D, GILARRANZ M A, et al. Activation of waste tire char by cyclic liquid-phase oxidation[J]. Fuel Processing Technology, 2014, 127: 157-162. |
| 66 | BHAUMIK Madhumita, RAJU Kumar, ARUNACHELLAN Iviwe, et al. High-performance supercapacitors based on S-doped polyaniline nanotubes decorated with Ni(OH)2 nanosponge and onion-like carbons derived from used car tyres[J]. Electrochimica Acta, 2020, 342: 136111. |
| 67 | 李成, 张斌, 任泽, 等. 废轮胎裂解技术能耗经济分析及建议[J].中国轮胎资源综合利用, 2018(7):36-41. |
| LI C, ZHANG B, REN Z, et al. Economic analysis and suggestions on energy consumption of waste tire cracking technology[J]. China Tire Resources Recycling, 2018(7): 36-41. | |
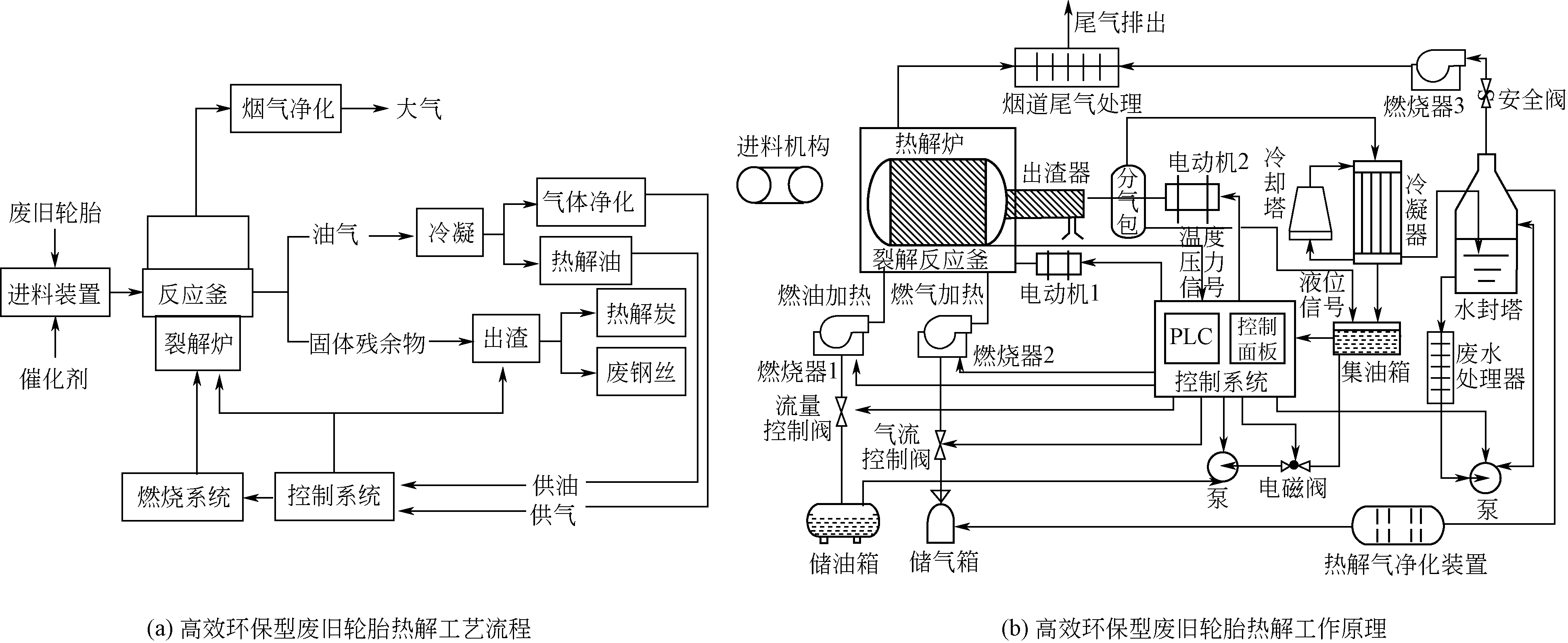
| 68 | 李德治, 姜莉莉, 周鑫. 高效环保型废旧轮胎裂解设备[J]. 橡胶工业, 2013, 60(8): 493-496. |
| LI D Z, JIANG L L, ZHOU X. Efficient and environment-friendly waste tire cracking equipment[J]. China Rubber Industry, 2013, 60(8): 493-496. |
| [1] | 张凤岐, 崔成东, 鲍学伟, 朱炜玄, 董宏光. 胺液吸收-分步解吸脱硫工艺的设计与评价[J]. 化工进展, 2023, 42(S1): 518-528. |
| [2] | 邵志国, 任雯, 许世佩, 聂凡, 许毓, 刘龙杰, 谢水祥, 李兴春, 王庆吉, 谢加才. 终温对油基钻屑热解产物分布和特性影响[J]. 化工进展, 2023, 42(9): 4929-4938. |
| [3] | 李志远, 黄亚继, 赵佳琪, 于梦竹, 朱志成, 程好强, 时浩, 王圣. 污泥与聚氯乙烯共热解重金属特性[J]. 化工进展, 2023, 42(9): 4947-4956. |
| [4] | 李海东, 杨远坤, 郭姝姝, 汪本金, 岳婷婷, 傅开彬, 王哲, 何守琴, 姚俊, 谌书. 炭化与焙烧温度对植物基铁碳微电解材料去除As(Ⅲ)性能的影响[J]. 化工进展, 2023, 42(7): 3652-3663. |
| [5] | 姚丽铭, 王亚琢, 范洪刚, 顾菁, 袁浩然, 陈勇. 餐厨垃圾处理现状及其热解技术研究进展[J]. 化工进展, 2023, 42(7): 3791-3801. |
| [6] | 张杉, 仲兆平, 杨宇轩, 杜浩然, 李骞. 磷酸盐改性高岭土对生活垃圾热解过程中重金属的富集[J]. 化工进展, 2023, 42(7): 3893-3903. |
| [7] | 侯殿保, 贺茂勇, 陈育刚, 杨海云, 李海民. 资源优化配置与循环经济在钾资源开发利用中的应用[J]. 化工进展, 2023, 42(6): 3197-3208. |
| [8] | 李若琳, 何少林, 苑宏英, 刘伯约, 纪冬丽, 宋阳, 刘博, 余绩庆, 徐英俊. 原位热解对油页岩物性及地下水水质影响探索[J]. 化工进展, 2023, 42(6): 3309-3318. |
| [9] | 李栋先, 王佳, 蒋剑春. 皂脚热解-催化气态加氢制备生物燃料[J]. 化工进展, 2023, 42(6): 2874-2883. |
| [10] | 王子杰, 陆树银, 赵梓良, 王宁, 顾煜炯. 供热改造对火电机组性能的影响分析[J]. 化工进展, 2023, 42(5): 2325-2331. |
| [11] | 王志伟, 郭帅华, 吴梦鸽, 陈颜, 赵俊廷, 李辉, 雷廷宙. 生物质与塑料催化共热解技术研究进展[J]. 化工进展, 2023, 42(5): 2655-2665. |
| [12] | 郭文杰, 翟玉玲, 陈文哲, 申鑫, 邢明. Al2O3-CuO/水混合纳米流体对流传热性能及热经济性分析[J]. 化工进展, 2023, 42(5): 2315-2324. |
| [13] | 梁贻景, 马岩, 卢展烽, 秦福生, 万骏杰, 王志远. La1-x Sr x MnO3钙钛矿涂层的抗结焦性能[J]. 化工进展, 2023, 42(4): 1769-1778. |
| [14] | 耿书阳, 姜泽毅, 张欣茹. 均混预热电石熔炼新工艺的能耗特征与热经济成本分析[J]. 化工进展, 2023, 42(4): 1787-1796. |
| [15] | 刘静, 林琳, 张健, 赵峰. 生物质基炭材料孔径调控及电化学性能研究进展[J]. 化工进展, 2023, 42(4): 1907-1916. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||