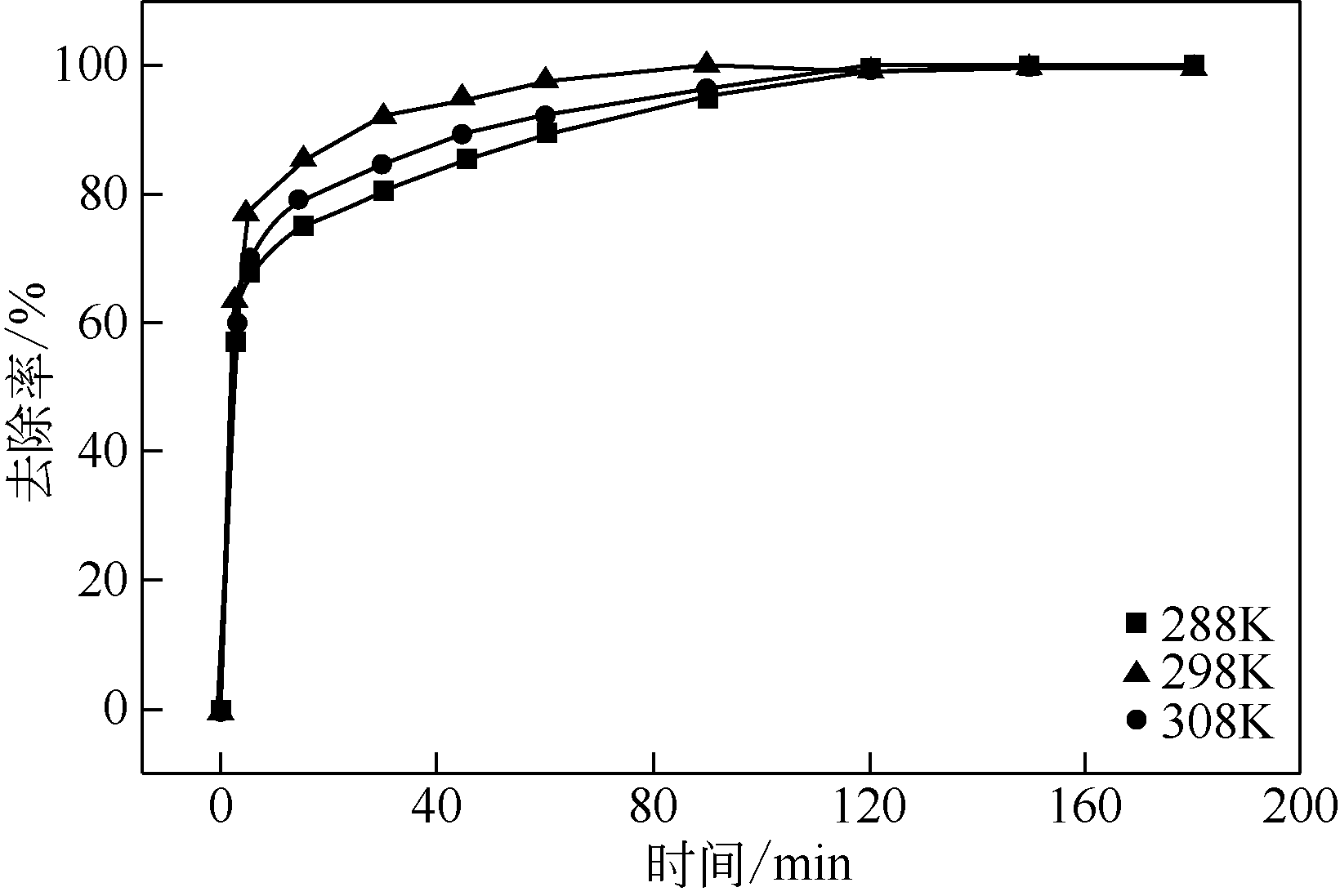
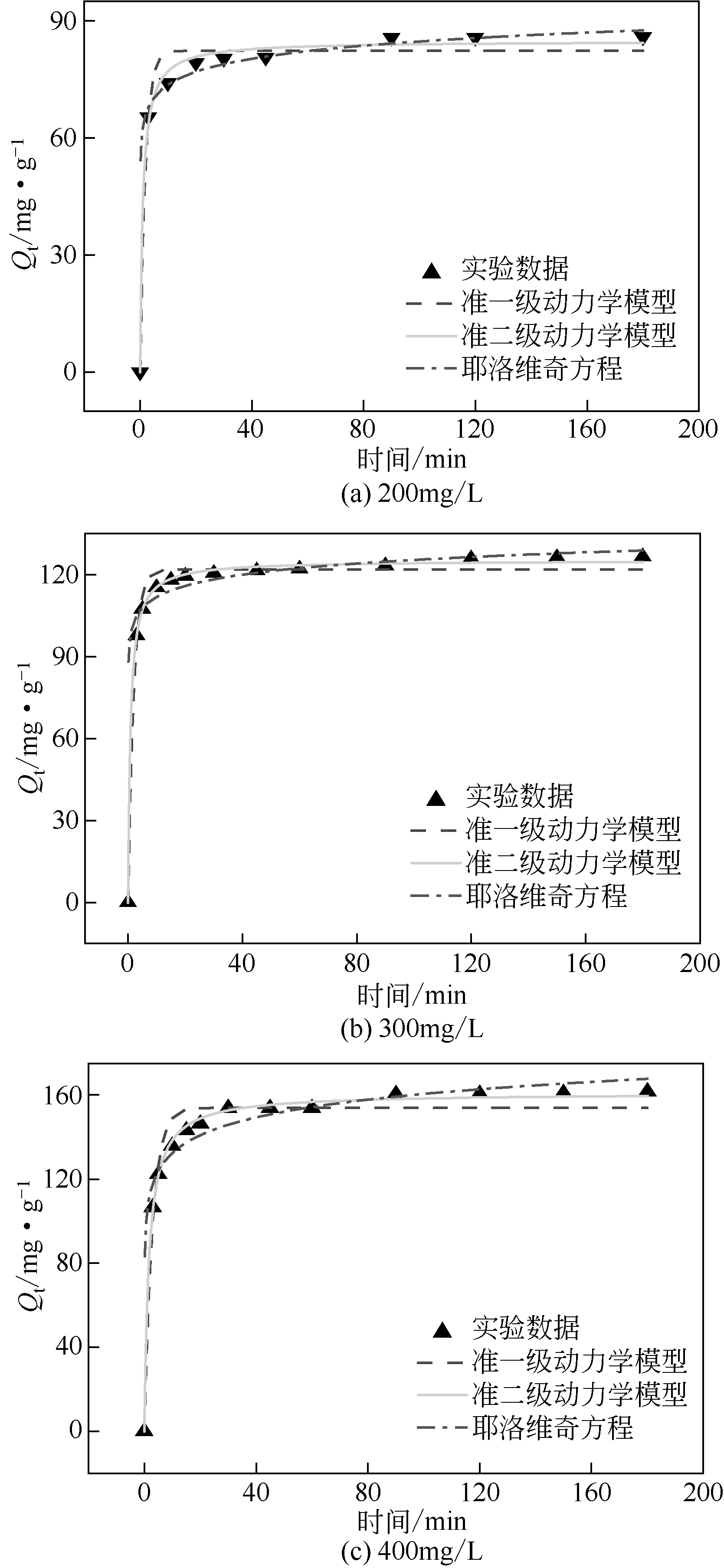
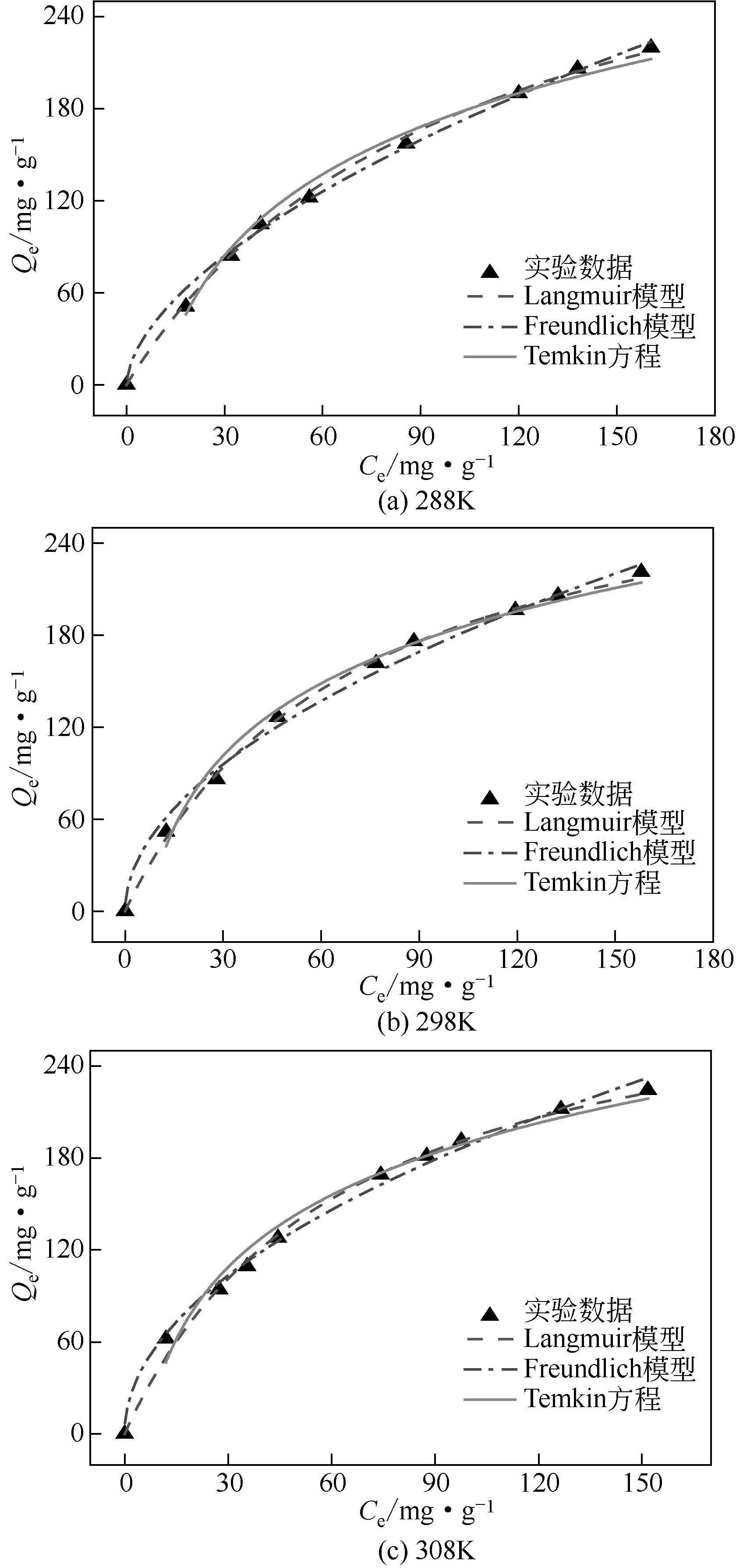
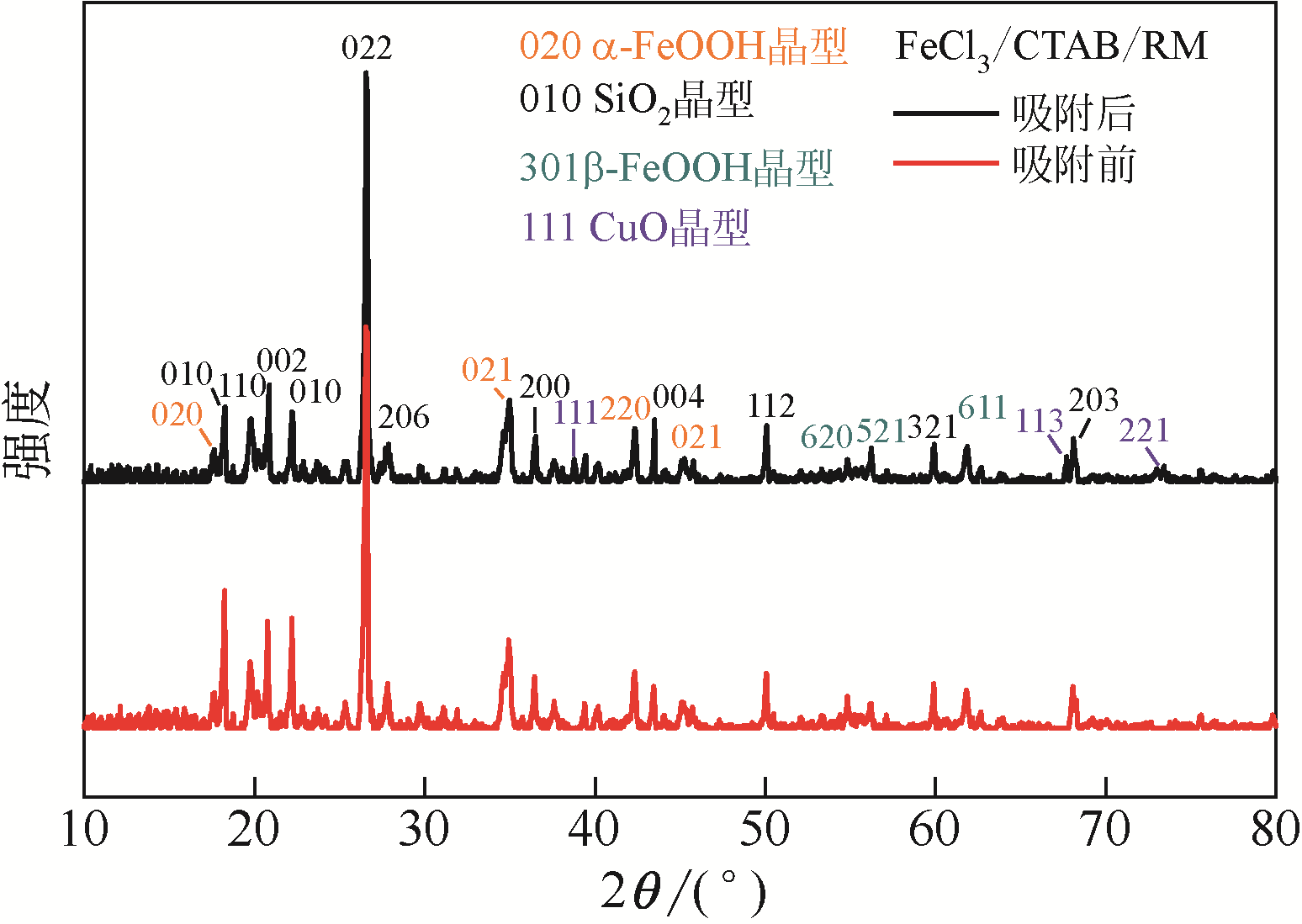
| 1 |
FAN L, LUO C, LÜ Z, et al. Preparation of magnetic modified chitosan and adsorption of Zn from aqueous solutions[J]. Colloids & Surfaces B: Biointerfaces, 2011, 88(2): 574-581.
|
| 2 |
余伟光, 黎吉辉, 王敦, 等. 香蕉茎秆生物炭的制备及其对铜离子的吸附特性[J]. 化工进展, 2017, 36(4): 1499-1505.
|
|
YU Weiguang, LI Jihui, WANG Dun, et al. The preparation of biochar from pre-oxidation of banana stem and its adsorption of Cu2+[J]. Chemical Industry and Engineering Progress, 2017, 36(4): 1499-1505.
|
| 3 |
BHATNAGAR A, VILAR V J, BOTELHO C M, et al. A review of the use of red mud as adsorbent for the removal of toxic pollutants from water and wastewater[J]. Environmental Technology, 2011, 32(3): 231-249.
|
| 4 |
SOYLAK M, MURAT I. A new coprecipitation methodology with lutetium hydroxide for preconcentration of heavy metal ions in herbal plant samples[J]. Journal of Aoac International, 2014, 97(4): 1189.
|
| 5 |
BULGARIU L, BULGARIU D. Selective extraction of Hg(Ⅱ), Cd(Ⅱ) and Zn(Ⅱ) ions from aqueous media by a green chemistry procedure using aqueous two-phase systems[J]. Separation & Purification Technology, 2013, 118(43): 209-216.
|
| 6 |
PENG W, LI H, LIU Y, et al. A review on heavy metal ions adsorption from water by graphene oxide and its composites[J]. Journal of Molecular Liquids, 2017, 230: 496-504.
|
| 7 |
文小年. 氧化铝工业废渣(赤泥)的吸附特性及其环境修复材料制备[D]. 桂林: 桂林理工大学, 2006.
|
|
WEN X N. Adsorption characteristics of alumina industrial waste(red mud) and preparation of environmental remediation materials[D]. Guilin: Guilin University of Technology, 2006.
|
| 8 |
潘嘉芬, 李梦红. 拜耳法赤泥陶粒改性及吸附废水中氟离子试验[J]. 有色金属(冶炼部分), 2015(2): 63-65.
|
|
PAN Jiafen, LI Menghong. Modification of porous ceramsite with red mud of Bayer process and adsorbing fluoride from waste water[J]. Nonferrous Metals (Extractive Metallurgy), 2015(2): 63-65.
|
| 9 |
LI D, DING Y, LI L, et al. Removal of hexavalent chromium by using red mud activated with cetyltrimethylammonium bromide[J]. Environmental Technology, 2015, 36(9): 1084-1090.
|
| 10 |
吴建锋, 方斌正, 徐晓虹, 等. 用于去除电镀废水中As(V)的赤泥基环境修复材料的研究[C]//第7届亚洲陶瓷技术研讨会, 中国, 江西, 2011.
|
|
WU J F, FANG B Z, XU X H, et al. Study on red mud environmental remediation materials for removal As(V) ions in electroplating wastewater[C]//The 7th Asian Ceramic Technology Seminar. Jiangxi, China, 2011.
|
| 11 |
VENKATESAN G, NARAYANAN S L. Synthesis of Fe2O3 coated and HCl treated bauxite ore waste for the adsorption of arsenic (Ⅲ) from aqueous solution: isotherm and kinetic models[J]. Chemical Engineering Communications, 2017, 205(1): 34-46.
|
| 12 |
CUI Y W, LI J, DU Z F, et al. Cr(Ⅵ) adsorption on red mud modified by lanthanum: performance, kinetics and mechanisms[J]. PloS One, 2016, 11(9): e0161780.
|
| 13 |
JIE C, NING W, MA H, et al. Facile Modification of a polythiophene/TiO2 composite using surfactants in an aqueous medium for an enhanced Pb(Ⅱ) adsorption and mechanism investigation[J]. Journal of Chemical & Engineering Data, 2017, 62(7): 2208-2221.
|
| 14 |
张多, 张盼月, 田帅, 等. 用于磷吸附的载铁(β-FeOOH)沸石制备及特性[J]. 环境工程学报, 2014, 8(2): 499-504.
|
|
ZHANG Duo, ZHANG Panyue, TIAN Shuai, et al. Preparation of β-FeOOH loaded-zeolite and its characteristics for phosphorus adsorption[J]. Chinese Journal of Environmental Engineering, 2014, 8(2): 499-504.
|
| 15 |
CUI H, YANG X, XU L, et al. Effects of goethite on the fractions of Cu, Cd, Pb, P and soil enzyme activity with hydroxyapatite in heavy metal-contaminated soil[J]. RSC Advances, 2017, 7(72): 45869-45877.
|
| 16 |
刘俊, 楼跃丰, 李军. 石英砂表面负载铁氧化物的方法和特性[J]. 化工进展, 2016, 35(2): 624-628.
|
|
LIU Jun, LOU Yuefeng, LI Jun. Method and characteristics of iron oxides coated on quartz sand surface[J]. Chemical Industry and Engineering Progress, 2016, 35(2): 624-628.
|
| 17 |
WEI W, CONG Haibing, XIONG Huixin. The properties of FeOOH precipitates formed in CTAB-rich FeCl3 solutions at various pH and ethanol contents[J]. Colloid & Polymer Science, 2018, 296(7): 1205-1212.
|
| 18 |
SAHU M K, MANDAL S, DASH S S, et al. Removal of Pb(Ⅱ) from aqueous solution by acid activated red mud[J]. Journal of Environmental Chemical Engineering, 2013, 1(4): 1315-1324.
|
| 19 |
高鹏杰. 氧化铝赤泥改性及对废水中Cr(Ⅵ)的吸附研究[D]. 郑州: 郑州大学, 2016.
|
|
GAO P J. Modification of alumina red mud and research on the adsorption of Cr(VI) in polluted water[D]. Zhengzhou: Zhengzhou University, 2016.
|
| 20 |
徐翔丽. 氨基功能化氧化石墨烯重金属吸附剂的制备与应用[D]. 长沙: 湖南大学, 2014.
|
|
XU Xiangli. The preparation and and characterization of amino-functionalized graphene oxide as heavy metal adsorbents [D]. Changsha: Hunan University, 2014.
|
| 21 |
石太宏, 吕灿, 左莉娜. 硅烷化改性沸石对重金属离子的吸附性能[J]. 环境工程学报, 2013, 7(3): 1045-1052.
|
|
SHI Taihong, Can LÜ, ZUO Lina. Adsorption of heavy metal ions by silylation modified zeolite[J]. Chinese Journal of Environmental Engineering, 2013, 7(3): 1045-1052.
|
| 22 |
柏珊珊. 介孔分子筛的表面修饰及其对重金属离子的吸附性能研究[D]. 哈尔滨: 哈尔滨工业大学, 2010.
|
|
BAI S S. Study on the modification of mesoporous molecular sieve and their adsorption properties of heavy metals[D]. Harbin: Harbin Institute of Technology, 2010.
|
| 23 |
韩毅, 王京刚, 唐明述. 赤泥氯化铁改性材料的制备及其表征[J]. 有色金属(选矿部分), 2004(2): 37-40.
|
|
HAN Yi, WANG Jinggang, TANG Mingshu. Modification and characterization of red mud activated by FeCl3[J]. Nonferrous Metals, 2004(2): 37-40.
|
| 24 |
LYU W, YU M, FENG J, et al. Highly crystalline polyaniline nanofibers coating with low-cost biomass for easy separation and high efficient removal of anionic dye ARG from aqueous solution[J]. Applied Surface Science, 2018, 458: 413-424.
|
| 25 |
TSAMO C, DJONGA P N D, DIKDIM J M D, et al. Kinetic and equilibrium studies of Cr(VI), Cu(Ⅱ) and Pb(Ⅱ) removal from aqueous solution using red mud, a low-cost adsorbent[J]. Arabian Journal for Science & Engineering, 2017, 43(5): 1-16.
|
| 26 |
李孟, 刘丹, 吴梓鸿. 赤泥基多孔陶瓷材料及其改性物除Cu2+研究[J]. 武汉理工大学学报, 2010, 32(18): 29-32.
|
|
LI Meng, LIU Dan, WU Zihong. Research on the removal of Cu2+ by red mud-based porous ceramic material and its modification[J]. Journal of Wuhan University of Technology, 2010, 32(18): 29-32.
|
| 27 |
PELLERA F, GIANNIS A, ANASTASIADOU K, et al. Adsorption of Cu(Ⅱ) ions from aqueous solutions using biochar prepared from agricultural byproducts[J]. Journal of Environmental Management, 2012, 96(1): 35-42.
|
| 28 |
RUI F P P, VALENTE A J M, BURROWS H D. The interaction of long chain sodium carboxylates and sodium dodecylsulfate with lead(Ⅱ) ions in aqueous solutions[J]. Journal of Colloid & Interface Science, 2014, 414(414C): 66-72.
|
 ),Yan GUO(
),Yan GUO( ),Yihui XI
),Yihui XI