化工进展 ›› 2020, Vol. 39 ›› Issue (2): 667-678.DOI: 10.16085/j.issn.1000-6613.2019-0777
秸秆类型及配比变化对污泥厌氧消化中微生物群落的影响
唐涛涛1( ),李江1,2(
),李江1,2( ),杨爱江1,2,杨钊1,向福亮1,袁华宇1
),杨爱江1,2,杨钊1,向福亮1,袁华宇1
- 1.贵州大学资源与环境工程学院,贵州 贵阳 550025
2.贵州大学环境工程规划设计研究所,贵州 贵阳 550025
-
收稿日期:2019-05-13出版日期:2020-02-05发布日期:2020-03-12 -
通讯作者:李江 -
作者简介:唐涛涛(1994—),男,硕士研究生,研究方向为有机污染控制。E-mail:1398455709@qq.com 。 -
基金资助:国家自然科学基金青年基金(51508120);贵州省重点学科建设计划(黔学位合字ZDXK[2016]11号)
Effects of straw type and ratio change on microbial community in anaerobic digestion of sludge
Taotao TANG1( ),Jiang LI1,2(
),Jiang LI1,2( ),Aijinag YANG1,2,Zhao YANG1,Fuliang XIANG1,Huayu YUAN1
),Aijinag YANG1,2,Zhao YANG1,Fuliang XIANG1,Huayu YUAN1
- 1.College of Resources and Environmental Engineering, Guizhou University, Guiyang 550025, Guizhou, China
2.Institute of Environmental Engineerting Planing and Designing, Guizhou University, Guiyang 550025, Guizhou, China
-
Received:2019-05-13Online:2020-02-05Published:2020-03-12 -
Contact:Jiang LI
摘要:
为探讨秸秆类型及配比变化对污泥厌氧消化体系中微生物群落结构的影响,采用16S rRNA高通量测序技术,对污泥-秸秆联合厌氧消化体系中微生物群落进行分析。结果表明:玉米秸秆的添加对体系中pH和挥发性脂肪酸(VFAs)的影响较大,尤其是乙酸。而小麦和水稻秸秆的添加对碱度的影响较大,但当配比增加时体系中的VFAs和乙酸浓度也会增加,特别是1∶1.5(挥发性固体质量比)。通过对微生物群落结构分析发现,秸秆类型及配比的变化能显著提高厌氧体系中水解菌和酸化菌的相对丰度(p<0.001),如深古菌门Bathyarchaeota、未识别的_c_深古菌门(norank_p_Bathyarchaeota)、拟杆菌门(Bacteroidetes)、阴沟单胞菌门(Cloacimonetes)、同力菌门(Synergistetes)、未分类的_p_阴沟单胞菌门(unclassified_p_Cloacimonetes)、未识别的_c_拟杆菌门_vadinHA17(norank_c_Bacteroidetes_vadinHA17)、(Christensenellaceae_R-7_group)、未识别的_f_紫单胞菌科(norank_f_ Porphyromonadaceae)、未分类的_f_瘤胃菌科(unclassified_f_Ruminococcaceae)、拟杆菌属(Bacteroides)、丙酸杆菌属(Prolixibacter)、长绳菌属(Longilinea)、纤绳菌属(Leptolinea)等菌群;但对体系中乙酸型产甲烷菌(Methanosaeta)和氢型产甲烷菌(甲烷螺菌属Methanospirillum、甲烷杆菌属Methanobacterium和甲烷短杆菌属Methanobrevibacter)的生长产生抑制作用。同时也发现秸秆的添加对甲基型产甲烷菌(Methanomassiliicoccus)的生长具有显著的促进作用(p<0.001),从而影响厌氧体系的产甲烷特性。
中图分类号:
引用本文
唐涛涛,李江,杨爱江,杨钊,向福亮,袁华宇. 秸秆类型及配比变化对污泥厌氧消化中微生物群落的影响[J]. 化工进展, 2020, 39(2): 667-678.
Taotao TANG,Jiang LI,Aijinag YANG,Zhao YANG,Fuliang XIANG,Huayu YUAN. Effects of straw type and ratio change on microbial community in anaerobic digestion of sludge[J]. Chemical Industry and Engineering Progress, 2020, 39(2): 667-678.
| 样品 | pH | 化学需氧量COD/mg·L-1 | 含水率/% | |
|---|---|---|---|---|
| 污泥 | 7.2±0.25 | 24257.07±2313.10 | 96.91±0.54 | 51.57±7.47 |
| 玉米 | — | — | 19.92±6.17 | 92.62±5.83 |
| 小麦 | — | — | 15.41±0.42 | 88.34±0.56 |
| 水稻 | — | — | 15.76±0.49 | 82.26±0.88 |
表1 污泥与秸秆基本性质
| 样品 | pH | 化学需氧量COD/mg·L-1 | 含水率/% | |
|---|---|---|---|---|
| 污泥 | 7.2±0.25 | 24257.07±2313.10 | 96.91±0.54 | 51.57±7.47 |
| 玉米 | — | — | 19.92±6.17 | 92.62±5.83 |
| 小麦 | — | — | 15.41±0.42 | 88.34±0.56 |
| 水稻 | — | — | 15.76±0.49 | 82.26±0.88 |
| 试验号 | 累积沼气产量/L | pH | 碱度/mg·L-1 | VFAs/mg·L-1 | VFAs/ALK | |
|---|---|---|---|---|---|---|
| CK | 52.43±1.67 | 7.31±0.16 | 324.43±13.28 | 1304.54±93.35 | 181.27±21.04 | 0.14±0.02 |
| C1 | 106.13±5.80 | 7.62±0.26 | 478.33±27.05 | 1860.25±59.02 | 203.50±20.45 | 0.11±0.01 |
| C2 | 163.02±3.99 | 6.94±0.23 | 293.42±7.00 | 1445.59±289.01 | 261.76±40.78 | 0.19±0.04 |
| C3 | 223.98±8.63 | 6.89±0.24 | 262.89±9.54 | 1367.77±305.71 | 295.66±31.38 | 0.22±0.04 |
| W1 | 93.82±3.71 | 7.76±0.13 | 449.94±17.56 | 1710.85±124.10 | 196.85±18.81 | 0.12±0.01 |
| W2 | 150.88±4.55 | 7.39±0.18 | 442.44±12.96 | 1669.67±125.13 | 207.02±24.85 | 0.12±0.02 |
| W3 | 211.5±5.17 | 7.51±0.20 | 536.0±38.96 | 2016.29±134.44 | 225.68±15.33 | 0.11±0.01 |
| R1 | 106.91±1.86 | 7.44±0.18 | 390.19±15.52 | 1707.19±69.69 | 202.21±22.55 | 0.12±0.02 |
| R2 | 149.99 ± 21.98 | 7.37±0.19 | 481.13±25.59 | 1869.41±77.10 | 211.83±21.05 | 0.11±0.01 |
| R3 | 211.68 ± 11.29 | 7.42±0.24 | 542.52±25.96 | 2044.33±142.87 | 237.29±21.79 | 0.12±0.01 |
| 试验号 | 乙酸/mg·L-1 | 丙酸/mg·L-1 | 丁酸/mg·L-1 | 异丁酸/mg·L-1 | 戊酸/mg·L-1 | |
| CK | 57.24±19.69 | 16.17±3.80 | 21.95±1.17 | 30.19±2.00 | 26.55±6.82 | |
| C1 | 80.79±20.06 | 17.80±2.08 | 23.87±4.44 | 29.87±1.04 | 23.11±0.96 | |
| C2 | 119.89±35.62 | 19.78±4.94 | 23.35±3.31 | 34.88±3.95 | 23.80±3.28 | |
| C3 | 156.26±26.44 | 16.64±3.82 | 22.46±1.82 | 34.23±4.45 | 23.40±1.74 | |
| W1 | 68.04±16.06 | 21.89±2.81 | 25.43±5.60 | 28.86±0.91 | 23.18±1.03 | |
| W2 | 77.85±15.72 | 22.61±7.06 | 23.76±2.38 | 29.03±0.94 | 24.93±1.61 | |
| W3 | 99.54±11.52 | 21.22±5.57 | 23.59±1.44 | 30.09±1.12 | 24.50±1.82 | |
| R1 | 70.37±20.28 | 21.61±3.38 | 23.76±3.83 | 32.37±6.62 | 24.04±2.68 | |
| R2 | 83.33±18.35 | 20.83±2.96 | 25.21±4.13 | 29.66±1.55 | 23.40±1.89 | |
| R3 | 107.55±19.54 | 20.60±6.84 | 24.10±2.82 | 31.07±4.41 | 25.97±8.09 |
表2 秸秆对消化污泥特性的影响
| 试验号 | 累积沼气产量/L | pH | 碱度/mg·L-1 | VFAs/mg·L-1 | VFAs/ALK | |
|---|---|---|---|---|---|---|
| CK | 52.43±1.67 | 7.31±0.16 | 324.43±13.28 | 1304.54±93.35 | 181.27±21.04 | 0.14±0.02 |
| C1 | 106.13±5.80 | 7.62±0.26 | 478.33±27.05 | 1860.25±59.02 | 203.50±20.45 | 0.11±0.01 |
| C2 | 163.02±3.99 | 6.94±0.23 | 293.42±7.00 | 1445.59±289.01 | 261.76±40.78 | 0.19±0.04 |
| C3 | 223.98±8.63 | 6.89±0.24 | 262.89±9.54 | 1367.77±305.71 | 295.66±31.38 | 0.22±0.04 |
| W1 | 93.82±3.71 | 7.76±0.13 | 449.94±17.56 | 1710.85±124.10 | 196.85±18.81 | 0.12±0.01 |
| W2 | 150.88±4.55 | 7.39±0.18 | 442.44±12.96 | 1669.67±125.13 | 207.02±24.85 | 0.12±0.02 |
| W3 | 211.5±5.17 | 7.51±0.20 | 536.0±38.96 | 2016.29±134.44 | 225.68±15.33 | 0.11±0.01 |
| R1 | 106.91±1.86 | 7.44±0.18 | 390.19±15.52 | 1707.19±69.69 | 202.21±22.55 | 0.12±0.02 |
| R2 | 149.99 ± 21.98 | 7.37±0.19 | 481.13±25.59 | 1869.41±77.10 | 211.83±21.05 | 0.11±0.01 |
| R3 | 211.68 ± 11.29 | 7.42±0.24 | 542.52±25.96 | 2044.33±142.87 | 237.29±21.79 | 0.12±0.01 |
| 试验号 | 乙酸/mg·L-1 | 丙酸/mg·L-1 | 丁酸/mg·L-1 | 异丁酸/mg·L-1 | 戊酸/mg·L-1 | |
| CK | 57.24±19.69 | 16.17±3.80 | 21.95±1.17 | 30.19±2.00 | 26.55±6.82 | |
| C1 | 80.79±20.06 | 17.80±2.08 | 23.87±4.44 | 29.87±1.04 | 23.11±0.96 | |
| C2 | 119.89±35.62 | 19.78±4.94 | 23.35±3.31 | 34.88±3.95 | 23.80±3.28 | |
| C3 | 156.26±26.44 | 16.64±3.82 | 22.46±1.82 | 34.23±4.45 | 23.40±1.74 | |
| W1 | 68.04±16.06 | 21.89±2.81 | 25.43±5.60 | 28.86±0.91 | 23.18±1.03 | |
| W2 | 77.85±15.72 | 22.61±7.06 | 23.76±2.38 | 29.03±0.94 | 24.93±1.61 | |
| W3 | 99.54±11.52 | 21.22±5.57 | 23.59±1.44 | 30.09±1.12 | 24.50±1.82 | |
| R1 | 70.37±20.28 | 21.61±3.38 | 23.76±3.83 | 32.37±6.62 | 24.04±2.68 | |
| R2 | 83.33±18.35 | 20.83±2.96 | 25.21±4.13 | 29.66±1.55 | 23.40±1.89 | |
| R3 | 107.55±19.54 | 20.60±6.84 | 24.10±2.82 | 31.07±4.41 | 25.97±8.09 |
| 样品名称 | OTU | Chao | ACE | Shannon | Simpson | Coverage |
|---|---|---|---|---|---|---|
| CK | 1301 | 1537.99 | 1515.78 | 5.28 | 0.017 | 0.9920 |
| C1 | 1613 | 1805.21 | 1811.09 | 5.39 | 0.023 | 0.9925 |
| C2 | 1595 | 1816.65 | 1785.31 | 4.98 | 0.043 | 0.9950 |
| C3 | 1349 | 1622.81 | 1631.66 | 5.15 | 0.025 | 0.9879 |
| W1 | 1572 | 1800.11 | 1781.41 | 5.45 | 0.016 | 0.9918 |
| W2 | 1628 | 1835.45 | 1833.47 | 5.44 | 0.022 | 0.9923 |
| W3 | 1346 | 1662.75 | 1658.87 | 4.90 | 0.030 | 0.9906 |
| R1 | 1608 | 1864.64 | 1819.15 | 5.54 | 0.021 | 0.9923 |
| R2 | 1627 | 1870.63 | 1849.14 | 5.37 | 0.019 | 0.9927 |
| R3 | 1642 | 1805.18 | 1816.58 | 5.35 | 0.025 | 0.9942 |
表3 不同实验组中细菌群落的丰度和多样性
| 样品名称 | OTU | Chao | ACE | Shannon | Simpson | Coverage |
|---|---|---|---|---|---|---|
| CK | 1301 | 1537.99 | 1515.78 | 5.28 | 0.017 | 0.9920 |
| C1 | 1613 | 1805.21 | 1811.09 | 5.39 | 0.023 | 0.9925 |
| C2 | 1595 | 1816.65 | 1785.31 | 4.98 | 0.043 | 0.9950 |
| C3 | 1349 | 1622.81 | 1631.66 | 5.15 | 0.025 | 0.9879 |
| W1 | 1572 | 1800.11 | 1781.41 | 5.45 | 0.016 | 0.9918 |
| W2 | 1628 | 1835.45 | 1833.47 | 5.44 | 0.022 | 0.9923 |
| W3 | 1346 | 1662.75 | 1658.87 | 4.90 | 0.030 | 0.9906 |
| R1 | 1608 | 1864.64 | 1819.15 | 5.54 | 0.021 | 0.9923 |
| R2 | 1627 | 1870.63 | 1849.14 | 5.37 | 0.019 | 0.9927 |
| R3 | 1642 | 1805.18 | 1816.58 | 5.35 | 0.025 | 0.9942 |
| 样品名称 | OTU | Chao | ACE | Shannon | Simpson | Coverage |
|---|---|---|---|---|---|---|
| CK | 59 | 60.11 | 60.83 | 1.82 | 0.285 | 0.9999 |
| C1 | 62 | 69.88 | 78.69 | 1.96 | 0.260 | 0.9997 |
| C2 | 48 | 50.00 | 50.68 | 1.65 | 0.366 | 0.9999 |
| C3 | 55 | 63.20 | 62.93 | 2.01 | 0.227 | 0.9997 |
| W1 | 110 | 119.50 | 120.98 | 2.01 | 0.237 | 0.9995 |
| W2 | 60 | 65.58 | 72.47 | 1.93 | 0.261 | 0.9997 |
| W3 | 60 | 73.00 | 67.90 | 1.65 | 0.376 | 0.9998 |
| R1 | 82 | 125.17 | 123.88 | 2.14 | 0.191 | 0.9995 |
| R2 | 92 | 99.56 | 104.77 | 2.35 | 0.147 | 0.9997 |
| R3 | 78 | 125.00 | 139.07 | 1.80 | 0.296 | 0.9993 |
表4 不同实验组中古菌群落的丰度和多样性
| 样品名称 | OTU | Chao | ACE | Shannon | Simpson | Coverage |
|---|---|---|---|---|---|---|
| CK | 59 | 60.11 | 60.83 | 1.82 | 0.285 | 0.9999 |
| C1 | 62 | 69.88 | 78.69 | 1.96 | 0.260 | 0.9997 |
| C2 | 48 | 50.00 | 50.68 | 1.65 | 0.366 | 0.9999 |
| C3 | 55 | 63.20 | 62.93 | 2.01 | 0.227 | 0.9997 |
| W1 | 110 | 119.50 | 120.98 | 2.01 | 0.237 | 0.9995 |
| W2 | 60 | 65.58 | 72.47 | 1.93 | 0.261 | 0.9997 |
| W3 | 60 | 73.00 | 67.90 | 1.65 | 0.376 | 0.9998 |
| R1 | 82 | 125.17 | 123.88 | 2.14 | 0.191 | 0.9995 |
| R2 | 92 | 99.56 | 104.77 | 2.35 | 0.147 | 0.9997 |
| R3 | 78 | 125.00 | 139.07 | 1.80 | 0.296 | 0.9993 |

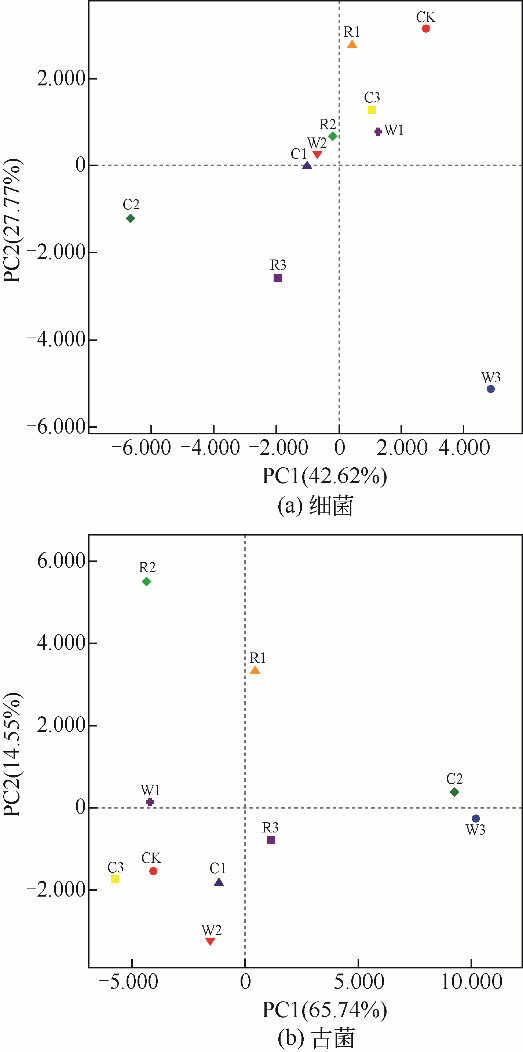
图2 细菌和古菌在门和属水平下的分类Bacteroidetes (拟杆菌门);Proteobacteria(变形菌门);Chloroflexi(绿弯菌门);Firmicutes(厚壁菌门);Actinobacteria(放线菌门);Acidobacteria(酸杆菌门);Verrucomicrobia(疣微菌门);Nitrospirae(硝化螺旋菌门);Caldiserica(嗜热丝菌门);Planctomycetes(浮霉菌门);Fibrobacteres(纤维秆菌门);Euryarchaeota(广古菌门)(门分类);Bacteroides(拟杆菌属);Nitrospira(硝化螺旋菌属);Woodsholea(木洞菌属);Syntrophobacter(互营杆菌属);Syntrophus(互营菌属);Smithella(史密斯氏菌属);Roseiflexus(花弯菌属); norank_f_Porphyromonadaceae(未识别的_f_紫单胞菌科);norank_f_Anaerolineaceae(未识别的_f_厌氧绳菌科);norank_f_Saprospiraceae(未识别的_f_腐螺旋菌科);norank_k_Acidobacteria(未识别的_k_酸杆菌门);norank_f_Xanthomonadaceae(未识别的_f_黄单胞菌科);unclassified_f_ Ruminococcaceae(未分类的_f_瘤胃菌科);unclassified_p_Chloroflexi(未分类的_p_绿弯菌门);unclassified_f_Anaerolineaceae(未分类的_f_厌氧绳菌科);norank_o_Acidimicrobiales(未识别的_o_酸微菌目);unclassified_f_Peptostreptococcaceae(未分类的_f_消化链球菌科);Methanosaeta (鬃毛甲烷菌属);Methanobacterium(甲烷杆菌属);Methanospirillum(甲烷螺菌属);Methanobrevibacter(甲烷短杆菌属)(属分类)
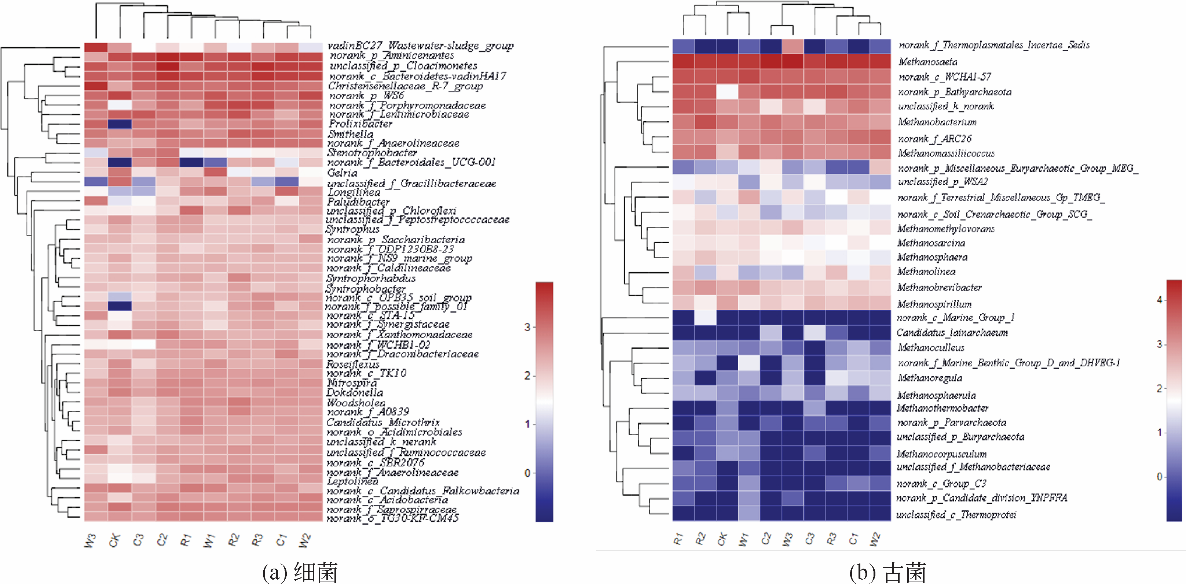
图3 各样本间的分布热图Leptolinea(纤绳菌属);Methanomethylovorans(甲烷食甲基菌属);Methanosarcina(甲烷八叠球菌属);Methanosphaera(甲烷球菌属);Methanolinea(甲烷绳菌属);Methanoculleus(甲烷囊菌属);Methanocorpusculum(甲烷粒菌属)(属分类)
| 环境因子 | VIF | |
|---|---|---|
| 筛选前 | 筛选后 | |
| pH | 51.81 | 7.15 |
| 氨氮( | 3631.04 | 4.34 |
| 乙酸 | 248838.3 | 3.96 |
| 丙酸 | 1474.49 | 2.42 |
| 丁酸 | 711.34 | 2.88 |
| 异丁酸 | 4888.83 | 4.67 |
表5 环境因子的VIF值
| 环境因子 | VIF | |
|---|---|---|
| 筛选前 | 筛选后 | |
| pH | 51.81 | 7.15 |
| 氨氮( | 3631.04 | 4.34 |
| 乙酸 | 248838.3 | 3.96 |
| 丙酸 | 1474.49 | 2.42 |
| 丁酸 | 711.34 | 2.88 |
| 异丁酸 | 4888.83 | 4.67 |
| 1 | 石祖梁, 李想, 王久臣, 等. 中国秸秆资源空间分布特征及利用模式[J].中国人口·资源与环境, 2018, 28(s1): 202-205. |
| SHI Zuliang, LI Xiang, WANG Jiucheng, et al. Thespatial distribution characteristics and utilization model of crop straw in China [J]. China Population, Resources and Environment, 2018, 28(s1): 202-205. | |
| 2 | 杭世珺, 傅涛, 戴晓虎, 等. 技术路线没有走通,产业没有融通,政策缺乏贯通污泥出路困境如何破?[J]. 环境经济, 2019(2): 34-39. |
| HANG Shijun, FU Tao, DAI Xiaohu, et al. The technical route has not been communicated, the industry has not been integrated, and the policy lacks the way to break through the sludge[J]. Environmental Economy, 2019(2): 34-39. | |
| 3 | DEVLIN D C, ESTEVES S R R, DINSDALE R M, et al. The effect of acid pretreatment on the anaerobic digestion and dewatering of waste activated sludge[J]. Bioresource Technology, 2011, 102(5): 4076-4082. |
| 4 | LI R, CHEN S, LI X. Anaerobic co-digestion of kitchen waste and cattle manure for methane production[J]. Energy Sources Part A: Recovery Utilization & Environmental Effects, 2009, 31(20): 1848-1856. |
| 5 | GELEGENIS J, GEORGAKAKIS D, ANGELIDAKI I, et al. Optimization of biogas production from olive-oil mill wastewater, by co-digesting with diluted poultry-manure[J]. Applied Energy, 2007, 84(6): 646-663. |
| 6 | XU S, SELVAM A, KARTHIKEYAN O P, et al. Responses of microbial community and acidogenic intermediates to different water regimes in a hybrid solid anaerobic digestion system treating food waste[J]. Bioresour. Technol., 2014, 168: 49-58. |
| 7 | NING J, ZHOU M D, PAN X F, et al. Simultaneous biogas and biogas slurry production from co-digestion of pig manure and corn straw: performance optimization and microbial community shift[J]. Bioresource Technology, 2019, 282: 37-47. |
| 8 | 任南琪,王爱杰. 厌氧生物技术原理与应用[M]. 北京: 化学工业出版社, 2004: 30-31. |
| REN N Q, WANG A J. The method and technology of anaerobic digestion[J]. Beijing: Chemistry Industry Press, 2004: 30-31. | |
| 9 | CALLAGHAN F J, WASE D A J, THAYANIYHY K, et al. Continuous co-digestion of cattle slurry with fruit and vegetable wastes and chicken manure[J]. Biomass Bioenergy, 2002, 22(1): 71-77. |
| 10 | CHEN Y D, ZHAO Z Y, ZOU H J, et al. Digestive performance of sludge with different crop straws in mesophilic anaerobic digestion[J]. Bioresource Technology, 2019, 289: 121595. |
| 11 | PARAWIRA W, MURTO M, READ J S, et al. Volatile fatty acid production during anaerobic mesophilic digestion of solid potato waste[J]. J. Chem. Technol. Biotechnol., 2004, 79: 673-677. |
| 12 | MIRON Y, ZEEMAN G, LIER J Bet al VAN. The role of sludge retention time in the hydrolysis and acidification of lipids, carbohydrates and proteins during digestion of primary sludge in CSTR systems[J]. Water Res., 2000, 34: 1705-1713. |
| 13 | BARLINDHAUG J, ØDEGAARD H. Thermal hydrolysis for the production of carbon source for denitrification[J]. Water Sci. Technol., 1996, 34: 371-378. |
| 14 | CHANDRA R, TAKEUCHI H, HASEGAWA T. Methane production from lignocellulosic agricultural crop wastes: a review in context to second generation of biofuel production[J]. Renew. Sust. Energ. Rev., 2012, 16(3): 1462-1476. |
| 15 | LI Y, JIN Y, LI H, et al. Kinetic studies on organic degradation and its impacts on improving methane production during anaerobic digestion of food waste[J]. Appl. Energy, 2018, 213: 136-147. |
| 16 | FITAMO T, TREU L, BOLDRIN A, et al. Microbial population dynamics in urban organic waste anaerobic co-digestion with mixed sludge during a change in feedstock composition and different hydraulic retention times[J]. Water Res., 2017, 118: 261-271. |
| 17 | JANG H M, HA H, KIM M S, et al. Effect of increased load of high-strength food wastewater in thermophilic and mesophilic anaerobic co-digestion of waste activated sludge on bacterial community structure[J]. Water Res., 2016, 99: 140-148. |
| 18 | LIU Y, WACHEMO A C, YUAN H R, et al. Anaerobic digestion performance and microbial community structure of corn stover in three-stage continuously stirred tank reactors[J]. Bioresource Technology, 2019, 287: 121339. |
| 19 | LU L, XING D F, REN N Q. Pyrosequencing reveals highly diverse microbial communities in microbial electrolysis cells involved in enhanced H2 production from waste activated sludge[J]. Water Research, 2012, 46(7): 2425-2434. |
| 20 | ZHENG X, CHENG Y G, LI X, et al. Pyrosequencing reveals the key microorganisms involved in sludge alkaline fermentation for efficient short-chain fatty acids production[J]. Environmental Science & Technology, 2013, 47(9): 4262-4268. |
| 21 | SRILES M E, HOLZAPFEL W H. Lactic acid bacteria of foods and their current taxonomy[J]. Int. J. Food Microbiol., 1997, 36(1): 1-29. |
| 22 | BALK M, WEIJMA J, STAMS A J M. Thermotoga lettingae sp. nov., a novel thermophilic, methanol-degrading bacterium isolated from a thermophilic anaerobic reactor[J]. Int. J. Syst. Evol. Microbiol., 2002, 52(4): 1361-1368. |
| 23 | DONG X, XIN Y, JIAN W, et al. Bifidobacterium thermacidophilum sp. nov., isolated from an anaerobic digester[J]. Int. J. Syst. Evol. Microbiol., 2000, 50(1): 119-125. |
| 24 | YAMADA T, SEKIGUCHI Y, HANADA S, et al. Anaerolinea thermolimosa sp. nov., Levilinea saccharolytica gen. nov., sp. nov. and Leptolinea tardivitalis gen. nov.,sp. nov., novel filamentous anaerobes, and description of the new classes Anaerolineae classis nov. and Caldilineae classis nov. in the bacterial phylum chloroflexi[J]. Int. J. Syst. Evol. Microbiol., 2006, 56(6): 1331-1340. |
| 25 | VREELAND R, LITCHFIELD C, MARTIN E. Halomonas elongata, a new genus and species of extremely salt-tolerant bacteria[J]. Int. J. Syst. Bacteriol., 1980, 30: 485-495. |
| 26 | YI J, DONG B, JIN J. Effect of increasing total solids contents on anaerobic digestion of food waste under mesophilic conditions: performance and microbial characteristics analysis[J]. PloS One, 2014, 9: e102548. |
| 27 | HIRAISHI A, IWASAKI M, SHINJO H. Terminal restriction pattern analysis of 16S rRNA genes for the characterization of bacterial communities of activated sludge[J]. J. Biosci. Bioeng., 2000, 90: 148-156. |
| 28 | YUE Z, CHEN R, YANG F, et al. Effects of dairy manure and corn stover co-digestion on anaerobic microbes and corresponding digestion performance[J]. Bioresour. Technol., 2013, 128: 65-71. |
| 29 | YANG Y, YU K, XIA Y, et al. Metagenomic analysis of sludge from full-scale anaerobic digesters operated in municipal wastewater treatment plants[J]. Appl. Microbiol. Biotechnol., 2014, 98: 5709-5718. |
| 30 | ZOU H, CHEN Y, SHI J, et al. Mesophilic anaerobic co-digestion of residual sludge with different lignocellulosic wastes in the batch digester[J]. Bioresour. Technol., 2018, 268: 371-381. |
| 31 | XIAN C Q, MENG M J, XIAO G W, et al. Response of treatment performance and microbial community structure to the temporary suspension of an industrial anaerobic bioreactor[J]. Science of the Total Environment, 2019, 646: 229-237. |
| 32 | FLINT H J, BAYER E A, RINCON M T, et al. Polysaccharide utilization by gut bacteria: potential for new insights from genomic analysis[J]. Nature Reviews Microbiology, 2008, 6(2): 121-131. |
| 33 | FONTES C M, GILBERT H J. Cellulosomes: highly efficient nanomachines designed to deconstruct plant cell wall complex carbohydrates[J]. Annual Review of Biochemistry, 2010, 79(1): 655-681. |
| 34 | KOH A, DE VADDER F, KOVATCHEVA-DATHARY P, et al. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites[J]. Cell, 2016, 165(6): 1332-1345. |
| 35 | BESTEN G DEN, EUNEN K VAN, GROEN A K, et al. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism[J]. The Journal of Lipid Research, 2013, 54(9): 2325-2340. |
| 36 | MORRISON M, MIRON J. Adhesion to cellulose by Ruminococcus albus: a combination of cellulosomes and Pil-proteins?[J]. FEMS Microbiol. Lett., 2000, 185(2): 109-115. |
| 37 | ZHANG L, CHUNG J, JIANG Q, et al. Characteristics of rumen microorganisms involved in anaerobic degradation of cellulose at various pH values[J]. RSC Adv., 2017, 7(64): 40303-40310. |
| 38 | ZHIVIN O, DASSA B, MORAIS S, et al. Unique organization and unprecedented diversity of the Bacteroides (Pseudobacteroides) cellulosolvens cellulosome system[J]. Biotechnol. Biofuels., 2017, 10: 211. |
| 39 | HE Y, LI M, PERUMAL V. Genomic and enzymatic evidence for acetogenesis among multiple lineages of the archaeal phylum Bathyarchaeota widespread in marine sediments[J]. Nat. Microbiol., 2016, 1(6): 16035. |
| 40 | BECKER K W, ELLING F J, YOSHINAGA M Y, et al. Unusual butane-and pentanetriol-based tetraether lipids in Methanomassiliicoccus luminyensis, a representative of the seventh order of methanogens[J]. Appl. Environ. Microbiol., 2016, 82(15): 4505-4516. |
| 41 | KRONINGER L, BERGER S, WELTE C, et al. Evidence for the involvement of two heterodisulfide reductases in the energy-conserving system of Methanomassiliicoccus luminyensis[J]. FEBS J., 2016, 283(3): 472-483. |
| 42 | MATA-ALVAREZ J, DOSTA J, ROMERO-GUIZA M S, et al. A critical review on anaerobic co-digestion achievements between 2010 and 2013[J]. Renew. Sust. Energ. Rev., 2014, 36: 412-427. |
| 43 | HOLMES D E, NEVIN K P, WOODARD T L, et al. Prolixibacter bellariivorans gen. nov., sp. nov., a sugar-fermenting, psychrotolerant anaerobe of the phylum Bacteroidetes, isolated from a marine-sediment fuel cell[J]. Int. J. Syst. Evol. Microbiol., 2007, 57(4): 701-707. |
| 44 | IINO T, Sakamoto M, Ohkuma M. Prolixibacter denitrificans sp. nov., an iron-corroding, facultatively aerobic, nitrate-reducing bacterium isolated from crude oil, and emended descriptions of the genus Prolixibacter and Prolixibacter bellariivorans[J]. Int. J. Syst. Evol. Microbiol., 2015, 65: 2865-2869. |
| 45 | MCILROY S J, KIRKEGAARD R H, DUEHOIM M S, et al. Culture-independent analyses reveal novel Anaerolineaceae as abundant primary fermenters in anaerobic digesters treating waste activated sludge[J]. Frontiers in Microbiology, 2017, 8: 1134. |
| 46 | YAMADA T, IMACHI H, OHASHI A, et al. Bellilinea caldifistulae gen. nov., sp. nov. and Longilinea arvoryzae gen. nov., sp. nov., strictly anaerobic, filamentous bacteria of the phylumchloroflexi isolated from methanogenic propionate-degrading consortia[J]. Int. J. Syst. Evol. Microbiol., 2007, 57(10): 2299-2306. |
| [1] | 李志远, 黄亚继, 赵佳琪, 于梦竹, 朱志成, 程好强, 时浩, 王圣. 污泥与聚氯乙烯共热解重金属特性[J]. 化工进展, 2023, 42(9): 4947-4956. |
| [2] | 史天茜, 石永辉, 武新颖, 张益豪, 秦哲, 赵春霞, 路达. Fe2+对厌氧氨氧化EGSB反应器运行性能的影响[J]. 化工进展, 2023, 42(9): 5003-5010. |
| [3] | 杨子育, 朱玲, 王文龙, 于超凡, 桑义敏. 阴燃法处理含油污泥的研究及应用进展[J]. 化工进展, 2023, 42(7): 3760-3769. |
| [4] | 郑昕, 贾里, 王彦霖, 张靖超, 陈世虎, 乔晓磊, 樊保国. 污泥与煤泥混烧对重金属固留特性的影响[J]. 化工进展, 2023, 42(6): 3233-3241. |
| [5] | 修浩然, 王云刚, 白彦渊, 邹立, 刘阳. 准东煤/市政污泥混燃燃烧特性及灰熔融行为分析[J]. 化工进展, 2023, 42(6): 3242-3252. |
| [6] | 詹咏, 王慧, 韦婷婷, 朱星宇, 王先恺, 陈思思, 董滨. Mn2+强化臭氧调理对生物处理工艺的污泥原位减量效果[J]. 化工进展, 2023, 42(6): 3253-3260. |
| [7] | 常占坤, 张弛, 苏冰琴, 张聪政, 王健, 权晓慧. H2S气态基质对污泥生物沥滤处理效能的影响[J]. 化工进展, 2023, 42(5): 2733-2743. |
| [8] | 杨自强, 李风海, 郭卫杰, 马名杰, 赵薇. 市政污泥热处理过程中磷迁移转化的研究进展[J]. 化工进展, 2023, 42(4): 2081-2090. |
| [9] | 赵佳琪, 黄亚继, 李志远, 丁雪宇, 祁帅杰, 张煜尧, 刘俊, 高嘉炜. 污泥和聚氯乙烯共热解三相产物特性[J]. 化工进展, 2023, 42(4): 2122-2129. |
| [10] | 张涵, 张肖静, 马冰冰, 佴灿, 刘烁烁, 马永鹏, 宋亚丽. 以城市废弃污泥为种泥启动厌氧氨氧化工艺的可行性[J]. 化工进展, 2023, 42(2): 1080-1088. |
| [11] | 郭宇晨, 刘庆林, 蒋金洋, 宗永忠, 王金伟, 李臻, 吕树祥. 含铬污泥资源化方法研究进展[J]. 化工进展, 2023, 42(2): 575-584. |
| [12] | 孙千千, 刘阵, 李瑞, 张溪, 杨明德, 吴玉龙. 低温水热耦合亚铁离子活化过硫酸盐提高剩余污泥的脱水性能[J]. 化工进展, 2023, 42(2): 595-602. |
| [13] | 段一航, 高宁博, 全翠. 水热处理对含油污泥热解特性及动力学影响[J]. 化工进展, 2023, 42(2): 603-613. |
| [14] | 杨娟娟, 何林, 贺常晴, 李鑫钢, 隋红. 含油污泥多相复合调质降黏破乳分离过程[J]. 化工进展, 2023, 42(2): 614-623. |
| [15] | 胡兆岩, 张景新, 何义亮. Fe负载污泥生物炭催化热解聚丙烯及产物特性[J]. 化工进展, 2023, 42(2): 631-640. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||