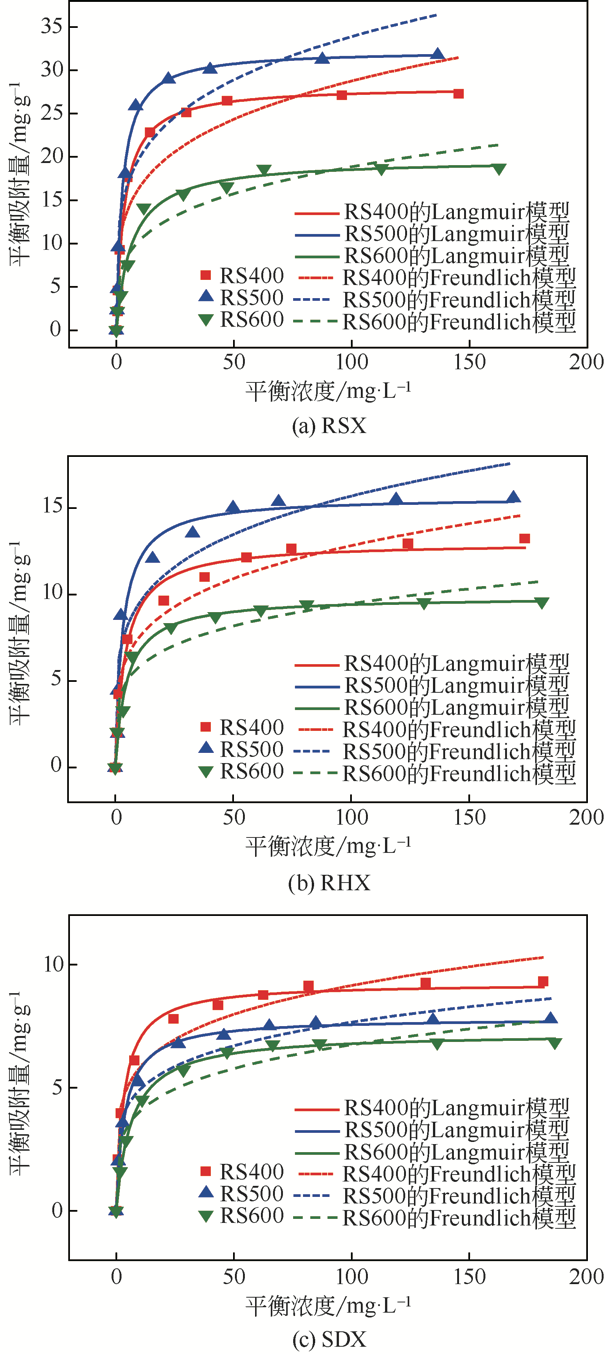
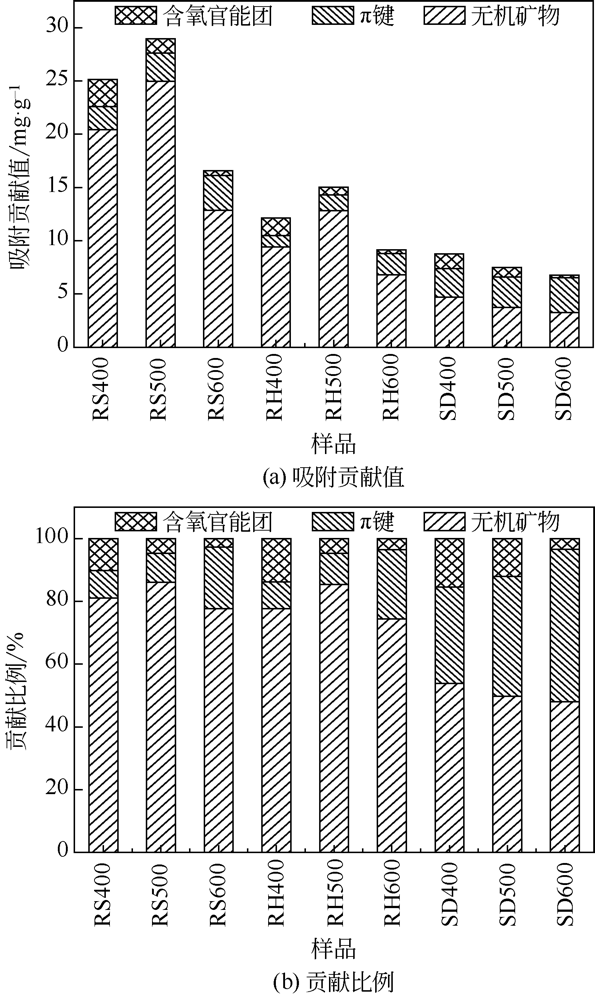
| 1 |
LI H , DONG X , SILVA E B DA , et al . Mechanisms of metal sorption by biochars: biochar characteristics and modifications[J]. Chemosphere, 2017, 178: 466-478.
|
| 2 |
何昱轩, 张黎明, 郭飞飞, 等 . 硅基吸附剂处理含镉废水的研究进展[J]. 化工进展, 2018, 37(2): 724-736.
|
|
HE Yuxuan , ZHANG Liming , GUO Feifei , et al . Advances in cadmium removal from wastewater by silica-based materials[J]. Chemical Industry and Engineering Progress, 2018, 37(2): 724-736.
|
| 3 |
刘金燕, 刘立华, 薛建荣, 等 . 重金属废水吸附处理的研究进展[J]. 环境化学, 2018, 37(9): 2016-2024.
|
|
LIU Jinyan , LIU Lihua , XUE Jianrong , et al . Research progress on treatment of heavy metal wastewater by adsorption[J]. Environmental Chemistry, 2018, 37(9): 2016-2024.
|
| 4 |
ZHOU N , CHEN H , XI J , et al . Biochars with excellent Pb(II) adsorption property produced from fresh and dehydrated banana peels via hydrothermal carbonization[J]. Bioresource Technology, 2017, 232: 204-210.
|
| 5 |
CUI X , FANG S , YAO Y , et al . Potential mechanisms of cadmium removal from aqueous solution by Canna indica derived biochar[J]. Science of the Total Environment, 2016, 562: 517-525.
|
| 6 |
AHMAD M , RAJAPAKSHA A U , LIM J E, et al . Biochar as a sorbent for contaminant management in soil and water: a review[J]. Chemosphere, 2014, 99: 19-33.
|
| 7 |
INYANG M I , GAO B , YAO Y , et al . A review of biochar as a low-cost adsorbent for aqueous heavy metal removal[J]. Critical Reviews in Environmental Science & Technology, 2016, 46(4): 406-433.
|
| 8 |
TAG A T, DUMAN G , UCAR S , et al . Effects of feedstock type and pyrolysis temperature on potential applications of biochar[J]. Journal of Analytical & Applied Pyrolysis, 2016, 120: 200-206.
|
| 9 |
ZHANG F , WANG X , YIN D , et al . Efficiency and mechanisms of Cd removal from aqueous solution by biochar derived from water hyacinth (Eichornia crassipes)[J]. Journal of Environmental Management, 2015, 153: 68-73.
|
| 10 |
LEE S M, DAVIS A P . Removal of Cu(Ⅱ) and Cd(Ⅱ) from aqueous solution by seafood processing waste sludge[J]. Water Research, 2001, 35(2): 534-540.
|
| 11 |
SUN J , LIAN F , LIU Z , et al . Biochars derived from various crop straws: characterization and Cd(Ⅱ) removal potential[J]. Ecotoxicology & Environmental Safety, 2014, 106(2): 226-231.
|
| 12 |
XU X , CAO X , LING Z , et al . Removal of Cu, Zn, and Cd from aqueous solutions by the dairy manure-derived biochar[J]. Environmental Science and Pollution Research, 2013, 20(1): 358-368.
|
| 13 |
陈再明, 方远, 徐义亮, 等 . 水稻秸秆生物碳对重金属Pb2+的吸附作用及影响因素[J]. 环境科学学报, 2012, 32(4): 769-776.
|
|
CHEN Zaiming , FANG Yuan , XU Yiliang , et al . Adsorption of Pb2+ by rice straw derived-biochar and its influential factors[J]. Acta Scientiae Circumstantiae, 2012, 32(4): 769-776.
|
| 14 |
WANG R , HUANG D , LIU Y , et al . Investigating the adsorption behavior and the relative distribution of Cd2+ sorption mechanisms on biochars by different feedstock[J]. Bioresource Technology, 2018, 261: 265-271.
|
| 15 |
ZHANG C , SHAN B , TANG W , et al . Comparison of cadmium and lead sorption by Phyllostachys pubescens biochar produced under a low-oxygen pyrolysis atmosphere[J]. Bioresource Technology, 2017, 238: 352-360.
|
| 16 |
王震宇, 刘国成, 李锋民, 等 . 不同热解温度生物炭对Cd(Ⅱ)的吸附特性[J]. 环境科学, 2014, 35(12): 4735-4744.
|
|
WANG Zhenyu , LIU Guocheng , LI Fengmin , et al . Adsorption of Cd(Ⅱ) varies with biochars derived at different pyrolysis temperature[J]. Environmental Science, 2014, 35(12): 4735-4744.
|
| 17 |
CHENG M , ZENG G , HUANG D , et al . Hydroxyl radicals based advanced oxidation processes (AOPs) for remediation of soils contaminated with organic compounds: a review[J]. Chemical Engineering Journal, 2016, 284: 582-598.
|
| 18 |
徐楠楠, 林大松, 徐应明, 等 . 玉米秸秆生物炭对Cd2+的吸附特性及影响因素[J]. 农业环境科学学报, 2014, 33(5): 958-964.
|
|
XU Nannan , LIN Dasong , XU Yingming , et al . Adsorption of aquatic Cd2+ by biochar obtained from corn stover[J]. Journal of Agro-Environment Science, 2014, 33(5): 958-964.
|
| 19 |
MARCO K , NICO P S , JOHNSON M G , et al . Dynamic molecular structure of plant biomass-derived black carbon (biochar)[J]. Environmental Science & Technology, 2010, 44(4): 1247-1253.
|
| 20 |
WANG Z , ZHENG H , LUO Y , et al . Characterization and influence of biochars on nitrous oxide emission from agricultural soil[J]. Environmental Pollution, 2013, 174(5): 289-296.
|
| 21 |
LIU L , FAN S . Removal of cadmium in aqueous solution using wheat straw biochar: effect of minerals and mechanism[J]. Environmental Science & Pollution Research, 2018, 25(9): 8688-8700.
|
 ),刘凌沁1(
),刘凌沁1( ),黄亚继1(
),黄亚继1( ),陶圣年2,秦文慧1,任海斌2
),陶圣年2,秦文慧1,任海斌2
 ),Lingqin LIU1(
),Lingqin LIU1( ),Yaji HUANG1(
),Yaji HUANG1( ),Shengnian TAO2,Wenhui QIN1,Haibin REN2
),Shengnian TAO2,Wenhui QIN1,Haibin REN2