化工进展 ›› 2019, Vol. 38 ›› Issue (04): 1984-1995.DOI: 10.16085/j.issn.1000-6613.2018-1257
沸石材料吸附水中放射性锶和铯的研究进展
- 天津大学环境科学与工程学院,天津 300350
-
收稿日期:2018-06-18修回日期:2018-08-10出版日期:2019-04-05发布日期:2019-04-05 -
通讯作者:张光辉 -
作者简介:张振国(1994—),女,硕士研究生,研究方向为膜法水处理。|张光辉,副教授,研究方向为膜法水处理。E-mail:<email>zgh@tju.edu.cn</email>。 -
基金资助:国家水体污染控制与治理科技重大专项(饮用水放射性污染控制技术及应急装备研发)(2015ZX07406006)
Progress in adsorption of radioactive strontium and cesium from aqueous solution on zeolite materials
Zhenguo ZHANG,Mingdong ZHANG,Ping GU,Guanghui ZHANG( )
)
- School of Environmental Science and Engineering, Tianjin University, Tianjin 300350, China
-
Received:2018-06-18Revised:2018-08-10Online:2019-04-05Published:2019-04-05 -
Contact:Guanghui ZHANG
摘要:
放射性锶和铯是核工业生产过程中重要的核裂变产物,也是放射性废水中含量较多的放射性污染物,具有较长的半衰期和持续的生物毒性。本文综述了近几年国内外采用天然沸石、合成沸石和复合沸石吸附去除水中放射性锶、铯的研究进展。重点阐述了海藻酸盐、聚丙烯腈、炭材料以及磁改性金属与沸石构成的复合吸附材料对水中放射性锶、铯的吸附。复合沸石可以解决沸石粒径小、难分离、稳定性差等难题,强化了沸石的实用性。从吸附平衡时间、最大吸附容量、酸碱耐受度等方面归纳了沸石材料对水中放射性锶、铯的吸附特性,并分析总结了三类沸石的优缺点。最后,针对沸石材料在处理放射性锶、铯废水中的应用,展望了未来的研究方向,指出可以在降低原水浓度、开发组合工艺和加强模型模拟等方面的研究进行完善,从而推动沸石材料未来在工程技术领域的应用。
中图分类号:
引用本文
张振国, 张铭栋, 顾平, 张光辉. 沸石材料吸附水中放射性锶和铯的研究进展[J]. 化工进展, 2019, 38(04): 1984-1995.
Zhenguo ZHANG, Mingdong ZHANG, Ping GU, Guanghui ZHANG. Progress in adsorption of radioactive strontium and cesium from aqueous solution on zeolite materials[J]. Chemical Industry and Engineering Progress, 2019, 38(04): 1984-1995.
| 沸石种类 | 主要实验条件 | 平衡时间/min | 最大吸附容量②/mg?g-1 | pH对吸附能力的影响 | 参考文献 |
|---|---|---|---|---|---|
| 塞尔维亚斜发沸石 | C 0=5~1000mg?L-1, m/V=5g?L-1, pH=5, 20℃ | 360 | 9.80 | 2~10时吸附稳定, >10时急剧增加 | [ |
| A型沸石 | C 0=100~1000mg?L-1, m/V =1g?L-1, pH=6, 25℃ | 90~120 | 303.00 | <5时受到抑制, 6~8时最高 | [ |
| Na A-X型沸石 | C 0=100~1000mg?L-1, m/V=1g?L-1, pH=7, 25℃ | 90 | 312.50 | 2~6时不断增加, 6~9时趋于平稳 | [ |
| 纳米Y型沸石 | C 0=50~1500mg?L-1, m/V =2g?L-1, 25℃ | 60① | 1342.34 | — | [ |
| MWCNT增强海藻酸钠A型沸石 | C 0=20~100mg?L-1②, m/V =1.375g?L-1, 25℃ | 700① | 107.50 | — | [ |
| PAN-沸石纳米复合材料 | C 0=43.81~4381mg?L-1, m/V =10g?L-1, 25℃ | 90 | 98.13 | 2~7时不断增加, >7时缓慢下降 | [ |
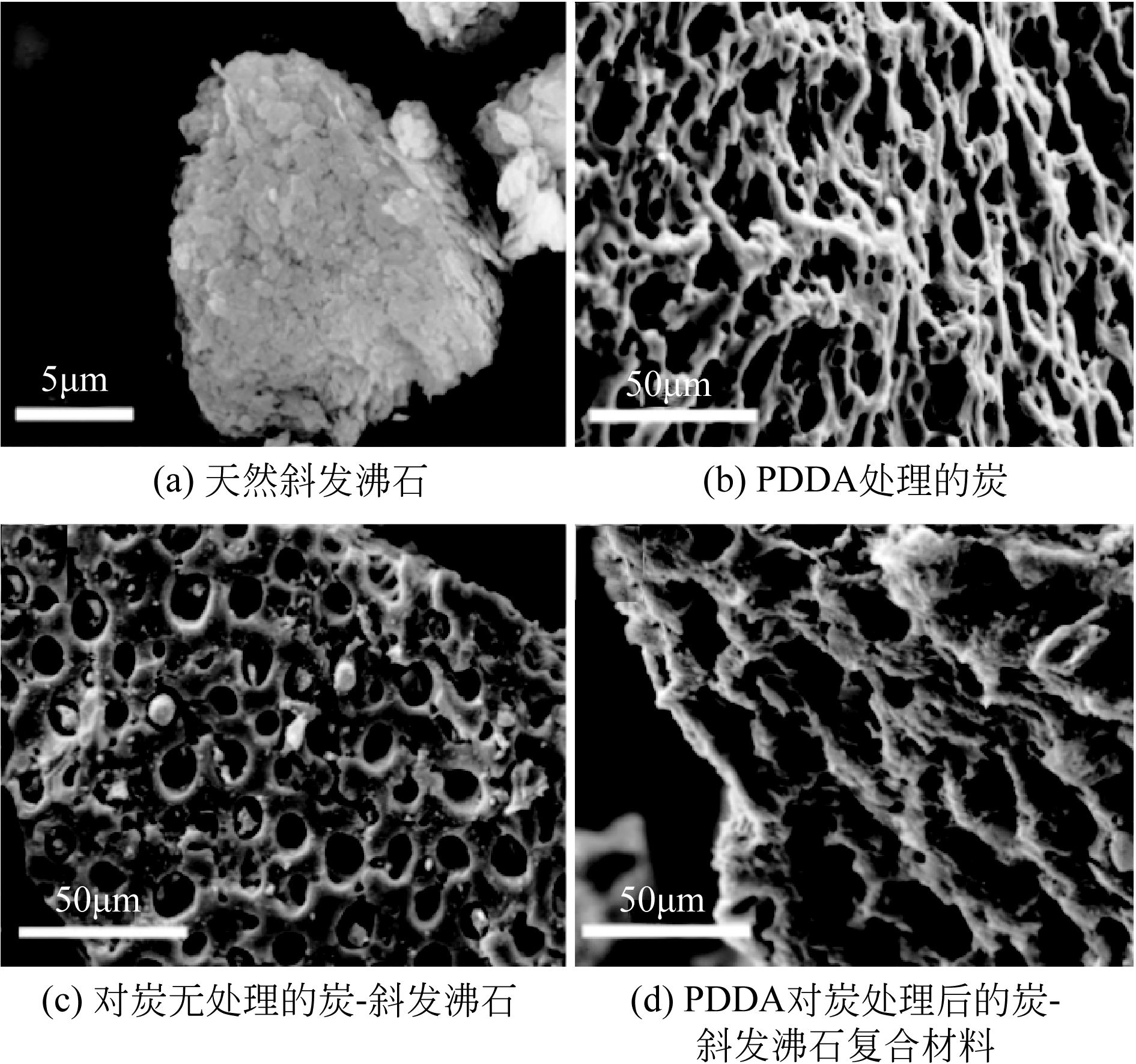
| 炭/天然斜发沸石复合材料 | C 0=50~700mg?L-1, m/V =2g?L-1, pH=5.7, 25℃ | 180 | 95.62 | 2~10时不断增加 | [ |
| 磁性斜发沸石 | C 0=50~400mg?L-1, m/V =5g?L-1, pH=4, 25℃ | 1200 | 20.58 | 2~10时不断增加, >10时急剧增加 | [ |
| 磁性纳米A型沸石 | C 0=87.62~8762mg?L-1, m/V =10g?L-1, 25℃ | 60① | 89.05 | 3~7时不断增加, 7~9时趋于平稳 | [ |
| 磁性纳米Y型沸石 | C 0=50~1000mg?L-1, m/V =2g?L-1, 25℃ | 120 | 228.63 | 4~8时不断增加 | [ |
表1 不同种类沸石对Sr2+的吸附特性
| 沸石种类 | 主要实验条件 | 平衡时间/min | 最大吸附容量②/mg?g-1 | pH对吸附能力的影响 | 参考文献 |
|---|---|---|---|---|---|
| 塞尔维亚斜发沸石 | C 0=5~1000mg?L-1, m/V=5g?L-1, pH=5, 20℃ | 360 | 9.80 | 2~10时吸附稳定, >10时急剧增加 | [ |
| A型沸石 | C 0=100~1000mg?L-1, m/V =1g?L-1, pH=6, 25℃ | 90~120 | 303.00 | <5时受到抑制, 6~8时最高 | [ |
| Na A-X型沸石 | C 0=100~1000mg?L-1, m/V=1g?L-1, pH=7, 25℃ | 90 | 312.50 | 2~6时不断增加, 6~9时趋于平稳 | [ |
| 纳米Y型沸石 | C 0=50~1500mg?L-1, m/V =2g?L-1, 25℃ | 60① | 1342.34 | — | [ |
| MWCNT增强海藻酸钠A型沸石 | C 0=20~100mg?L-1②, m/V =1.375g?L-1, 25℃ | 700① | 107.50 | — | [ |
| PAN-沸石纳米复合材料 | C 0=43.81~4381mg?L-1, m/V =10g?L-1, 25℃ | 90 | 98.13 | 2~7时不断增加, >7时缓慢下降 | [ |
| 炭/天然斜发沸石复合材料 | C 0=50~700mg?L-1, m/V =2g?L-1, pH=5.7, 25℃ | 180 | 95.62 | 2~10时不断增加 | [ |
| 磁性斜发沸石 | C 0=50~400mg?L-1, m/V =5g?L-1, pH=4, 25℃ | 1200 | 20.58 | 2~10时不断增加, >10时急剧增加 | [ |
| 磁性纳米A型沸石 | C 0=87.62~8762mg?L-1, m/V =10g?L-1, 25℃ | 60① | 89.05 | 3~7时不断增加, 7~9时趋于平稳 | [ |
| 磁性纳米Y型沸石 | C 0=50~1000mg?L-1, m/V =2g?L-1, 25℃ | 120 | 228.63 | 4~8时不断增加 | [ |
| 沸石种类 | 主要实验条件 | 平衡时间 /min | 最大吸附容量 /mg?g-1 | pH对吸附能力的影响 | 参考文献 |
|---|---|---|---|---|---|
| 塞尔维亚斜发沸石 | C 0=5~1000mg?L-1, m/V =5g?L-1, pH=5, 20℃ | 360 | 49.02③ | 2~3时缓慢增加, 3~12时保持恒定 | [ |
| 天然斜发沸石 | C 0=13.29g?L-1, m/V=10g?L-1, pH=5, 25℃ [K+]= 0.0119mol?L-1 | — | 168.80② | 3~9时变化不大 | [ |
| 天然菱沸石 | C 0=0~13290mg?L-1, m/V=10g?L-1, pH=5, 25℃, [K+]= 0.0119mol?L-1 | 360 | 275.10② | 3~9时变化不大 | [ |
| 天然丝光沸石 | C 0=0~13290mg?L-1, m/V=10g?L-1, pH=5, 25℃ [K+]= 0.0119mol?L-1 | 360 | 256.50② | 3~9时变化不大 | [ |
| 斜发沸石ZO | C 0=7.9~3946.5mg?L-1, m/V =10g?L-1, pH=7, 30℃ | 60 | 236.31③ | 3~9时影响不大 | [ |
| 斜发沸石ZCh | C 0=7.9~3946.5mg?L-1, m/V =10g?L-1, pH=7, 30℃ | 60 | 170.35③ | 3~9时影响不大 | [ |
| A型沸石 | C 0=100~1000mg?L-1, m/V=1g?L-1, pH=6, 25℃ | 90~120 | 207.47③ | <5时受到抑制, 6~8时最高 | [ |
| Na A-X型沸石 | C 0=100~1000mg?L-1, m/V =1g?L-1, pH=7, 25℃ | 40② | 205.46③ | 2~6时不断增加, 6~8时趋于平稳 | [ |
| 纳米Y型沸石 | C 0=50~1500mg?L-1, m/V =2g?L-1, 25℃ | 60② | 893.09③ | — | [ |
| MWCNT增强的海藻酸钠A型沸石 | C 0=50~250mg?L-1②, m/V =1.375g?L-1, 25℃ | 400② | 113.60③ | — | [ |
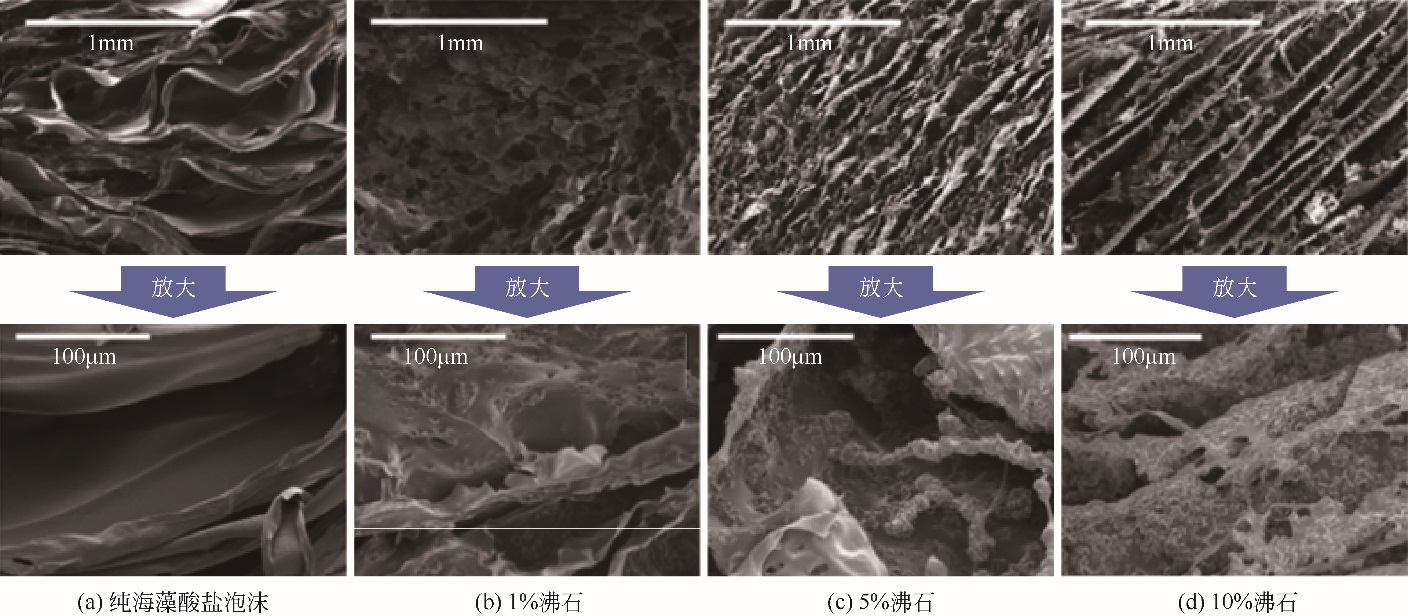
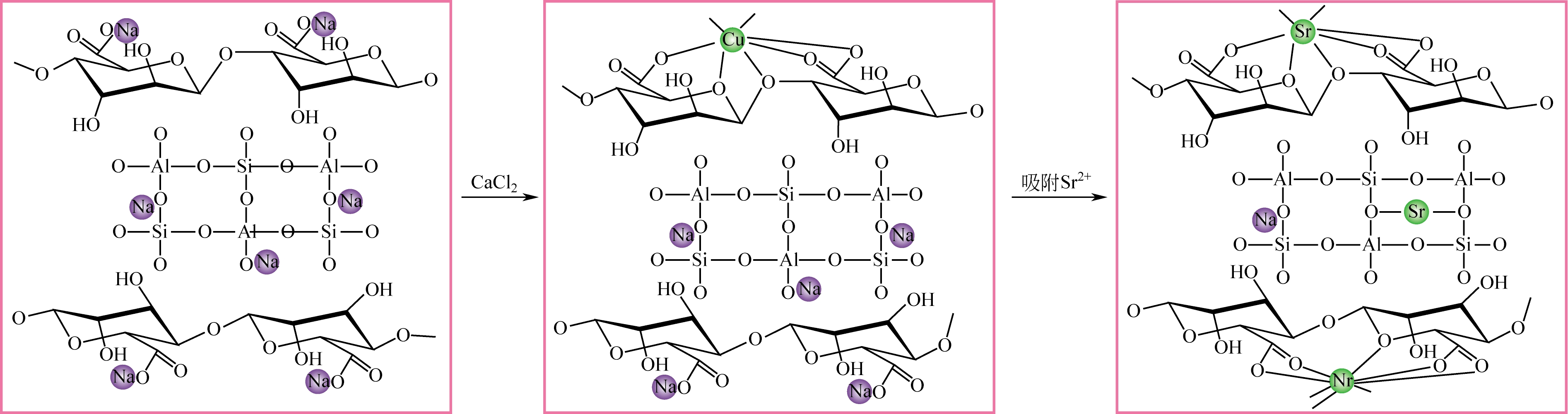
| PAN-沸石纳米复合材料 | C 0=132.9~13290mg?L-1, m/V =10g?L-1, 25℃ | 90 | 214.10③ | 2~7时不断增加, >7时缓慢下降 | [ |
| 炭/天然斜发沸石复合材料 | C 0=400mg?L-1, m/V =5g?L-1, pH=5, 25℃ | 120 | 27.70② | 2~10时不断增加 | [ |
| 磁性纳米A型沸石 | C 0=87.62~8762mg?L-1, m/V =10g?L-1, 25℃ | 80② | 229.30③ | 3~7时不断增加, 7~9时趋于平稳 | [ |
| 磁性纳米Y型沸石 | C 0=50~1000mg?L-1, m/V =2g?L-1, 25℃ | 120 | 298.50③ | 4~6时不断增加, 6~8时缓慢下降 | [ |
表2 不同种类沸石对Cs+的吸附特性
| 沸石种类 | 主要实验条件 | 平衡时间 /min | 最大吸附容量 /mg?g-1 | pH对吸附能力的影响 | 参考文献 |
|---|---|---|---|---|---|
| 塞尔维亚斜发沸石 | C 0=5~1000mg?L-1, m/V =5g?L-1, pH=5, 20℃ | 360 | 49.02③ | 2~3时缓慢增加, 3~12时保持恒定 | [ |
| 天然斜发沸石 | C 0=13.29g?L-1, m/V=10g?L-1, pH=5, 25℃ [K+]= 0.0119mol?L-1 | — | 168.80② | 3~9时变化不大 | [ |
| 天然菱沸石 | C 0=0~13290mg?L-1, m/V=10g?L-1, pH=5, 25℃, [K+]= 0.0119mol?L-1 | 360 | 275.10② | 3~9时变化不大 | [ |
| 天然丝光沸石 | C 0=0~13290mg?L-1, m/V=10g?L-1, pH=5, 25℃ [K+]= 0.0119mol?L-1 | 360 | 256.50② | 3~9时变化不大 | [ |
| 斜发沸石ZO | C 0=7.9~3946.5mg?L-1, m/V =10g?L-1, pH=7, 30℃ | 60 | 236.31③ | 3~9时影响不大 | [ |
| 斜发沸石ZCh | C 0=7.9~3946.5mg?L-1, m/V =10g?L-1, pH=7, 30℃ | 60 | 170.35③ | 3~9时影响不大 | [ |
| A型沸石 | C 0=100~1000mg?L-1, m/V=1g?L-1, pH=6, 25℃ | 90~120 | 207.47③ | <5时受到抑制, 6~8时最高 | [ |
| Na A-X型沸石 | C 0=100~1000mg?L-1, m/V =1g?L-1, pH=7, 25℃ | 40② | 205.46③ | 2~6时不断增加, 6~8时趋于平稳 | [ |
| 纳米Y型沸石 | C 0=50~1500mg?L-1, m/V =2g?L-1, 25℃ | 60② | 893.09③ | — | [ |
| MWCNT增强的海藻酸钠A型沸石 | C 0=50~250mg?L-1②, m/V =1.375g?L-1, 25℃ | 400② | 113.60③ | — | [ |
| PAN-沸石纳米复合材料 | C 0=132.9~13290mg?L-1, m/V =10g?L-1, 25℃ | 90 | 214.10③ | 2~7时不断增加, >7时缓慢下降 | [ |
| 炭/天然斜发沸石复合材料 | C 0=400mg?L-1, m/V =5g?L-1, pH=5, 25℃ | 120 | 27.70② | 2~10时不断增加 | [ |
| 磁性纳米A型沸石 | C 0=87.62~8762mg?L-1, m/V =10g?L-1, 25℃ | 80② | 229.30③ | 3~7时不断增加, 7~9时趋于平稳 | [ |
| 磁性纳米Y型沸石 | C 0=50~1000mg?L-1, m/V =2g?L-1, 25℃ | 120 | 298.50③ | 4~6时不断增加, 6~8时缓慢下降 | [ |
| 1 | KITTO M E , MARRANTINO J C , FIELMAN E M ,et al .Long-term monitoring of radioactivity in fish from New York waters[J].Journal of Environmental Radioactivity, 2015,146:44-50. |
| 2 | GHANDHI S A , WEBER W , MELO D ,et al .Effect of 90Sr internal emitter on gene expression in mouse blood[J].BMC Genomics, 2015,16(1): 586. |
| 3 | STRAND P , SUNDELL-BERGMAN S , BROWN J E ,et al .On the divergences in assessment of environmental impacts from ionising radiation following the Fukushima accident[J]. Journal of Environmental Radioactivity, 2017,169/170: 159-173. |
| 4 | ALIYU A S , NIKOLAOS E , TIMOTHY A M ,et al .An overview of current knowledge concerning the health and environmental consequences of the Fukushima Daiichi Nuclear Power Plant (FDNPP) accident[J].Environment International, 2015,7(85): 213-228. |
| 5 | BUESSELER K O , DAI M , AOYAMA M ,et al .Fukushima Daiichi-derived radionuclides in the ocean-transport[J]. Annua Review of Marine Science, 2016,9: 173-203. |
| 6 | BUESSELER K , AOYAMA M , FUKASAWA M .Impacts of the Fukushima Nclear Power Plants on marine radioactivity[J]. Environmental Science & Technology, 2011,45(23): 9931-9935. |
| 7 | HODKIN D J , STEWART D I , GRAHAM J T ,et al .Coprecipitation of 14C and Sr with carbonate precipitates: the importance of reaction kinetics and recrystallization pathways[J]. Science of the Total Environment, 2016, 562: 335-343. |
| 8 | WU L , ZHANG G , WANG Q ,et al .Removal of strontium from liquid waste using a hydraulic pellet co-precipitation microfiltration (HPC-MF) process[J].Desalination, 2014,349: 31-38. |
| 9 | ROGERS H , BOWERS J , GATES-ANDERSON D .An isotope dilution-precipitation process for removing radioactive cesium from wastewater[J]. Journal of Hazardous Materials, 2012,243: 124-129. |
| 10 | LAUCHNOR E G , SCHULTZ L N , BUGNI S ,et al .Bacterially induced calcium carbonate precipitation and strontium coprecipitation in a porous media flow system[J]. Environmental Science & Technology, 2013,47(3): 1557-1564. |
| 11 | SUN T , ZHENG Z , CHEN J ,et al .Efficient co-extraction of strontium and cesium from nitric acid medium by mixtures of di-tert-butylcyclohexano-18-crown-6 and 1,3-di(2-propoxy)calix[4]arene-crown-6 in n-octanol[J].Separation Science and Technology, 2017,53(3): 503-512. |
| 12 | RAUT D R , MOHAPATRA P K .Simultaneous extraction of Cs and Sr from synthetic high level waste solutions using a solvent containing chlorinated dicarbollide and PEG-400 in PTMS[J]. Journal of Radioanalytical and Nuclear Chemistry, 2014,299(1): 75-80. |
| 13 | XU C , WANG J , CHEN J .Solvent extraction of strontium and cesium: a review of recent progress[J]. Solvent Extraction and Ion Exchange, 2012,30(6): 623-650. |
| 14 | SHARMA J N , KUMAR A , KUMAR V ,et al .Process development for separation of cesium from acidic nuclear waste solution using 1,3-dioctyloxycalix[4]arene-crown-6 plus isodecyl alcohol/n-dodecane solvent[J].Separation and Purification Technology, 2014,135:176-182. |
| 15 | COMBERNOUX N , SCHRIVE L , LABED V ,et al .Treatment of radioactive liquid effluents by reverse osmosis membranes: from lab-scale to pilot-scale[J].Water Research, 2017,123:311-320. |
| 16 | DANG T T H , LI C , CHOO K .Comparison of low-pressure reverse osmosis filtration and polyelectrolyte-enhanced ultrafiltration for the removal of Co and Sr from nuclear plant wastewater[J].Separation and Purification Technology, 2016,157:209-214. |
| 17 | DING S , YANG Y , LI C ,et al .The effects of organic fouling on the removal of radionuclides by reverse osmosis membranes[J]. Water Research, 2016,95:174-184. |
| 18 | ZHANG L , LU Y , LIU Y ,et al .High flux MWCNTs-interlinked GO hybrid membranes survived in cross-flow filtration for the treatment of strontium-containing wastewater[J].Journal of Hazardous Materials, 2016, 320: 187-193. |
| 19 | YIN Y , WANG J , YANG X ,et al .Removal of strontium ions by immobilized saccharomyces cerevisiae in magnetic chitosan microspheres[J]. Nuclear Engineering and Technology, 2017,49(6):172-177. |
| 20 | SINGH S , EAPEN S , THORAT V ,et al .Phytoremediation of 137cesium and 90strontium from solutions and low-level nuclear waste by Vetiveria zizanoides[J]. Ecotoxicology and Environmental Safety, 2008,69(2):306-311. |
| 21 | QI X H , DU K Z , FENG M L ,et al .A two-dimensionally microporous thiostannate with superior Cs+ and Sr2+ ion-exchange property[J].Journal of Materials Chemistry A, 2015,3(10):5665-5673. |
| 22 | ZHANG L , WEI J Y , ZHAO X .Adsorption characteristics of strontium on synthesized antimony silicate[J].Chemical Engineering Journal, 2015,277:378-387. |
| 23 | VILLARD A , SIBOULET B , TOQUER G ,et al .Strontium selectivity in sodium nonatitanate Na4Ti9O20·xH2O[J]. Journal of Hazardous Materials, 2015,283:432-438. |
| 24 | NAEIMI S , FAGHIHIAN H .Performance of novel adsorbent prepared by magnetic metal-organic framework (MOF) modified by potassium nickel hexacyanoferrate for removal of Cs+ from aqueous solution[J].Separation and Purification Technology, 2017,175:255-265. |
| 25 | ALBY D , CHARNAY C , HERAN M ,et al .Recent developments in nanostructured inorganic materials for sorption of cesium and strontium: Synthesis and shaping, sorption capacity, mechanisms, and selectivity—A review[J].Journal of Hazardous Materials, 2018,344:511-530. |
| 26 | SARMA D , MALLIAKAS C D , SUBRAHMANYAM K S ,et al .K2 x Sn4− x S8− x (x=0.65~1): a new metal sulfide for rapid and selective removal of Cs+, Sr2+ and UO2 2+ ions[J].Chemical Science, 2016,7(2): 1121-1132. |
| 27 | ZHANG L , WEI J , ZHAO X ,et al .Removal of strontium(Ⅱ) and cobalt(Ⅱ) from acidic solution by manganese antimonate[J].Chemical Engineering Journal, 2016, 302: 733-743. |
| 28 | BAEK W ,HA S, HONG S ,et al .Cation exchange of cesium and cation selectivity of natural zeolites: chabazite, stilbite, and heulandite[J]. Microporous and Mesoporous Materials, 2018, 264: 159-166. |
| 29 | RAJEC P , DOMIANOVÁ K .Cesium exchange reaction on natural and modified clinoptilolite zeolites[J].Journal of Radioanalytical and Nuclear Chemistry, 2008,275(3): 503-508. |
| 30 | SMIČIKLAS I , DIMOVIĆ S , PLEĆAŠ I .Removal of Cs+, Sr2+ and Co2+ from aqueous solutions by adsorption on natural clinoptilolite[J].Applied Clay Science, 2007,35(1/2): 139-144. |
| 31 | BORAI E H , HARJULA R , MALINEN L ,et al .Efficient removal of cesium from low-level radioactive liquid waste using natural and impregnated zeolite minerals[J].Journal of Hazardous Materials, 2009,172(1): 416-422. |
| 32 | CORTÉS-MARTÍNEZ R , OLGUÍN M T , SOLACHE-RÍOS M . Cesium sorption by clinoptilolite-rich tuffs in batch and fixed-bed systems[J].Desalination, 2010,258(1/2/3): 164-170. |
| 33 | OSMANLIOGLU A E .Treatment of radioactive liquid waste by sorption on natural zeolite in Turkey[J].Journal of Hazardous Materials, 2006,137(1): 332-335. |
| 34 | RABIDEAU A J , BENSCHOTEN J VAN , PATEL A ,et al .Performance assessment of a zeolite treatment wall for removing Sr-90 from groundwater[J].Journal of Contaminant Hydrology, 2005,79(1/2): 1-24. |
| 35 | SENECA S M , RABIDEAU A J .Natural zeolite permeable treatment wall for removing Sr-90 from groundwater.[J].Environmental Science & Technology, 2013,3(47): 1550-1556. |
| 36 | 余少青,张春明,陈晓秋,等 .日本福岛核电站事故后高浓度放射性废水处理系统介绍及其应用启示[J].辐射防护, 2013,33(5):294-299. |
| YU S Q , ZHANG C M , CHEN X Q ,et al .Introduction and application of high concentration radioactive wastewater treatment system after accident at Fukushima Nuclear Power Plants in Japan[J].Radiation Protection, 2013, 33(5): 294-299. | |
| 37 | TSUKADA T , UOZUMI K , HIJIKATA T ,et al .Early construction and operation of highly contaminated water treatment system in Fukushima Daiichi Nuclear Power Station(Ⅰ)-Ion exchange properties of KURION herschelite in simulating contaminated water[J].Journal of Nuclear Science and Technology, 2014,51(7/8): 886-893. |
| 38 | KULPRATHIPANJA S .Zeolites in industrial separation and catalysis[J].Focus on Catalysts, 2010(7):8. |
| 39 | MERCEILLE A , WEINZAEPFEL E , BARRÉ Y ,et al .The sorption behaviour of synthetic sodium nonatitanate and zeolite A for removing radioactive strontium from aqueous wastes[J]. Separation and Purification Technology, 2012,33(96): 81-88. |
| 40 | 罗洁 .粉煤灰基沸石对Sr、Cs的吸附及其固化研究[D].绵阳:西南科技大学, 2016. |
| LUO J .Study on the sorption and solidification of strontium and cesium by the synthesized zeolite from fly ash[D].Mianyang:Southwest University of Science and Technology, 2016. | |
| 41 | EL-RAHMAN K M ABD , EL-KAMASH A M , EL-SOUROUGY M R ,et al .Thermodynamic modeling for the removal of Cs+, Sr2+, Ca2+ and Mg2+ ions from aqueous waste solutions using zeolite A[J].Journal of Radioanalytical and Nuclear Chemistry, 2006,268(2): 221-230. |
| 42 | EL-KAMASH A M .Evaluation of zeolite A for the sorptive removal of Cs+ and Sr2+ ions from aqueous solutions using batch and fixed bed column operations[J].Journal of Hazardous Materials, 2008,151(2/3):432-445. |
| 43 | EL-RAHMAN K M ABD , EL-SOUROUGY M R , ABDEL-MONEM N M ,et al .Modeling the sorption kinetics of cesium and strontium ions on zeolite A[J]. Journal of Radioanalytical and Nuclear Chemistry, 2006,2(7): 21-27. |
| 44 | ELKAMASH A M , ZAKI A A , GELEEL M E .Modeling batch kinetics and thermodynamics of zinc and cadmium ions removal from waste solutions using synthetic zeolite A[J].Journal of Hazardous Materials, 2005,127(1/2/3): 211-220. |
| 45 | EL-NAGGAR M R , EL-KAMASH A M , EL-DESSOUKY M I ,et al .Two-step method for preparation of Na A-X zeolite blend from fly ash for removal of cesium ions[J].Journal of Hazardous Materials, 2008,154(1/2/3):963-972. |
| 46 | IBRAHIM H A , EL-KAMASH A M , HANAFY M ,et al .Examination of the use of synthetic zeolite Na A-X blend as backfill material in a radioactive waste disposal facility: thermodynamic approach[J].Chemical Engineering Journal, 2008,144(1): 67-74. |
| 47 | ABDEL RAHMAN R O , IBRAHIM H A , ABDEL MONEM N M .Long-term performance of zeolite Na A-X blend as backfill material in near surface disposal vault[J]. Chemical Engineering Journal, 2009,149(1/2/3): 143-152. |
| 48 | RAHMAN R O A , IBRAHIM H A , HANAFY M ,et al .Assessment of synthetic zeolite Na A-X as sorbing barrier for strontium in a radioactive disposal facility[J].Chemical Engineering Journal, 2010,157(1): 100-112. |
| 49 | WEN T , WU X L , LIU M C ,et al .Efficient capture of strontium from aqueous solutions using graphene oxide-hydroxyapatite nanocomposites[J].Dalion Transactions, 2014,43(20): 7464-7472. |
| 50 | YANG H , SUN L , ZHAI J ,et al . In situ controllable synthesis of magnetic Prussian blue/graphene oxide nanocomposites for removal of radioactive cesium in water[J].Journal of Materials Chemistry A, 2014, 2(2): 326-332. |
| 51 | METWALLY S S , GHALY M , EL-SHERIEF E A . Physicochemical properties of synthetic nano-birnessite and its enhanced scavenging of Co2+ and Sr2+ ions from aqueous solutions[J].Materials Chemistry and Physics, 2017,193: 63-72. |
| 52 | ZARE F , GHAEDI M , DANESHFAR A ,et al .Efficient removal of radioactive uranium from solvent phase using AgOH-MWCNTs nanoparticles: kinetic and thermodynamic study[J].Chemical Engineering Journal, 2015,273: 296-306. |
| 53 | LEE K,KIM K, PARK M ,et al .Novel application of nanozeolite for radioactive cesium removal from high-salt wastewater[J].Water Research, 2016,95: 134-141. |
| 54 | LEE K, PARK M ,KIM J,et al .Equilibrium, kinetic and thermodynamic study of cesium adsorption onto nanocrystalline mordenite from high-salt solution[J].Chemosphere, 2016, 150:765-771. |
| 55 | TORAD N L , NAITO M , TATAMI J ,et al .Highly crystallized nanometer-sized zeolite A with large Cs adsorption capability for the decontamination of water[J].Chemistry—an Asian Journal, 2014,9(3): 759-763. |
| 56 | ABDEL MOAMEN O A , ISMAIL I M , ABDELMONEM N ,et al .Factorial design analysis for optimizing the removal of cesium and strontium ions on synthetic nano-sized zeolite[J].Journal of the Taiwan Institute of Chemical Engineers, 2015, 55: 133-144. |
| 57 | SAID B , GRANDJEAN A , BARRE Y ,et al .LTA zeolite monoliths with hierarchical trimodal porosity as highly efficient microreactors for strontium capture in continuous flow[J].Microporous and Mesoporous Materials, 2016,232: 39-52. |
| 58 | HONG H J ,KIM B G,RYU J .Preparation of highly stable zeolite-alginate foam composite for strontium(Sr-90) removal from seawater and evaluation of Sr adsorption performance[J].Journal of Environmental Management, 2018,205: 192-200. |
| 59 | ZHANG Y , LIN L X , HU S ,et al .Core-shell zeolite@Alg-Ca particles for removal of strontium from aqueous solutions[J].RSC Advances, 2016,6(78): 73959-73973. |
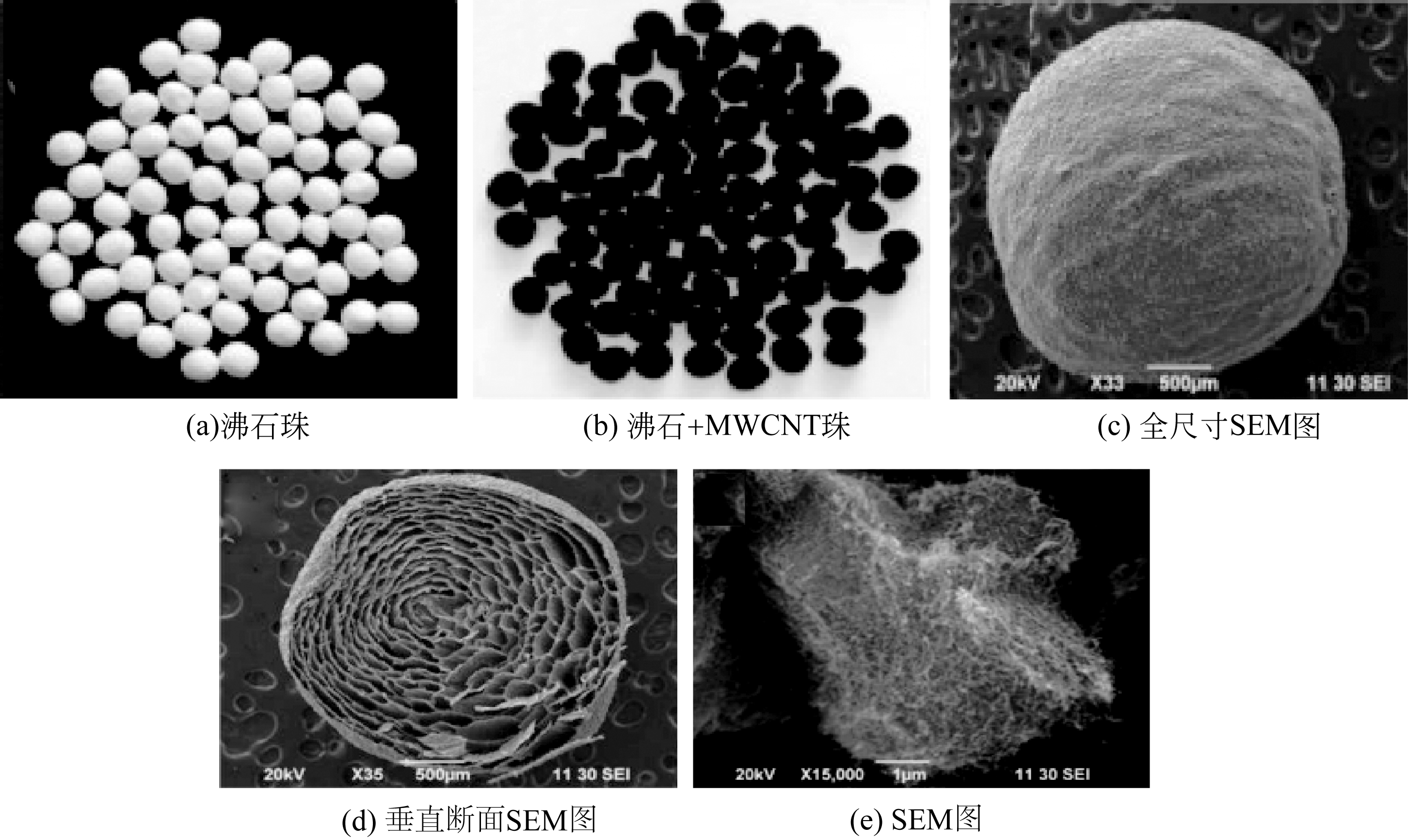
| 60 | VIPIN A K , SUN L B F .Removal of Cs+ and Sr2+ from water using MWCNT reinforced zeolite-A beads[J].Microporous and Mesoporous Materials, 2016, 224: 84-88. |
| 61 | KAYGUN A , AKYIL S .Study of the behaviour of thorium adsorption on PAN/zeolite composite adsorbent[J].Journal of Hazardous Materials, 2007,147(1/2): 357-362. |
| 62 | YUSAN S , ERENTURK S .Adsorption characterization of strontium on PAN/zeolite composite adsorbent[J].World Journal of Nuclear Science and Technology, 2011,1(1): 6-12. |
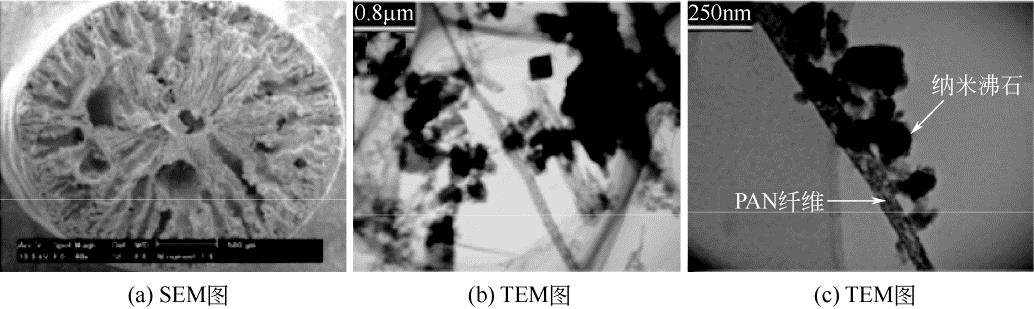
| 63 | FAGHIHIAN H , MOZHGAN I , MOHAMMAD M ,et al .Preparation of a novel PAN-zeolite nanocomposite for removal of Cs+ and Sr2+ from aqueous solutions: kinetic, equilibrium, and thermodynamic studies[J].Chemical Engineering Journal, 2013, 222: 41-48. |
| 64 | FAGHIHIAN H , IRAVANI M , MOAYED M , et al . A novel polyacrylonitrile-zeolite nanocomposite to clean Cs and Sr from radioactive waste[J]. Environmental Chemistry Letters, 2013,11(3): 277-282. |
| 65 | HARO-DEL RIO D A DE , AL-JOUBORI S , KONTOGIANNIS O ,et al .The removal of caesium ions using supported clinoptilolite[J].Journal of Hazardous Materials, 2015, 289: 181. |
| 66 | AL-JUBOURI S M , CURRY N A , HOLMES S M .Hierarchical porous structured zeolite composite for removal of ionic contaminants from waste streams and effective encapsulation of hazardous waste[J].Journal of Hazardous Materials, 2016, 320: 241-251. |
| 67 | HUSNAIN S M ,UM W, WOOJIN-LEE W ,et al .Magnetite-based adsorbents for sequestration of radionuclides:a review[J]. RSC Advances, 2018,8(5): 2521-2540. |
| 68 | WANG W , FENG Q , LIU K ,et al .A novel magnetic 4A zeolite adsorbent synthesised from kaolinite type pyrite cinder (KTPC)[J].Solid State Sciences, 2015,39: 52-58. |
| 69 | HUANG Y , WANG W , FENG Q ,et al .Preparation of magnetic clinoptilolite/CoFe2O4 composites for removal of Sr2+ from aqueous solutions: Kinetic,equilibrium, and thermodynamic studies[J].Journal of Saudi Chemical Society, 2017,21(1): 58-66. |
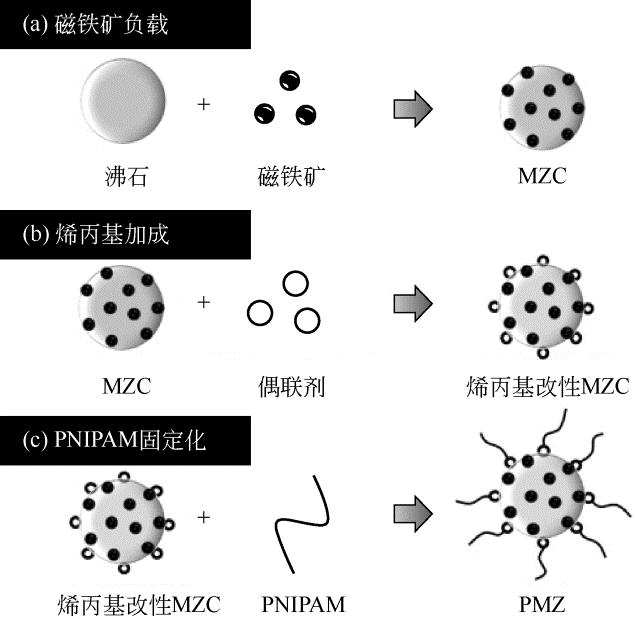
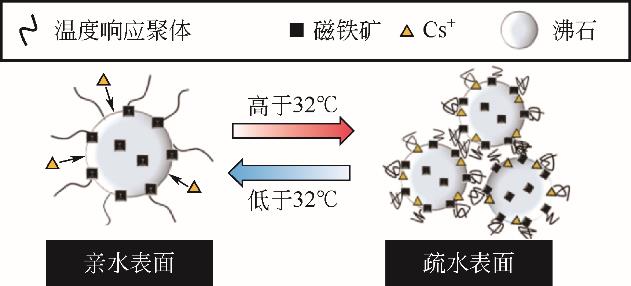
| 70 | NAKAMURA A , SUGAWARA K , NAKAJIMA S ,et al .Adsorption of Cs ions using a temperature-responsive polymer/magnetite/zeolite composite adsorbent and separation of the adsorbent from water using high-gradient magnetic separation[J].Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2017,527: 63-69. |
| 71 | FAGHIHIAN H , MOAYED M , FIROOZ A ,et al .Synthesis of a novel magnetic zeolite nanocomposite for removal of Cs+ and Sr2+ from aqueous solution: Kinetic, equilibrium, and thermodynamic studies[J]. Journal of Colloid and Interface Science, 2013,393: 445-451. |
| 72 | FAGHIHIAN H , MOHAMMAD M , ALIREZA F ,et al .Evaluation of a new magnetic zeolite composite for removal of Cs+ and Sr2+ from aqueous solutions: Kinetic, equilibrium and thermodynamic studies[J].Comptes Rendus Chimie, 2014,17(2): 108-117. |
| 73 | MOAMEN O A A , IBRAHIM H A , ABDELMONEM N ,et al .Thermodynamic analysis for the sorptive removal of cesium and strontium ions onto synthesized magnetic nano zeolite[J].Microporous and Mesoporous Materials, 2016,223: 187-195. |
| 74 | SONG D , PARK S , KANG H W , et al .Recovery of lithium(Ⅰ), strontium(Ⅱ), and lanthanum (Ⅲ) using Ca-Alginate beads[J]. Journal of Chemical & Engineering Data, 2013,58(9): 2455-2464. |
| 75 | HONG H ,RYU J, PARK I ,et al .Investigation of the strontium (Sr(Ⅱ)) adsorption of an alginate microsphere as a low-cost adsorbent for removal and recovery from seawater[J]. Journal of Environmental Management, 2016,165: 263-270. |
| 76 | KIM H,KIM M,LEE W,et al .Rapid removal of radioactive cesium by polyacrylonitrile nanofibers containing Prussian blue[J].Journal of Hazardous Materials, 2018,347: 106-113. |
| 77 | WANG Y , LI J , YANG J E ,et al .Granulous KMS-1/PAN composite for Cs+ removal[J].RSC Advances, 2015,5(111): 91431-91435. |
| 78 | CAKIR P , INAN S , ALTAS Y .Investigation of strontium and uranium sorption onto zirconium-antimony oxide/polyacrylonitrile (Zr-Sb oxide/PAN) composite using experimental design[J].Journal of Hazardous Materials, 2014,271: 108-119. |
| 79 | HUSNAIN S M ,UM W, CHANG Y Y ,et al .Recyclable superparamagnetic adsorbent based on mesoporous carbon for sequestration of radioactive cesium[J].Chemical Engineering Journal, 2017,308: 798-808. |
| 80 | CHEN L L , ZHAO D L , CHEN S H ,et al .One-step fabrication of amino functionalized magnetic graphene oxide composite for uranium(Ⅵ) removal[J].Journal of Colloid and Interface Science, 2016,472: 99-107. |
| 81 | DING C C , CHEN W C , SUN Y B ,et al .Novel fungus-Fe3O4 bio-nanocomposites as high performance adsorbents for the removal of radionuclides[J].Journal of Hazardous Materials, 2015,295: 127-137. |
| 82 | LI D , EGODAWATTE S , KAPLAN D I ,et al . Functionalized magnetic mesoporous silica nanoparticles for U removal from low and high pH groundwater[J]. Journal of Hazardous Materials, 2016,317: 494-502. |
| 83 | ZHANG X Y , GU P , ZHOU S S ,et al .Enhanced removal of iodide ions by nano Cu2O/Cu modified activated carbon from simulated wastewater with improved countercurrent two-stage adsorption[J].Science of the Total Environment, 2018,626: 612-620. |
| [1] | 王胜岩, 邓帅, 赵睿恺. 变电吸附二氧化碳捕集技术研究进展[J]. 化工进展, 2023, 42(S1): 233-245. |
| [2] | 崔守成, 徐洪波, 彭楠. 两种MOFs材料用于O2/He吸附分离的模拟分析[J]. 化工进展, 2023, 42(S1): 382-390. |
| [3] | 陈崇明, 陈秋, 宫云茜, 车凯, 郁金星, 孙楠楠. 分子筛基CO2吸附剂研究进展[J]. 化工进展, 2023, 42(S1): 411-419. |
| [4] | 许春树, 姚庆达, 梁永贤, 周华龙. 共价有机框架材料功能化策略及其对Hg(Ⅱ)和Cr(Ⅵ)的吸附性能研究进展[J]. 化工进展, 2023, 42(S1): 461-478. |
| [5] | 顾永正, 张永生. HBr改性飞灰对Hg0的动态吸附及动力学模型[J]. 化工进展, 2023, 42(S1): 498-509. |
| [6] | 郭强, 赵文凯, 肖永厚. 增强流体扰动强化变压吸附甲硫醚/氮气分离的数值模拟[J]. 化工进展, 2023, 42(S1): 64-72. |
| [7] | 葛亚粉, 孙宇, 肖鹏, 刘琦, 刘波, 孙成蓥, 巩雁军. 分子筛去除VOCs的研究进展[J]. 化工进展, 2023, 42(9): 4716-4730. |
| [8] | 杨莹, 侯豪杰, 黄瑞, 崔煜, 王兵, 刘健, 鲍卫仁, 常丽萍, 王建成, 韩丽娜. 利用煤焦油中酚类物质Stöber法制备碳纳米球用于CO2吸附[J]. 化工进展, 2023, 42(9): 5011-5018. |
| [9] | 潘宜昌, 周荣飞, 邢卫红. 高效分离同碳数烃的先进微孔膜:现状与挑战[J]. 化工进展, 2023, 42(8): 3926-3942. |
| [10] | 张振, 李丹, 陈辰, 吴菁岚, 应汉杰, 乔浩. 吸附树脂对唾液酸的分离纯化[J]. 化工进展, 2023, 42(8): 4153-4158. |
| [11] | 王兰江, 梁瑜, 汤琼, 唐明兴, 李学宽, 刘雷, 董晋湘. 快速热解铂前体合成高分散的Pt/HY催化剂及其萘深度加氢性能[J]. 化工进展, 2023, 42(8): 4159-4166. |
| [12] | 姜晶, 陈霄宇, 张瑞妍, 盛光遥. 载锰生物炭制备及其在环境修复中应用研究进展[J]. 化工进展, 2023, 42(8): 4385-4397. |
| [13] | 于静文, 宋璐娜, 刘砚超, 吕瑞东, 武蒙蒙, 冯宇, 李忠, 米杰. 一种吲哚基超交联聚合物In-HCP对水中碘的吸附作用[J]. 化工进展, 2023, 42(7): 3674-3683. |
| [14] | 李艳玲, 卓振, 池亮, 陈曦, 孙堂磊, 刘鹏, 雷廷宙. 氮掺杂生物炭的制备与应用研究进展[J]. 化工进展, 2023, 42(7): 3720-3735. |
| [15] | 白亚迪, 邓帅, 赵睿恺, 赵力, 杨英霞. 变温吸附碳捕集机组标准化测试方案探讨及性能实验[J]. 化工进展, 2023, 42(7): 3834-3846. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||