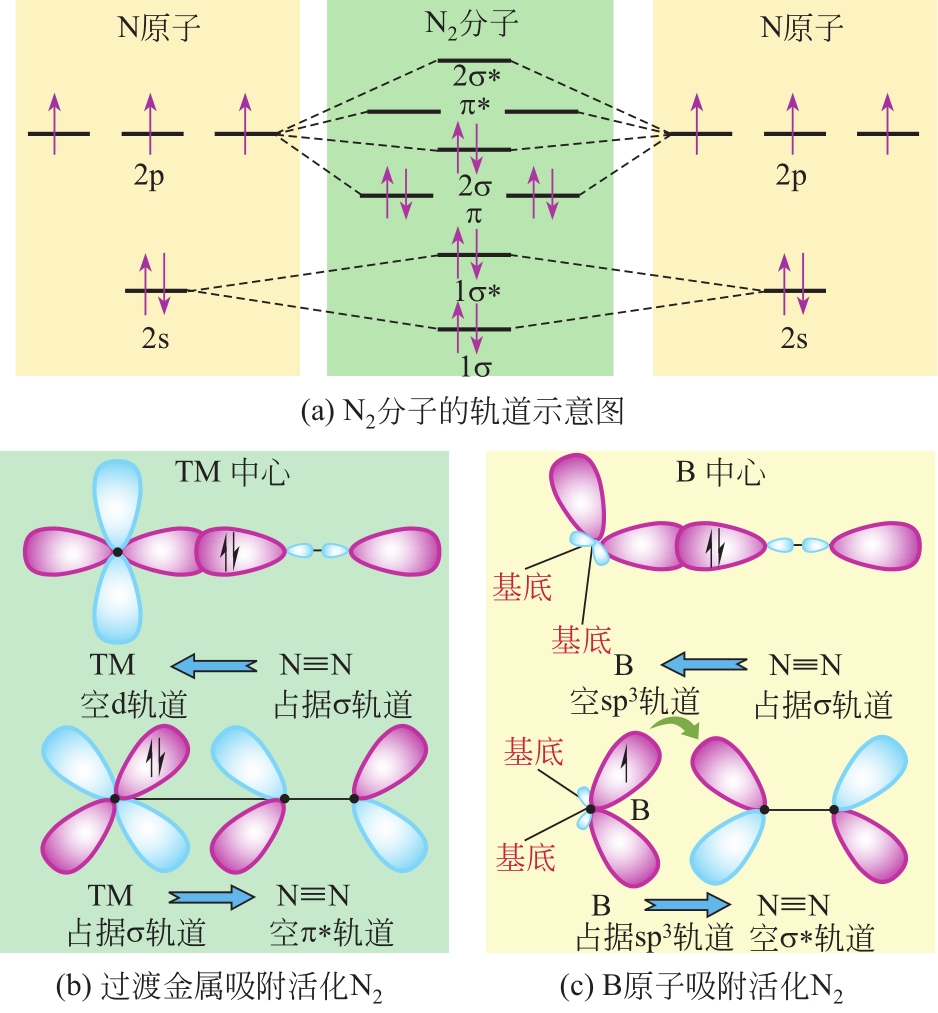
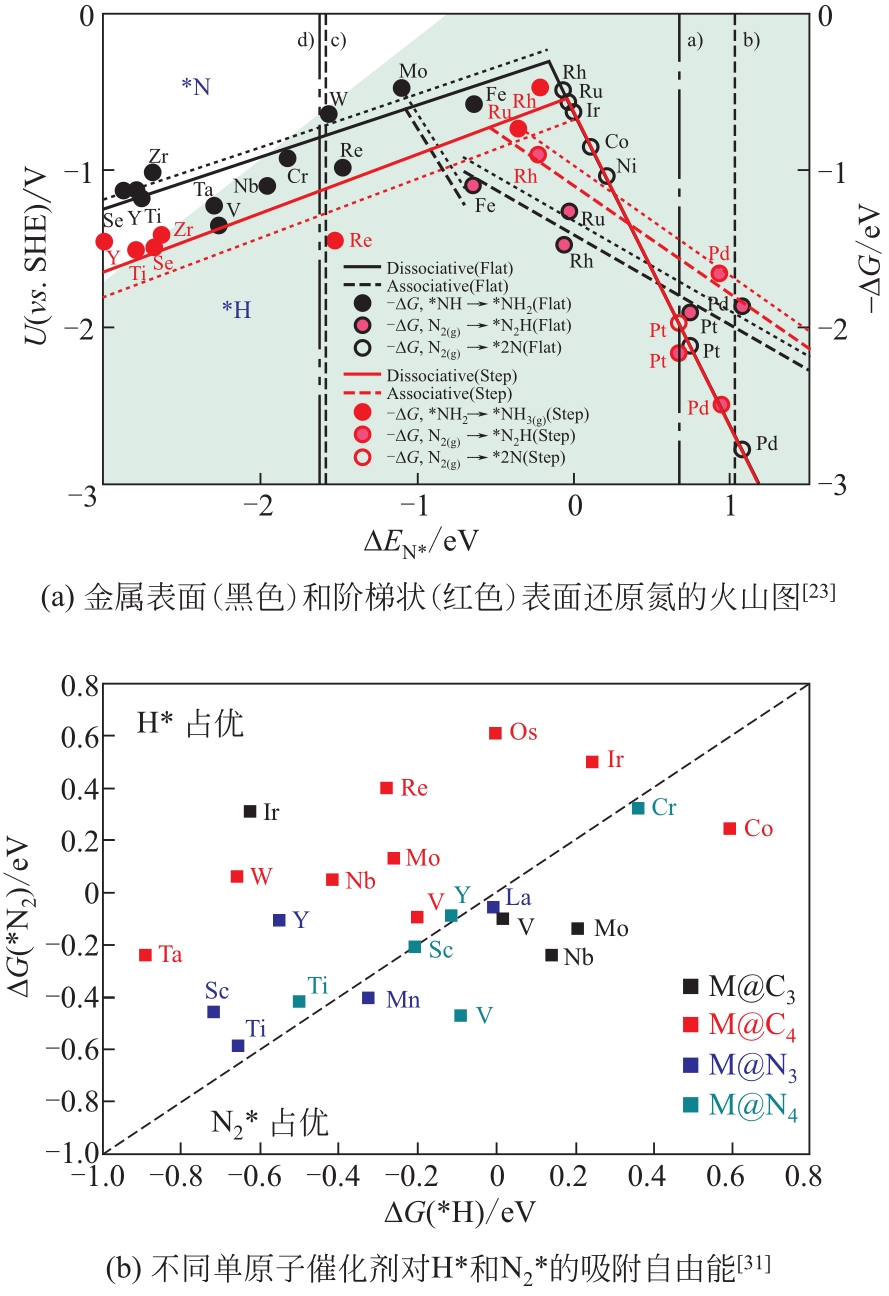
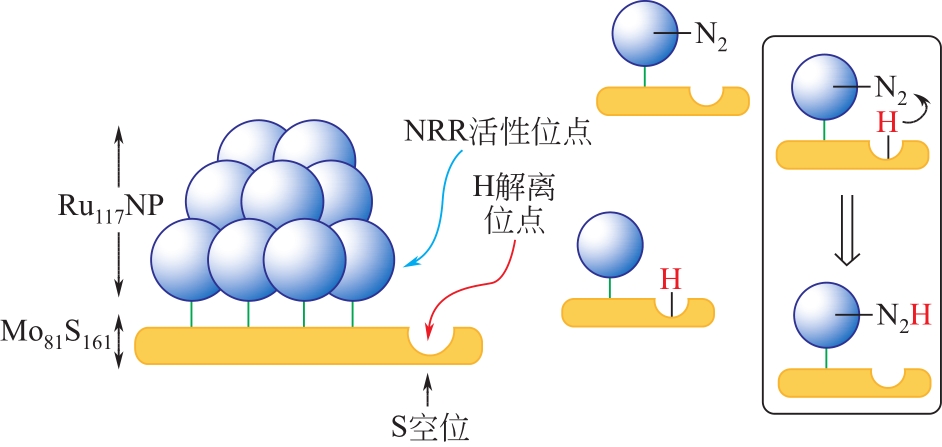
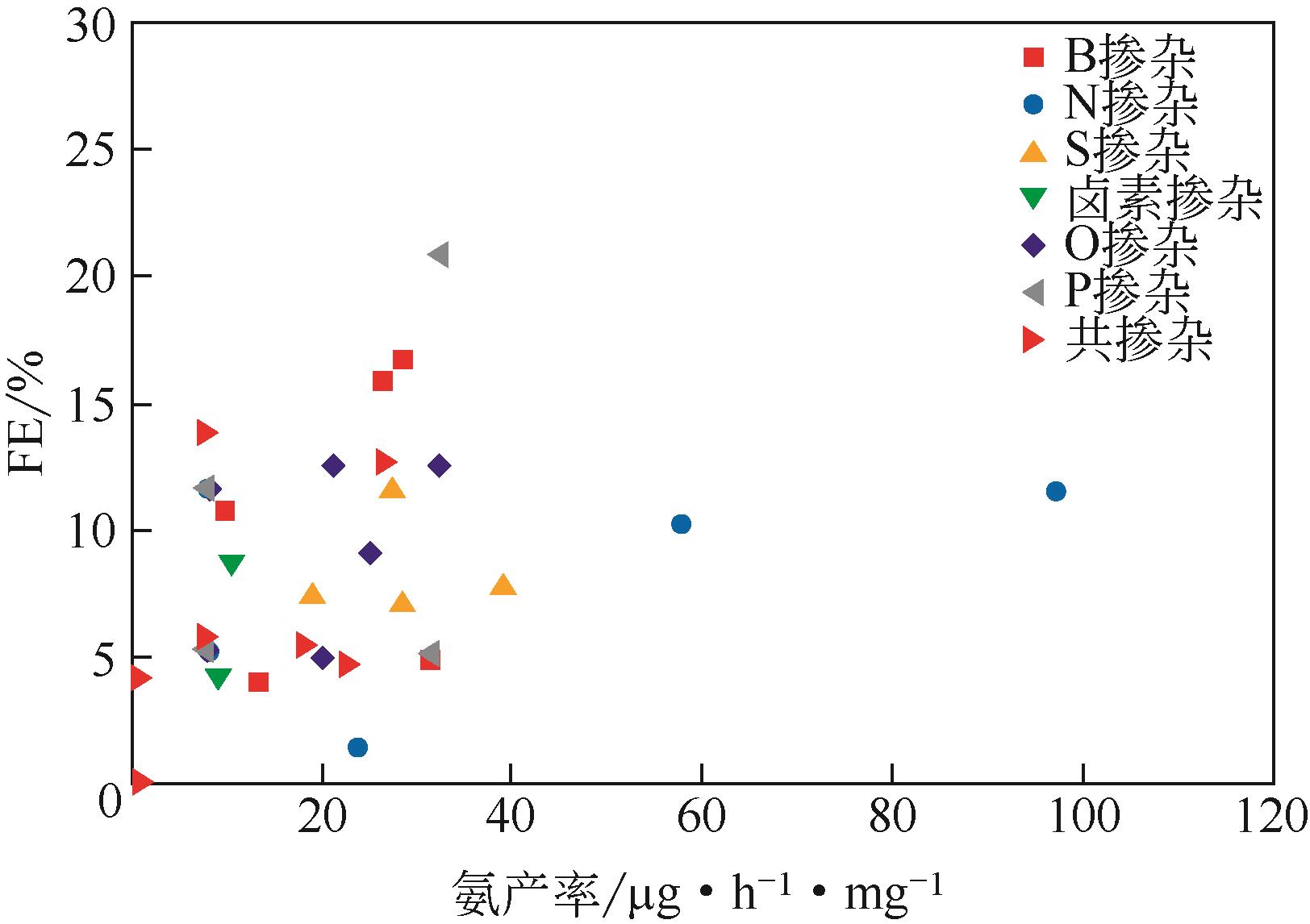
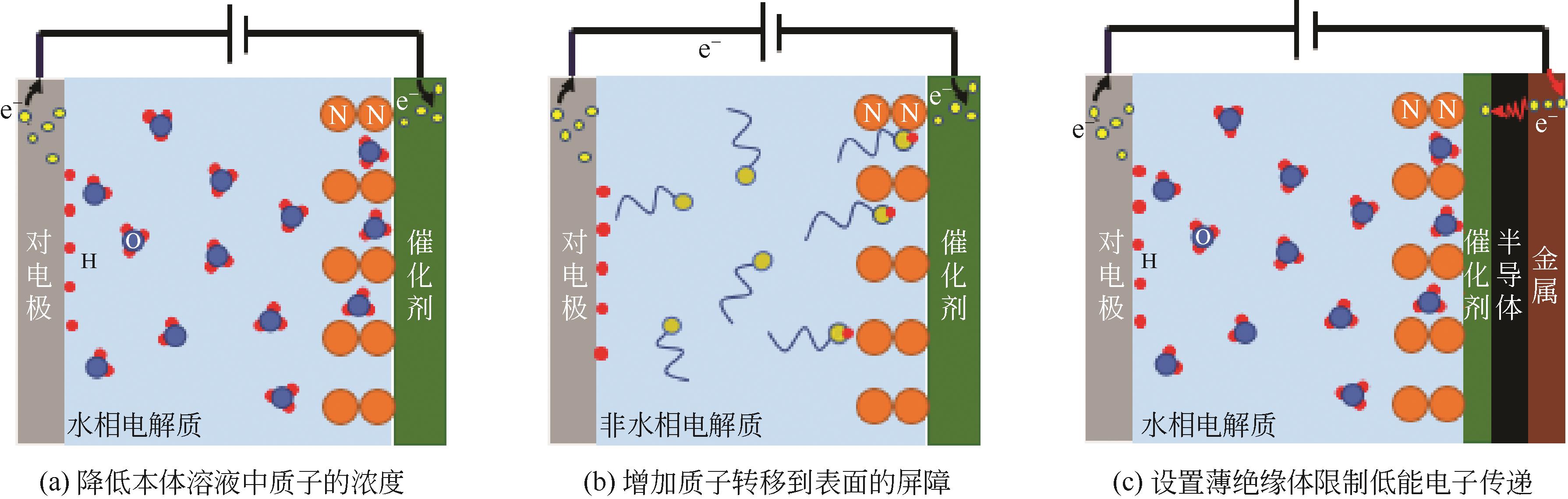
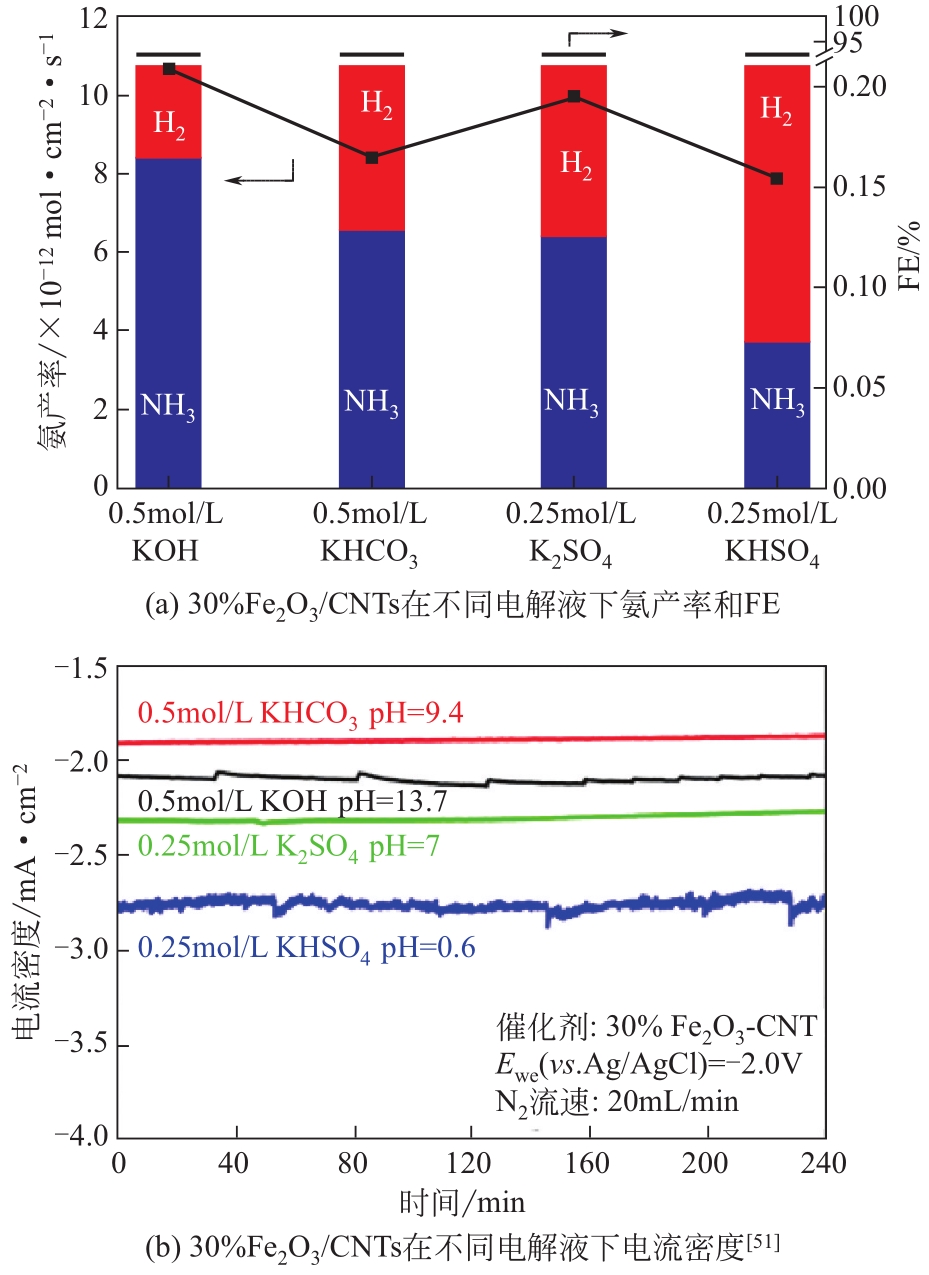
Chemical Industry and Engineering Progress ›› 2021, Vol. 40 ›› Issue (12): 6670-6687.DOI: 10.16085/j.issn.1000-6613.2020-2520
• Industrial catalysis • Previous Articles Next Articles
Research progress of inhibiting hydrogen evolution in electro-catalytic ammonia synthesis
ZHANG Ting( ), SUN Xiaohong, YU Hongbing, DONG Heng(
), SUN Xiaohong, YU Hongbing, DONG Heng( )
)
- College of Environmental Science and Engineering, Nankai University, TianJin 300350, China
-
Received:2020-12-17Revised:2021-03-08Online:2021-12-21Published:2021-12-05 -
Contact:DONG Heng
电催化氮气还原合成氨反应中抑制水解析氢竞争的研究进展
- 南开大学环境科学与工程学院,天津 300350
-
通讯作者:董恒 -
作者简介:张婷(1995—),女,硕士研究生,研究方向为电化学。E-mail:zhangt@nankai.edu.cn 。 -
基金资助:国家自然科学基金(51708300)
CLC Number:
Cite this article
ZHANG Ting, SUN Xiaohong, YU Hongbing, DONG Heng. Research progress of inhibiting hydrogen evolution in electro-catalytic ammonia synthesis[J]. Chemical Industry and Engineering Progress, 2021, 40(12): 6670-6687.
张婷, 孙晓红, 于宏兵, 董恒. 电催化氮气还原合成氨反应中抑制水解析氢竞争的研究进展[J]. 化工进展, 2021, 40(12): 6670-6687.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2020-2520
| 项目 | 反应方程式 | E0(vs. SHE)/V |
|---|---|---|
| R1 | N2+e- | -3.37(pH=0) |
| R2 | N2+H++e- | -3.20(pH=0) |
| R3 | N2+2H++2e- | -1.10(pH=0) |
| R4 | N2+4H++4e- | -0.36(pH=0) |
| R5 | N2+6H++6e- | 0.148(pH=0) |
| R6 | 2H++2e- | 0(pH=0) |
| R7 | N2+6H2O+6e- | -0.763(pH=14) |
| R8 | 2H2O+2e- | -0.828(pH=14) |
| 项目 | 反应方程式 | E0(vs. SHE)/V |
|---|---|---|
| R1 | N2+e- | -3.37(pH=0) |
| R2 | N2+H++e- | -3.20(pH=0) |
| R3 | N2+2H++2e- | -1.10(pH=0) |
| R4 | N2+4H++4e- | -0.36(pH=0) |
| R5 | N2+6H++6e- | 0.148(pH=0) |
| R6 | 2H++2e- | 0(pH=0) |
| R7 | N2+6H2O+6e- | -0.763(pH=14) |
| R8 | 2H2O+2e- | -0.828(pH=14) |
| 催化剂 | 电解液 | 过电位/V | FE/% | 氨产率 | 参考文献 |
|---|---|---|---|---|---|
| 贵金属 | |||||
| Au纳米笼 | 0.5mol/L LiClO4 | -0.4 | 30.2 | 3.9μg?h-1?cm-2 | [ |
| Au纳米棒 | 0.1mol/L KOH | -0.2 | 4 | 1.648μg?h-1?cm-2 | [ |
| Ru纳米粒子 | 0.01mol/L HCl | 0.01 | 5.4 | 约0.2μg?h-1?cm-2 | [ |
| Rh纳米片 | 0.1mol/L KOH | -0.2 | 21.7 | 23.88μg?h-1?cm-2 | [ |
| Pd/C | 0.1mol/L PBS | -0.1 | 8.2 | 4.5μg?h-1?mg-1 | [ |
| Au/TiO2 | 0.1mol/L HCl | -0.2 | 8.11 | 21.4μg?h-1?mg-1 | [ |
| Pt/C | H+/Li+/NH4+ | -1.2 | 0.83 | 47.2μg?h-1?cm-2 | [ |
| 过渡金属及其杂化物 | |||||
| Bi纳米片 | 0.1mol/L Na2SO4 | -0.8 | 10.46 | (2.54±0.16)μg?cm-2?h-1 | [ |
| Bi4V2O11/CeO2 | 0.1mol/L HCl | -0.2 | 10.16 | 23.21μg??h-1?mg-1 | [ |
| Fe3S4纳米片 | 0.1mol/L HCl | -0.4 | 6.45 | 75.4μg?h-1?mg-1 | [ |
| Fe/Fe3O4 | 0.1mol/L PBS | -0.3 | 8.29 | 0.19μg?h-1?cm-2 | [ |
| 30% Fe2O3-CNT | 0.5mol/L KOH | -0.2 | 0.16 | 0.65μg?h-1?cm-2 | [ |
| β-FeOOH | 0.5mol/L LiClO4 | -0.7 | 6.7 | 23.32μg?h-1?mg-1 | [ |
| MoO3纳米片 | 0.1mol/L HCl | -0.3 | 1.9 | 29.43μg?h-1?mg-1 | [ |
| Mo2C纳米棒 | 0.1mol/L HCl | -0.3 | 8.13 | 95.1μg?h-1?mg-1 | [ |
| MoS2 | 0.1mol/L Na2SO4 | -0.5 | 1.17 | 8.08×10-11mol?s-1?cm-2 | [ |
| Mo2N | 0.1mol/L HCl | -0.3 | 4.5 | 78.4μg?h-1?mg-1 | [ |
| MoN | 0.1mol/L HCl | -0.3 | 1.15 | 3.01×10-10mol?s-1?cm-2 | [ |
| MoS2纳米花 | 0.1mol/L Na2SO4 | -0.4 | 8.34 | 29.28μg?h-1?mg-1 | [ |
| Mo2C | 0.5mol/L Li2SO4 | -0.3 | 1.1 | 11.3μg?h-1?mg-1 | [ |
| MoSe2纳米球 | 0.1mol/L Na2SO4 | -0.6 | 14.2 | 11.2μg?h-1?mg-1 | [ |
| SnO2 | 0.1mol/L Na2SO4 | -0.7 | 2.17 | 0.53μmmol?cm-2?h-1 | [ |
| Nb2O5 | 0.1mol/L HCl | -0.55 | 9.26 | 43.6μg?h-1?mg-1 | [ |
| NbO2纳米颗粒 | 0.05mol/L H2SO4 | -0.65 | 32 | 11.6μg?h-1?mg-1 | [ |
| TiO2 | 0.1mol/L HCl | 0.15 | 9.17 | 1.24×10-10mol?s-1?cm-2 | [ |
| Fe-TiO2 | 0.5mol/L LiClO4 | -0.4 | 25.6 | 25.47μg?h-1?mg-1 | [ |
| TiO2/Ti | 0.1mol/L Na2SO4 | -0.7 | 2.5 | 5.60μg?h-1?mg-1 | [ |
| Cr-CeO2 | 0.1mol/L Na2SO4 | -0.4 | 3.7 | 16.4μg?h-1?mg-1 | [ |
| Cu-CeO2 | 0.1mol/L Na2SO4 | -0.45 | 19.1 | 5.3×10-10mol?s-1?cm-2 | [ |
| MnO | 0.1mol/L Na2SO4 | -0.39 | 8.02 | 1.11×10-10mol?s-1?cm-2 | [ |
| WO3纳米片 | 0.1mol/L HCl | -0.3 | 7 | 17.28μg?h-1?mg-1 | [ |
| Cr2O3 | 0.1mol/L Na2SO4 | -0.9 | 6.78 | 25.3μg?h-1?mg-1 | [ |
| W2N3纳米片 | 0.1mol/L KOH | -0.2 | 11.67 | (11.66±0.98)μg?h-1?mg-1 | [ |
| NiN3 | 0.5mol/L LiClO4 | -0.8 | — | 115μg?cm-2?h-1 | [ |
| LaF3 | 0.5mol/L LiClO4 | -0.45 | 16 | 55.9μg?h-1?mg-1 | [ |
| VN/Ti | 0.1mol/L HCl | -0.5 | 2.25 | 5.14μg?h-1?cm-2 | [ |
| VN | 0.05mol/L H2SO4 | -0.1 | 6 | 20.2μg?h-1?cm-2 | [ |
| 单金属原子催化剂(SACs) | |||||
| ISAS-Fe/NC | 0.5mol/L LiSO4 | -0.4 | 18.6 | (62.9±2.7)μg?h-1?mg-1 | [ |
| Cu SAC[N] | 0.1mol/L KOH | -0.35 | 13.8 | 53.3μg?h-1?mg-1 | [ |
| Au/C3N4 | 5mmol/L H2SO4 | -0.1 | 11.1 | 1.3mg?h-1?mg-1 | [ |
| Au-TiO2 | 0.1mol/L HCl | -0.2 | 8.11 | 21.4μg?mg-1?h-1 | [ |
| Ru/NC | 0.1mol/L HCl | -0.21 | 8 | 3.665mg?h-1?mg-1 | [ |
| Ru-ZrO2/NC | 0.1mol/L HCl | -0.1 | 21 | 约1mg?h-1?mg-1 | [ |
| 碳基催化剂 | |||||
| B4C | 0.1mol/L HCl | -0.2 | 10.1 | 26.57μg?h-1?mg-1 | [ |
| B掺杂石墨烯 | 0.05mol/L H2SO4 | -0.75 | 4.83 | 9.8mg?h-1?cm-2 | [ |
| N掺杂多孔碳 | 0.1mol/L KOH | -0.85 | 3.52 | 57.8μg?h-1?cm-2 | [ |
| N掺杂的碳纳米片 | 0.1mol/L HCl | -0.5 | 7.1 | (97.18±7.13)mg?h-1?cm-2 | [ |
| S掺杂三维石墨烯 | 0.1mol/L HCl | -0.85 | 7.07 | 38.81μg?h-1?mg-1 | [ |
| S掺杂碳纳米球 | 0.1mol/L Na2SO4 | -0.5 | 6.9 | 19.07μg?h-1?mg-1 | [ |
| S掺杂石墨烯 | 0.1mol/L HCl | -0.5 | 5.89 | 27.3μg?h-1?mg-1 | [ |
| Cl掺杂石墨 | 0.1mol/L HCl | -0.45 | 8.7 | 10.7μg?h-1cm-2 | [ |
| F掺杂石墨烯 | 0.1mol/L KOH | -0.7 | 4.2 | 9.3μg?h-1?mg-1 | [ |
| F掺杂多孔碳 | 0.1mol/L HCl | -0.2 | 54.8 | 197.7μg?mg-1?h-1 | [ |
| O掺杂石墨烯 | 0.1mol/L HCl | -0.45 | 12.6 | 21.3μg?h-1?mg-1 | [ |
| 黑磷纳米片 | 0.01mol/L HCl | -0.6 | 5.07 | 31.37μg?h-1?mg-1 | [ |
| P掺杂石墨烯 | 0.1mol/L KOH | -0.65 | 20.82 | 32.33μg?h-1?mg-1 | [ |
| N,S-石墨烯 | 0.5mol/L LiClO4 | -0.6 | 5.8 | 7.7μg?h-1?mg-1 | [ |
| 金属+碳基 | |||||
| Au/CeOx-RGO | 0.1mol/L KOH | -0.2 | 10.1 | 24.7μg?h-1?mg-1 | [ |
| FeP2-rGO | 0.1mol/L HCl | -0.4 | 21.99 | 22.13μg?h-1?mg-1 | [ |
| TA-rGO | 0.1mol/L Na2SO4 | -0.75 | 4.83 | 17.02μg?h-1?mg-1 | [ |
| MoS2/石墨烯 | 0.1mol/L LiClO4 | -0.45 | 4.58 | 24.82μg?h-1?mg-1 | [ |
| TiO2-rGO | 0.1mol/L HCl | -0.9 | 3.3 | 15.13μg?h-1?mg-1 | [ |
| Mn3O4-rGO | 0.1mol/L Na2SO4 | -0.85 | 3.52 | 17.4μg?h-1?mg-1 | [ |
| Cr2O3-rGO | 0.1mol/L Na2SO4 | -0.6 | 7.33 | 33.3μg?h-1?mg-1 | [ |
| SnO2/RGO | 0.1mol/L HCl | -0.5 | 7.1 | 25.6μg?h-1?mg-1 | [ |
| 催化剂 | 电解液 | 过电位/V | FE/% | 氨产率 | 参考文献 |
|---|---|---|---|---|---|
| 贵金属 | |||||
| Au纳米笼 | 0.5mol/L LiClO4 | -0.4 | 30.2 | 3.9μg?h-1?cm-2 | [ |
| Au纳米棒 | 0.1mol/L KOH | -0.2 | 4 | 1.648μg?h-1?cm-2 | [ |
| Ru纳米粒子 | 0.01mol/L HCl | 0.01 | 5.4 | 约0.2μg?h-1?cm-2 | [ |
| Rh纳米片 | 0.1mol/L KOH | -0.2 | 21.7 | 23.88μg?h-1?cm-2 | [ |
| Pd/C | 0.1mol/L PBS | -0.1 | 8.2 | 4.5μg?h-1?mg-1 | [ |
| Au/TiO2 | 0.1mol/L HCl | -0.2 | 8.11 | 21.4μg?h-1?mg-1 | [ |
| Pt/C | H+/Li+/NH4+ | -1.2 | 0.83 | 47.2μg?h-1?cm-2 | [ |
| 过渡金属及其杂化物 | |||||
| Bi纳米片 | 0.1mol/L Na2SO4 | -0.8 | 10.46 | (2.54±0.16)μg?cm-2?h-1 | [ |
| Bi4V2O11/CeO2 | 0.1mol/L HCl | -0.2 | 10.16 | 23.21μg??h-1?mg-1 | [ |
| Fe3S4纳米片 | 0.1mol/L HCl | -0.4 | 6.45 | 75.4μg?h-1?mg-1 | [ |
| Fe/Fe3O4 | 0.1mol/L PBS | -0.3 | 8.29 | 0.19μg?h-1?cm-2 | [ |
| 30% Fe2O3-CNT | 0.5mol/L KOH | -0.2 | 0.16 | 0.65μg?h-1?cm-2 | [ |
| β-FeOOH | 0.5mol/L LiClO4 | -0.7 | 6.7 | 23.32μg?h-1?mg-1 | [ |
| MoO3纳米片 | 0.1mol/L HCl | -0.3 | 1.9 | 29.43μg?h-1?mg-1 | [ |
| Mo2C纳米棒 | 0.1mol/L HCl | -0.3 | 8.13 | 95.1μg?h-1?mg-1 | [ |
| MoS2 | 0.1mol/L Na2SO4 | -0.5 | 1.17 | 8.08×10-11mol?s-1?cm-2 | [ |
| Mo2N | 0.1mol/L HCl | -0.3 | 4.5 | 78.4μg?h-1?mg-1 | [ |
| MoN | 0.1mol/L HCl | -0.3 | 1.15 | 3.01×10-10mol?s-1?cm-2 | [ |
| MoS2纳米花 | 0.1mol/L Na2SO4 | -0.4 | 8.34 | 29.28μg?h-1?mg-1 | [ |
| Mo2C | 0.5mol/L Li2SO4 | -0.3 | 1.1 | 11.3μg?h-1?mg-1 | [ |
| MoSe2纳米球 | 0.1mol/L Na2SO4 | -0.6 | 14.2 | 11.2μg?h-1?mg-1 | [ |
| SnO2 | 0.1mol/L Na2SO4 | -0.7 | 2.17 | 0.53μmmol?cm-2?h-1 | [ |
| Nb2O5 | 0.1mol/L HCl | -0.55 | 9.26 | 43.6μg?h-1?mg-1 | [ |
| NbO2纳米颗粒 | 0.05mol/L H2SO4 | -0.65 | 32 | 11.6μg?h-1?mg-1 | [ |
| TiO2 | 0.1mol/L HCl | 0.15 | 9.17 | 1.24×10-10mol?s-1?cm-2 | [ |
| Fe-TiO2 | 0.5mol/L LiClO4 | -0.4 | 25.6 | 25.47μg?h-1?mg-1 | [ |
| TiO2/Ti | 0.1mol/L Na2SO4 | -0.7 | 2.5 | 5.60μg?h-1?mg-1 | [ |
| Cr-CeO2 | 0.1mol/L Na2SO4 | -0.4 | 3.7 | 16.4μg?h-1?mg-1 | [ |
| Cu-CeO2 | 0.1mol/L Na2SO4 | -0.45 | 19.1 | 5.3×10-10mol?s-1?cm-2 | [ |
| MnO | 0.1mol/L Na2SO4 | -0.39 | 8.02 | 1.11×10-10mol?s-1?cm-2 | [ |
| WO3纳米片 | 0.1mol/L HCl | -0.3 | 7 | 17.28μg?h-1?mg-1 | [ |
| Cr2O3 | 0.1mol/L Na2SO4 | -0.9 | 6.78 | 25.3μg?h-1?mg-1 | [ |
| W2N3纳米片 | 0.1mol/L KOH | -0.2 | 11.67 | (11.66±0.98)μg?h-1?mg-1 | [ |
| NiN3 | 0.5mol/L LiClO4 | -0.8 | — | 115μg?cm-2?h-1 | [ |
| LaF3 | 0.5mol/L LiClO4 | -0.45 | 16 | 55.9μg?h-1?mg-1 | [ |
| VN/Ti | 0.1mol/L HCl | -0.5 | 2.25 | 5.14μg?h-1?cm-2 | [ |
| VN | 0.05mol/L H2SO4 | -0.1 | 6 | 20.2μg?h-1?cm-2 | [ |
| 单金属原子催化剂(SACs) | |||||
| ISAS-Fe/NC | 0.5mol/L LiSO4 | -0.4 | 18.6 | (62.9±2.7)μg?h-1?mg-1 | [ |
| Cu SAC[N] | 0.1mol/L KOH | -0.35 | 13.8 | 53.3μg?h-1?mg-1 | [ |
| Au/C3N4 | 5mmol/L H2SO4 | -0.1 | 11.1 | 1.3mg?h-1?mg-1 | [ |
| Au-TiO2 | 0.1mol/L HCl | -0.2 | 8.11 | 21.4μg?mg-1?h-1 | [ |
| Ru/NC | 0.1mol/L HCl | -0.21 | 8 | 3.665mg?h-1?mg-1 | [ |
| Ru-ZrO2/NC | 0.1mol/L HCl | -0.1 | 21 | 约1mg?h-1?mg-1 | [ |
| 碳基催化剂 | |||||
| B4C | 0.1mol/L HCl | -0.2 | 10.1 | 26.57μg?h-1?mg-1 | [ |
| B掺杂石墨烯 | 0.05mol/L H2SO4 | -0.75 | 4.83 | 9.8mg?h-1?cm-2 | [ |
| N掺杂多孔碳 | 0.1mol/L KOH | -0.85 | 3.52 | 57.8μg?h-1?cm-2 | [ |
| N掺杂的碳纳米片 | 0.1mol/L HCl | -0.5 | 7.1 | (97.18±7.13)mg?h-1?cm-2 | [ |
| S掺杂三维石墨烯 | 0.1mol/L HCl | -0.85 | 7.07 | 38.81μg?h-1?mg-1 | [ |
| S掺杂碳纳米球 | 0.1mol/L Na2SO4 | -0.5 | 6.9 | 19.07μg?h-1?mg-1 | [ |
| S掺杂石墨烯 | 0.1mol/L HCl | -0.5 | 5.89 | 27.3μg?h-1?mg-1 | [ |
| Cl掺杂石墨 | 0.1mol/L HCl | -0.45 | 8.7 | 10.7μg?h-1cm-2 | [ |
| F掺杂石墨烯 | 0.1mol/L KOH | -0.7 | 4.2 | 9.3μg?h-1?mg-1 | [ |
| F掺杂多孔碳 | 0.1mol/L HCl | -0.2 | 54.8 | 197.7μg?mg-1?h-1 | [ |
| O掺杂石墨烯 | 0.1mol/L HCl | -0.45 | 12.6 | 21.3μg?h-1?mg-1 | [ |
| 黑磷纳米片 | 0.01mol/L HCl | -0.6 | 5.07 | 31.37μg?h-1?mg-1 | [ |
| P掺杂石墨烯 | 0.1mol/L KOH | -0.65 | 20.82 | 32.33μg?h-1?mg-1 | [ |
| N,S-石墨烯 | 0.5mol/L LiClO4 | -0.6 | 5.8 | 7.7μg?h-1?mg-1 | [ |
| 金属+碳基 | |||||
| Au/CeOx-RGO | 0.1mol/L KOH | -0.2 | 10.1 | 24.7μg?h-1?mg-1 | [ |
| FeP2-rGO | 0.1mol/L HCl | -0.4 | 21.99 | 22.13μg?h-1?mg-1 | [ |
| TA-rGO | 0.1mol/L Na2SO4 | -0.75 | 4.83 | 17.02μg?h-1?mg-1 | [ |
| MoS2/石墨烯 | 0.1mol/L LiClO4 | -0.45 | 4.58 | 24.82μg?h-1?mg-1 | [ |
| TiO2-rGO | 0.1mol/L HCl | -0.9 | 3.3 | 15.13μg?h-1?mg-1 | [ |
| Mn3O4-rGO | 0.1mol/L Na2SO4 | -0.85 | 3.52 | 17.4μg?h-1?mg-1 | [ |
| Cr2O3-rGO | 0.1mol/L Na2SO4 | -0.6 | 7.33 | 33.3μg?h-1?mg-1 | [ |
| SnO2/RGO | 0.1mol/L HCl | -0.5 | 7.1 | 25.6μg?h-1?mg-1 | [ |
| 1 | SHIPMAN M A, SYMES M D. Recent progress towards the electrosynthesis of ammonia from sustainable resources[J]. Catalysis Today, 2017, 286(1): 57-68. |
| 2 | Xianwei LYU, WENG Chenchen, YUAN Zhongyong. Ambient ammonia electrosynthesis: current status, challenges, and perspectives[J]. ChemSusChem, 2020, 13(12): 3061-3078. |
| 3 | PICKETT C J, TALARMIN J. Electrosynthesis of ammonia[J]. Nature, 1985, 317(6038): 652-653. |
| 4 | FURUYA N, YOSHIBA H. Electroreduction of nitrogen to ammonia on gas-diffusion electrodes loaded with inorganic catalyst[J]. Journal of Electroanalytical Chemistry & Interfacial Electrochemistry, 1990, 291(1/2): 269-272. |
| 5 | BAO Di, ZHANG Qi, MENG Fanlu, et al. Electrochemical reduction of N2 under ambient conditions for artificial N2 fixation and renewable energy storage using N2/NH3 cycle[J]. Advanced Materials, 2017, 29(3): 1604799-1604803. |
| 6 | WANG Ziqiang, LI Yinghao, YU Hongjie, et al. Ambient electrochemical synthesis of ammonia from nitrogen and water catalyzed by flower-like gold microstructures[J]. ChemSusChem, 2018, 17(32): 3480-3485. |
| 7 | WANG D B, AZOFRA L M, HARB M, et al. Energy efficient nitrogen reduction to ammonia at low overpotential in aqueous electrolyte under ambient conditions[J]. ChemSusChem, 2018, 11(24): 3416-3422. |
| 8 | KORDALI V, KYRIACOU G, LAMBROU C. Electrochemical synthesis of ammonia at atmospheric pressure and low temperature in a solid polymer electrolyte cell[J]. Chemical Communications, 2000, 17: 1673-1684. |
| 9 | MANJUNATHA R, SCHECHTER A. Electrochemical synthesis of ammonia using ruthenium-platinum alloy at ambient pressure and low temperature[J]. Electrochemistry Communications, 2018, 90(2): 96-100. |
| 10 | LIU Huimin, HAN Shuhe, ZHAO Yue, et al. Surfactant-free atomically ultrathin rhodium nanosheet nanoassemblies for efficient nitrogen electroreduction[J]. Journal of Materials Chemistry A, 2018, 6(7): 3211-3217. |
| 11 | WANG Jun, YU Liang, HU Lin, et al. Ambient ammonia synthesis via palladium-catalyzed electrohydrogenation of dinitrogen at low overpotential[J]. Nature Communications, 2018, 9(1): 1795-1806. |
| 12 | WANG Hong, WANG Lu, WANG Qiang, et al. Ambient electrosynthesis of ammonia: electrode porosity and composition engineering[J]. Angew. Chem. Int. Ed., 2018, 57(38): 12360-12364. |
| 13 | ZHANG Shengbo, ZHAO Cuijiao, LIU Yanyan, et al. Cu doping in CeO2 to form multiple oxygen vacancies for dramatically enhanced ambient N2 reduction performance[J]. Chem. Commun., 2019, 55(20): 2952-2955. |
| 14 | XU Bo, XIA Li, ZHOU Fuling, et al. Enhancing electrocatalytic N2 reduction to NH3 by CeO2 nanorod with oxygen vacancies[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(3): 2889-2893. |
| 15 | HAN Jingrui, JI Xuqiang, REN Xiang, et al. MoO3 nanosheets for efficient electrocatalytic N2 fixation to NH3[J]. Journal of Materials Chemistry A, 2018, 6(27): 12974-12977. |
| 16 | KONG Wenhan, ZHANG Rong, ZHANG Xiaoxue, et al. WO3 nanosheets rich in oxygen vacancies for enhanced electrocatalytic N2 reduction to NH3[J]. Nanoscale, 2019, 11(41): 19274-19277. |
| 17 | Fang LYU, ZHAO Shunzheng, GUO Ruijie, et al. Nitrogen-coordinated single Fe sites for efficient electrocatalytic N2 fixation in neutral media[J]. Nano Energy, 2019, 61(13): 420-427. |
| 18 | ZANG Wenjie, YANG Tong, ZOU Haiyuan, et al. Copper single atoms anchored in porous nitrogen-doped carbon as efficient pH-universal catalysts for the nitrogen reduction reaction[J]. ACS Catalysis, 2019, 2019(38): 2944-2956. |
| 19 | WANG Xiaoqian, WANG Wenyu, QIAO Man, et al. Atomically dispersed Au1 catalyst towards efficient electrochemical synthesis of ammonia[J]. Science Bulletin, 2018, 63(19): 1246-1253. |
| 20 | ZHAO Jingxiang, CHEN Zhongfang. Single Mo atom supported on defective boron nitride monolayer as an efficient electrocatalyst for nitrogen fixation: a computational study[J]. J. Am. Chem. Soc., 2017, 139(36): 12480-12487. |
| 21 | LING Chongyi, BAI Xiaowan, YANG Yixin. et al. Single molybdenum atom anchored on N-doped carbon as a promising electrocatalyst for nitrogen reduction into ammonia at ambient conditions[J]. The Journal of Physical Chemistry C, 2018, 122(29): 16842-16847. |
| 22 | TANG Cheng, QIAO Shizhang. How to explore ambient electrocatalytic nitrogen reduction reliably and insightfully[J]. Chemical Society Reviews, 2019, 48(23): 2366-2380. |
| 23 | SKULASON E, BLIGAARD T, GUDMUNDSDOTTIR S, et al. A theoretical evaluation of possible transition metal electro-catalysts for N2 reduction[J]. Physical Chemistry Chemical Physics, 2012, 14(3): 1235-1245. |
| 24 | SHI Li, LI Qiang, LING Chongyi, et al. Metal-free electrocatalyst for reducing nitrogen to ammonia using a Lewis acid pair[J]. Journal of Materials Chemistry A, 2019, 7(9): 4865-4871. |
| 25 | LÉGARÉ M A, BÉLANGER-CHABOT G, DEWHURST R D, et al. Nitrogen fixation and reduction at boron[J]. Science, 2018, 359(12): 896-900. |
| 26 | LING Chongyi, NIU Xianghong, LI Qiang, et al. Metal-free single atom catalyst for N2 fixation driven by visible light[J]. Journal of the American Chemical Society, 2018, 140(43): 14161-14168. |
| 27 | GUO Wenhan, ZHANG Kexin, LIANG Zibin, et al. Electrochemical nitrogen fixation and utilization: theories, advanced catalyst materials and system design[J]. Chem. Soc. Rev., 2019, 48(24): 5658-5716. |
| 28 | YANG Xuan, NASH Jared, ANIBAL Jacob, et al. Mechanistic insights into electrochemical nitrogen reduction reaction on vanadium nitride nanoparticles[J]. Journal of the American Chemical Society, 2018, 140(41): 13387-13391. |
| 29 | ABGHOUI Y, SKÚLASON E. Onset potentials for different reaction mechanisms of nitrogen activation to ammonia on transition metal nitride electro-catalysts[J]. Catalysis Today, 2017, 286(2): 69-77. |
| 30 | SINGH A R, ROHR B A, SCHWALBE J A, et al. Electrochemical ammonia synthesis—the selectivity challenge[J]. ACS Catalysis, 2016, 7(1): 706-709. |
| 31 | CHOI Changhyeok, BACK S, KIM N, et al. Suppression of hydrogen evolution reaction in electrochemical N2 reduction using single-atom catalysts: a computational guideline[J]. ACS Catalysis, 2018, 8(8): 7517-7525. |
| 32 | MARTÍN A J, SHINAGAWA T, PÉREZ-RAMÍREZ J. Electrocatalytic reduction of nitrogen: from haber-bosch to ammonia artificial leaf[J]. Chem, 2019, 5(2): 263-283. |
| 33 | NASH J, YANG Xuan, ANIBAL Jaced, et al. Electrochemical nitrogen reduction reaction on noble metal catalysts in proton and hydroxide exchange membrane electrolyzers[J]. Journal of the Electrochemical Society, 2017, 164(14): F1712-F1716. |
| 34 | HOWALT J G, VEGGE T. Electrochemical ammonia production on molybdenum nitride nanoclusters[J]. Physical Chemistry Chemical Physics, 2013, 15(48): 20957-20965. |
| 35 | CHU Ke, LIU Yaping, LI Yubiao, et al. Electronically coupled SnO2 quantum dots and graphene for efficient nitrogen reduction reaction[J]. ACS Applied Materials & Interfaces, 2019, 11(35): 758-763. |
| 36 | ZHANG Ling, REN Xiang, LUO Yonglan, et al. Ambient NH3 synthesis via electrochemical reduction of N2 over cubic sub-micron SnO2 particles[J]. Chemical Communications, 2018, 54(92): 12966-12969. |
| 37 | CHU Ke, LIU Yaping, LI Yubiao, et al. Efficient electrocatalytic N2 reduction on CoO quantum dots[J]. Journal of Materials Chemistry A, 2019, 7(9): 4389-4394. |
| 38 | HAN Jingrui, LIU Zaichun, MA Yongjun, et al. Ambient N2 fixation to NH3 at ambient conditions: using Nb2O5 nanofiber as a high-performance electrocatalyst[J]. Nano Energy, 2018, 52(15): 264-270. |
| 39 | ZHU Xiaojuan, LIU Zaichun, LIU Qin, et al. Efficient and durable N2 reduction electrocatalysis under ambient conditions: beta-FeOOH nanorods as a non-noble-metal catalyst[J]. Chemical Communications, 2018, 54(80): 11332-11335. |
| 40 | LI Laiquan, TANG Cheng, XIA Bingquan, et al. Two-dimensional mosaic bismuth nanosheets for highly selective ambient electrocatalytic nitrogen reduction[J]. ACS Catalysis, 2019, 9(4): 2902-2908. |
| 41 | QIN Qing, ZHAO Yun, SCHMALLEGGER Max, et al. Enhanced electrocatalytic N2 reduction via partial anion substitutionin titanium oxide-carbon composites[J]. Angewandte Chemie, 2019, 131(37): 13235-13240. |
| 42 | ZHAO Lu, KUANG Xuan, CHEN Cheng, et al. Boosting electrocatalytic nitrogen fixation via energy-efficient anodic oxidation of sodium gluconate[J]. Chemical Communications, 2019, 55(68): 10170-10173. |
| 43 | SONG Zhongxin, ZHANG Lei, Kieran DOYLE-DAVIS, et al. Recent advances in MOF-derived single atom catalysts for electrochemical applications[J]. Advanced Energy Materials, 2020, 10(38): 2001561-2001567. |
| 44 | ZHANG Ya, QIU Weibin, MA Yongjun, et al. High-performance electrohydrogenation of N2 to NH3 catalyzed by multishelled hollow Cr2O3 microspheres under ambient conditions[J]. ACS Catalysis, 2018, 8(9): 8540-8544. |
| 45 | NAZEMI M, PANIKKANVALAPPIL S R, EL-SAYED M A. Enhancing the rate of electrochemical nitrogen reduction reaction for ammonia synthesis under ambient conditions using hollow gold nanocages[J]. Nano Energy, 2018, 49(39): 316-323. |
| 46 | YANG Liuxin, WANG Hui, WANG Xin, et al. Flower-like hollow MoSe2 nanospheres as efficient earth-abundant electrocatalysts for nitrogen reduction reaction under ambient conditions[J]. Inorg. Chem., 2020, 59(17): 12941-12946. |
| 47 | WAN Yuchi, XU Jichu, Rutiao LYU. Heterogeneous electrocatalysts design for nitrogen reduction reaction under ambient conditions[J]. Materials Today, 2019, 27(1): 69-90. |
| 48 | ZHANG Rong, REN Xiang, SHI Xifeng, et al. Enabling effective electrocatalytic N2 conversion to NH3 by the TiO2 nanosheets array under ambient conditions[J]. ACS Appl. Mater. Interfaces, 2018, 10(34): 28251-28255. |
| 49 | ZHANG Ling, XIE Xiaoying, WANG Huanbo, et al. Boosting electrocatalytic N2 reduction by MnO2 with oxygen vacancies[J]. Chemical Communications, 2019, 55(32): 4627-4630. |
| 50 | LI Lei, WANG Xingyong, GUO Haoran, et al. Theoretical screening of single transition metal atoms embedded in MXene defects as superior electrocatalyst of nitrogen reduction reaction[J]. Small Methods, 2019, 3(11): 1900337-1900342. |
| 51 | CHEN Shiming, PERATHONER Siglinda, AMPELLI Claudio, et al. Room-temperature electrocatalytic synthesis of NH3 from H2O and N2 in a gas-liquid-solid three-phase reactor[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(8): 7393-7400. |
| 52 | LYU C, YAN Chunshuang, CHEN Gang, et al. An amorphous noble-metal-free electrocatalyst that enables nitrogen fixation under ambient conditions[J]. Angewandte Chemie International Edition, 2018, 57(21): 6073-6076. |
| 53 | LI Hong, TSAI Charlie, Ai Leen KOH, et al. Activating and optimizing MoS2 basal planes for hydrogen evolution through the formation of strained sulphur vacancies[J]. Nature Materials, 2016, 15(1): 48-53. |
| 54 | SURYANTO B H R, WANG Dabin, AZOFRA L M, et al. MoS2 polymorphic engineering enhances selectivity in the electrochemical reduction of nitrogen to ammonia[J]. ACS Energy Letters, 2018, 4(2): 430-435. |
| 55 | LYU C, QIAN Yumin, YAN Chunshuang, et al. Defect engineering metal-free polymeric carbon nitride electrocatalyst for effective nitrogen fixation under ambient conditions[J]. Angewandte Chemie International Edition, 2018, 57(32): 10246-10250. |
| 56 | JIN Huanyu, LI Laiquan, LIU Xin, et al. Nitrogen vacancies on 2D layered W2N3: a stable and efficient active site for nitrogen reduction reaction[J]. Adv. Mater., 2019, 31(32): 1902709-1902716. |
| 57 | ZHAO Shenlong, LU Xunyu, WANG Lianzhou, et al. Carbon-based metal-free catalysts for electrocatalytic reduction of nitrogen for synthesis of ammonia at ambient conditions[J]. Adv. Mater., 2019, 31(13): 1805367-1805374. |
| 58 | ZHANG Xiaoxue, WU Tongwei, WANG Huanbo, et al. Boron nanosheet: an elemental two-dimensional (2D) material for ambient electrocatalytic N2-to-NH3 fixation in neutral media[J]. ACS Catalysis, 2019, 9(5): 4609-4615. |
| 59 | CHEN Chen, YAN Dafang, WANG Yu, et al. B-N pairs enriched defective carbon nanosheets for ammonia synthesis with high efficiency[J]. Small, 2019, 15(7): e1805029. |
| 60 | YU Xiaomin, HAN Peng, WEI Zengxi, et al. Boron-doped graphene for electrocatalytic N2 reduction[J]. Joule, 2018, 2(8): 1610-1622. |
| 61 | LIU Yanming, SU Yan, QUAN Xie, et al. Facile ammonia synthesis from electrocatalytic N2 reduction under ambient conditions on N-doped porous carbon[J]. ACS Catalysis, 2018, 8(2): 1186-1191. |
| 62 | CHEN Hongyu, ZHU Xiaojuan, HUANG Hong, et al. Sulfur dots-graphene nanohybrid: a metal-free electrocatalyst for efficient N2-to-NH3 fixation under ambient conditions[J]. Chemical Communications, 2019, 55(21): 3152-3155. |
| 63 | XIA Li, YANG Jiajia, WANG Huanbo, et al. Sulfur-doped graphene for efficient electrocatalytic N2-to-NH3 fixation[J]. Chemical Communications, 2019, 55(23): 3371-3374. |
| 64 | WU Tongwei, LI Xinyi, ZHU Xiaojuan, et al. P-doped graphene toward enhanced electrocatalytic N2 reduction[J]. Chemical Communications, 2020, 56(12): 1831-1834. |
| 65 | ZHANG Rong, JI Ling, KONG Wang, et al. Electrocatalytic N2-to-NH3 conversion with high faradaic efficiency enabled using a Bi nanosheet array[J]. Chemical Communications, 2019, 55(36): 5263-5266. |
| 66 | WANG Ting, XIA Li, YANG Jiajia, et al. Electrocatalytic N2-to-NH3 conversion using oxygen-doped graphene: experimental and theoretical studies[J]. Chemical Communications, 2019, 55(52): 7502-7505. |
| 67 | LIU Yan, LI Qiuyao, GUO Xu, et al. A highly efficient metal-free electrocatalyst of F-doped porous carbon toward N2 electroreduction[J]. Adv. Mater., 2020, 32(24): 1907690-1907695. |
| 68 | ZOU Haiyuan, RONG Weifeng, LONG Baihua, et al. Corrosion-induced Cl-doped ultrathin graphdiyne toward electrocatalytic nitrogen reduction at ambient conditions[J]. ACS Catalysis, 2019, 9(12): 10649-10655. |
| 69 | SHI Miaomiao, BAO Di, WULAN B, et al. Au sub-nanoclusters on TiO2 toward highly efficient and selective electrocatalyst for N2 conversion to NH3 at ambient conditions[J]. Advanced Materials, 2017, 29(17): 1606550-1606555. |
| 70 | LAN Rong, IRVINE J T S, TAO Shanwen. Synthesis of ammonia directly from air and water at ambient temperature and pressure[J]. Sci. Rep., 2013, 3(1): 1-7. |
| 71 | ZHAO Xinhui, LAN Xue, YU Dongkun, et al. Deep eutectic-solvothermal synthesis of nanostructured Fe3S4 for electrochemical N2 fixation under ambient conditions[J]. Chemical Communications, 2018, 54(92): 13010-13013. |
| 72 | HU Lin, KHANIYA Asim, WANG Jun, et al. Ambient electrochemical ammonia synthesis with high selectivity on Fe/Fe oxide catalyst[J]. ACS Catalysis, 2018, 8(10): 9312-9319. |
| 73 | REN Xiang, ZHAO Jinxiu, WEI Qin, et al. High-performance N2-to-NH3 conversion electrocatalyzed by Mo2C nanorod[J]. ACS Cent. Sci., 2019, 5(1): 116-121. |
| 74 | ZHANG Ling, JI Xuqiang, REN Xiang, et al. Electrochemical ammonia synthesis via nitrogen reduction reaction on a MoS2 catalyst: theoretical and experimental studies[J]. Adv. Mater., 2018, 30(28): e1800191. |
| 75 | REN Xiang, CUI Guanwei, CHEN Liang, et al. Electrochemical N2 fixation to NH3 under ambient conditions: Mo2N nanorod as a highly efficient and selective catalyst[J]. Chemical Communications, 2018, 54(61): 8474-8477. |
| 76 | ZHANG Ling, JI Xuqiang, REN Xiang, et al. Efficient electrochemical N2 reduction to NH3 on MoN nanosheets array under ambient conditions[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(8): 9550-9554. |
| 77 | CHENG Hui, DING Liangxin, CHEN Gaofeng, et al. Molybdenum carbide nanodots enable efficient electrocatalytic nitrogen fixation under ambient conditions[J]. Adv. Mater., 2018, 30(46): 1803694-1803697. |
| 78 | HUANG Linsong, WU Jiawen, HAN Peng,et al. NbO2 electrocatalyst toward 32% faradaic efficiency for N2 fixation[J]. Small Methods, 2019, 3(6): 1800386. |
| 79 | YANG Li, WU Tongwei, ZHANG Rong, et al. Insights into defective TiO2 in electrocatalytic N2 reduction: combining theoretical and experimental studies[J]. Nanoscale, 2019, 11(4): 1555-1562. |
| 80 | WU Tongwei, ZHU Xiaojuan, XING Zhe, et al. Greatly improving electrochemical N2 reduction over TiO2 nanoparticles by iron doping[J]. Angewandte Chemie, 2019, 58(51): 18449-18453. |
| 81 | WANG Zao, GONG Feng, ZHANG Ling, et al. Electrocatalytic hydrogenation of N2 to NH3 by MnO: experimental and theoretical investigations[J]. Adv. Sci., 2019, 6(1): 1801182-1801190. |
| 82 | HAN Lili, LIU Xijun, CHEN Jinping, et al. Atomically dispersed molybdenum catalysts for efficient ambient nitrogen fixation[J]. Angewandte Chemie International Edition, 2019, 58(8): 2321-2325. |
| 83 | LI Peipei, LIU Zaichun, WU Tongwei, et al. Ambient electrocatalytic N2 reduction to NH3 by metal fluorides[J]. Journal of Materials Chemistry A, 2019, 7(30): 17761-17765. |
| 84 | TAO Hengcong, CHOI Changhyeok, DING Liangxin, et al. Nitrogen fixation by Ru single-atom electrocatalytic reduction[J]. Chem, 2019, 5(1): 204-214. |
| 85 | TAO Huan, LI Na, ZHANG Zhao, et al. Erlotinib protects LPS-induced acute lung injury in mice by inhibiting EGFR/TLR4 signaling pathway[J]. Shock, 2019, 51(1): 131-138. |
| 86 | ZHANG Ya, QIU Weibin, Ma Yongjun, et al. High-performance artificial nitrogen fixation at ambient conditions using a metal-free electrocatalyst[J]. Nature Communications, 2018, 9(2): 3485. |
| 87 | MUKHERJEE S, CULLEN D A, KARAKALOS Stavros, et al. Metal-organic framework-derived nitrogen-doped highly disordered carbon for electrochemical ammonia synthesis using N2 and H2O in alkaline electrolytes[J]. Nano Energy, 2018, 48(5): 217-226. |
| 88 | SONG Yang, JOHNSON D, PENG Rui, et al. A physical catalyst for the electrolysis of nitrogen to ammonia[J]. Science Advances, 2018, 4(4): e1700336. |
| 89 | ZHANG Lipeng, NIU Jianbing, LI Mingtao, et al. Catalytic mechanisms of sulfur-doped graphene as efficient oxygen reduction reaction catalysts for fuel cells[J]. The Journal of Physical Chemistry C, 2014, 118(7): 3545-3553. |
| 90 | LIU Lin, LIU Yuhong, LIU Chaoxiang, et al. Potential effect and accumulation of veterinary antibiotics in Phragmites australis under hydroponic conditions[J]. Ecological Engineering, 2013, 53(1):138-143. |
| 91 | ZHAO Jinxiu, YANG Jiajia, LEI Ji, et al. Defect-rich fluorographene nanosheets for artificial N2 fixation under ambient conditions[J]. Chemical Communications, 2019, 55(29): 4266-4269. |
| 92 | LIU Yan, LI Qiuyao, GUO Xu, et al. A highly efficient metal free electrocatalyst of F doped porous carbon toward N2 electroreduction[J]. Advanced Materials, 2020, 32(24): 1907690. |
| 93 | ZHANG Lili, DING LiangXin, CHEN Gaofeng, et al. Ammonia synthesis under ambient conditions: selective electroreduction of dinitrogen to ammonia on black phosphorus nanosheets[J]. Angewandte Chemie International Edition, 2019, 58(9): 2612-2616. |
| 94 | TIAN Ye, XU Dazhong, CHU Ke, et al. Metal-free N, S co-doped graphene for efficient and durable nitrogen reduction reaction[J]. Journal of Materials Science, 2019, 54(12): 9088-9097. |
| 95 | LI Sijia, BAO Di, SHI Miaomiao, et al. Amorphizing of Au nanoparticles by CeOx-RGO hybrid support towards highly efficient electrocatalyst for N2 reduction under ambient conditions[J]. Adv. Mater., 2017, 29(33): 1700001-1700006. |
| 96 | ZHU Xiaojuan, WU Tongwei, JI Lei, et al. Unusual electrochemical N2 reduction activity in an earth-abundant iron catalyst via phosphorous modulation[J]. Chemical Communications, 2020, 56(5): 731-734. |
| 97 | SONG Yanyan, WANG Ting, SUN Junwei, et al. Enhanced electrochemical N2 reduction to NH3 on reduced graphene oxide by tannic acid modification[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(17): 14368-14372. |
| 98 | LI Xianghong, REN Xiang, LIU Xuejing, et al. A MoS2 nanosheet-reduced graphene oxide hybrid: an efficient electrocatalyst for electrocatalytic N2 reduction to NH3 under ambient conditions[J]. Journal of Materials Chemistry A, 2019, 7(6): 2524-2528. |
| 99 | HUANG Hong, GONG Feng, WANG Yuan, et al. Mn3O4 nanoparticles reduced graphene oxide composite: an efficient electrocatalyst for artificial N2 fixation to NH3 at ambient conditions[J]. Nano Research, 2019, 12(5): 1093-1098. |
| 100 | XIA Li, LI Baihai, ZHANG Ya, et al. Cr2O3 nanoparticle-reduced graphene oxide hybrid: a highly active electrocatalyst for N2 reduction at ambient conditions[J]. Inorg. Chem., 2019, 58(4): 2257-2260. |
| 101 | WANG Weikang, ZHANG Haimin, ZHANG Shengbo, et al. Potassium-ion-assisted regeneration of active cyano groups in carbon nitride nanoribbons: visible-light-driven photocatalytic nitrogen reduction[J]. Angewandte Chemie International Edition, 2019, 58(46): 16644-16650. |
| 102 | LI Yan, KONG Yan, HOU Yang, et al. In situ growth of nitrogen-doped carbon-coated γ-Fe2O3 nanoparticles on carbon fabric for electrochemical N2 fixation[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(9): 8853-8859. |
| 103 | YANDULOV Dmitry V, SCHROCK Richard R. et al. Catalytic reduction of dinitrogen to ammonia at a single molybdenum center[J]. Science, 2003, 301(5629): 76-78. |
| 104 | ZHOU Fengling, AZOFRA Luis Miguel, Muata ALI, et al. Electro-synthesis of ammonia from nitrogen at ambient temperature and pressure in ionic liquids[J]. Energy & Environmental Science, 2017, 10(12): 2516-2520. |
| 105 | LICHT S, CUI Baochen, WANG Baohui, et al. Ammonia synthesis by N2 and steam electrolysis in molten hydroxide suspensions of nanoscale Fe2O3[J]. Science, 2014, 345(6197): 637-640. |
| 106 | LIU Ruiquan, XIE Yahong, WANG Jide, et al. Synthesis of ammonia at atmospheric pressure with Ce0.8M0.2O2-δ (M = La, Y, Gd, Sm) and their proton conduction at intermediate temperature[J]. Solid State Ionics, 2006, 177(1/2): 73-76. |
| 107 | PANG Fangjie, WANG Fei, YANG Liting, et al. Hierarchical nanoporous Pd1Ag1 alloy enables efficient electrocatalytic nitrogen reduction under ambient conditions[J]. Chemical Communications, 2019, 55(68): 10108-10111. |
| 108 | CHEN Shiming, PERATHONER Siglinda, AMPELLI Claudio, et al. Electrocatalytic synthesis of ammonia at room temperature and atmospheric pressure from water and nitrogen on a carbon-nanotube-based electrocatalyst[J]. Angewandte Chemie International Edition, 2017, 56(10): 2699-2703. |
| 109 | THOMSEN L B, NIELSEN G, VENDELBO S B, et al. Ultralarge area MoS tunnel devices for electron emission[J]. Physical Review B, 2007, 76(15): 155315. |
| [1] | ZHANG Mingyan, LIU Yan, ZHANG Xueting, LIU Yake, LI Congju, ZHANG Xiuling. Research progress of non-noble metal bifunctional catalysts in zinc-air batteries [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 276-286. |
| [2] | SHI Yongxing, LIN Gang, SUN Xiaohang, JIANG Weigeng, QIAO Dawei, YAN Binhang. Research progress on active sites in Cu-based catalysts for CO2 hydrogenation to methanol [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 287-298. |
| [3] | XIE Luyao, CHEN Songzhe, WANG Laijun, ZHANG Ping. Platinum-based catalysts for SO2 depolarized electrolysis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 299-309. |
| [4] | YANG Xiazhen, PENG Yifan, LIU Huazhang, HUO Chao. Regulation of active phase of fused iron catalyst and its catalytic performance of Fischer-Tropsch synthesis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 310-318. |
| [5] | HU Xi, WANG Mingshan, LI Enzhi, HUANG Siming, CHEN Junchen, GUO Bingshu, YU Bo, MA Zhiyuan, LI Xing. Research progress on preparation and sodium storage properties of tungsten disulfide composites [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 344-355. |
| [6] | ZHANG Jie, BAI Zhongbo, FENG Baoxin, PENG Xiaolin, REN Weiwei, ZHANG Jingli, LIU Eryong. Effect of PEG and its compound additives on post-treatment of electrolytic copper foils [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 374-381. |
| [7] | WANG Lele, YANG Wanrong, YAO Yan, LIU Tao, HE Chuan, LIU Xiao, SU Sheng, KONG Fanhai, ZHU Canghai, XIANG Jun. Influence of spent SCR catalyst blending on the characteristics and deNO x performance for new SCR catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 489-497. |
| [8] | DENG Liping, SHI Haoyu, LIU Xiaolong, CHEN Yaoji, YAN Jingying. Non-noble metal modified vanadium titanium-based catalyst for NH3-SCR denitrification simultaneous control VOCs [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 542-548. |
| [9] | LAI Shini, JIANG Lixia, LI Jun, HUANG Hongyu, KOBAYASHI Noriyuki. Research progress of ammonia blended fossil fuel [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4603-4615. |
| [10] | CHENG Tao, CUI Ruili, SONG Junnan, ZHANG Tianqi, ZHANG Yunhe, LIANG Shijie, PU Shi. Analysis of impurity deposition and pressure drop increase mechanisms in residue hydrotreating unit [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4616-4627. |
| [11] | WANG Jingang, ZHANG Jianbo, TANG Xuejiao, LIU Jinpeng, JU Meiting. Research progress on modification of Cu-SSZ-13 catalyst for denitration of automobile exhaust gas [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4636-4648. |
| [12] | WANG Peng, SHI Huibing, ZHAO Deming, FENG Baolin, CHEN Qian, YANG Da. Recent advances on transition metal catalyzed carbonylation of chlorinated compounds [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4649-4666. |
| [13] | ZHANG Qi, ZHAO Hong, RONG Junfeng. Research progress of anti-toxicity electrocatalysts for oxygen reduction reaction in PEMFC [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4677-4691. |
| [14] | GE Quanqian, XU Mai, LIANG Xian, WANG Fengwu. Research progress on the application of MOFs in photoelectrocatalysis [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4692-4705. |
| [15] | WANG Weitao, BAO Tingyu, JIANG Xulu, HE Zhenhong, WANG Kuan, YANG Yang, LIU Zhaotie. Oxidation of benzene to phenol over aldehyde-ketone resin based metal-free catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4706-4715. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||