Chemical Industry and Engineering Progress ›› 2021, Vol. 40 ›› Issue (3): 1215-1225.DOI: 10.16085/j.issn.1000-6613.2020-1933
• Special column:Green biomanufacturing • Previous Articles Next Articles
Advances in the molecular modification and application of D-amino acid oxidase
JU Shuyun1,2( ), WU Jianping1,2, YANG Lirong1,2(
), WU Jianping1,2, YANG Lirong1,2( )
)
- 1.College of Chemical and Biological Engineering, Zhejiang University, Hangzhou 310027, Zhejiang, China
2.Hangzhou Global Scientific and Technological Innovation Center, Zhejiang University, Hangzhou 311200, Zhejiang, China
-
Received:2020-09-22Online:2021-03-17Published:2021-03-05 -
Contact:YANG Lirong
D-氨基酸氧化酶的分子改造及应用研究进展
- 1.浙江大学化学工程与生物工程学院,浙江 杭州 310027
2.浙江大学杭州国际科创中心,浙江 杭州 311200
-
通讯作者:杨立荣 -
作者简介:居述云(1988—),男,博士,研究方向为生物催化与转化。E-mail:jushuyun@zju.edu.cn 。 -
基金资助:国家重点研发计划(2019YFA09005000)
CLC Number:
Cite this article
JU Shuyun, WU Jianping, YANG Lirong. Advances in the molecular modification and application of D-amino acid oxidase[J]. Chemical Industry and Engineering Progress, 2021, 40(3): 1215-1225.
居述云, 吴坚平, 杨立荣. D-氨基酸氧化酶的分子改造及应用研究进展[J]. 化工进展, 2021, 40(3): 1215-1225.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2020-1933
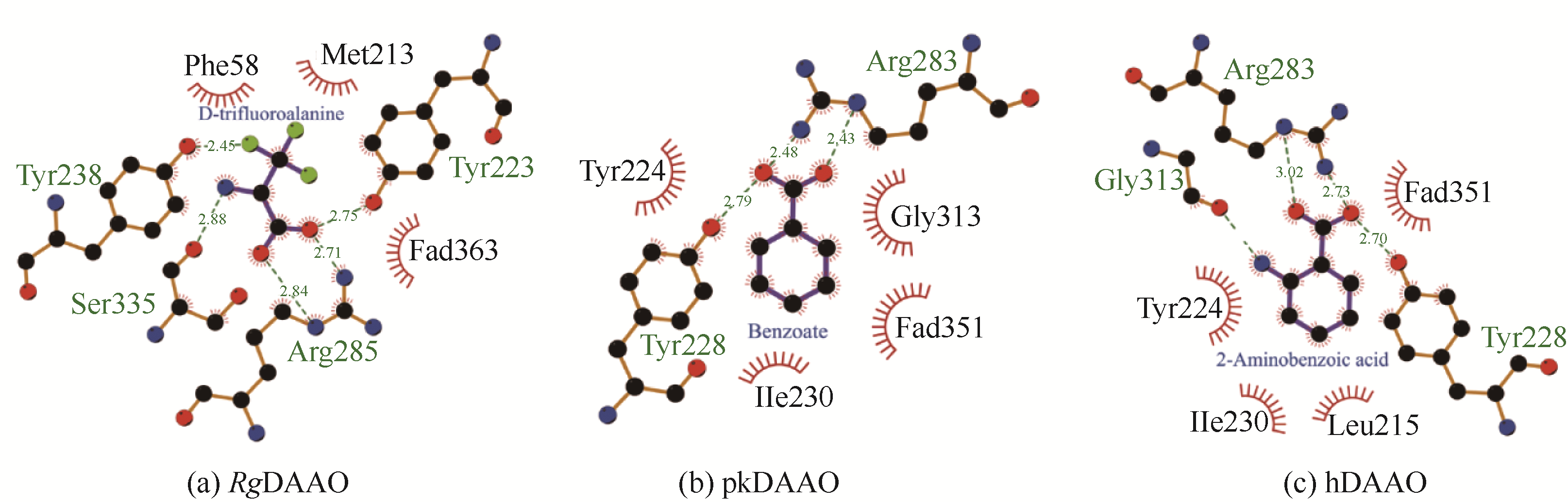
| 参数 | RgDAAO | pkDAAO | hDAAO |
|---|---|---|---|
| 单亚基氨基酸数量 | 368 | 347 | 347 |
| 与RgDAAO的序列一致性 | 100% | 28.73% | 27.73% |
| 亚基分子量 | 40000 | 39600 | 39600 |
| 亚基聚合状态 | 同源二聚体+2FAD | 同源二聚体+2FAD | 同源二聚体+2FAD |
| 配体 | D-三氟丙氨酸 | 苯甲酸 | 2-氨基苯甲酸 |
| 与配体相互作用的关键氨基酸残基 | Y223,Y238,R285,S335, M213,F58 | Y224,Y228,R283, G313,I230 | Y224,Y228,R283,G313, I230,L215 |
| PDB登录号 | 1C0L | 1VE9 | 2E4A |
| 参数 | RgDAAO | pkDAAO | hDAAO |
|---|---|---|---|
| 单亚基氨基酸数量 | 368 | 347 | 347 |
| 与RgDAAO的序列一致性 | 100% | 28.73% | 27.73% |
| 亚基分子量 | 40000 | 39600 | 39600 |
| 亚基聚合状态 | 同源二聚体+2FAD | 同源二聚体+2FAD | 同源二聚体+2FAD |
| 配体 | D-三氟丙氨酸 | 苯甲酸 | 2-氨基苯甲酸 |
| 与配体相互作用的关键氨基酸残基 | Y223,Y238,R285,S335, M213,F58 | Y224,Y228,R283, G313,I230 | Y224,Y228,R283,G313, I230,L215 |
| PDB登录号 | 1C0L | 1VE9 | 2E4A |
| 原始酶 | 改造策略 | 突变体 | 底物 | kcat /min-1 | Km /mmol·L-1 | (kcat/Km) /mL·mol-1·min-1 | 参考 文献 |
|---|---|---|---|---|---|---|---|
| RgDAAO | 改变底物结合口袋处氨基酸残基的极性和带电性质,稳定底物结合构象 | M213R |  | 235(29) | 2(18) | 118(1.6) | [ |
 | 975(60) | 33(77.3) | 29.5(0.8) | ||||
 | 630(5000) | 17.8(0.8) | 35.4(6100) | ||||
| 减小底物结合口袋处氨基酸残基的空间位阻,扩大底物结合口袋 | M213G |  | 870(125) | 0.03(0.04) | 29000(3100) | [ | |
 | 31(6) | 0.03(0.01) | 1035(600) | ||||
 | 92(53) | 0.05(0.33) | 1840(160) | ||||
 | 950(180) | 1.6(2.1) | 600(90) | [ | |||
| 定向进化 | L118H |  | 3620(3900) | 0.4(0.9) | 8415(4330) | [ | |
 | 31(40) | 12.8(33.1) | 2.4(1.2) | ||||
T60A/Q144R /K152E |  | 53(40) | 7.9(33.1) | 6.7(1.2) | |||
| TvDAAO | 基于同源建模,改变底物结合口袋处氨基酸残基的极性,稳定底物结合构象 | F54Y |  | 2200(370) | 4.8(1.6) | 470(230) | [ |
| pkDAAO | 突变与底物(配体)羧基相互作用的精氨酸残基,重塑底物结合口袋 | Y228L/R283G |  | 444.0(-) | 7.0(-) | 63.4(-) | [ |
 | 46.8(-) | 1.5(-) | 31.2(-) | [ | |||
| 减小底物结合口袋处氨基酸残基的空间位阻,扩大底物结合口袋 | Y228L/R283G /F242I |  | 180.0(-) | 3.6(-) | 50.0(-) | [ | |
| I230A/R283G |  | 360.6(-) | 2.94(-) | 122.7(-) | [ |
| 原始酶 | 改造策略 | 突变体 | 底物 | kcat /min-1 | Km /mmol·L-1 | (kcat/Km) /mL·mol-1·min-1 | 参考 文献 |
|---|---|---|---|---|---|---|---|
| RgDAAO | 改变底物结合口袋处氨基酸残基的极性和带电性质,稳定底物结合构象 | M213R |  | 235(29) | 2(18) | 118(1.6) | [ |
 | 975(60) | 33(77.3) | 29.5(0.8) | ||||
 | 630(5000) | 17.8(0.8) | 35.4(6100) | ||||
| 减小底物结合口袋处氨基酸残基的空间位阻,扩大底物结合口袋 | M213G |  | 870(125) | 0.03(0.04) | 29000(3100) | [ | |
 | 31(6) | 0.03(0.01) | 1035(600) | ||||
 | 92(53) | 0.05(0.33) | 1840(160) | ||||
 | 950(180) | 1.6(2.1) | 600(90) | [ | |||
| 定向进化 | L118H |  | 3620(3900) | 0.4(0.9) | 8415(4330) | [ | |
 | 31(40) | 12.8(33.1) | 2.4(1.2) | ||||
T60A/Q144R /K152E |  | 53(40) | 7.9(33.1) | 6.7(1.2) | |||
| TvDAAO | 基于同源建模,改变底物结合口袋处氨基酸残基的极性,稳定底物结合构象 | F54Y |  | 2200(370) | 4.8(1.6) | 470(230) | [ |
| pkDAAO | 突变与底物(配体)羧基相互作用的精氨酸残基,重塑底物结合口袋 | Y228L/R283G |  | 444.0(-) | 7.0(-) | 63.4(-) | [ |
 | 46.8(-) | 1.5(-) | 31.2(-) | [ | |||
| 减小底物结合口袋处氨基酸残基的空间位阻,扩大底物结合口袋 | Y228L/R283G /F242I |  | 180.0(-) | 3.6(-) | 50.0(-) | [ | |
| I230A/R283G |  | 360.6(-) | 2.94(-) | 122.7(-) | [ |
| 1 | 郭姣洁, 薛永常, 徐书景, 等. D-氨基酸氧化酶研究进展[J]. 中国生物工程杂志, 2010, 30(11): 106-111. |
| GUO Jiaojie, XUE Yongchang, XU Shujing, et al. D-amino acid oxidase update and review[J]. China Biotechnology, 2010, 30(11): 106-111. | |
| 2 | KREBS H A. Metabolism of amino-acids: deamination of amino-acids[J]. Biochemical Journal, 1935, 29(7): 1620-1644. |
| 3 | SIMONETTA M P, VANONI M A, CASALIN P. Purification and properties of D-amino acid oxidase, an inducible flavoenzyme from Rhodotorula gracilis[J]. Biochimica et Biophysica Acta: Protein Structure and Molecular Enzymology, 1987, 914(2): 136-142. |
| 4 | GONZÁLEZ F J, MONTES J, MARTIN F, et al. Molecular cloning of TvDAO1, a gene encoding a D-amino acid oxidase from Trigonopsis variabilis and its expression in Saccharomyces cerevisiae and Kluyveromyces lactis[J]. Yeast, 1997, 13(15): 1399-1408. |
| 5 | TAKAHASHI S, ABE K, KERA Y. Bacterial D-amino acid oxidases: recent findings and future perspectives[J]. Bioengineered, 2015, 6(4): 237-241. |
| 6 | POLLEGIONI L, PIUBELLI L, SACCHI S, et al. Physiological functions of D-amino acid oxidases: from yeast to humans[J]. Cellular and Molecular Life Sciences, 2007, 64(11): 1373-1394. |
| 7 | POLLEGIONI L, MOLLA G. New biotech applications from evolved D-amino acid oxidases[J]. Trends in Biotechnology, 2011, 29(6): 276-283. |
| 8 | BATALLA P, MARTIN A, LOPEZ M A, et al. Enzyme-based microfluidic chip coupled to graphene electrodes for the detection of D-amino acid enantiomer-biomarkers[J]. Analytical Chemistry, 2015, 87(10): 5074-5078. |
| 9 | UMHAU S, POLLEGIONI L, MOLLA G, et al. The X-ray structure of D-amino acid oxidase at very high resolution identifies the chemical mechanism of flavin-dependent substrate dehydrogenation[J]. Proceedings of the National Academy of Sciences, 2000, 97(23): 12463-12468. |
| 10 | POLLEGIONI L, DIEDERICHS K, MOLLA G, et al. Yeast D-amino acid oxidase: structural basis of its catalytic properties[J]. Journal of Molecular Biology, 2002, 324(3): 535-546. |
| 11 | MATTEVI A, CURTI B. Crystal structure of D-amino acid oxidase: a case of active site mirror-image convergent evolution with flavocytochrome b2[J]. Proceedings of the National Academy of Sciences, 1996, 93(15): 7496-7501. |
| 12 | MIZUTANI H, MIYAHARA I, HIROTSU K, et al. Three-dimensional structure of porcine kidney D-amino acid oxidase at 3.0Å resolution[J]. Journal of Biochemistry, 1996, 120(1): 14-17. |
| 13 | KAWAZOE T, TSUGE H, IMAGAWA T, et al. Structural basis of D-DOPA oxidation by D-amino acid oxidase: alternative pathway for dopamine biosynthesis[J]. Biochemical and Biophysical Research Communications, 2007, 355(2): 385-391. |
| 14 | POLLEGIONI L, MOLLA G, SACCHI S, et al. Properties and applications of microbial D-amino acid oxidases: current state and perspectives[J]. Applied Microbiology and Biotechnology, 2008, 78(1): 1-16. |
| 15 | 赵冉冉, 冯利伟, 张金秀, 等. D-氨基酸氧化酶结构的研究进展[J]. 生物技术通报, 2013(12): 27-35. |
| ZHAO Ranran, FENG Liwei, ZHANG Jinxiu, et al. Research progress on the crystal structure of D-amino acid oxidase[J]. Biotechnology Bulletin, 2013(12): 27-35. | |
| 16 | WALSH C T, SCHONBRUNN A, ABELES R H. Studies on the mechanism of action of D-amino acid oxidase. evidence for removal of substrate-hydrogen as a proton[J]. Journal of Biological Chemistry, 1971, 246(22): 6855-6866. |
| 17 | HERSH L B, JORNS M S. Use of 5-deazaFAD to study hydrogen transfer in the D-amino acid oxidase reaction[J]. Journal of Biological Chemistry, 1975, 250(22): 8728-8734. |
| 18 | DIJKMAN W P, DE GONZALO G, MATTEVI A, et al. Flavoprotein oxidases: classification and applications[J]. Applied Microbiology and Biotechnology, 2013, 97(12): 5177-88. |
| 19 | HARRIS C M, MOLLA G, PILONE M S, et al. Studies on the reaction mechanism of Rhodotorula gracilis D-amino-acid oxidase. role of the highly conserved Tyr-223 on substrate binding and catalysis[J]. Journal of Biological Chemistry, 1999, 274(274): 36233-36240. |
| 20 | SACCHI S, LORENZI S, MOLLA G, et al. Engineering the substrate specificity of D-amino acid oxidase[J]. Journal of Biological Chemistry, 2002, 277(30): 27510-27516. |
| 21 | YASUKAWA K, NAKANO S, ASANO Y. Tailoring D-amino acid oxidase from the pig kidney to R-stereoselective amine oxidase and its use in the deracemization of alpha-methylbenzylamine[J]. Angewandte Chemie: International Edition, 2014, 53(17): 4428-4431. |
| 22 | CALIGIURI A, D'ARRIGO P, ROSINI E, et al. Enzymatic conversion of unnatural amino acids by yeast D-amino acid oxidase[J]. Advanced Synthesis & Catalysis, 2006, 348(15): 2183-2190. |
| 23 | CALIGIURI A, D’ARRIGO P, ROSINI E, et al. Activity of yeast D-amino acid oxidase on aromatic unnatural amino acids[J]. Journal of Molecular Catalysis B: Enzymatic, 2008, 50(4): 93-98. |
| 24 | TRIMMER E E, WANNINAYAKE U S, FITZPATRICK P F. Mechanistic studies of an amine oxidase derived from D-amino acid oxidase[J]. Biochemistry, 2017, 56(14): 2024-2030. |
| 25 | NAKANO S, YASUKAWA K, TOKIWA T, et al. Origin of stereoselectivity and substrate/ligand recognition in an FAD-dependent R-selective amine oxidase[J]. Journal of Physical Chemistry B, 2016, 120: 10736-10743 |
| 26 | YASUKAWA K, MOTOJIMA F, ONO A, et al. Expansion of the substrate specificity of porcine kidney D-amino acid oxidase for S-stereoselective oxidation of 4-Cl-benzhydrylamine[J]. ChemCatChem, 2018, 10: 1-7. |
| 27 | WONG K S, FONG W P, TSANG P W. A single Phe54Tyr substitution improves the catalytic activity and thermostability of Trigonopsis variabilis D-amino acid oxidase[J]. New Biotechnology, 2010, 27(1): 78-84. |
| 28 | SACCHI S, ROSINI E, MOLLA G, et al. Modulating D-amino acid oxidase substrate specificity: production of an enzyme for analytical determination of all D-amino acids by directed evolution[J]. Protein Engineering, Design & Selection, 2004, 17(6): 517-525. |
| 29 | BAKKE M, SETOYAMA C, MIURA R, et al. Thermostabilization of porcine kidney D-amino acid oxidase by a single amino acid substitution[J]. Biotechnology and Bioengineering, 2006, 93(5): 1023-1027. |
| 30 | VOLPATO G, RODRIGUES R C, FERNANDEZ-LAFUENTE R. Use of enzymes in the production of semi-synthetic penicillins and cephalosporins: drawbacks and perspectives[J]. Current Medical Chemistry, 2010, 17(32): 3855-3873. |
| 31 | CONTI G, POLLEGIONI L, ROSINI E. One-pot conversion of cephalosporin C by using an optimized two-enzyme process[J]. Catalysis Science & Technology, 2015, 5(3): 1854-1863. |
| 32 | TAN Q, QIU J, LUO X, et al. Progress in one-pot bioconversion of cephalosporin C to 7-aminocephalosporanic acid[J]. Current Pharmaceutical Biotechnology, 2018, 19: 30-42. |
| 33 | HU L, MAGESH S, CHEN L, et al. Discovery of a small-molecule inhibitor and cellular probe of Keap1-Nrf2 protein-protein interaction[J]. Bioorganic & Medicinal Chemistry Letters, 2013, 23(10): 3039-3043. |
| 34 | KOTHA S, DEODHAR D, KHEDKAR P. Diversity-oriented synthesis of medicinally important 1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid (Tic) derivatives and higher analogs[J]. Organic & Biomolecular Chemistry,2014, 12: 9054-9091. |
| 35 | PARK E, KIM M, J-S SHIN. One-pot conversion of L-threonine into L-homoalanine: biocatalytic production of an unnatural amino acid from a natural one[J]. Advanced Synthesis & Catalysis, 2010, 352(18): 3391-3398. |
| 36 | ENRIGHT A, F-R ALEXANDRE, ROFF G, et al. Stereoinversion of β- and γ-substituted α-amino acids using a chemo-enzymatic oxidation–reduction procedure[J]. Chemical Communications, 2003, 20: 2636-2637. |
| 37 | BEARD T M, TURNER N J. Deracemisation and stereoinversion of α-amino acids using D-amino acid oxidase and hydride reducing agents[J]. Chemical Communications, 2002, 3: 246-247. |
| 38 | ALEXANDRE F R, PANTALEONE D P, TAYLOR P P, et al. Amine-boranes: effective reducing agents for the deracemisation of DL-amino acids using L-amino acid oxidase from Proteus myxofaciens[J]. Tetrahedron Letters, 2002, 43(4): 707-710. |
| 39 | CHEN Y, GOLDBERG S L, HANSON R L, et al. Enzymatic preparation of an (S)-amino acid from a racemic amino acid[J]. Organic Process Research & Development, 2011, 15(1): 241-248. |
| 40 | JU S Y, QIAN M X, XU G, et al. Chemoenzymatic approach to (S)-1,2,3,4-tetrahydroisoquinoline carboxylic acids employing D-amino acid oxidase[J]. Advanced Synthesis & Catalysis, 2019, 361(13): 3191-3199. |
| 41 | FINDRIK Z, VASIC-RACKI D. Biotransformation of D-methionine into L-methionine in the cascade of four enzymes[J]. Biotechnology and Bioengineering, 2007, 98(5): 956-967. |
| 42 | QI Y, YANG T, ZHOU J, et al. Development of a multi-enzymatic desymmetrization and its application for the biosynthesis of L-norvaline from DL-norvaline[J]. Process Biochemistry, 2017, 55: 104-109. |
| 43 | GREEN B M, GRADLEY M L. Methods for making L-glufosinate: US 20170253897 A1[P]. 2017-09-07. |
| 44 | Y-M SEO, MATHEW S, H-S BEA, et al. Deracemization of unnatural amino acid: homoalanine using D-amino acid oxidase and ω-transaminase[J]. Organic & Biomolecular Chemistry, 2012, 10(12): 2482-2485. |
| 45 | LIU W, TANG D, SHI R, et al. Efficient production of S‐adenosyl‐L‐methionine from DL‐methionine in metabolic engineered Saccharomyces cerevisiae[J]. Biotechnology and Bioengineering, 2019, 116(12): 3312-3323. |
| 46 | YASUDA M, UEDA M, MURAMATSU H, et al. Enzymatic synthesis of cyclic amino acids by N-methyl-L-amino acid dehydrogenase from Pseudomonas putida[J]. Tetrahedron: Asymmetry, 2006, 17(12): 1775-1779. |
| 47 | JU S Y, QIAN M X, LI J, et al. A biocatalytic redox cascade approach for one-pot deracemization of carboxyl-substituted tetrahydroisoquinolines by stereoinversion[J]. Green Chemistry, 2019, 21(20): 5579-5585. |
| 48 | ORREGO A H, LÓPEZ-GALLEGO F, ESPAILLAT A, et al. One-step synthesis of alpha-keto acids from racemic amino acids by a versatile immobilized multienzyme cell-free system[J]. ChemCatChem, 2018, 10: 3002-3011. |
| 49 | MOLLA G, MELIS R, POLLEGIONI L. Breaking the mirror: L-amino acid deaminase, a novel stereoselective biocatalyst[J]. Biotechnology Advances, 2017, 35(6): 657-668. |
| 50 | HOSSAIN G S, LI J, SHIN H D, et al. L-amino acid oxidases from microbial sources: types, properties, functions, and applications[J]. Applied Microbiology and Biotechnology, 2014, 98(4): 1507-1515. |
| 51 | POLLEGIONI L, MOTTA P, MOLLA G. L-amino acid oxidase as biocatalyst: a dream too far?[J]. Applied Microbiology and Biotechnology, 2013, 97(21): 9323-9341. |
| 52 | SONG W, XU X, GAO C, et al. Open gate of corynebacterium glutamicum threonine deaminase for efficient synthesis of bulky α-keto acids[J]. ACS Catalysis, 2020, 10: 9994-10004. |
| [1] | LI Huahua, LI Yihang, JIN Beichen, LI Longxin, CHENG Shao’an. Research progress of Anammox bio-electrochemical coupling wastewater treatment system [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2678-2690. |
| [2] | MENG Lingding, MAO Menglei, LIAO Qiyong, MENG Zihui, LIU Wenfang. Recent advance in stability of carbonic anhydrase and formate dehydrogenase [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 436-447. |
| [3] | GAO Bo, FENG Xudong, LI Chun. Visual and high-throughput method for detecting the activity of aspartate transcarbamylase [J]. Chemical Industry and Engineering Progress, 2022, 41(4): 2054-2059. |
| [4] | ZHANG Yan, WANG Wei, XIE Rui, JU Xiaojie, LIU Zhuang, CHU Liangyin. Controllable fabrication of polymeric microparticles loaded with enzyme@ZIF-8 [J]. Chemical Industry and Engineering Progress, 2022, 41(4): 2022-2028. |
| [5] | LI Qingyuan, WANG Chao, XU Shipei, ZHANG Xueqin, QIU Mingjian, LIU Mengyao, CONG Mengxiao. Research progress on reaction process and catalysts for PBS precursor of 1,4-butanediol synthesis [J]. Chemical Industry and Engineering Progress, 2022, 41(11): 5771-5782. |
| [6] | LU Zeping, PEI Xinhua, XUE Yu, ZHANG Xiaoguang, HU Yi. Chemical modification of porcine pancreatic lipase with betaine ionic liquid to improve its enzymatic properties [J]. Chemical Industry and Engineering Progress, 2022, 41(11): 6045-6052. |
| [7] | LI Qing, LIU Wujun, GUO Xiaojia, WANG Qian, ZHAO Zongbao. Chiral NAD analogs as cofactors for biocatalysis [J]. Chemical Industry and Engineering Progress, 2021, 40(9): 5214-5221. |
| [8] | ZHANG Xiaojian, LIU Qian, LIU Zhiqiang, ZHENG Yuguo. Stereoselective carbonyl reductases and their application in chiral alcohols synthesis [J]. Chemical Industry and Engineering Progress, 2021, 40(3): 1142-1160. |
| [9] | Zhufan LIN, Shao’an CHENG, Zhengzhong MAO, Ruonan GU, Jiawei YANG. Recent advances in the construction and influencing factors of bio-electrochemical nitrogen removal systems [J]. Chemical Industry and Engineering Progress, 2020, 39(9): 3766-3776. |
| [10] | Cheng ZHU,Guochao XU,Wei DAI,Jieyu ZHOU,Ye NI. Effect of position 127 on the activity and enantioselectivity of alcohol dehydrogenase KpADH [J]. Chemical Industry and Engineering Progress, 2019, 38(12): 5504-5511. |
| [11] | Tian JIANG, Xudong FENG, Yan LI, Chu LI. The biocatalysis and enzyme modification of substrate specificity [J]. Chemical Industry and Engineering Progress, 2019, 38(01): 606-614. |
| [12] | YU Bo, LIU Chao, LIU Jindong, DING Wanyu, CHAI Weiping. Preparation of mesoporous zirconium phosphate and its catalytic performace in the preparation of cellulose from glucose [J]. Chemical Industry and Engineering Progress, 2018, 37(06): 2236-2241. |
| [13] | YAN Xingchen, ZHAO Qianru, WANG Kaifeng, GUO Yuxin, JIANG Ling, HUANG He. Auto-induced expression of trehalose synthetase and novel process for catalytic production of trehalose [J]. Chemical Industry and Engineering Progress, 2018, 37(05): 1949-1955. |
| [14] | WANG Rui, XU Yaohui, WANG Kewei, WU Minchen. Expression of PvEH3,a Phaseolus vulgaris epoxide hydrolase,and synthesis of chiral vicinal diols [J]. Chemical Industry and Engineering Progress, 2018, 37(05): 1933-1939. |
| [15] | ZHOU Ya, YANG Chun. Degradation of 4-chloronitrobenzene by bioelectrochemical system [J]. Chemical Industry and Engineering Progress, 2018, 37(01): 375-380. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||