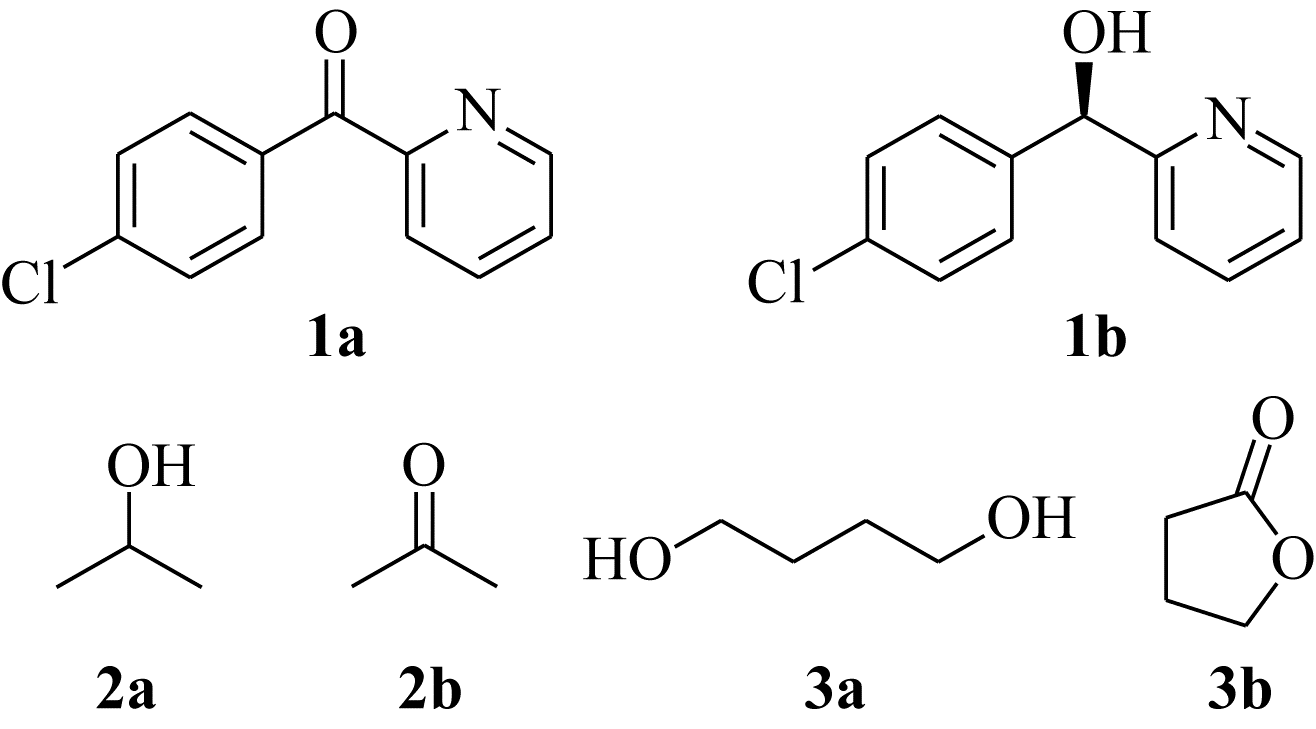
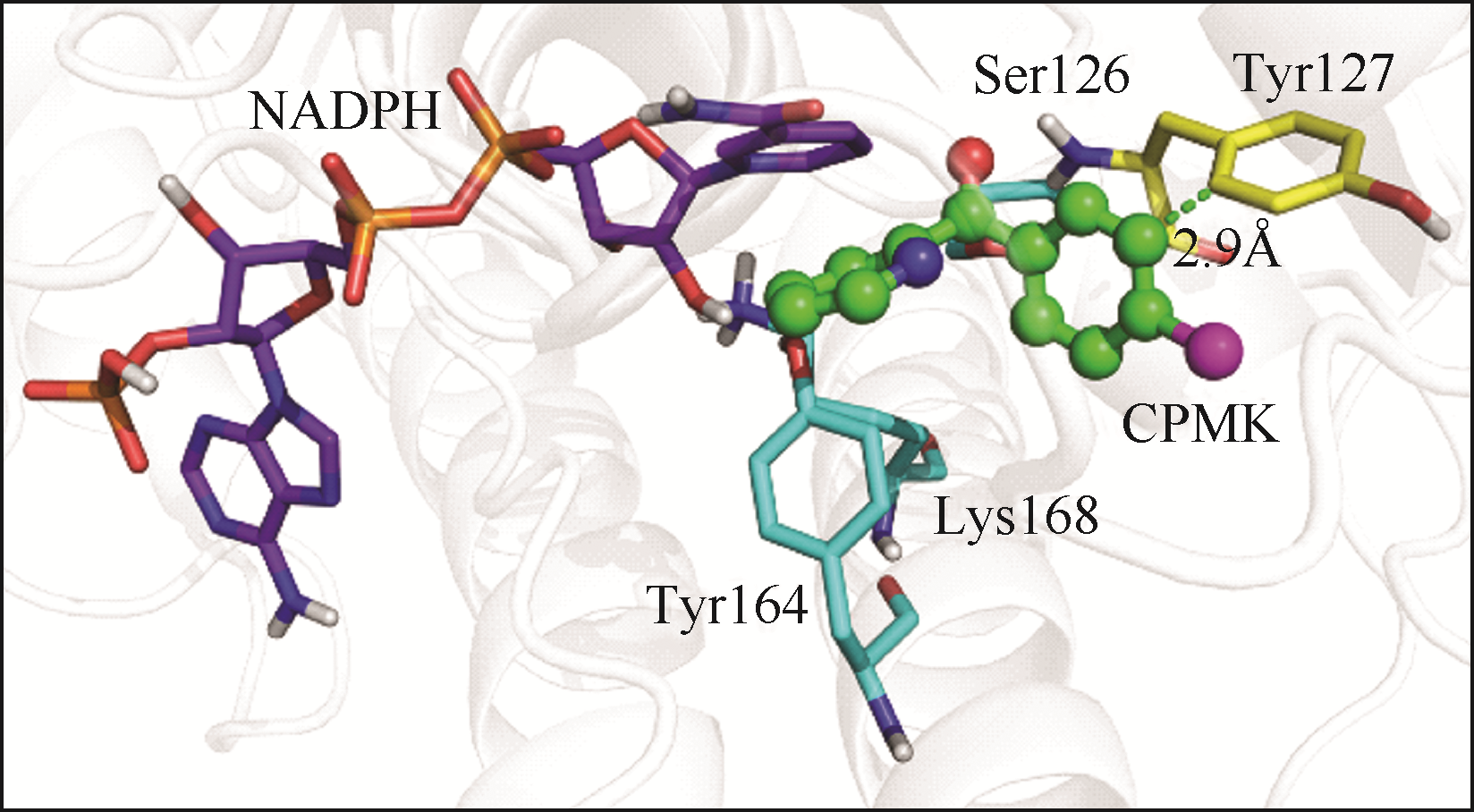
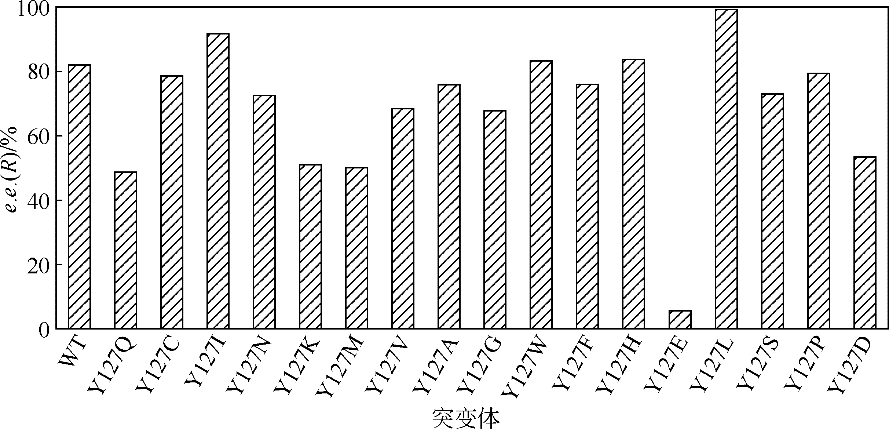
Chemical Industry and Engineering Progress ›› 2019, Vol. 38 ›› Issue (12): 5504-5511.DOI: 10.16085/j.issn.1000-6613.2019-0472
• Biochemical and pharmaceutical engineering • Previous Articles Next Articles
Effect of position 127 on the activity and enantioselectivity of alcohol dehydrogenase KpADH
Cheng ZHU( ),Guochao XU,Wei DAI,Jieyu ZHOU,Ye NI(
),Guochao XU,Wei DAI,Jieyu ZHOU,Ye NI( )
)
- School of Biotechnology, Jiangnan University, Wuxi 214122, Jiangsu, China
-
Received:2019-03-28Online:2019-12-05Published:2019-12-05 -
Contact:Ye NI
醇脱氢酶KpADH的127位点对催化活性和对映选择性的影响
- 江南大学生物工程学院,江苏 无锡 214122
-
通讯作者:倪晔 -
作者简介:朱诚(1994—),男,硕士研究生,研究方向为生物催化与酶工程。E-mail:6160206048@vip.jiangnan.edu.cn 。 -
基金资助:国家轻工技术与工程一流学科计划(LITE2018-07);国家自然科学基金(21506073)
CLC Number:
Cite this article
Cheng ZHU,Guochao XU,Wei DAI,Jieyu ZHOU,Ye NI. Effect of position 127 on the activity and enantioselectivity of alcohol dehydrogenase KpADH[J]. Chemical Industry and Engineering Progress, 2019, 38(12): 5504-5511.
朱诚,许国超,戴威,周婕妤,倪晔. 醇脱氢酶KpADH的127位点对催化活性和对映选择性的影响[J]. 化工进展, 2019, 38(12): 5504-5511.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2019-0472
| 引物名称 | 引物序列(5’-3’) | 引物序列(5’-3’) |
|---|---|---|
| Y127Q | PF: ATGACTGCTTCT | PR: CATAATTGAAGC |
| Y127C | PF: ATGACTGCTTCT | PR: CATAATTGAAGC |
| Y127I | PF: ATGACTGCTTCT | PR: CATAATTGAAGC |
| Y127N | PF: ATGACTGCTTCT | PR: CATAATTGAAGC |
| Y127K | PF: ATGACTGCTTCT | PR: CATAATTGAAGC |
| Y127M | PF: ATGACTGCTTCT | PR: CATAATTGAAGC |
| Y127V | PF: ATGACTGCTTCT | PR: CATAATTGAAGC |
| Y127A | PF: ATGACTGCTTCT | PR: CATAATTGAAGC |
| Y127G | PF: ATGACTGCTTCT | PR: CATAATTGAAGC |
| Y127W | PF: ATGACTGCTTCT | PR: CATAATTGAAGC |
| Y127F | PF: ATGACTGCTTCT | PR: CATAATTGAAGC |
| Y127H | PF: ATGACTGCTTCT | PR: CATAATTGAAGC |
| Y127E | PF: ATGACTGCTTCT | PR: CATAATTGAAGC |
| Y127L | PF: ATGACTGCTTCT | PR: CATAATTGAAGC |
| Y127S | PF: ATGACTGCTTCT | PR: CATAATTGAAGC |
| Y127P | PF: ATGACTGCTTCT | PR: CATAATTGAAGC |
| Y127D | PF: ATGACTGCTTCT | PR: CATAATTGAAGC |
| Y127R | PF: ATGACTGCTTCT | PR: CATAATTGAAGC |
| Y127T | PF: ATGACTGCTTCT | PR: CATAATTGAAGC |
| 引物名称 | 引物序列(5’-3’) | 引物序列(5’-3’) |
|---|---|---|
| Y127Q | PF: ATGACTGCTTCT | PR: CATAATTGAAGC |
| Y127C | PF: ATGACTGCTTCT | PR: CATAATTGAAGC |
| Y127I | PF: ATGACTGCTTCT | PR: CATAATTGAAGC |
| Y127N | PF: ATGACTGCTTCT | PR: CATAATTGAAGC |
| Y127K | PF: ATGACTGCTTCT | PR: CATAATTGAAGC |
| Y127M | PF: ATGACTGCTTCT | PR: CATAATTGAAGC |
| Y127V | PF: ATGACTGCTTCT | PR: CATAATTGAAGC |
| Y127A | PF: ATGACTGCTTCT | PR: CATAATTGAAGC |
| Y127G | PF: ATGACTGCTTCT | PR: CATAATTGAAGC |
| Y127W | PF: ATGACTGCTTCT | PR: CATAATTGAAGC |
| Y127F | PF: ATGACTGCTTCT | PR: CATAATTGAAGC |
| Y127H | PF: ATGACTGCTTCT | PR: CATAATTGAAGC |
| Y127E | PF: ATGACTGCTTCT | PR: CATAATTGAAGC |
| Y127L | PF: ATGACTGCTTCT | PR: CATAATTGAAGC |
| Y127S | PF: ATGACTGCTTCT | PR: CATAATTGAAGC |
| Y127P | PF: ATGACTGCTTCT | PR: CATAATTGAAGC |
| Y127D | PF: ATGACTGCTTCT | PR: CATAATTGAAGC |
| Y127R | PF: ATGACTGCTTCT | PR: CATAATTGAAGC |
| Y127T | PF: ATGACTGCTTCT | PR: CATAATTGAAGC |
| 突变体 | CPMK/U·mg-1 | 疏水参数lgP |
|---|---|---|
| WTKpADH | 14.6±0.7 | -1.3 |
| Y127Q | 4.77±0.2 | -3.5 |
| Y127C | 15.7±0.5 | 2.5 |
| Y127I | 84.0±1.8 | 4.5 |
| Y127N | 0.834±0.3 | -3.5 |
| Y127K | 15.6±0.4 | -3.9 |
| Y127M | 15.4±0.5 | 1.9 |
| Y127V | 95.0±7.6 | 4.2 |
| Y127A | 2.82±0.2 | 1.8 |
| Y127G | 0.175±0.01 | -0.4 |
| Y127W | 1.41±0.1 | -0.9 |
| Y127F | 7.08±0.2 | 2.8 |
| Y127H | 2.74±0.1 | -3.2 |
| Y127E | 5.14±0.1 | -3.5 |
| Y127L | 5.27±1.0 | 3.8 |
| Y127S | 2.59±0.2 | -0.8 |
| Y127P | <0.1 | -1.6 |
| Y127D | <0.1 | -3.5 |
| Y127R | <0.1 | -4.5 |
| Y127T | <0.1 | -0.7 |
| 突变体 | CPMK/U·mg-1 | 疏水参数lgP |
|---|---|---|
| WTKpADH | 14.6±0.7 | -1.3 |
| Y127Q | 4.77±0.2 | -3.5 |
| Y127C | 15.7±0.5 | 2.5 |
| Y127I | 84.0±1.8 | 4.5 |
| Y127N | 0.834±0.3 | -3.5 |
| Y127K | 15.6±0.4 | -3.9 |
| Y127M | 15.4±0.5 | 1.9 |
| Y127V | 95.0±7.6 | 4.2 |
| Y127A | 2.82±0.2 | 1.8 |
| Y127G | 0.175±0.01 | -0.4 |
| Y127W | 1.41±0.1 | -0.9 |
| Y127F | 7.08±0.2 | 2.8 |
| Y127H | 2.74±0.1 | -3.2 |
| Y127E | 5.14±0.1 | -3.5 |
| Y127L | 5.27±1.0 | 3.8 |
| Y127S | 2.59±0.2 | -0.8 |
| Y127P | <0.1 | -1.6 |
| Y127D | <0.1 | -3.5 |
| Y127R | <0.1 | -4.5 |
| Y127T | <0.1 | -0.7 |
| 突变体 | 1,4-丁二醇/U·g-1 | 异丙醇/U·mg-1 |
|---|---|---|
| WTKpADH | 57.8±0.5 | 14.9±1.5 |
| Y127Q | 29.2±1.6 | 2.06±0.1 |
| Y127C | 173±8.0 | 1.58±0.1 |
| Y127I | 37.1±1.1 | 21.8±0.3 |
| Y127N | 30.2±0.8 | 0.987±0.1 |
| Y127K | 143±11.0 | 12.0±0.5 |
| Y127M | 147±5.0 | 11.5±0.1 |
| Y127V | 124±5.0 | 15.7±0.4 |
| Y127A | 93.0±1.9 | 5.64±0.2 |
| Y127G | 8.89±1.4 | 0.202±0.01 |
| Y127W | 30.7±0.1 | 1.83±0.1 |
| Y127F | 157±3.0 | 18.8±0.7 |
| Y127H | 48.1±1.2 | 4.19±0.4 |
| Y127E | 6.15±0.1 | 0.221±0.01 |
| Y127L | 91.4±5.3 | 14.9±0.6 |
| Y127S | 31.5±2.3 | 5.76±0.1 |
| Y127P | 0.495±0.1 | <0.1 |
| Y127D | 0.913±0.1 | <0.1 |
| Y127R | 2.75±0.5 | 0.233±0.01 |
| Y127T | <0.1 | <0.1 |
| 突变体 | 1,4-丁二醇/U·g-1 | 异丙醇/U·mg-1 |
|---|---|---|
| WTKpADH | 57.8±0.5 | 14.9±1.5 |
| Y127Q | 29.2±1.6 | 2.06±0.1 |
| Y127C | 173±8.0 | 1.58±0.1 |
| Y127I | 37.1±1.1 | 21.8±0.3 |
| Y127N | 30.2±0.8 | 0.987±0.1 |
| Y127K | 143±11.0 | 12.0±0.5 |
| Y127M | 147±5.0 | 11.5±0.1 |
| Y127V | 124±5.0 | 15.7±0.4 |
| Y127A | 93.0±1.9 | 5.64±0.2 |
| Y127G | 8.89±1.4 | 0.202±0.01 |
| Y127W | 30.7±0.1 | 1.83±0.1 |
| Y127F | 157±3.0 | 18.8±0.7 |
| Y127H | 48.1±1.2 | 4.19±0.4 |
| Y127E | 6.15±0.1 | 0.221±0.01 |
| Y127L | 91.4±5.3 | 14.9±0.6 |
| Y127S | 31.5±2.3 | 5.76±0.1 |
| Y127P | 0.495±0.1 | <0.1 |
| Y127D | 0.913±0.1 | <0.1 |
| Y127R | 2.75±0.5 | 0.233±0.01 |
| Y127T | <0.1 | <0.1 |
| 1 | FARINA V, REEVES J T, SENANAYAKE C H, et al. Asymmetric synthesis of active pharmaceutical ingredients[J]. Chemical Reviews, 2006, 106(7): 2734-2793. |
| 2 | SCHMIDT F, STEMMLER R T, RUDOLPH J, et al. Catalytic asymmetric approaches towards enantiomerically enriched diarylmethanols and diarylmethylamines[J]. Chemical Society Reviews, 2006, 35(5): 454-470. |
| 3 | SUI Y Z, ZHANG X C, WU J W, et al. CuII-catalyzed asymmetric hydrosilylation of diaryl- and aryl heteroaryl ketones: application in the enantioselective synthesis of orphenadrine and neobenodine[J]. Chemistry: A European Journal, 2012, 18(24): 7486-7492. |
| 4 | HOLLMANN F, ARENDS I W, HOLTMANN D. Enzymatic reduction for the chemist[J]. Green Chemistry, 2011, 13(9): 2285-2314. |
| 5 | 谌容, 王秋岩, 殷晓浦, 等. 醇脱氢酶不对称还原制备手性醇的研究进展[J].化工进展, 2011, 30(7): 1562-1569. |
| KAN R, WANG Q Y, YIN X P, et al. Progress of asymmetric reduction with alcohol dehydrogenase for preparation of chiral alcohols, [J].Chemical Industry and Engineering Progress, 2011, 30(7): 1562-1569. | |
| 6 | TRUPPO M D, POLLARD D, DEVINE P. Enzyme-catalyzed enantioselective diaryl ketone reductions[J]. Organic Letters, 2007, 9(2): 335-338. |
| 7 | NI Y, ZHOU J Y, SUN Z H. Production of a key chiral intermediate of betahistine with a newly isolated Kluyveromyces sp. in an aqueous two-phase system[J]. Process Biochemistry, 2012, 47(7): 1042-1048. |
| 8 | ZHU D M, YANG Y, MAJKOWICZ S, et al. Inverting the enantioselectivity of a carbonyl reductase via substrate-enzyme docking guiding point mutation[J]. Organic Letters, 2008, 10(4): 525-528. |
| 9 | LI H M, ZHU D M, HUA L, et al. Enantioselective reduction of diaryl ketones catalyzed by a carbonyl reductase from Sporobolomyces salmonicolor and its mutant enzymes[J]. Advanced Synthesis & Catalysis, 2009, 351(4): 583-588. |
| 10 | 唐铭烩, 许国超, 倪晔. 双芳基酮还原酶的基因挖掘及催化性质[J].食品与生物技术学报, 2018, 37(3): 240-249. |
| TANG M H, XU G C, NI Y. Genime mining and characterization of diaryl ketone reductase from kluyveromyces polysporus[J]. Journal of Food Science and Biotechnology, 2018, 37(3): 240-249. | |
| 11 | XU G C, WANG Y, TANG M H, et al. Hydroclassified combinatorial saturation mutagenesis: reshaping substrate binding pockets of KpADH for enantioselective reduction of bulky-bulky ketones[J]. ACS Catalysis, 2018, 8(9): 8336-8345. |
| 12 | ZHOU J Y, WANG Y, XU G C, et al. Structural insight into enantioselective inversion of an alcohol dehydrogenase reveals a “polar gate” in stereorecognition of diaryl ketones[J]. Journal of the American Chemical Society, 2018, 140(39): 12645-12654. |
| 13 | ARNOLD F H. Combinatorial and computational challenges for biocatalyst design[J]. Nature, 2001, 409(6817): 253-257. |
| 14 | BRANNIGAN J A, WILKINSON A J. Protein engineering 20 years on[J]. Nature Reviews Molecular Cell Biology, 2002, 3(12): 964-970. |
| 15 | 姜恬, 冯旭东, 李岩, 等. 底物特异性的生物催化与酶设计改造[J].化工进展, 2019, 38(1): 606-614. |
| JIANG T, FENG X D, LI Y, et al. The biocatalysis and enzyme modification of substrate specificity[J]. Chemical Industry and Engineering Progress, 2019, 38(1): 606-614. | |
| 16 | 冯旭东, 吕波, 李春, 等. 酶分子稳定性改造研究进展[J].化工学报, 2016, 67(1): 277-284. |
| FENG X D, LÜ B, LI C,et al. Advances in enzyme stability modification[J]. CIESC Journal, 2016, 67(1): 277-284. | |
| 17 | BETTS M J, RUSSELL R B. Bioinformatics for geneticists[M]. New York: Wiley, 2003: 291-315. |
| 18 | SUN Z T, LONSDALE R, KONG X D, et al. Reshaping an enzyme binding pocket for enhanced and inverted stereoselectivity: use of smallest amino acid alphabets in directed evolution[J]. Angewandte Chemie: International Edition, 2015, 54(42): 12410-12415. |
| 19 | KARA S, SPICKERMANN D, SCHEITTWIESER J H, et al. More efficient redox biocatalysis by utilising 1,4-butanediol as a ‘smart cosubstrate’[J]. Green Chemistry, 2013, 15(2): 330-335. |
| [1] | LI Huahua, LI Yihang, JIN Beichen, LI Longxin, CHENG Shao’an. Research progress of Anammox bio-electrochemical coupling wastewater treatment system [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2678-2690. |
| [2] | MENG Lingding, MAO Menglei, LIAO Qiyong, MENG Zihui, LIU Wenfang. Recent advance in stability of carbonic anhydrase and formate dehydrogenase [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 436-447. |
| [3] | GAO Bo, FENG Xudong, LI Chun. Visual and high-throughput method for detecting the activity of aspartate transcarbamylase [J]. Chemical Industry and Engineering Progress, 2022, 41(4): 2054-2059. |
| [4] | ZHANG Yan, WANG Wei, XIE Rui, JU Xiaojie, LIU Zhuang, CHU Liangyin. Controllable fabrication of polymeric microparticles loaded with enzyme@ZIF-8 [J]. Chemical Industry and Engineering Progress, 2022, 41(4): 2022-2028. |
| [5] | LI Qingyuan, WANG Chao, XU Shipei, ZHANG Xueqin, QIU Mingjian, LIU Mengyao, CONG Mengxiao. Research progress on reaction process and catalysts for PBS precursor of 1,4-butanediol synthesis [J]. Chemical Industry and Engineering Progress, 2022, 41(11): 5771-5782. |
| [6] | LU Zeping, PEI Xinhua, XUE Yu, ZHANG Xiaoguang, HU Yi. Chemical modification of porcine pancreatic lipase with betaine ionic liquid to improve its enzymatic properties [J]. Chemical Industry and Engineering Progress, 2022, 41(11): 6045-6052. |
| [7] | LI Qing, LIU Wujun, GUO Xiaojia, WANG Qian, ZHAO Zongbao. Chiral NAD analogs as cofactors for biocatalysis [J]. Chemical Industry and Engineering Progress, 2021, 40(9): 5214-5221. |
| [8] | ZHANG Xiaojian, LIU Qian, LIU Zhiqiang, ZHENG Yuguo. Stereoselective carbonyl reductases and their application in chiral alcohols synthesis [J]. Chemical Industry and Engineering Progress, 2021, 40(3): 1142-1160. |
| [9] | JU Shuyun, WU Jianping, YANG Lirong. Advances in the molecular modification and application of D-amino acid oxidase [J]. Chemical Industry and Engineering Progress, 2021, 40(3): 1215-1225. |
| [10] | Zhufan LIN, Shao’an CHENG, Zhengzhong MAO, Ruonan GU, Jiawei YANG. Recent advances in the construction and influencing factors of bio-electrochemical nitrogen removal systems [J]. Chemical Industry and Engineering Progress, 2020, 39(9): 3766-3776. |
| [11] | Tian JIANG, Xudong FENG, Yan LI, Chu LI. The biocatalysis and enzyme modification of substrate specificity [J]. Chemical Industry and Engineering Progress, 2019, 38(01): 606-614. |
| [12] | YU Bo, LIU Chao, LIU Jindong, DING Wanyu, CHAI Weiping. Preparation of mesoporous zirconium phosphate and its catalytic performace in the preparation of cellulose from glucose [J]. Chemical Industry and Engineering Progress, 2018, 37(06): 2236-2241. |
| [13] | YAN Xingchen, ZHAO Qianru, WANG Kaifeng, GUO Yuxin, JIANG Ling, HUANG He. Auto-induced expression of trehalose synthetase and novel process for catalytic production of trehalose [J]. Chemical Industry and Engineering Progress, 2018, 37(05): 1949-1955. |
| [14] | WANG Rui, XU Yaohui, WANG Kewei, WU Minchen. Expression of PvEH3,a Phaseolus vulgaris epoxide hydrolase,and synthesis of chiral vicinal diols [J]. Chemical Industry and Engineering Progress, 2018, 37(05): 1933-1939. |
| [15] | ZHOU Ya, YANG Chun. Degradation of 4-chloronitrobenzene by bioelectrochemical system [J]. Chemical Industry and Engineering Progress, 2018, 37(01): 375-380. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||