Chemical Industry and Engineering Progress ›› 2020, Vol. 39 ›› Issue (3): 1129-1136.DOI: 10.16085/j.issn.1000-6613.2019-1075
• Biochemical and pharmaceutical engineering • Previous Articles Next Articles
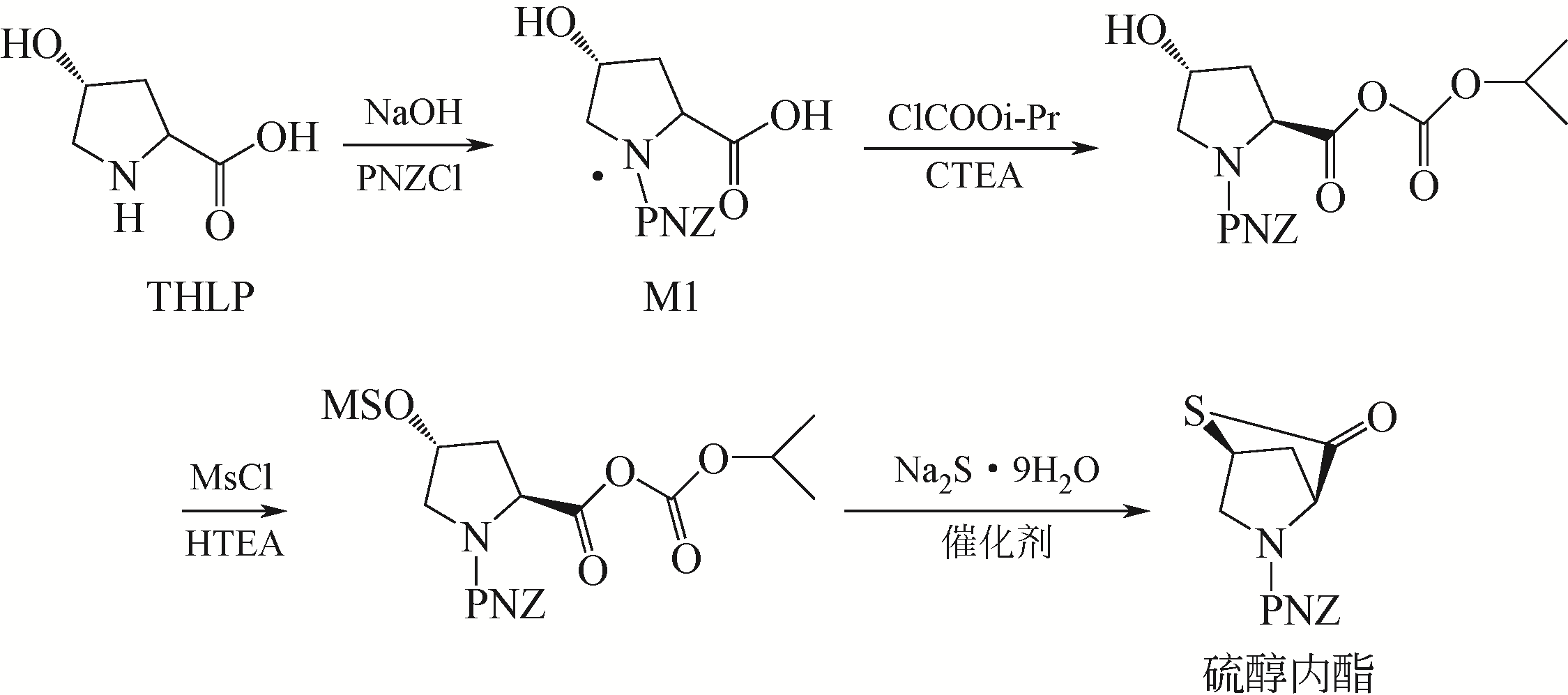
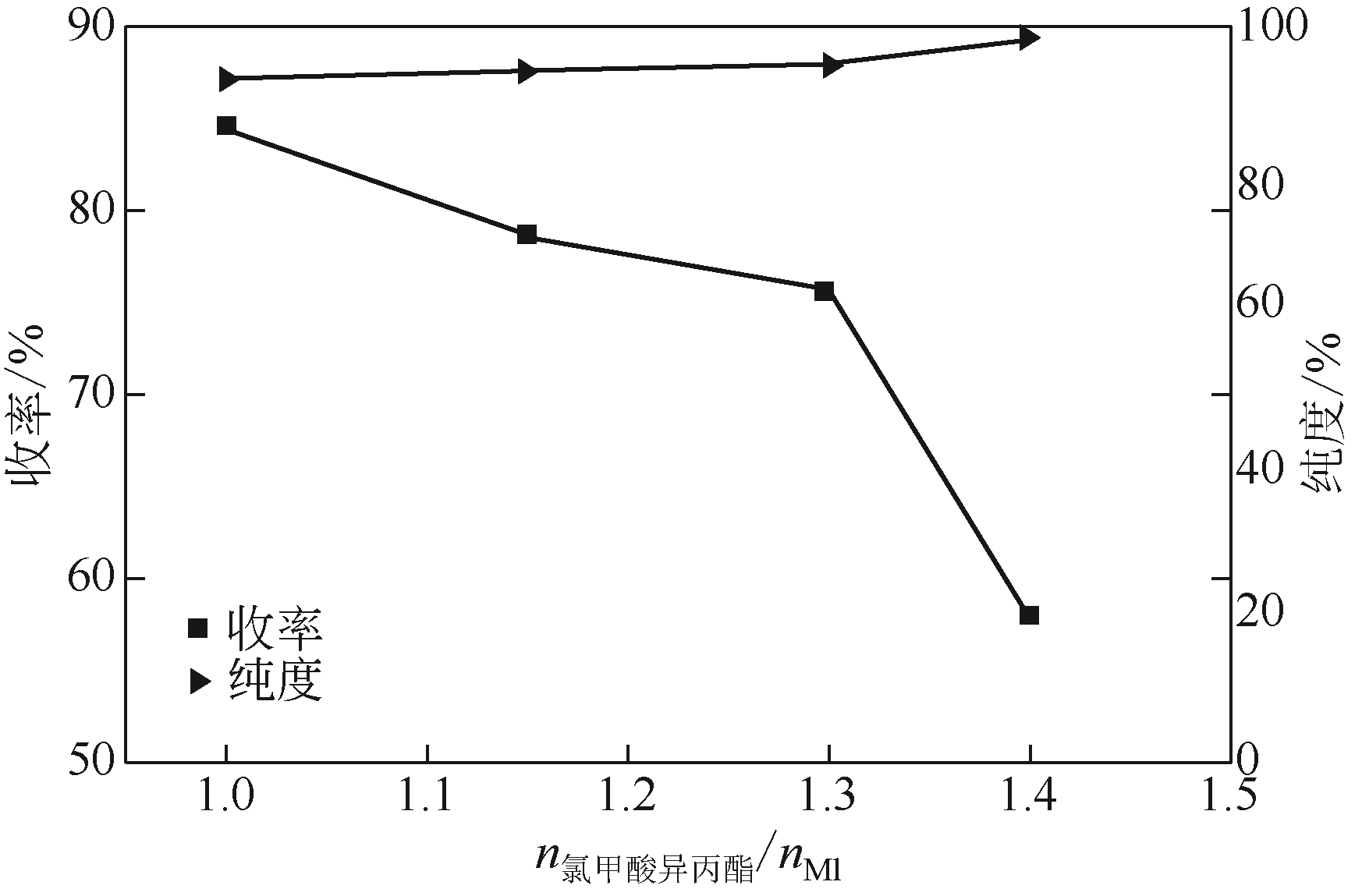
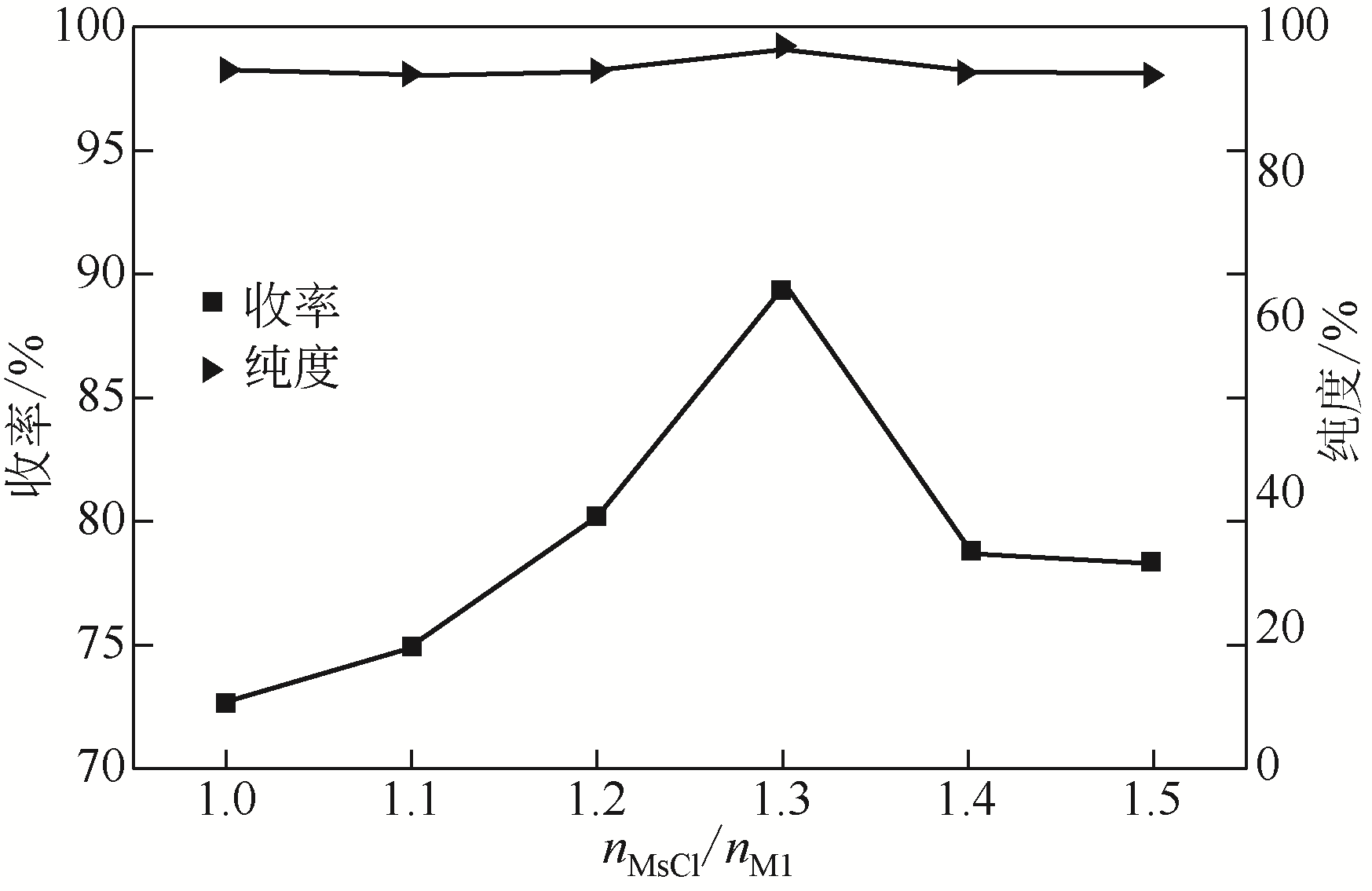
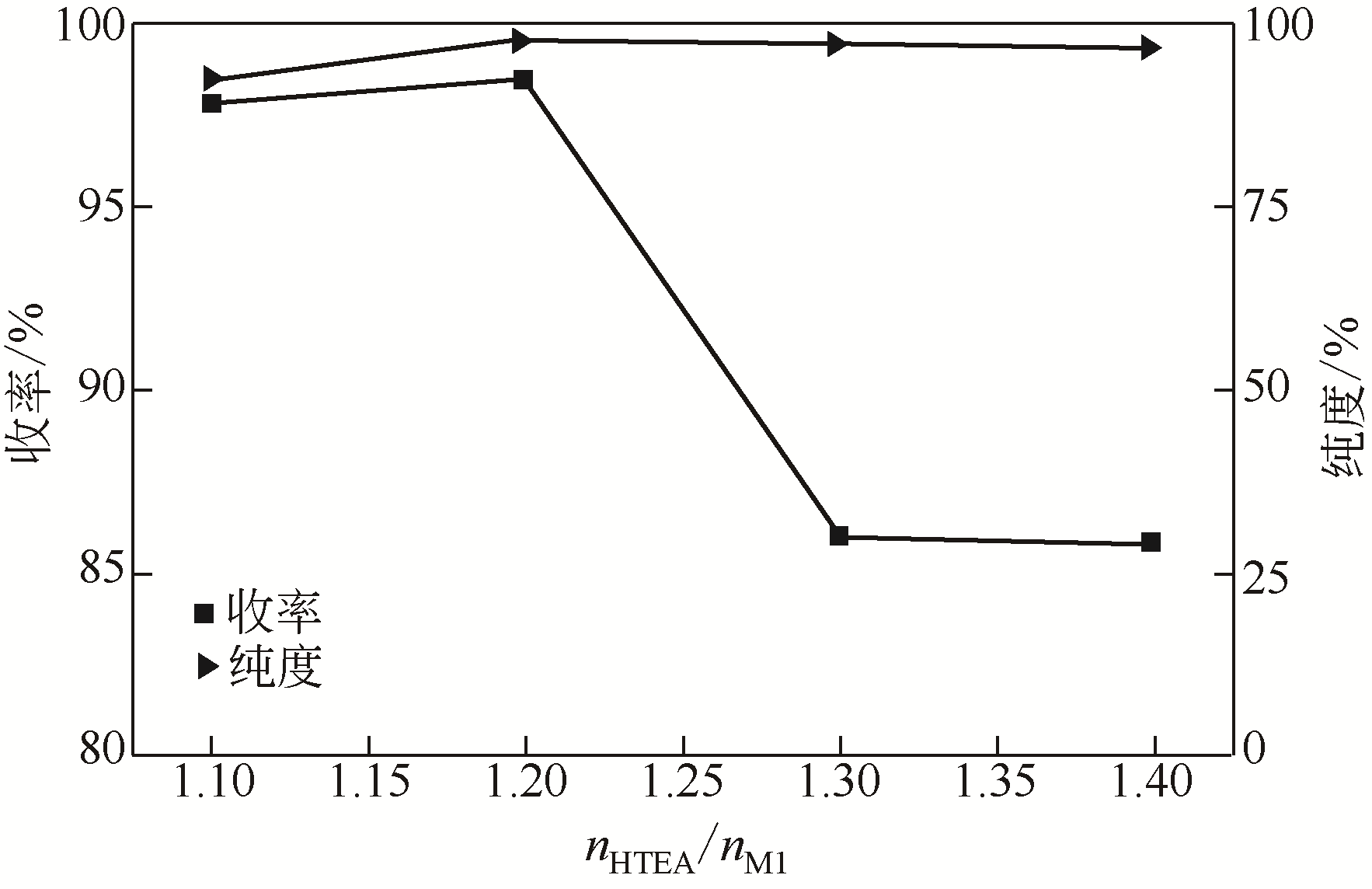
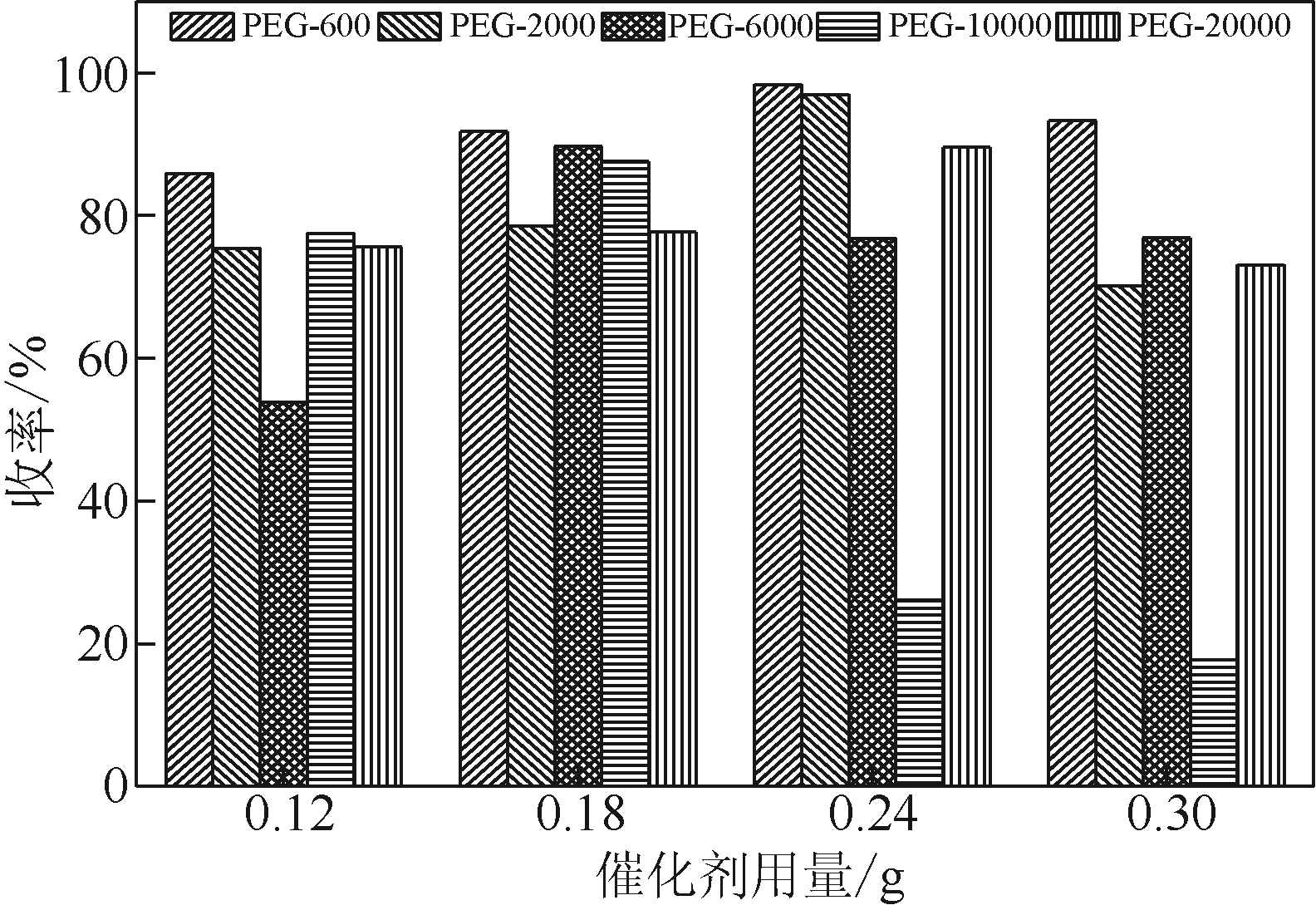
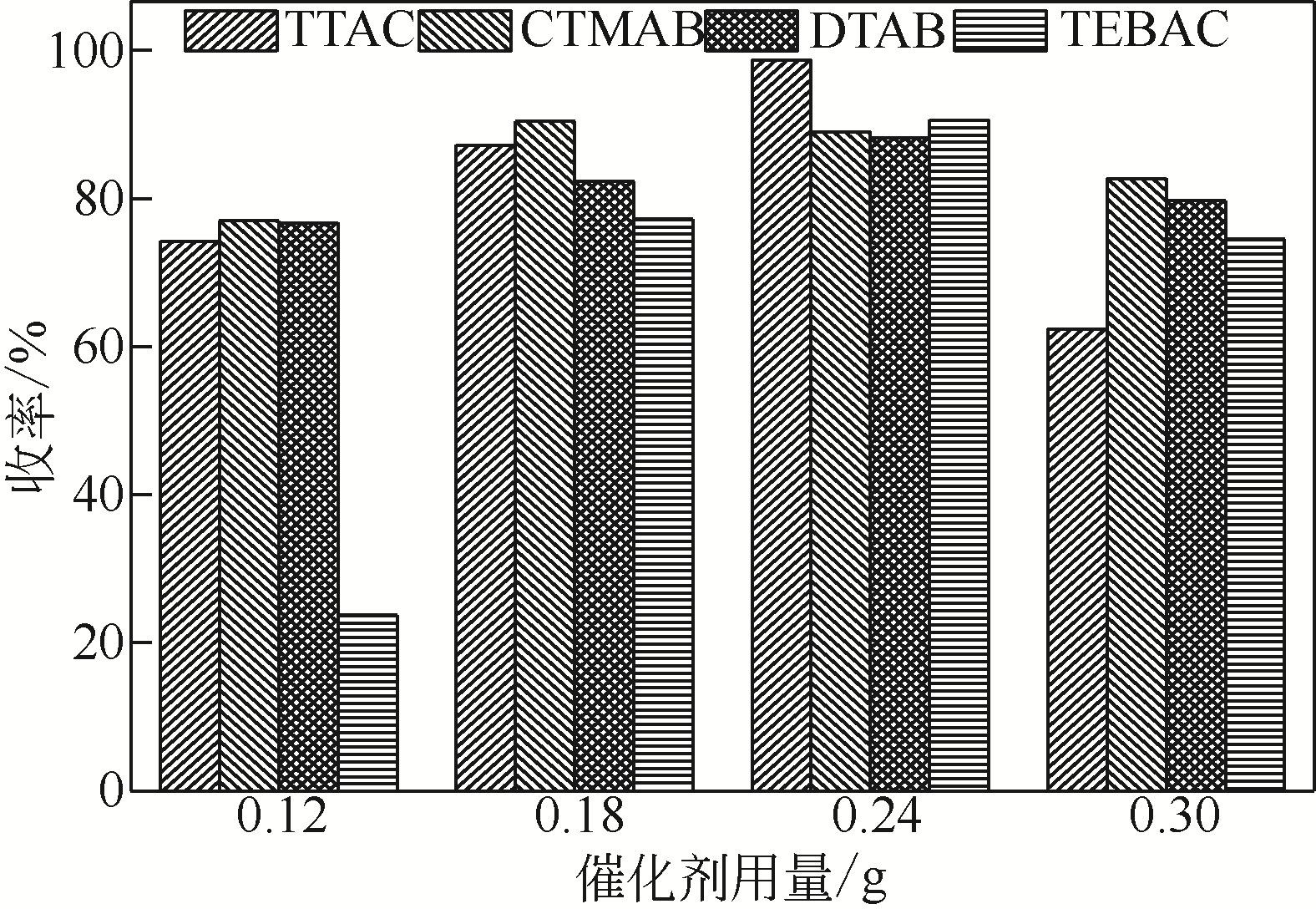
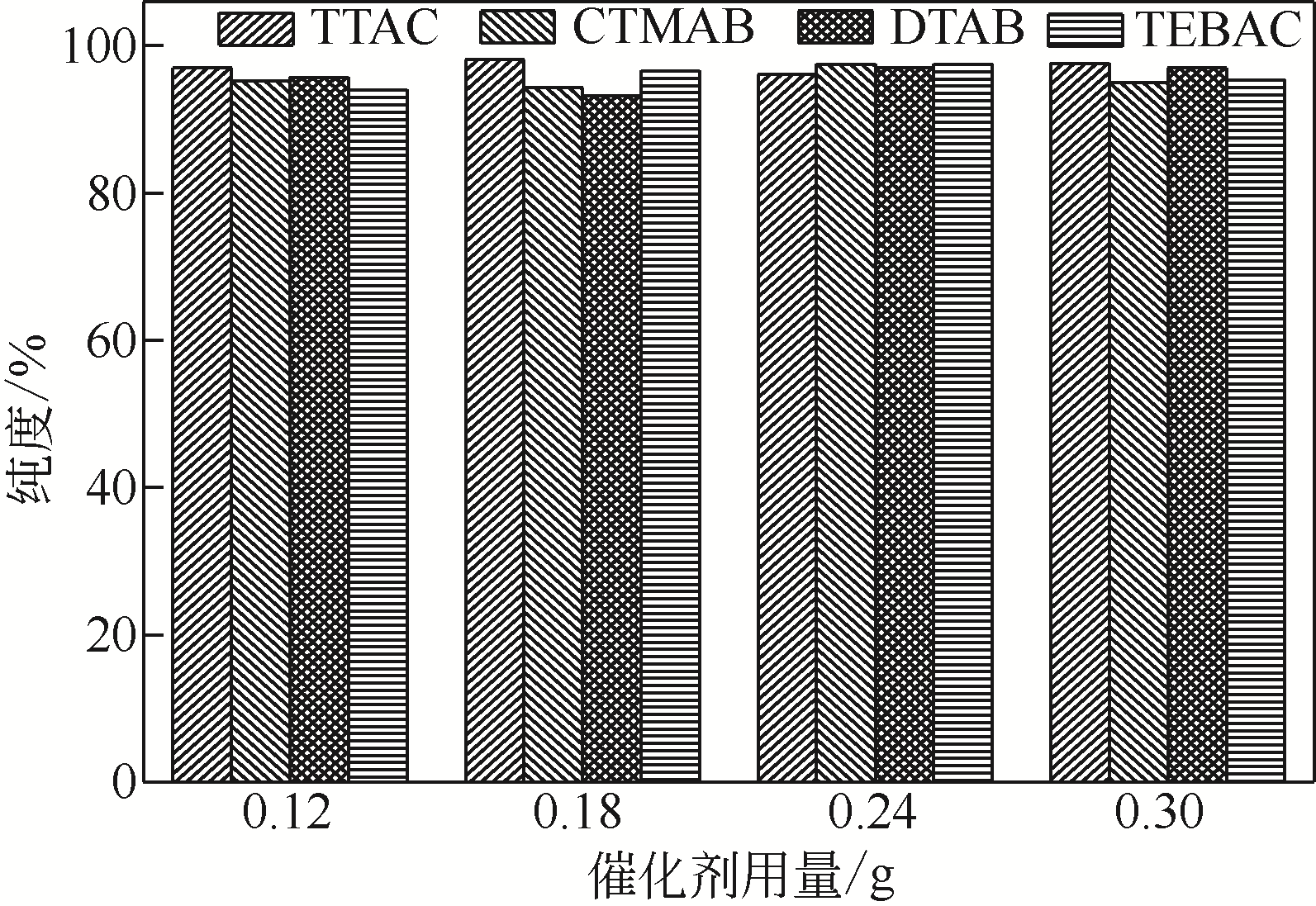
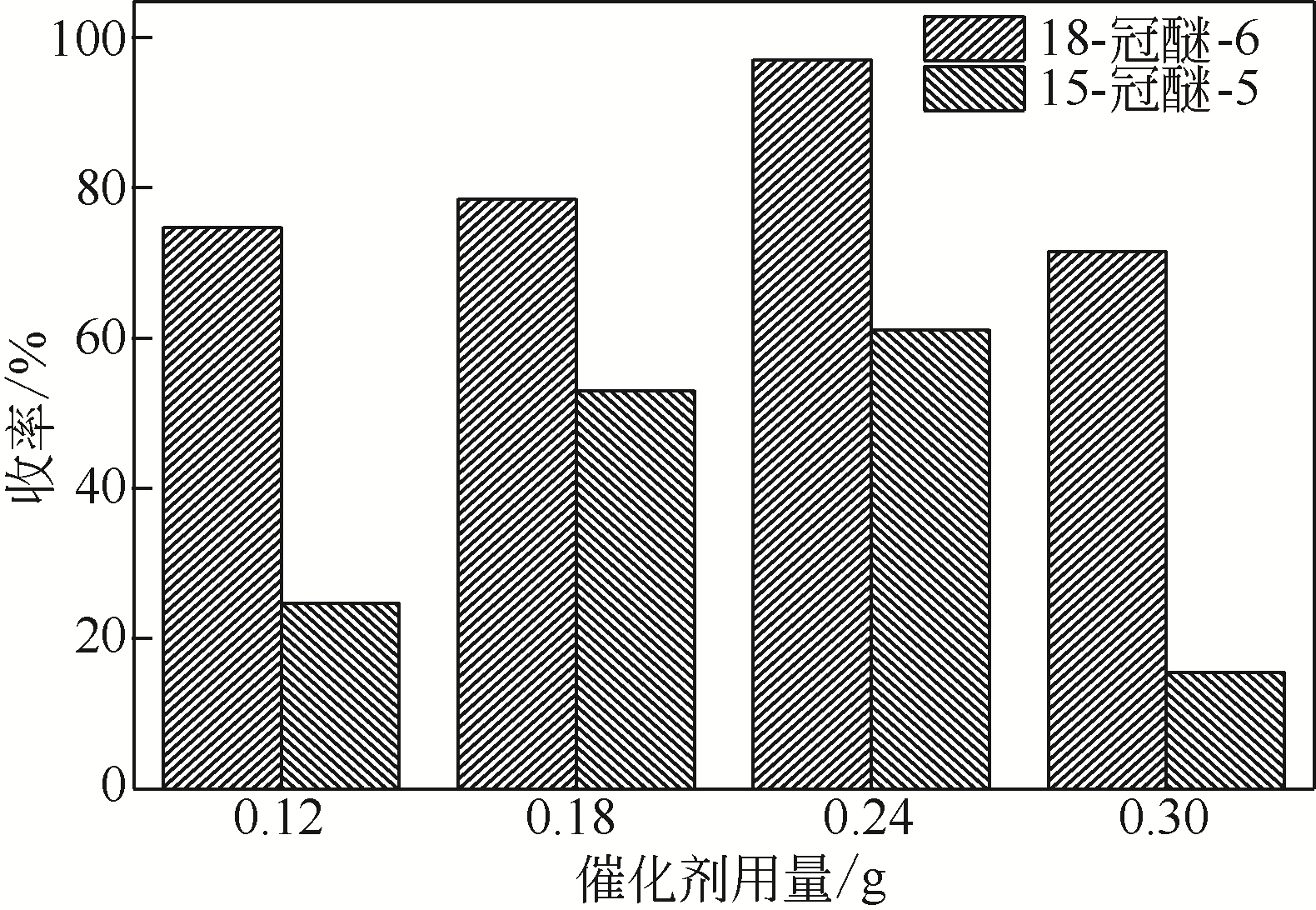
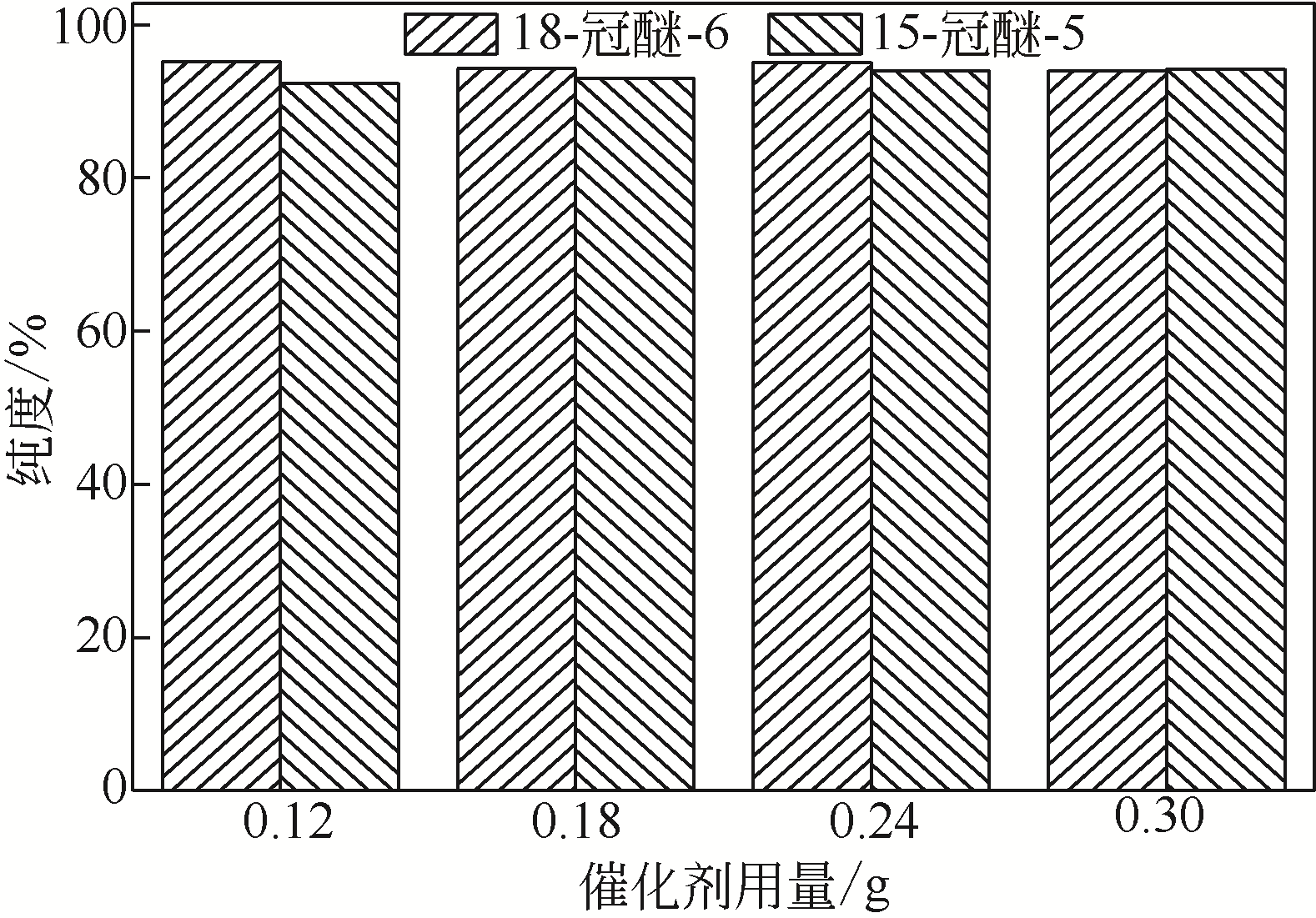
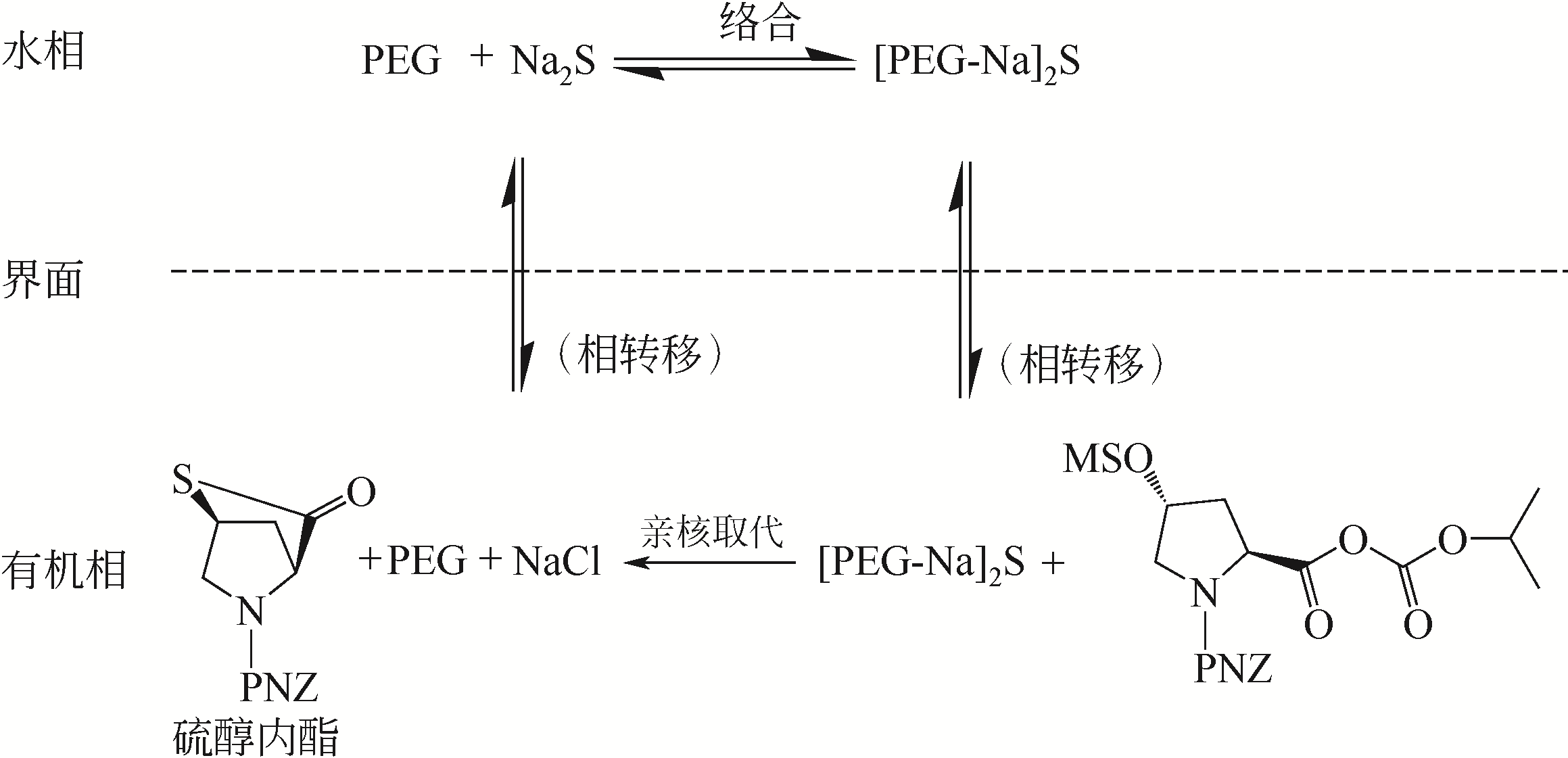
Synthesis of meropenem side chain intermediates with high yield
Bin LI1,2,3( ),Jixing SHI1,Shuang JIANG1,2(
),Jixing SHI1,Shuang JIANG1,2( ),Tianyong ZHANG1,2,3(
),Tianyong ZHANG1,2,3( ),Xiaokang LI1,Minghao ZHOU1,Yiwei LIU1
),Xiaokang LI1,Minghao ZHOU1,Yiwei LIU1
- 1.Tianjin Key Laboratory of Applied Catalysis Science and Technology, School of Chemical Engineering and Technology, Tianjin University, Tianjin 300354, China
2.Collaborative Innovation Center of Chemical Science and Engineering(Tianjin), Tianjin 300072, China
3.Tianjin Engineering Research Center of Functional Fine Chemicals, Tianjin 300354, China
高收率美罗培南侧链中间体的合成
李彬1,2,3( ),史继星1,姜爽1,2(
),史继星1,姜爽1,2( ),张天永1,2,3(
),张天永1,2,3( ),李小康1,周明浩1,刘艺炜1
),李小康1,周明浩1,刘艺炜1
- 1.天津大学化工学院,天津市应用催化科学与工程重点实验室,天津 300354
2.天津化学化工协同创新中心,天津 300072
3.天津市功能精细化学品技术工程中心,天津 300354