Chemical Industry and Engineering Progress ›› 2021, Vol. 40 ›› Issue (10): 5535-5546.DOI: 10.16085/j.issn.1000-6613.2020-2069
• Industrial catalysis • Previous Articles Next Articles
Recent advance in directing synthesis of aromatic hydrocarbon from syngas via oxygenated compound intermediates
SHANG Yunshan1( ), WANG Qianjin2, YANG Jiayi3, YUAN Delin1, ZHANG Fan1, LIU Hua1, XING Aihua1(
), WANG Qianjin2, YANG Jiayi3, YUAN Delin1, ZHANG Fan1, LIU Hua1, XING Aihua1( ), JI Shengfu4
), JI Shengfu4
- 1.National Institute of Clean-and-Low-Carbon Energy Beijing, Beijing 102209, China
2.School of Chemical & Environmental Engineering, China University of Mining and Technology-Beijing, Beijing 100083, China
3.Shenhua Ningxia Coal Industry Group Co. , Ltd. , Yinchuan 751400, Ningxia, China
4.School of Chemical Engineering, Beijing University of Chemical Technology, Beijing 100029, China
-
Received:2020-10-14Revised:2021-01-07Online:2021-10-25Published:2021-10-10 -
Contact:XING Aihua
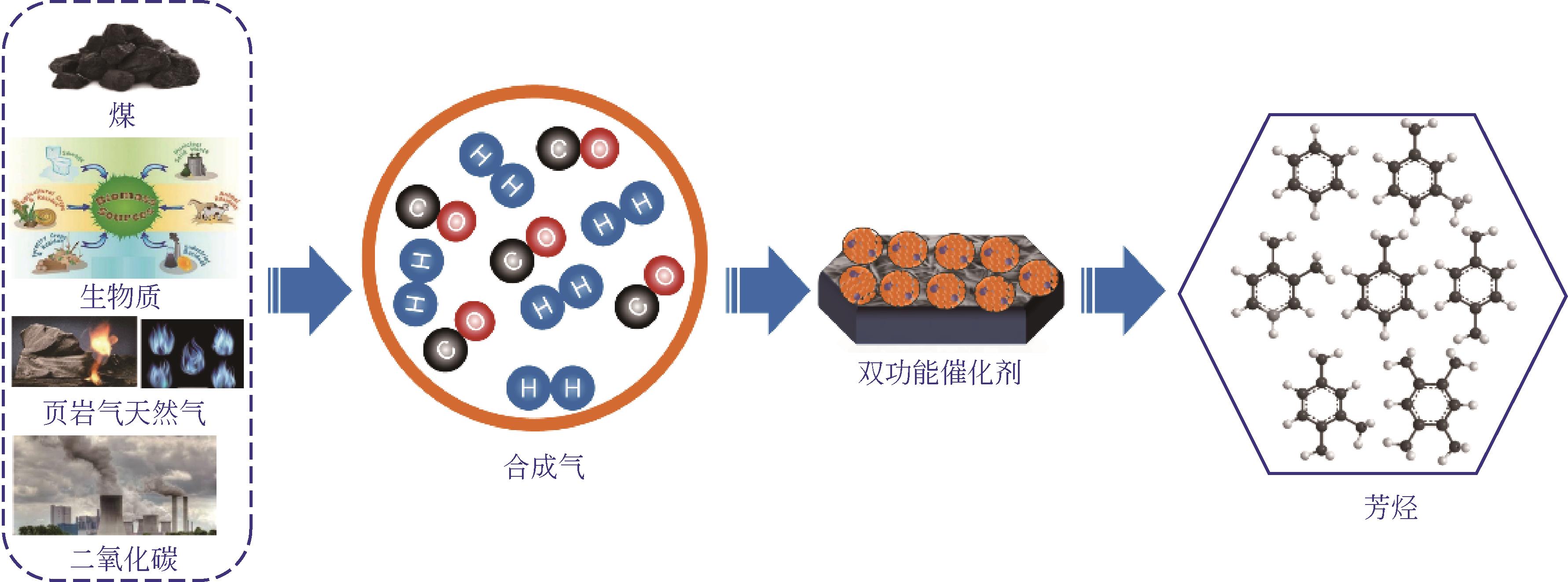
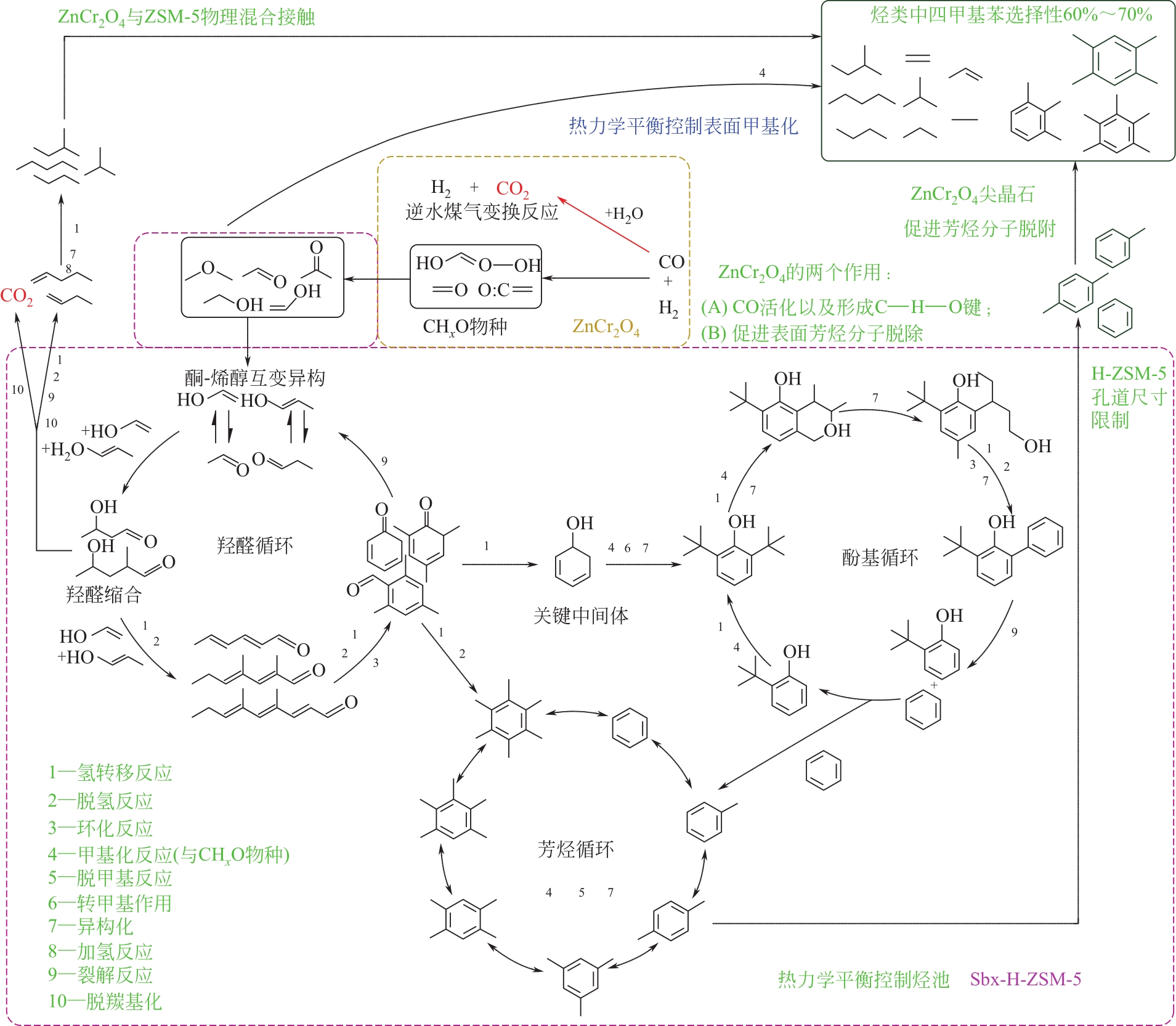
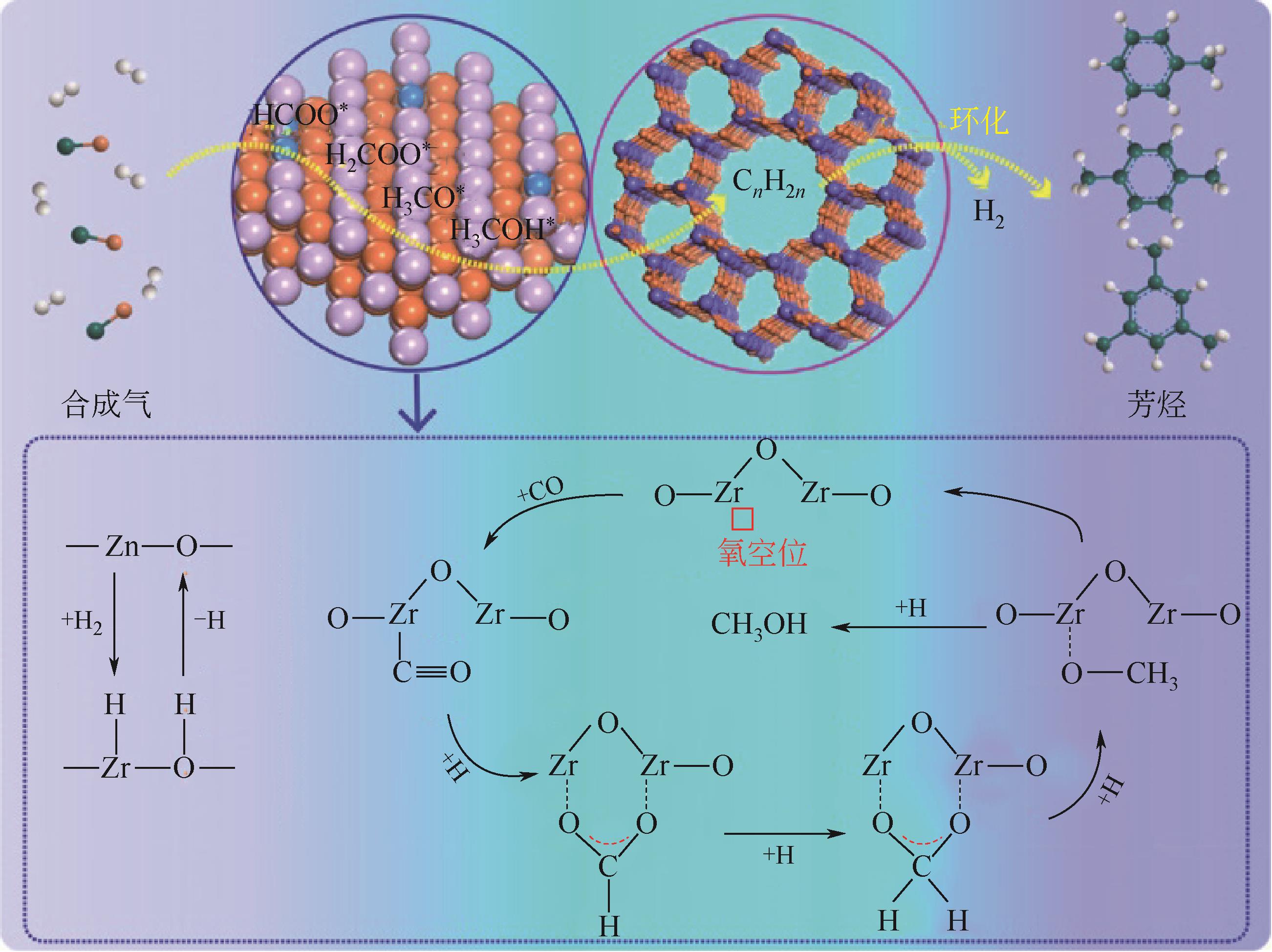
合成气经含氧化合物中间体一步法制芳烃研究进展
尚蕴山1( ), 王前进2, 杨加义3, 袁德林1, 张凡1, 刘华1, 邢爱华1(
), 王前进2, 杨加义3, 袁德林1, 张凡1, 刘华1, 邢爱华1( ), 季生福4
), 季生福4
- 1.北京低碳清洁能源研究院,北京 102209
2.中国矿业大学(北京)化学与环境工程学院,北京 100083
3.宁夏 煤业有限责任公司煤制油分公司,宁夏 银川 751400
4.北京化工大学化学工程学院,北京 100029
-
通讯作者:邢爱华 -
作者简介:尚蕴山(1986—),男,博士,研究方向为合成气一步法制高附加值烃类催化剂开发。E-mail:yunshan.shang@chnenergy.com.cn 。
CLC Number:
Cite this article
SHANG Yunshan, WANG Qianjin, YANG Jiayi, YUAN Delin, ZHANG Fan, LIU Hua, XING Aihua, JI Shengfu. Recent advance in directing synthesis of aromatic hydrocarbon from syngas via oxygenated compound intermediates[J]. Chemical Industry and Engineering Progress, 2021, 40(10): 5535-5546.
尚蕴山, 王前进, 杨加义, 袁德林, 张凡, 刘华, 邢爱华, 季生福. 合成气经含氧化合物中间体一步法制芳烃研究进展[J]. 化工进展, 2021, 40(10): 5535-5546.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2020-2069
| 氧化物 | 优点 | 缺点 |
|---|---|---|
| CuO-ZnO-Al2O3 | 活性高,CO转化率高,经济实惠 | 无法高温长周期运行,高温不可逆失活 |
| ZnCr2O4 | 活性适中,经济实惠 | Cr有毒,Zn在反应过程逐渐脱除晶体结构,稳定性差 |
| 金属掺杂ZrO2 | 热稳定好,潜在的再生性好 | 活性相对较低,价格昂贵,成本高 |
| 纳米ZnO | 经济、环保 | 活性低 |
| 氧化物 | 优点 | 缺点 |
|---|---|---|
| CuO-ZnO-Al2O3 | 活性高,CO转化率高,经济实惠 | 无法高温长周期运行,高温不可逆失活 |
| ZnCr2O4 | 活性适中,经济实惠 | Cr有毒,Zn在反应过程逐渐脱除晶体结构,稳定性差 |
| 金属掺杂ZrO2 | 热稳定好,潜在的再生性好 | 活性相对较低,价格昂贵,成本高 |
| 纳米ZnO | 经济、环保 | 活性低 |
| 催化剂组成 | 反应条件 | 转化率 /% | 选择性/% | 选择性(扣除CO2)/% | 中间体 | 参考 文献 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H2/CO比 | 压力 /MPa | 温度 /K | 空速 /mL·h-1·g-1 | CO2 | CH4 | C2~C4 | C5+ | 芳烃 | ||||
| ZnCr2O4&ZSM-5 | 1 | 8.3 | 700 | 1780 | 44.10 | 34.00 | 2.50 | 14.50 | 37.7 | 36.68 | —③ | [ |
| ZnCr2O4&ZSM-5 | 1 | 4 | 623 | 1500 | 16.00 | 48.00 | 1.70 | 15.10 | 2.7 | 73.90 | 乙烯酮 | [ |
| ZnCr2O4&Zn-Z5@S1 | 2.1 | 5 | 673 | 20.7 | 55.00 | 55.00 | 4.40 | 29.70 | 62.00 | 甲醇 | [ | |
| ZnCr2O4&ZSM-5 | 1 | 2 | 668 | 4000 | 11.10 | 40.70 | 2.50 | 24.10 | 72.40 | — | [ | |
| ZnCr2O4&Zn-ZSM-5 | 1 | 2 | 668 | 4000 | 13.20 | 40.00 | 2.10 | 24.40 | 73.50 | |||
| ZrO2&ZSM-5 | 1 | 3 | 673 | 500 | 26.20 | 12.50 | 24.50 | 38.20 | 37.4 | 53.00 | — | [ |
| Zn-ZrO2&ZSM-5 | 2 | 3 | 673 | 500 | 21.00 | 2.00 | <3.00 | 22.60 | 2.0 | 80.00 | 甲醇 | [ |
| CeZrO2&HZSM-5 | 1 | 2 | 653 | 3500 | 8.10 | 83.10 | 甲醇、C2+醇、 C6+烯烃 | [ | ||||
| CeZrO2&HZSM-5 | 1 | 2 | 723 | 3500 | 22.40 | 56.30 | ||||||
| Mo-ZrO2&H-ZSM-5 | 2 | 4 | 673 | 3000 | 22.00 | 41.00 | 3.40 | 18.00 | 2.7 | 76.00 | 甲醇 | [ |
| ZrO2&ZSM-5 | 1 | 6 | 623 | 3000 | 11.67 | 42.64 | 2.98 | 31.52 | 65.50 | 94.89① | 甲醇 | [ |
| ZrO2&ZSM-5 | 1 | 6 | 673 | 1200 | 21.59 | 44.27 | 22.90 | 22.07 | 55.30 | 95.13① | 甲醇 | [ |
| ZnZrO2@C&ZSM-5 | 2 | 3 | 633 | 3600 | 35.2 | 3.40 | 73.1 | 甲醇 | [ | |||
| ZnCr2O4&ZSM-5 | 1 | 2 | 668 | 4000 | 11.00 | 48.50 | 2.80 | 12.70 | 82.50 | 甲醇/乙烯酮 | [ | |
| CAZ&ZSM-5 | 1 | 2 | 633 | 500 | 71.90 | 86.80① | 甲醇 | [ | ||||
| CZA/Co-Nb&HZSM-5 | 2 | 4 | 603 | 1813 | 95.69 | 11.61 | 30.59 | 46.65 | 甲醇 | [ | ||
| CZA/Co-Nb&HZSM-5 | 2 | 4 | 623 | 1500 | 94.79 | 12.73 | 33.52 | 41.08 | ||||
| CZA&Nb-Ni-ZSM-5 | 2 | 4 | 603 | 1813 | 99.00 | 46.55 | ||||||
| ZnO&ZSM-5 | 1 | 3 | 603 | 750 | 14.80 | 80.10 | 甲醇 | [ | ||||
| MnCrOx&ZSM-5-USY | 1 | 4 | 703 | 1658 | 13.30 | 46.20 | 6.70 | 22.00 | 87.70② | — | [ | |
| 催化剂组成 | 反应条件 | 转化率 /% | 选择性/% | 选择性(扣除CO2)/% | 中间体 | 参考 文献 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H2/CO比 | 压力 /MPa | 温度 /K | 空速 /mL·h-1·g-1 | CO2 | CH4 | C2~C4 | C5+ | 芳烃 | ||||
| ZnCr2O4&ZSM-5 | 1 | 8.3 | 700 | 1780 | 44.10 | 34.00 | 2.50 | 14.50 | 37.7 | 36.68 | —③ | [ |
| ZnCr2O4&ZSM-5 | 1 | 4 | 623 | 1500 | 16.00 | 48.00 | 1.70 | 15.10 | 2.7 | 73.90 | 乙烯酮 | [ |
| ZnCr2O4&Zn-Z5@S1 | 2.1 | 5 | 673 | 20.7 | 55.00 | 55.00 | 4.40 | 29.70 | 62.00 | 甲醇 | [ | |
| ZnCr2O4&ZSM-5 | 1 | 2 | 668 | 4000 | 11.10 | 40.70 | 2.50 | 24.10 | 72.40 | — | [ | |
| ZnCr2O4&Zn-ZSM-5 | 1 | 2 | 668 | 4000 | 13.20 | 40.00 | 2.10 | 24.40 | 73.50 | |||
| ZrO2&ZSM-5 | 1 | 3 | 673 | 500 | 26.20 | 12.50 | 24.50 | 38.20 | 37.4 | 53.00 | — | [ |
| Zn-ZrO2&ZSM-5 | 2 | 3 | 673 | 500 | 21.00 | 2.00 | <3.00 | 22.60 | 2.0 | 80.00 | 甲醇 | [ |
| CeZrO2&HZSM-5 | 1 | 2 | 653 | 3500 | 8.10 | 83.10 | 甲醇、C2+醇、 C6+烯烃 | [ | ||||
| CeZrO2&HZSM-5 | 1 | 2 | 723 | 3500 | 22.40 | 56.30 | ||||||
| Mo-ZrO2&H-ZSM-5 | 2 | 4 | 673 | 3000 | 22.00 | 41.00 | 3.40 | 18.00 | 2.7 | 76.00 | 甲醇 | [ |
| ZrO2&ZSM-5 | 1 | 6 | 623 | 3000 | 11.67 | 42.64 | 2.98 | 31.52 | 65.50 | 94.89① | 甲醇 | [ |
| ZrO2&ZSM-5 | 1 | 6 | 673 | 1200 | 21.59 | 44.27 | 22.90 | 22.07 | 55.30 | 95.13① | 甲醇 | [ |
| ZnZrO2@C&ZSM-5 | 2 | 3 | 633 | 3600 | 35.2 | 3.40 | 73.1 | 甲醇 | [ | |||
| ZnCr2O4&ZSM-5 | 1 | 2 | 668 | 4000 | 11.00 | 48.50 | 2.80 | 12.70 | 82.50 | 甲醇/乙烯酮 | [ | |
| CAZ&ZSM-5 | 1 | 2 | 633 | 500 | 71.90 | 86.80① | 甲醇 | [ | ||||
| CZA/Co-Nb&HZSM-5 | 2 | 4 | 603 | 1813 | 95.69 | 11.61 | 30.59 | 46.65 | 甲醇 | [ | ||
| CZA/Co-Nb&HZSM-5 | 2 | 4 | 623 | 1500 | 94.79 | 12.73 | 33.52 | 41.08 | ||||
| CZA&Nb-Ni-ZSM-5 | 2 | 4 | 603 | 1813 | 99.00 | 46.55 | ||||||
| ZnO&ZSM-5 | 1 | 3 | 603 | 750 | 14.80 | 80.10 | 甲醇 | [ | ||||
| MnCrOx&ZSM-5-USY | 1 | 4 | 703 | 1658 | 13.30 | 46.20 | 6.70 | 22.00 | 87.70② | — | [ | |
| 1 | CHANG C D, SILVESTRI A J. The conversion of methanol and other O-compounds to hydrocarbons over zeolite catalysts[J]. Journal of Catalysis, 1977, 47(2): 249-259. |
| 2 | CHANG C D, LANG W H, SILVESTRI A J. Synthesis gas conversion to aromatic hydrocarbons[J]. Journal of Catalysis, 1979, 56(2): 268-273. |
| 3 | YAN Q, DOAN P T, TOGHIANI H, et al. Synthesis gas to hydrocarbons over CuO-CoO-Cr2O3/H-ZSM-5 bifunctional catalysts[J]. The Journal of Physical Chemistry C, 2008, 112(31): 11847-11858. |
| 4 | YANG J, PAN X, JIAO F, et al. Direct conversion of syngas to aromatics[J]. Chemical Communications, 2017, 53(81): 11146-11149. |
| 5 | ARSLAN M T, QURESHI B A, GILANI S Z A, et al. Single-step conversion of H2-deficient syngas into high yield of tetramethylbenzene[J]. ACS Catalysis, 2019, 9(3): 2203-2212. |
| 6 | XU Y, LIU J, WANG J, et al. Selective conversion of syngas to aromatics over Fe3O4@MnO2 and hollow HZSM-5 bifunctional catalysts[J]. ACS Catalysis, 2019, 9(6): 5147-5156. |
| 7 | ZHAO B, ZHAI P, WANG P, et al. Direct transformation of syngas to aromatics over Na-Zn-Fe5C2 and hierarchical HZSM-5 tandem catalysts[J]. Chem., 2017, 3(2): 323-333. |
| 8 | XU Y, WANG J, MA G, et al. Hollow zeolite nanoparticles combined with Fe3O4@MnO2 tandem catalyst for converting syngas to aromatics-rich gasoline[J]. ACS Applied Nano Materials, 2020, 3(3): 2857-2866. |
| 9 | SUN T, LIN T, AN Y, et al. Syngas conversion to aromatics over the Co2C-based catalyst and HZSM-5 via a tandem system[J]. Industrial & Engineering Chemistry Research, 2020, 59(10): 4419-4427. |
| 10 | LI M, NAWAZ M A, SONG G, et al. Influential role of elemental migration in a composite iron-zeolite catalyst for the synthesis of aromatics from syngas[J]. Industrial & Engineering Chemistry Research, 2020, 59(19): 9043-9054. |
| 11 | WANG Y, ZHAN W, CHEN Z, et al. Advanced 3D hollow-out ZnZrO@C combined with hierarchical zeolite for highly active and selective CO hydrogenation to aromatics[J]. ACS Catalysis, 2020, 10(13): 7177-7187. |
| 12 | YANG X, SUN T, MA J, et al. The Influence of intimacy on the ‘iterative reactions’ during OX-ZEO process for aromatic production[J]. Journal of Energy Chemistry, 2019, 35: 60-65. |
| 13 | YANG J, GONG K, MIAO D, et al. Enhanced aromatic selectivity by the sheet-like ZSM-5 in syngas conversion[J]. Journal of Energy Chemistry, 2019, 35: 44-48. |
| 14 | LIU C, LIU S, ZHOU H, et al. Selective conversion of syngas to aromatics over metal oxide/HZSM-5 catalyst by matching the activity between CO hydrogenation and aromatization[J]. Applied Catalysis A: General, 2019, 585: 117206. |
| 15 | ZHANG P, TAN L, YANG G, et al. One-pass selective conversion of syngas to para-xylene[J]. Chemical Science, 2017, 8(12): 7941-7946. |
| 16 | SONG W, HOU Y, CHEN Z, et al. Process simulation of the syngas-to-aromatics processes: technical economics aspects[J]. Chemical Engineering Science, 2020, 212: 115328. |
| 17 | YANG X, SU X, CHEN D, et al. Direct conversion of syngas to aromatics: a review of recent studies[J]. Chinese Journal of Catalysis, 2020, 41(4): 561-573. |
| 18 | KASIPANDI S, BAE J W. Recent advances in direct synthesis of value-added aromatic chemicals from syngas by cascade reactions over bifunctional catalysts[J]. Advanced Materials, 2019, 31(34): 1803390. |
| 19 | NIMZ M, LIETZ G, VöLTER J, et al. Direct conversion of syngas to aromatics on FePd/SiO2 catalyst[J]. Catalysis Letters, 1988, 1(4): 93-98. |
| 20 | FU Y, NI Y, ZHU W, et al. Enhancing syngas-to-aromatics performance of ZnO&H-ZSM-5 composite catalyst via Mn modulation[J]. Journal of Catalysis, 2020, 383: 97-102. |
| 21 | 杨成, 张成华, 许健, 等. 氧化锆催化合成气直接转化制芳烃[J]. 燃料化学学报, 2016, 44(7): 837-844. |
| YANG Cheng, ZHANG Chenghua, XU Jian, et al. One-step catalytic conversion of syngas to aromatics over ZrO2 catalyst[J]. Journal of Fuel Chemistry and Technology, 2016, 44(7): 837-844. | |
| 22 | LIU J, HE Y, YAN L, et al. Nano-sized ZrO2 derived from metal-organic frameworks and their catalytic performance for aromatic synthesis from syngas[J]. Catalysis Science & Technology, 2019, 9(11): 2982-2992. |
| 23 | LIU J, HE Y, YAN L, et al. Nano-ZrO2 as hydrogenation phase in bi-functional catalyst for syngas aromatization[J]. Fuel, 2020, 263: 116803. |
| 24 | GILANI S Z A, LU L, ARSLAN M T, et al. Two-way desorption coupling to enhance the conversion of syngas into aromatics by MnO/H-ZSM-5[J]. Catalysis Science & Technology, 2020, 10: 3366-3375. |
| 25 | YANG T, CHENG L, LI N, et al. Effect of metal active sites on the product distribution over composite catalysts in the direct synthesis of aromatics from syngas[J]. Industrial & Engineering Chemistry Research, 2017, 56(41): 11763-11772. |
| 26 | CHENG K, ZHOU W, KANG J, et al. Bifunctional catalysts for one-step conversion of syngas into aromatics with excellent selectivity and stability[J]. Chem., 2017, 3(2): 334-347. |
| 27 | HUANG Z, WANG S, QIN F, et al. Ceria-zirconia/zeolite bifunctional catalyst for highly selective conversion of syngas into aromatics[J]. ChemCatChem, 2018, 10(20): 4519-4524. |
| 28 | ZHOU W, SHI S, WANG Y, et al. Selective conversion of syngas to aromatics over a Mo-ZrO2/H-ZSM-5 bifunctional catalyst[J]. ChemCatChem, 2019, 11(6): 1681-1688. |
| 29 | MIAO D, DING Y, YU T, et al. Selective synthesis of benzene, toluene, and xylenes from syngas[J]. ACS Catalysis, 2020: 7389-7397. |
| 30 | SANTOS V P, POLLEFEYT G, YANCEY D F, et al. Direct conversion of syngas to light olefins (C2-C3) over a tandem catalyst CrZn-SAPO-34: tailoring activity and stability by varying the Cr/Zn ratio and calcination temperature[J]. Journal of Catalysis, 2020, 381, 108-120. |
| 31 | ZHOU C, SHI J, ZHOU W, et al. Highly active ZnO-ZrO2 aerogels integrated with H-ZSM-5 for aromatics synthesis from carbon dioxide[J]. ACS Catalysis, 2020, 10(1): 302-310. |
| 32 | ZHOU W, ZHOU C, YIN H, et al. Direct conversion of syngas into aromatics over a bifunctional catalyst: inhibiting net CO2 release[J]. Chemical Communications, 2020, 56(39): 5239-5242. |
| 33 | SONG H, LAUDENSCHLEGER D, CAREY J J, et al. Spinel-structured ZnCr2O4 with excess Zn is the active ZnO/Cr2O3 catalyst for high-temperature methanol synthesis[J]. ACS Catalysis, 2017, 7(11): 7610-7622. |
| 34 | HUANG Y, QIAN W, MA H, et al. Impact of Zn/Cr ratio on ZnCrOx-SAPO-34 bifunctional catalyst for direct conversion of syngas to light olefins[J]. International Journal of Chemical and Molecular Engineering, 2018, 12(10): 557-563. |
| 35 | WANG X, CAO R, CHEN K, et al. Synthesis gas conversion to lower olefins over ZnCr-SAPO-34 catalysts: role of ZnO-ZnCr2O4 interface[J]. ChemCatChem, 2020, 12(17): 4387-4395. |
| 36 | KOUVA S, HONKALA K, LEFFERTS L, et al. Review: monoclinic zirconia, its surface sites and their interaction with carbon monoxide[J]. Catalysis Science & Technology, 2015, 5(7): 3473-3490. |
| 37 | PIERO G D, TRIFIRO F, VACCARI A. Non-stoicheiometric Zn-Cr spinel as active phase in the catalytic synthesis of methanol[J]. Journal of the Chemical Society, Chemical Communications, 1984(10): 656-658. |
| 38 | TIAN S, DING S, YANG Q, et al. The role of non-stoichiometric spinel for iso-butanol formation from biomass syngas over Zn-Cr based catalysts [J]. RSC Advances, 2017, 7(33): 20135-20145. |
| 39 | BERTOLDI M, FUBINI B, GIAMELLO E, et al. Structure and reactivity of zinc-chromium mixed oxides. Part 1. The role of non-stoichiometry on bulk and surface properties[J]. Journal of the Chemical Society, Faraday Transactions 1: Physical Chemistry in Condensed Phases, 1988, 84(5): 1405-1421. |
| 40 | ARSLAN M T, ALI B, GILANI S Z A, et al. Selective conversion of syngas into tetramethylbenzene via an aldol-aromatic mechanism[J]. ACS Catalysis, 2020, 10(4): 2477-2488. |
| 41 | TAN L, WANG F, ZHANG P, et al. Design of a core-shell catalyst: an effective strategy for suppressing side reactions in syngas for direct selective conversion to light olefins[J]. Chemical Science, 2020, 11(16): 4097-4105. |
| 42 | FUJIMOTO K, KUDO Y, TOMINAGA H O. Synthesis gas conversion utilizing mixed catalyst composed of CO reducing catalyst and solid acid: Ⅱ. Direct synthesis of aromatic hydrocarbons from synthesis gas[J]. Journal of Catalysis, 1984, 87(1): 136-143. |
| 43 | JIAO F, LI J, PAN X, et al. Selective conversion of syngas to light olefins[J]. Science, 2016, 351(6277): 1065-1068. |
| 44 | MA Y, CAI D, LI Y, et al. The influence of straight pore blockage on the selectivity of methanol to aromatics in nanosized Zn/ZSM-5: an atomic Cs-corrected stem analysis study[J]. RSC Advances, 2016, 6(78): 74797-74801. |
| 45 | EL-MALKI E M, SANTEN R A VAN, SACHTLER W M H. Introduction of Zn, Ga, and Fe into HZSM-5 cavities by sublimation: identification of acid sites[J]. The Journal of Physical Chemistry B, 1999, 103(22): 4611-4622. |
| 46 | ONO Y, ADACHI H, SENODA Y. Selective conversion of methanol into aromatic hydrocarbons over zinc-exchanged ZSM-5 zeolites[J]. Journal of the Chemical Society, Faraday Transactions 1: Physical Chemistry in Condensed Phases, 1988, 84(4): 1091-1099. |
| 47 | YU B, DING C, WANG J, et al. Dual effects of zinc species on active sites in bifunctional composite catalysts Zr/H[Zn]ZSM-5 for alkylation of benzene with syngas[J]. The Journal of Physical Chemistry C, 2019, 123(31): 18993-19004. |
| 48 | 冯丽梅, 徐亚荣, 张力, 等. 甲醇芳构化反应的热力学研究[J]. 石化技术与应用, 2017, 35(2): 101-105. |
| FENG Limei, XU Yarong, ZHANG Li, et al. Study on thermodynamics of methanol to aromatic reaction[J]. Petrochemical Technology & Application, 2017, 35(2): 101-105. | |
| 49 | CHEN Zhiyang, NI Youming, ZHI Yuchun, et al. Coupling of methanol and carbon monoxide over H-ZSM-5 to form aromatics[J]. Angewandte Chemie International Edition, 2018, 57(38): 12549-12553. |
| [1] | YANG Xiazhen, PENG Yifan, LIU Huazhang, HUO Chao. Regulation of active phase of fused iron catalyst and its catalytic performance of Fischer-Tropsch synthesis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 310-318. |
| [2] | ZHENG Qian, GUAN Xiushuai, JIN Shanbiao, ZHANG Changming, ZHANG Xiaochao. Photothermal catalysis synthesis of DMC from CO2 and methanol over Ce0.25Zr0.75O2 solid solution [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 319-327. |
| [3] | WANG Zhengkun, LI Sifang. Green synthesis of gemini surfactant decyne diol [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 400-410. |
| [4] | WANG Lele, YANG Wanrong, YAO Yan, LIU Tao, HE Chuan, LIU Xiao, SU Sheng, KONG Fanhai, ZHU Canghai, XIANG Jun. Influence of spent SCR catalyst blending on the characteristics and deNO x performance for new SCR catalyst [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 489-497. |
| [5] | DENG Liping, SHI Haoyu, LIU Xiaolong, CHEN Yaoji, YAN Jingying. Non-noble metal modified vanadium titanium-based catalyst for NH3-SCR denitrification simultaneous control VOCs [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 542-548. |
| [6] | GENG Yuanze, ZHOU Junhu, ZHANG Tianyou, ZHU Xiaoyu, YANG Weijuan. Homogeneous/heterogeneous coupled combustion of heptane in a partially packed bed burner [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4514-4521. |
| [7] | WANG Jinhang, HE Yong, SHI Lingli, LONG Zhen, LIANG Deqing. Progress of gas hydrate anti-agglomerants [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4587-4602. |
| [8] | GAO Yanjing. Analysis of international research trend of single-atom catalysis technology [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4667-4676. |
| [9] | XU Zhongshuo, ZHOU Panpan, WANG Yuhui, HUANG Wei, SONG Xinshan. Advances in sulfur iron ore mediated autotrophic denitrification [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4863-4871. |
| [10] | LI Dongze, ZHANG Xiang, TIAN Jian, HU Pan, YAO Jie, ZHU Lin, BU Changsheng, WANG Xinye. Research progress of NO x reduction by carbonaceous substances for denitration in cement kiln [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4882-4893. |
| [11] | SONG Weitao, SONG Huiping, FAN Zhenlian, FAN Biao, XUE Fangbin. Research progress of fly ash in anti-corrosion coatings [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4894-4904. |
| [12] | WANG Chen, BAI Haoliang, KANG Xue. Performance study of high power UV-LED heat dissipation and nano-TiO2 photocatalytic acid red 26 coupling system [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4905-4916. |
| [13] | LI Xin, YANG Zao, ZHONG Xinru, HAN Haoxuan, ZHUANG Xuning, BAI Jianfeng, DONG Bin, XU Zuxin. Binding mechanism of Pb2+ onto humic acids from sludge hyper-thermophilic composting [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4957-4966. |
| [14] | YANG Han, ZHANG Yibo, LI Qi, ZHANG Jun, TAO Ying, YANG Quanhong. Practical carbon anodes for sodium-ion batteries: progress and challenge [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4029-4042. |
| [15] | HUANG Yufei, LI Ziyi, HUANG Yangqiang, JIN Bo, LUO Xiao, LIANG Zhiwu. Research progress on catalysts for photocatalytic CO2 and CH4 reforming [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4247-4263. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||