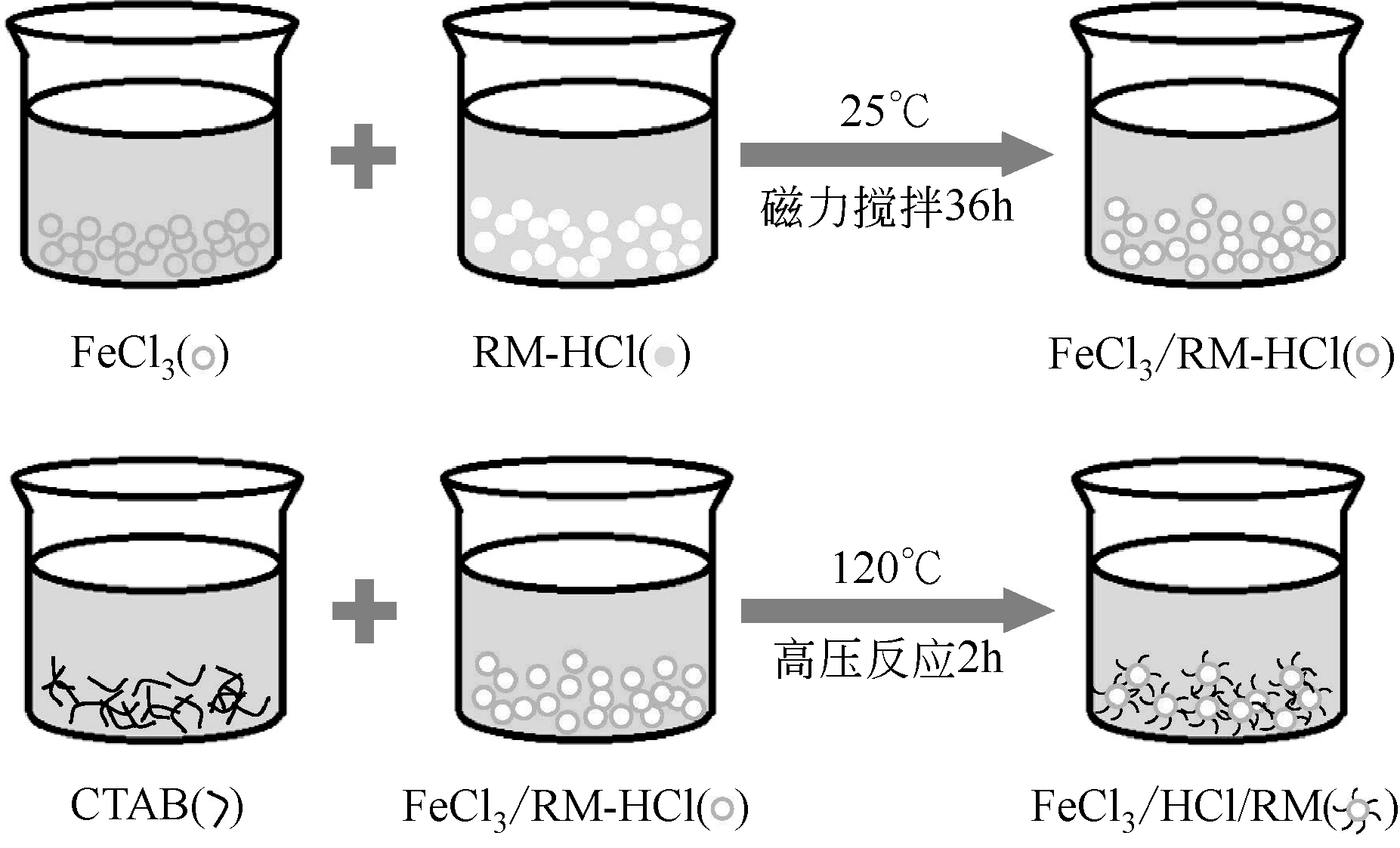
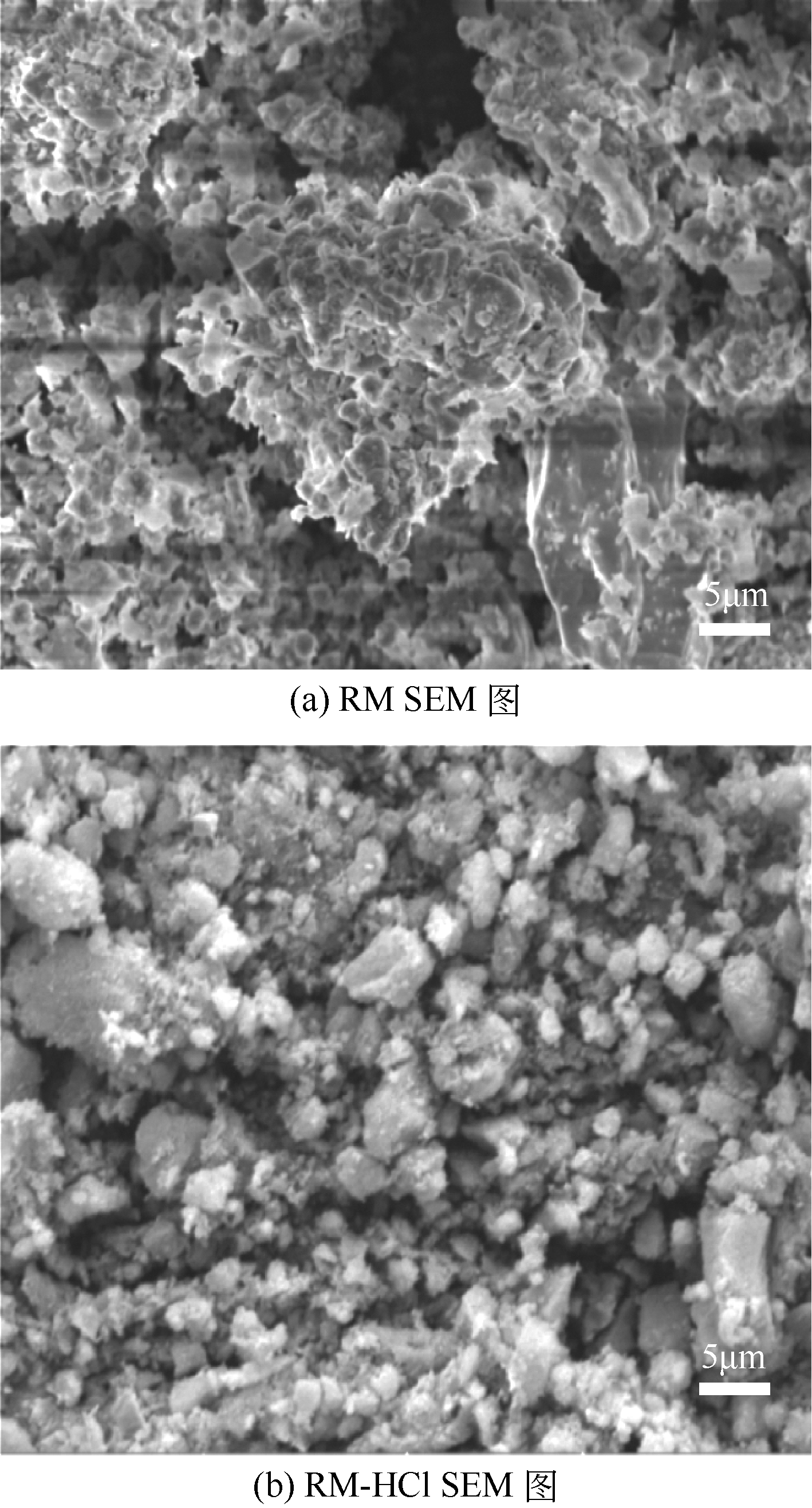
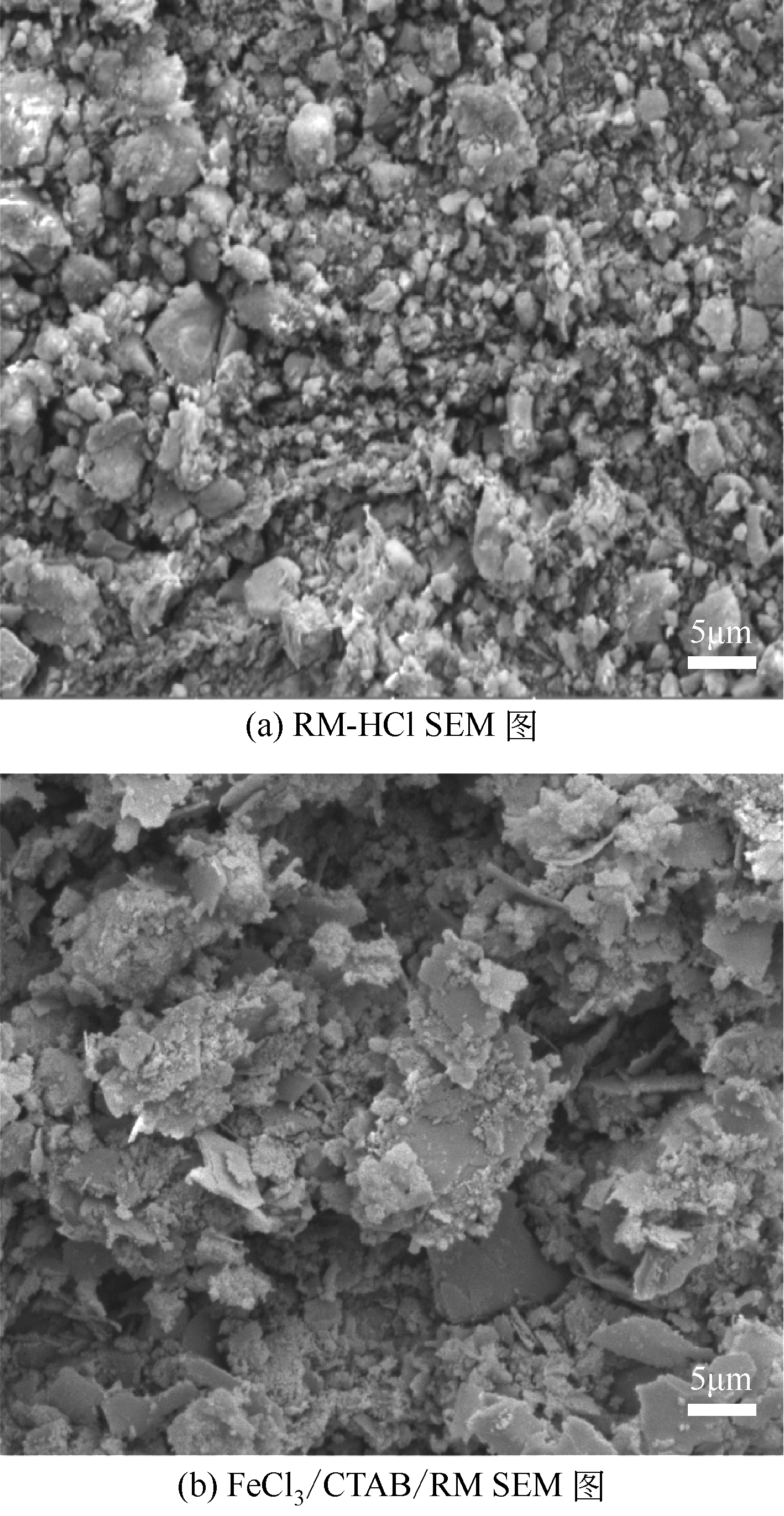
Chemical Industry and Engineering Progress ›› 2020, Vol. 39 ›› Issue (2): 776-789.DOI: 10.16085/j.issn.1000-6613.2019-0788
• Resources and environmental engineering • Previous Articles Next Articles
Adsorption and mechanism of copper ions in water by red mud modified with FeCl3 and hexadecyl trimethyl ammonium bromide (CTAB)
Jianglong LIU( ),Yan GUO(
),Yan GUO( ),Yihui XI
),Yihui XI
- Department of Environmental Engineering and Technology, Xi’an Jiaotong University, Xi’an 710049, Shaanxi, China
-
Received:2019-05-14Online:2020-03-12Published:2020-02-05 -
Contact:Yan GUO
FeCl3和十六烷基三甲基溴化铵改性赤泥对水中铜离子的吸附性能和机理
- 西安交通大学环境科学与工程系,陕西 西安 710049
-
通讯作者:郭焱 -
作者简介:刘江龙(1995—),男,硕士研究生,研究方向为废水处理及固体废弃物资源化处理技术。E-mail:245650606@qq.com 。
CLC Number:
Cite this article
Jianglong LIU,Yan GUO,Yihui XI. Adsorption and mechanism of copper ions in water by red mud modified with FeCl3 and hexadecyl trimethyl ammonium bromide (CTAB)[J]. Chemical Industry and Engineering Progress, 2020, 39(2): 776-789.
刘江龙,郭焱,席艺慧. FeCl3和十六烷基三甲基溴化铵改性赤泥对水中铜离子的吸附性能和机理[J]. 化工进展, 2020, 39(2): 776-789.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2019-0788
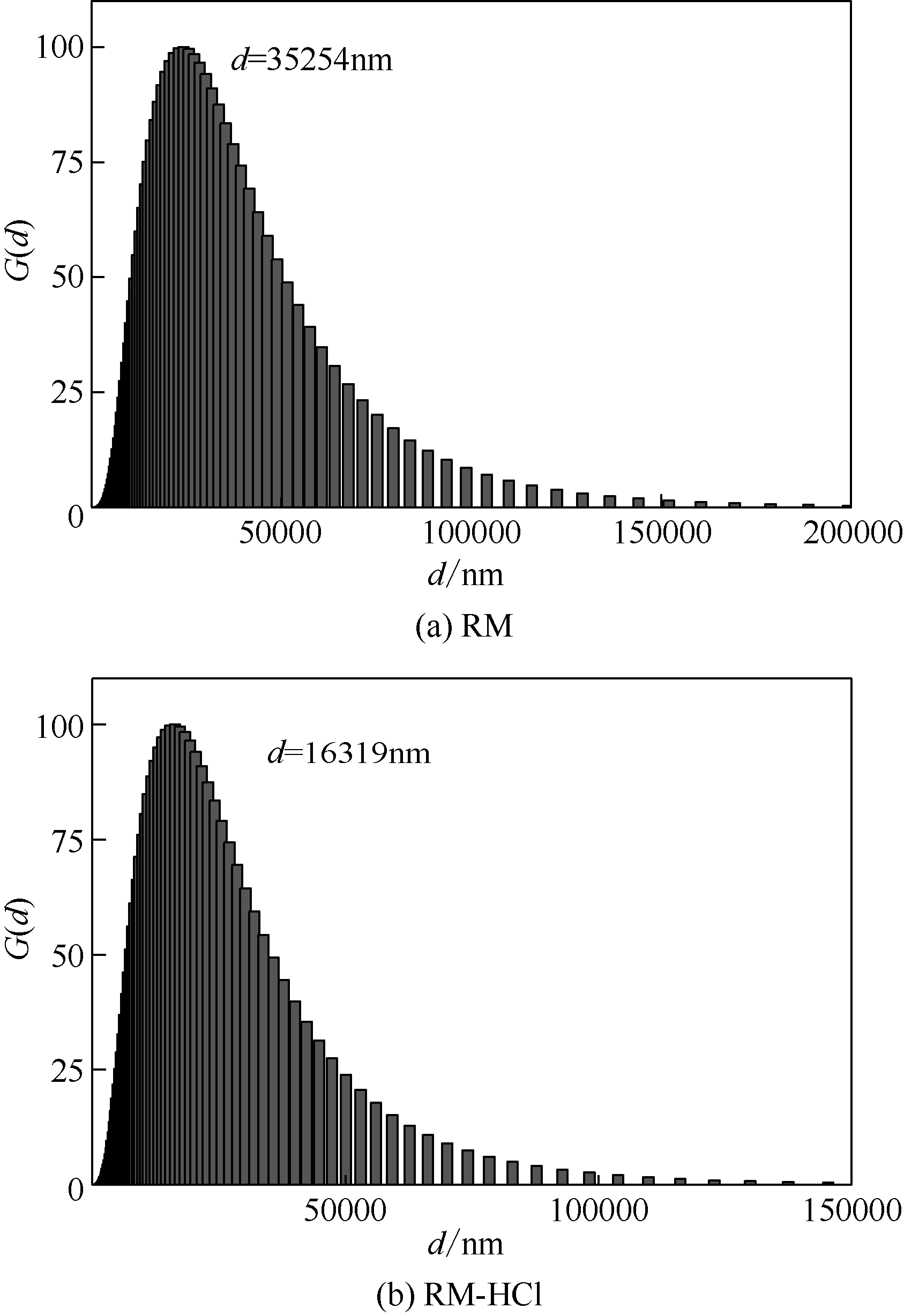
| 液固比 | 盐酸浓度/mol·L-1 | 反应时间/h | 粒径/μm |
|---|---|---|---|
| 8∶1 | 12 | 1 | 17.8 |
| 8∶1 | 6 | 0.5 | 19.5 |
| 6∶1 | 12 | 1 | 19.9 |
| 6∶1 | 6 | 0.5 | 21.7 |
| 5∶1 | 12 | 1 | 16.3 |
| 5∶1 | 6 | 0.5 | 22.6 |
| 液固比 | 盐酸浓度/mol·L-1 | 反应时间/h | 粒径/μm |
|---|---|---|---|
| 8∶1 | 12 | 1 | 17.8 |
| 8∶1 | 6 | 0.5 | 19.5 |
| 6∶1 | 12 | 1 | 19.9 |
| 6∶1 | 6 | 0.5 | 21.7 |
| 5∶1 | 12 | 1 | 16.3 |
| 5∶1 | 6 | 0.5 | 22.6 |
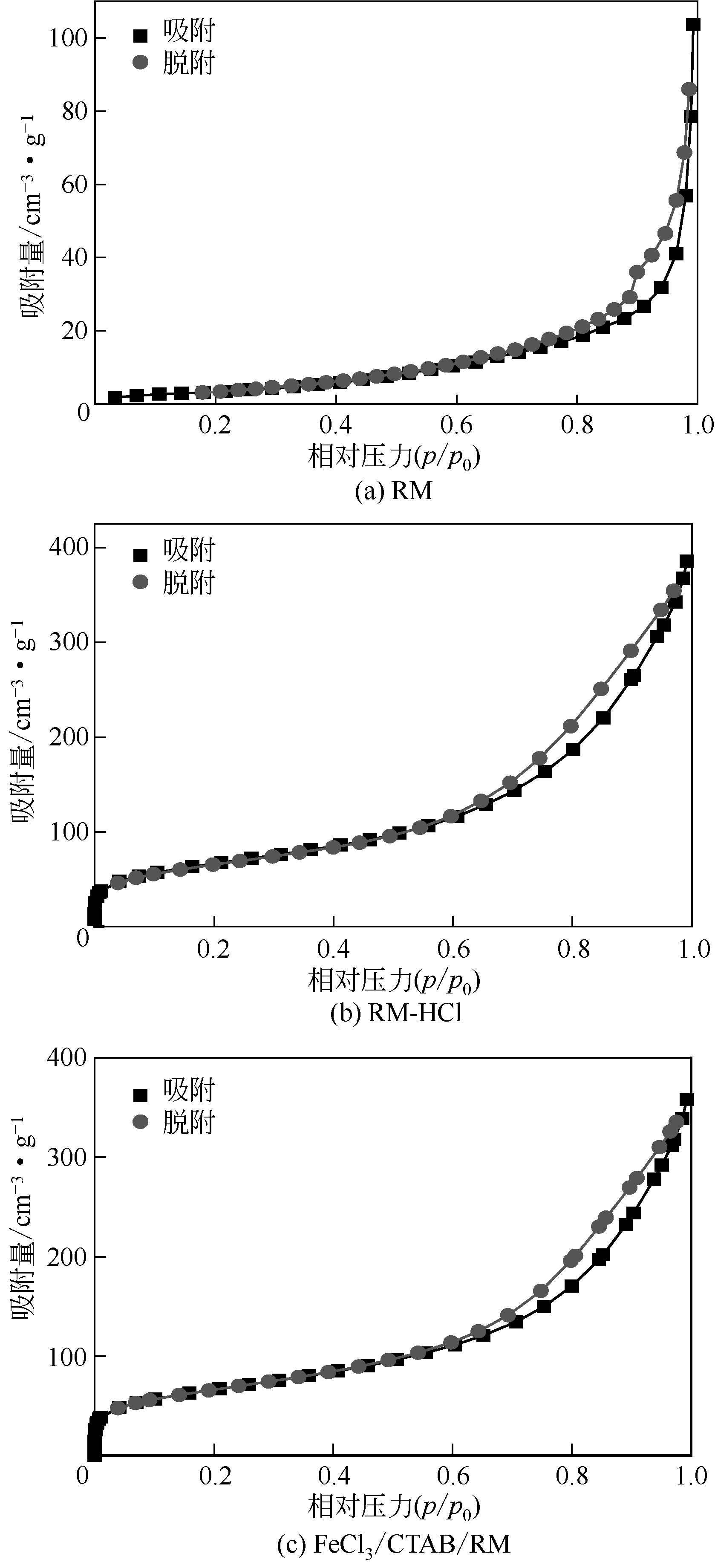
| 样品 | SBET/m2·g-1 | V/cm3·g-1 | R/nm |
|---|---|---|---|
| RM | 12.97 | 0.16 | 24.86 |
| RM-HCl | 233.09 | 0.59 | 9.37 |
| FeCl3/CTAB/RM | 201.2 | 0.38 | 10.11 |
| 样品 | SBET/m2·g-1 | V/cm3·g-1 | R/nm |
|---|---|---|---|
| RM | 12.97 | 0.16 | 24.86 |
| RM-HCl | 233.09 | 0.59 | 9.37 |
| FeCl3/CTAB/RM | 201.2 | 0.38 | 10.11 |
| 成分 | RM | RM-HCl | FeCl3/CTAB/RM |
|---|---|---|---|
| C | 1 | 0.9 | 1.97 |
| N | 0.45 | 0.17 | 0.24 |
| H | 0.90 | 0.81 | 1.52 |
| 成分 | RM | RM-HCl | FeCl3/CTAB/RM |
|---|---|---|---|
| C | 1 | 0.9 | 1.97 |
| N | 0.45 | 0.17 | 0.24 |
| H | 0.90 | 0.81 | 1.52 |
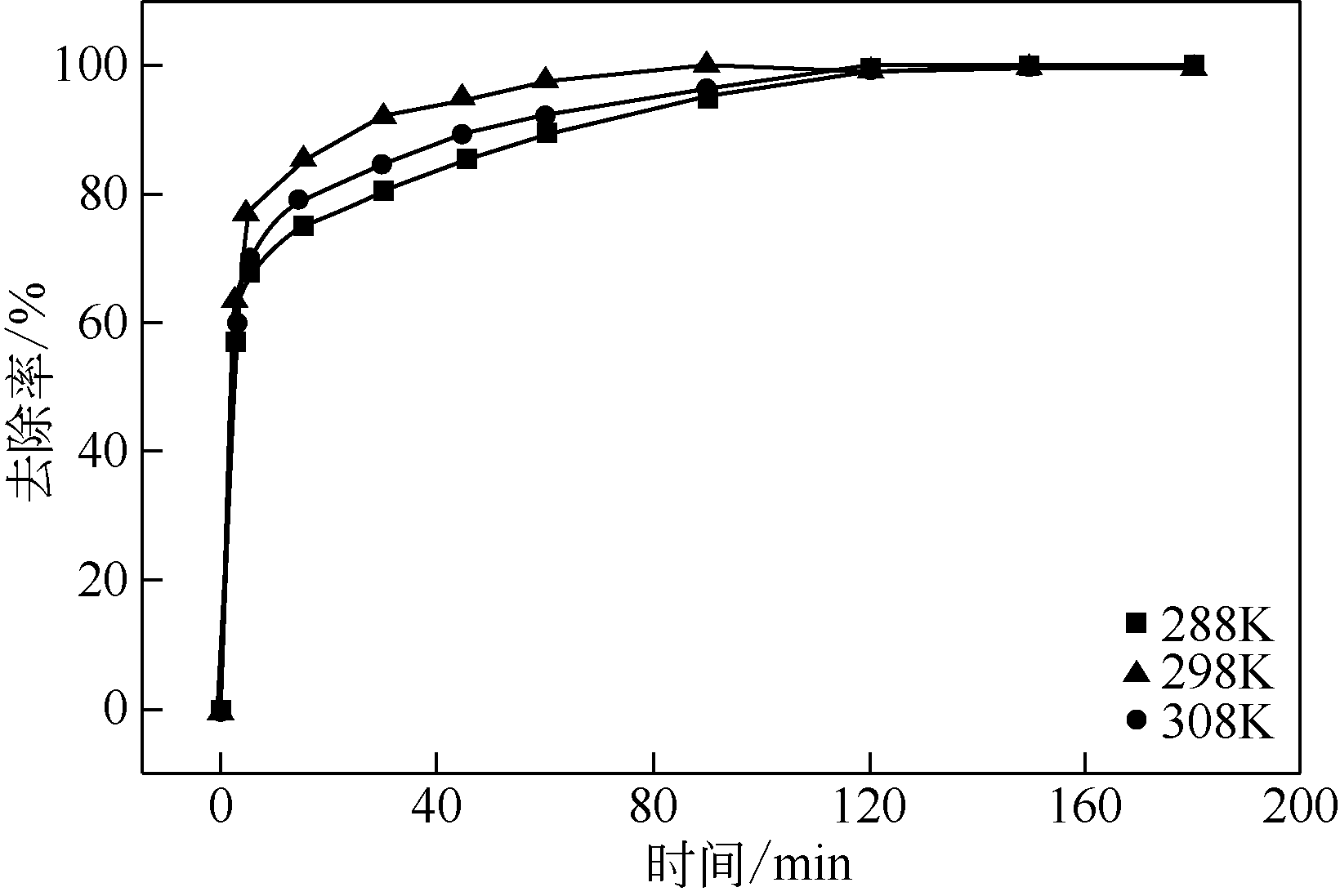
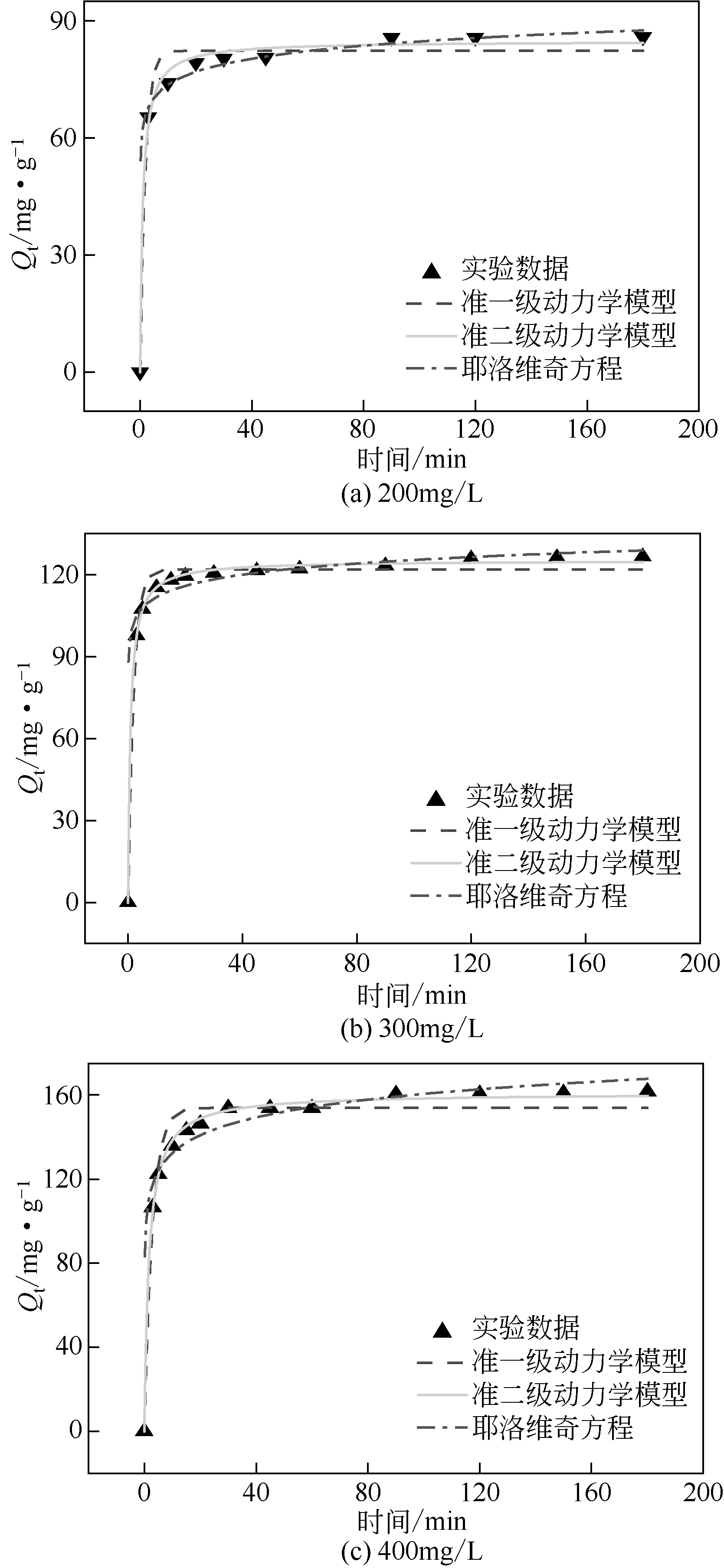
| C0/mg·L-1 | Qe,exp/mg·g-1 | 准一级动力学模型 | 准二级动力学模型 | Simple Elovich方程 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| k1/min-1 | Qe,cal/mg·g-1 | R2 | k2/min-1 | Qe,cal/mg·g-1 | R2 | α/mg·g-1·min-1 | β/mg·g-1 | R2 | ||
| 200 | 86 | 0.513 | 82.36 | 0.972 | 0.011 | 86.7 | 0.994 | 62.54 | 4.82 | 0.936 |
| 300 | 126.6 | 0.497 | 121.84 | 0.989 | 0.009 | 127.03 | 0.998 | 98.31 | 5.88 | 0.854 |
| 400 | 161.6 | 0.341 | 153.89 | 0.972 | 0.002 | 163.85 | 0.997 | 104.39 | 12.19 | 0.887 |
| C0/mg·L-1 | Qe,exp/mg·g-1 | 准一级动力学模型 | 准二级动力学模型 | Simple Elovich方程 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| k1/min-1 | Qe,cal/mg·g-1 | R2 | k2/min-1 | Qe,cal/mg·g-1 | R2 | α/mg·g-1·min-1 | β/mg·g-1 | R2 | ||
| 200 | 86 | 0.513 | 82.36 | 0.972 | 0.011 | 86.7 | 0.994 | 62.54 | 4.82 | 0.936 |
| 300 | 126.6 | 0.497 | 121.84 | 0.989 | 0.009 | 127.03 | 0.998 | 98.31 | 5.88 | 0.854 |
| 400 | 161.6 | 0.341 | 153.89 | 0.972 | 0.002 | 163.85 | 0.997 | 104.39 | 12.19 | 0.887 |
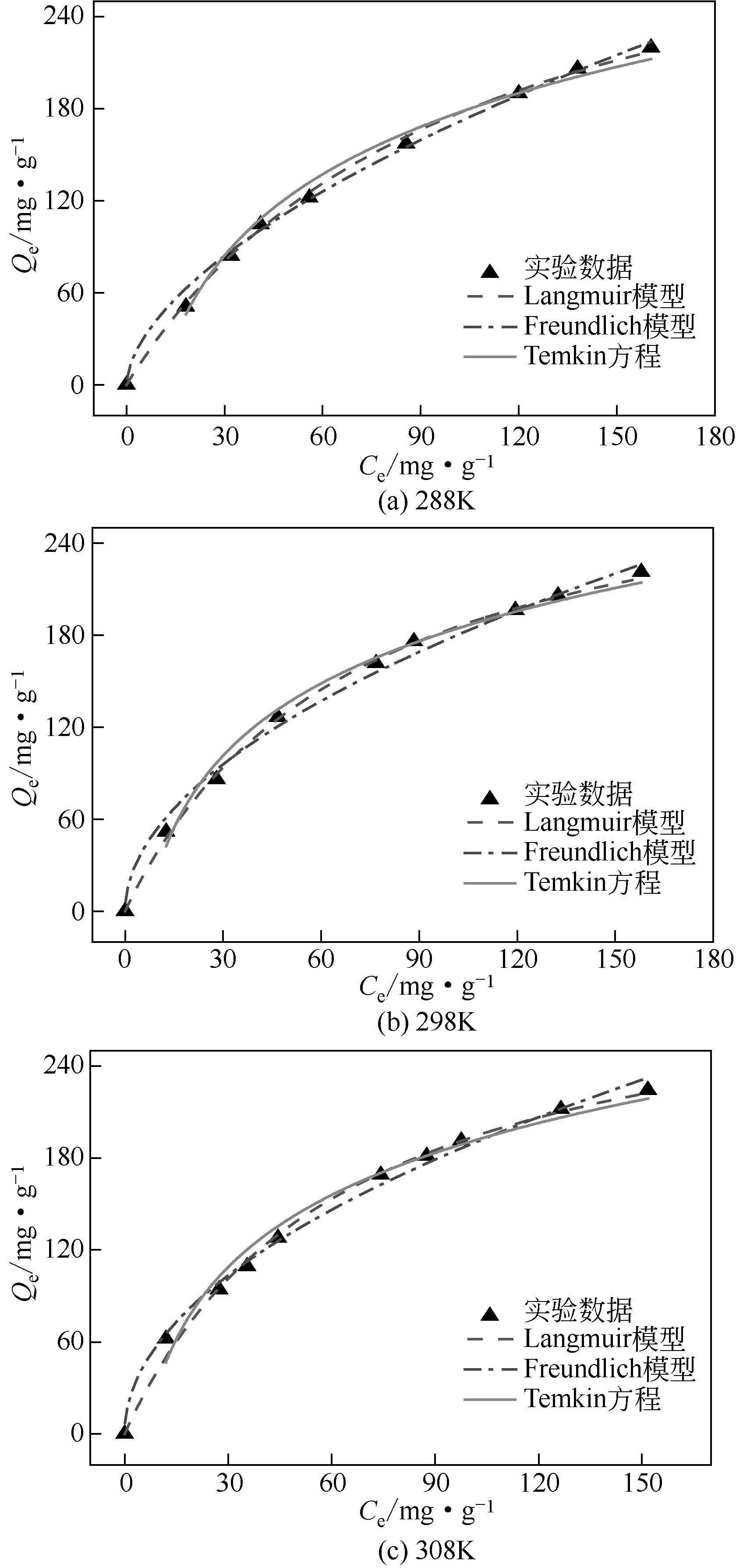
| T/K | Langmuir模型 | Freundlich模型 | Temkin方程 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Qm,cal/mg·g-1 | KL/L·mg-1 | R2 | Qm,exp/mg·g-1 | KF/(mg·g-1)(mg·L-1)-n | 1/n | R2 | bT/J·mol-1 | KT/L·mg-1 | R2 | |
| 288 | 304.9 | 0.01 | 0.998 | 219.8 | 11.59 | 0.58 | 0.996 | 31.34 | 9.97 | 0.982 |
| 298 | 313.6 | 0.014 | 0.998 | 221 | 16.5 | 0.52 | 0.985 | 36.43 | 6.89 | 0.986 |
| 308 | 315.7 | 0.016 | 0.996 | 224.2 | 18.92 | 0.5 | 0.991 | 37.77 | 6.05 | 0.975 |
| T/K | Langmuir模型 | Freundlich模型 | Temkin方程 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Qm,cal/mg·g-1 | KL/L·mg-1 | R2 | Qm,exp/mg·g-1 | KF/(mg·g-1)(mg·L-1)-n | 1/n | R2 | bT/J·mol-1 | KT/L·mg-1 | R2 | |
| 288 | 304.9 | 0.01 | 0.998 | 219.8 | 11.59 | 0.58 | 0.996 | 31.34 | 9.97 | 0.982 |
| 298 | 313.6 | 0.014 | 0.998 | 221 | 16.5 | 0.52 | 0.985 | 36.43 | 6.89 | 0.986 |
| 308 | 315.7 | 0.016 | 0.996 | 224.2 | 18.92 | 0.5 | 0.991 | 37.77 | 6.05 | 0.975 |
| T/K | ΔH0/kJ·mol-1 | ΔS0/J·mol-1·K-1 | ΔG0/kJ·mol-1 |
|---|---|---|---|
| 288 | -0.77 | ||
| 298 | 37.82 | 137.8 | -1.56 |
| 308 | -1.92 |
| T/K | ΔH0/kJ·mol-1 | ΔS0/J·mol-1·K-1 | ΔG0/kJ·mol-1 |
|---|---|---|---|
| 288 | -0.77 | ||
| 298 | 37.82 | 137.8 | -1.56 |
| 308 | -1.92 |
| 吸附剂 | 实验方法 | 吸附量/mg·g-1 | 参考文献 |
|---|---|---|---|
| FeCl3/CTAB/RM | 12mol·L-1 HCl+0.5mol·L-1 FeCl3+0.5%CTAB | 221 | 本文 |
| RM/HCl | 2.25mol·L-1 HCl | 78.13 | [ |
| RM/壳聚糖 | 0.01g·mL-1壳聚糖 | 7.2 | [ |
| RM/NaHSO4 | 0.32mol·L-1 NaHSO4 | 200 | [ |
| 吸附剂 | 实验方法 | 吸附量/mg·g-1 | 参考文献 |
|---|---|---|---|
| FeCl3/CTAB/RM | 12mol·L-1 HCl+0.5mol·L-1 FeCl3+0.5%CTAB | 221 | 本文 |
| RM/HCl | 2.25mol·L-1 HCl | 78.13 | [ |
| RM/壳聚糖 | 0.01g·mL-1壳聚糖 | 7.2 | [ |
| RM/NaHSO4 | 0.32mol·L-1 NaHSO4 | 200 | [ |
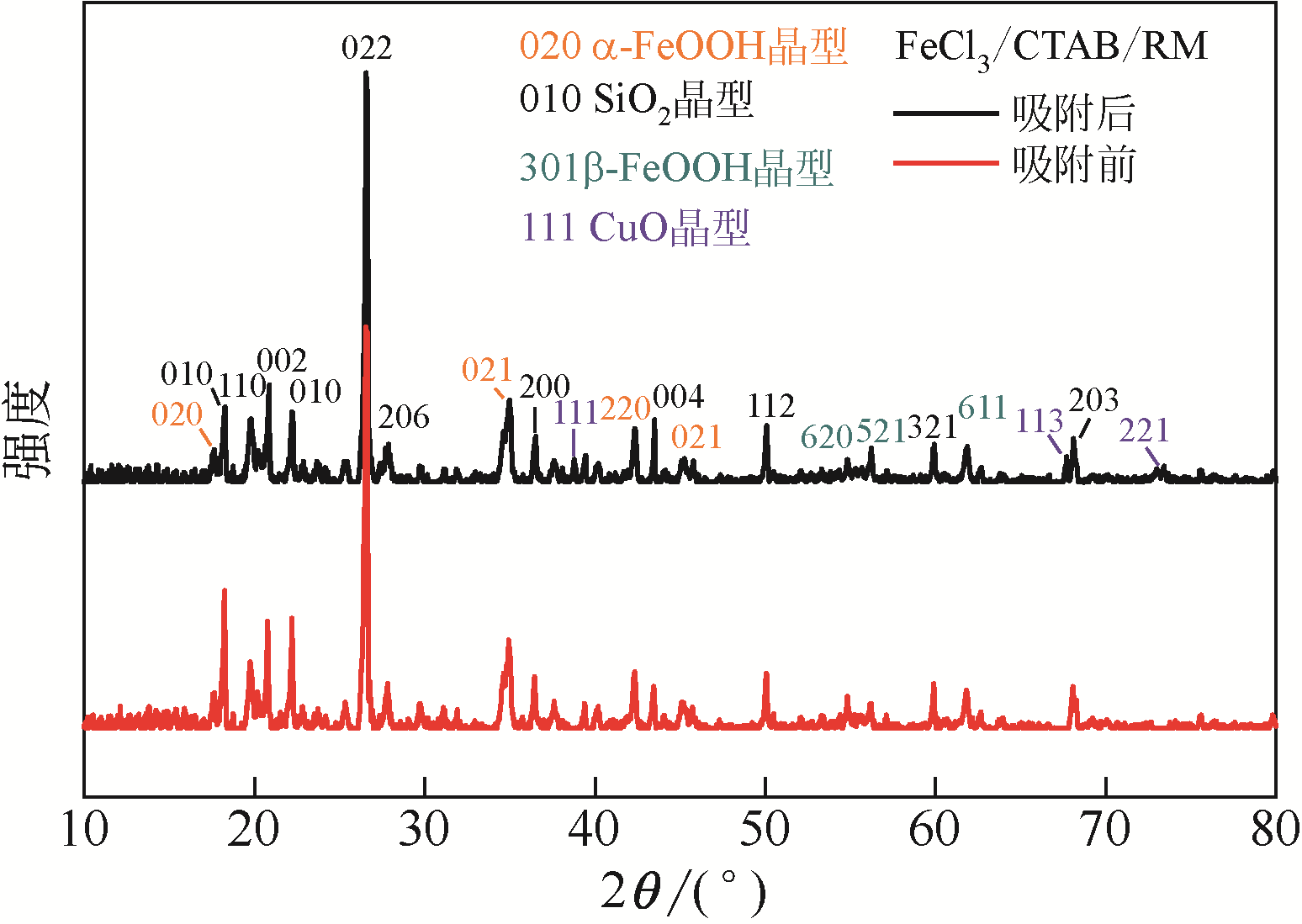
| 样品 | C | N | O | Si | Fe | Cl | Cu |
|---|---|---|---|---|---|---|---|
| RM | 5.57 | 1.66 | 66.84 | 25.84 | 0.05 | 0.03 | 0 |
| RM-HCl | 7.65 | 1.61 | 45.82 | 44.68 | 0.23 | 0 | 0 |
| FeCl3/CTAB/RM | 29.86 | 2.45 | 27.13 | 35.51 | 3.1 | 1.64 | 0 |
| FeCl3/CTAB/RM-Cu | 25.54 | 2.32 | 30.95 | 36.25 | 2.7 | 0.58 | 1.05 |
| 样品 | C | N | O | Si | Fe | Cl | Cu |
|---|---|---|---|---|---|---|---|
| RM | 5.57 | 1.66 | 66.84 | 25.84 | 0.05 | 0.03 | 0 |
| RM-HCl | 7.65 | 1.61 | 45.82 | 44.68 | 0.23 | 0 | 0 |
| FeCl3/CTAB/RM | 29.86 | 2.45 | 27.13 | 35.51 | 3.1 | 1.64 | 0 |
| FeCl3/CTAB/RM-Cu | 25.54 | 2.32 | 30.95 | 36.25 | 2.7 | 0.58 | 1.05 |
| 1 | FAN L, LUO C, LÜ Z, et al. Preparation of magnetic modified chitosan and adsorption of Zn from aqueous solutions[J]. Colloids & Surfaces B: Biointerfaces, 2011, 88(2): 574-581. |
| 2 | 余伟光, 黎吉辉, 王敦, 等. 香蕉茎秆生物炭的制备及其对铜离子的吸附特性[J]. 化工进展, 2017, 36(4): 1499-1505. |
| YU Weiguang, LI Jihui, WANG Dun, et al. The preparation of biochar from pre-oxidation of banana stem and its adsorption of Cu2+[J]. Chemical Industry and Engineering Progress, 2017, 36(4): 1499-1505. | |
| 3 | BHATNAGAR A, VILAR V J, BOTELHO C M, et al. A review of the use of red mud as adsorbent for the removal of toxic pollutants from water and wastewater[J]. Environmental Technology, 2011, 32(3): 231-249. |
| 4 | SOYLAK M, MURAT I. A new coprecipitation methodology with lutetium hydroxide for preconcentration of heavy metal ions in herbal plant samples[J]. Journal of Aoac International, 2014, 97(4): 1189. |
| 5 | BULGARIU L, BULGARIU D. Selective extraction of Hg(Ⅱ), Cd(Ⅱ) and Zn(Ⅱ) ions from aqueous media by a green chemistry procedure using aqueous two-phase systems[J]. Separation & Purification Technology, 2013, 118(43): 209-216. |
| 6 | PENG W, LI H, LIU Y, et al. A review on heavy metal ions adsorption from water by graphene oxide and its composites[J]. Journal of Molecular Liquids, 2017, 230: 496-504. |
| 7 | 文小年. 氧化铝工业废渣(赤泥)的吸附特性及其环境修复材料制备[D]. 桂林: 桂林理工大学, 2006. |
| WEN X N. Adsorption characteristics of alumina industrial waste(red mud) and preparation of environmental remediation materials[D]. Guilin: Guilin University of Technology, 2006. | |
| 8 | 潘嘉芬, 李梦红. 拜耳法赤泥陶粒改性及吸附废水中氟离子试验[J]. 有色金属(冶炼部分), 2015(2): 63-65. |
| PAN Jiafen, LI Menghong. Modification of porous ceramsite with red mud of Bayer process and adsorbing fluoride from waste water[J]. Nonferrous Metals (Extractive Metallurgy), 2015(2): 63-65. | |
| 9 | LI D, DING Y, LI L, et al. Removal of hexavalent chromium by using red mud activated with cetyltrimethylammonium bromide[J]. Environmental Technology, 2015, 36(9): 1084-1090. |
| 10 | 吴建锋, 方斌正, 徐晓虹, 等. 用于去除电镀废水中As(V)的赤泥基环境修复材料的研究[C]//第7届亚洲陶瓷技术研讨会, 中国, 江西, 2011. |
| WU J F, FANG B Z, XU X H, et al. Study on red mud environmental remediation materials for removal As(V) ions in electroplating wastewater[C]//The 7th Asian Ceramic Technology Seminar. Jiangxi, China, 2011. | |
| 11 | VENKATESAN G, NARAYANAN S L. Synthesis of Fe2O3 coated and HCl treated bauxite ore waste for the adsorption of arsenic (Ⅲ) from aqueous solution: isotherm and kinetic models[J]. Chemical Engineering Communications, 2017, 205(1): 34-46. |
| 12 | CUI Y W, LI J, DU Z F, et al. Cr(Ⅵ) adsorption on red mud modified by lanthanum: performance, kinetics and mechanisms[J]. PloS One, 2016, 11(9): e0161780. |
| 13 | JIE C, NING W, MA H, et al. Facile Modification of a polythiophene/TiO2 composite using surfactants in an aqueous medium for an enhanced Pb(Ⅱ) adsorption and mechanism investigation[J]. Journal of Chemical & Engineering Data, 2017, 62(7): 2208-2221. |
| 14 | 张多, 张盼月, 田帅, 等. 用于磷吸附的载铁(β-FeOOH)沸石制备及特性[J]. 环境工程学报, 2014, 8(2): 499-504. |
| ZHANG Duo, ZHANG Panyue, TIAN Shuai, et al. Preparation of β-FeOOH loaded-zeolite and its characteristics for phosphorus adsorption[J]. Chinese Journal of Environmental Engineering, 2014, 8(2): 499-504. | |
| 15 | CUI H, YANG X, XU L, et al. Effects of goethite on the fractions of Cu, Cd, Pb, P and soil enzyme activity with hydroxyapatite in heavy metal-contaminated soil[J]. RSC Advances, 2017, 7(72): 45869-45877. |
| 16 | 刘俊, 楼跃丰, 李军. 石英砂表面负载铁氧化物的方法和特性[J]. 化工进展, 2016, 35(2): 624-628. |
| LIU Jun, LOU Yuefeng, LI Jun. Method and characteristics of iron oxides coated on quartz sand surface[J]. Chemical Industry and Engineering Progress, 2016, 35(2): 624-628. | |
| 17 | WEI W, CONG Haibing, XIONG Huixin. The properties of FeOOH precipitates formed in CTAB-rich FeCl3 solutions at various pH and ethanol contents[J]. Colloid & Polymer Science, 2018, 296(7): 1205-1212. |
| 18 | SAHU M K, MANDAL S, DASH S S, et al. Removal of Pb(Ⅱ) from aqueous solution by acid activated red mud[J]. Journal of Environmental Chemical Engineering, 2013, 1(4): 1315-1324. |
| 19 | 高鹏杰. 氧化铝赤泥改性及对废水中Cr(Ⅵ)的吸附研究[D]. 郑州: 郑州大学, 2016. |
| GAO P J. Modification of alumina red mud and research on the adsorption of Cr(VI) in polluted water[D]. Zhengzhou: Zhengzhou University, 2016. | |
| 20 | 徐翔丽. 氨基功能化氧化石墨烯重金属吸附剂的制备与应用[D]. 长沙: 湖南大学, 2014. |
| XU Xiangli. The preparation and and characterization of amino-functionalized graphene oxide as heavy metal adsorbents [D]. Changsha: Hunan University, 2014. | |
| 21 | 石太宏, 吕灿, 左莉娜. 硅烷化改性沸石对重金属离子的吸附性能[J]. 环境工程学报, 2013, 7(3): 1045-1052. |
| SHI Taihong, Can LÜ, ZUO Lina. Adsorption of heavy metal ions by silylation modified zeolite[J]. Chinese Journal of Environmental Engineering, 2013, 7(3): 1045-1052. | |
| 22 | 柏珊珊. 介孔分子筛的表面修饰及其对重金属离子的吸附性能研究[D]. 哈尔滨: 哈尔滨工业大学, 2010. |
| BAI S S. Study on the modification of mesoporous molecular sieve and their adsorption properties of heavy metals[D]. Harbin: Harbin Institute of Technology, 2010. | |
| 23 | 韩毅, 王京刚, 唐明述. 赤泥氯化铁改性材料的制备及其表征[J]. 有色金属(选矿部分), 2004(2): 37-40. |
| HAN Yi, WANG Jinggang, TANG Mingshu. Modification and characterization of red mud activated by FeCl3[J]. Nonferrous Metals, 2004(2): 37-40. | |
| 24 | LYU W, YU M, FENG J, et al. Highly crystalline polyaniline nanofibers coating with low-cost biomass for easy separation and high efficient removal of anionic dye ARG from aqueous solution[J]. Applied Surface Science, 2018, 458: 413-424. |
| 25 | TSAMO C, DJONGA P N D, DIKDIM J M D, et al. Kinetic and equilibrium studies of Cr(VI), Cu(Ⅱ) and Pb(Ⅱ) removal from aqueous solution using red mud, a low-cost adsorbent[J]. Arabian Journal for Science & Engineering, 2017, 43(5): 1-16. |
| 26 | 李孟, 刘丹, 吴梓鸿. 赤泥基多孔陶瓷材料及其改性物除Cu2+研究[J]. 武汉理工大学学报, 2010, 32(18): 29-32. |
| LI Meng, LIU Dan, WU Zihong. Research on the removal of Cu2+ by red mud-based porous ceramic material and its modification[J]. Journal of Wuhan University of Technology, 2010, 32(18): 29-32. | |
| 27 | PELLERA F, GIANNIS A, ANASTASIADOU K, et al. Adsorption of Cu(Ⅱ) ions from aqueous solutions using biochar prepared from agricultural byproducts[J]. Journal of Environmental Management, 2012, 96(1): 35-42. |
| 28 | RUI F P P, VALENTE A J M, BURROWS H D. The interaction of long chain sodium carboxylates and sodium dodecylsulfate with lead(Ⅱ) ions in aqueous solutions[J]. Journal of Colloid & Interface Science, 2014, 414(414C): 66-72. |
| [1] | WANG Shengyan, DENG Shuai, ZHAO Ruikai. Research progress on carbon dioxide capture technology based on electric swing adsorption [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 233-245. |
| [2] | CUI Shoucheng, XU Hongbo, PENG Nan. Simulation analysis of two MOFs materials for O2/He adsorption separation [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 382-390. |
| [3] | CHEN Chongming, CHEN Qiu, GONG Yunqian, CHE Kai, YU Jinxing, SUN Nannan. Research progresses on zeolite-based CO2 adsorbents [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 411-419. |
| [4] | LI Shilin, HU Jingze, WANG Yilin, WANG Qingji, SHAO Lei. Research progress in separation and extraction of high value components by electrodialysis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 420-429. |
| [5] | XU Chunshu, YAO Qingda, LIANG Yongxian, ZHOU Hualong. Research progress on functionalization strategies of covalent organic frame materials and its adsorption properties for Hg(Ⅱ) and Cr(Ⅵ) [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 461-478. |
| [6] | GU Yongzheng, ZHANG Yongsheng. Dynamic behavior and kinetic model of Hg0 adsorption by HBr-modified fly ash [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 498-509. |
| [7] | ZHAO Jingchao, TAN Ming. Effect of surfactants on the reduction of industrial saline wastewater by electrodialysis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 529-535. |
| [8] | GUO Qiang, ZHAO Wenkai, XIAO Yonghou. Numerical simulation of enhancing fluid perturbation to improve separation of dimethyl sulfide/nitrogen via pressure swing adsorption [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 64-72. |
| [9] | WANG Jinhang, HE Yong, SHI Lingli, LONG Zhen, LIANG Deqing. Progress of gas hydrate anti-agglomerants [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4587-4602. |
| [10] | GE Yafen, SUN Yu, XIAO Peng, LIU Qi, LIU Bo, SUN Chengying, GONG Yanjun. Research progress of zeolite for VOCs removal [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4716-4730. |
| [11] | LI Weihua, YU Qianwen, YIN Junquan, WU Yinkai, SUN Yingjie, WANG Yan, WANG Huawei, YANG Yufei, LONG Yuyang, HUANG Qifei, GE Yanchen, HE Yiyang, ZHAO Lingyan. Leaching behavior of heavy metals from broken ton bags filled with fly ash in acid rain environment [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4917-4928. |
| [12] | LI Zhiyuan, HUANG Yaji, ZHAO Jiaqi, YU Mengzhu, ZHU Zhicheng, CHENG Haoqiang, SHI Hao, WANG Sheng. Characterization of heavy metals during co-pyrolysis of sludge with PVC [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4947-4956. |
| [13] | YANG Ying, HOU Haojie, HUANG Rui, CUI Yu, WANG Bing, LIU Jian, BAO Weiren, CHANG Liping, WANG Jiancheng, HAN Lina. Coal tar phenol-based carbon nanosphere prepared by Stöber method for adsorption of CO2 [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 5011-5018. |
| [14] | ZHANG Zhen, LI Dan, CHEN Chen, WU Jinglan, YING Hanjie, QIAO Hao. Separation and purification of salivary acids with adsorption resin [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4153-4158. |
| [15] | JIANG Jing, CHEN Xiaoyu, ZHANG Ruiyan, SHENG Guangyao. Research progress of manganese-loaded biochar preparation and its application in environmental remediation [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4385-4397. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||