Chemical Industry and Engineering Progress ›› 2025, Vol. 44 ›› Issue (5): 2724-2732.DOI: 10.16085/j.issn.1000-6613.2024-1844
• Renewable energy utilization • Previous Articles
Catalyst evolved by stepwise dehydroxylation/decarbonization method achieves efficient methanol decomposition to produce hydrogen
- SINOPEC Nanjing Research Institute of Chemical Industry Co. , Ltd. , Nanjing 210048, Jiangsu, China
-
Received:2024-11-12Revised:2025-04-10Online:2025-05-20Published:2025-05-25
分步脱羟/脱碳催化剂实现高效裂解甲醇制氢
- 中石化南京化工研究院有限公司,江苏 南京 210048
-
作者简介:何志勇(1975—),男,博士,研究方向为精细化工和工业催化。E-mail:hezy.nhgs@sinopec.com。 -
基金资助:中国石油化工集团有限公司资助项目(123048)
CLC Number:
Cite this article
HE Zhiyong. Catalyst evolved by stepwise dehydroxylation/decarbonization method achieves efficient methanol decomposition to produce hydrogen[J]. Chemical Industry and Engineering Progress, 2025, 44(5): 2724-2732.
何志勇. 分步脱羟/脱碳催化剂实现高效裂解甲醇制氢[J]. 化工进展, 2025, 44(5): 2724-2732.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2024-1844
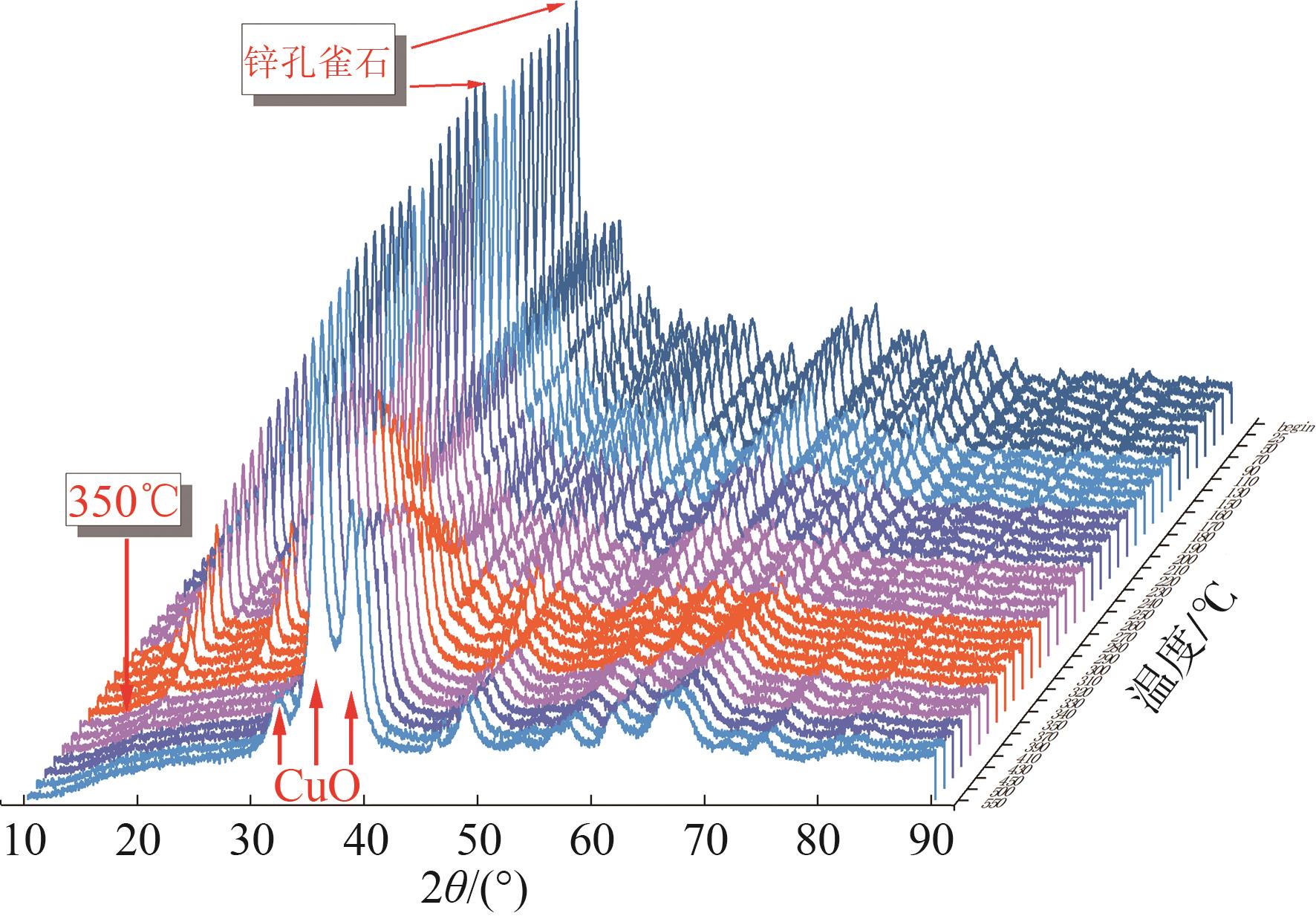
| 温度/℃ | CuO粒径①/nm |
|---|---|
| 300 | 6.8 |
| 350 | 8.8 |
| 400 | 9.3 |
| 450 | 9.8 |
| 500 | 10.1 |
| 550 | 10.6 |
| 温度/℃ | CuO粒径①/nm |
|---|---|
| 300 | 6.8 |
| 350 | 8.8 |
| 400 | 9.3 |
| 450 | 9.8 |
| 500 | 10.1 |
| 550 | 10.6 |
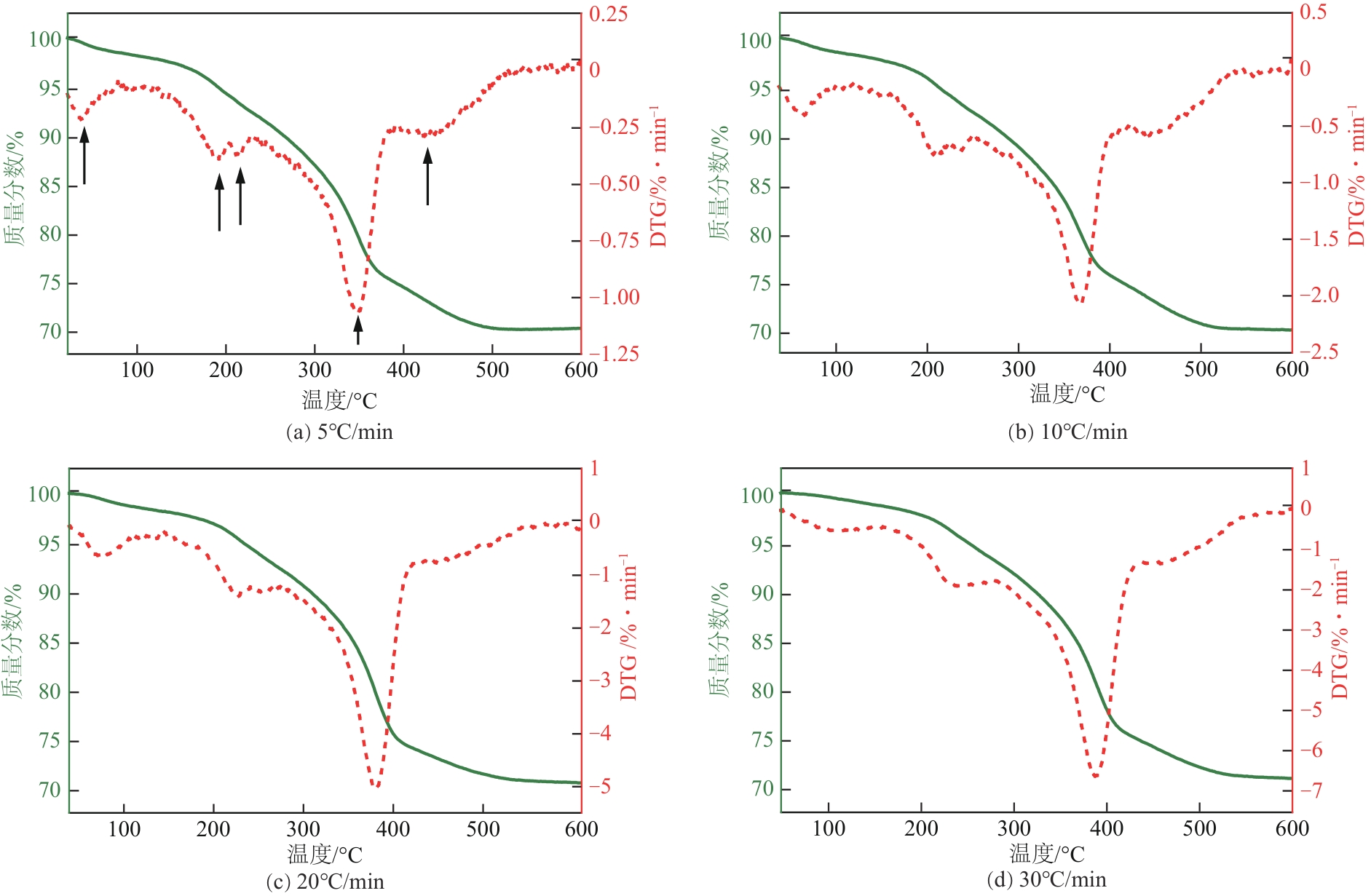
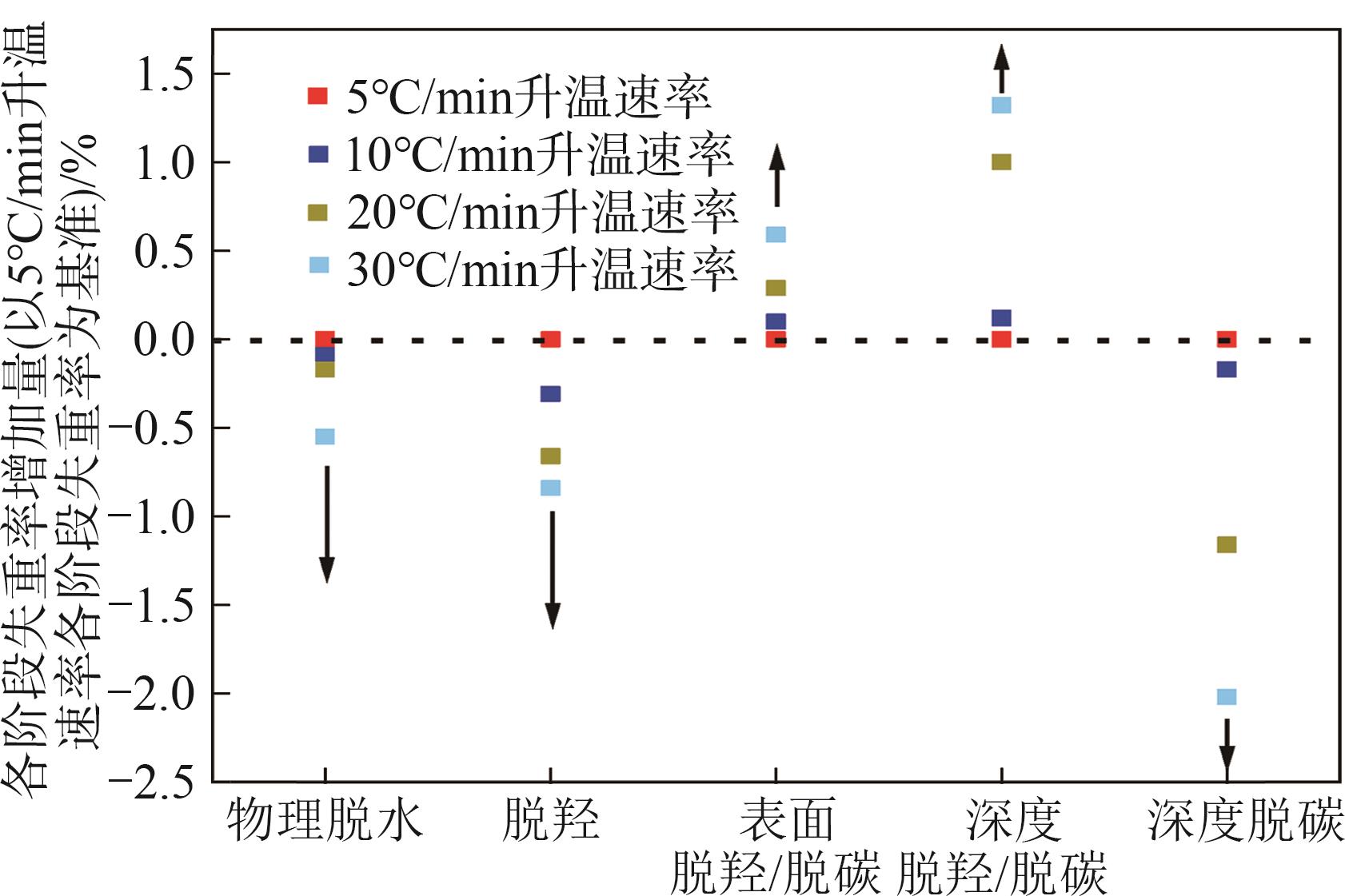
| 升温速率 /℃·min-1 | 失重峰1 (物理吸附水) | 失重峰2 (脱羟) | 失重峰3 (表面脱羟/脱碳) | 失重峰4 (深度脱羟/脱碳) | 失重峰5 (深度脱碳) | 总失重 /% | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 温度/℃ | 失重/% | 温度/℃ | 失重/% | 温度/℃ | 失重/% | 温度/℃ | 失重/% | 温度/℃ | 失重/% | |||||||||
| 5 | 47 | 1.96 | 198 | 4.23 | 218 | 1.82 | 351 | 17.37 | 425 | 4.89 | 29.91 | |||||||
| 10 | 58 | 1.87 | 206 | 3.92 | 231 | 1.92 | 365 | 17.49 | 437 | 4.72 | 30.01 | |||||||
| 20 | 70 | 1.79 | 218 | 3.57 | 244 | 2.11 | 372 | 18.37 | 454 | 3.73 | 29.41 | |||||||
| 30 | 93 | 1.41 | 222 | 3.39 | 246 | 2.41 | 381 | 18.69 | 453 | 2.87 | 29.01 | |||||||
| 升温速率 /℃·min-1 | 失重峰1 (物理吸附水) | 失重峰2 (脱羟) | 失重峰3 (表面脱羟/脱碳) | 失重峰4 (深度脱羟/脱碳) | 失重峰5 (深度脱碳) | 总失重 /% | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 温度/℃ | 失重/% | 温度/℃ | 失重/% | 温度/℃ | 失重/% | 温度/℃ | 失重/% | 温度/℃ | 失重/% | |||||||||
| 5 | 47 | 1.96 | 198 | 4.23 | 218 | 1.82 | 351 | 17.37 | 425 | 4.89 | 29.91 | |||||||
| 10 | 58 | 1.87 | 206 | 3.92 | 231 | 1.92 | 365 | 17.49 | 437 | 4.72 | 30.01 | |||||||
| 20 | 70 | 1.79 | 218 | 3.57 | 244 | 2.11 | 372 | 18.37 | 454 | 3.73 | 29.41 | |||||||
| 30 | 93 | 1.41 | 222 | 3.39 | 246 | 2.41 | 381 | 18.69 | 453 | 2.87 | 29.01 | |||||||
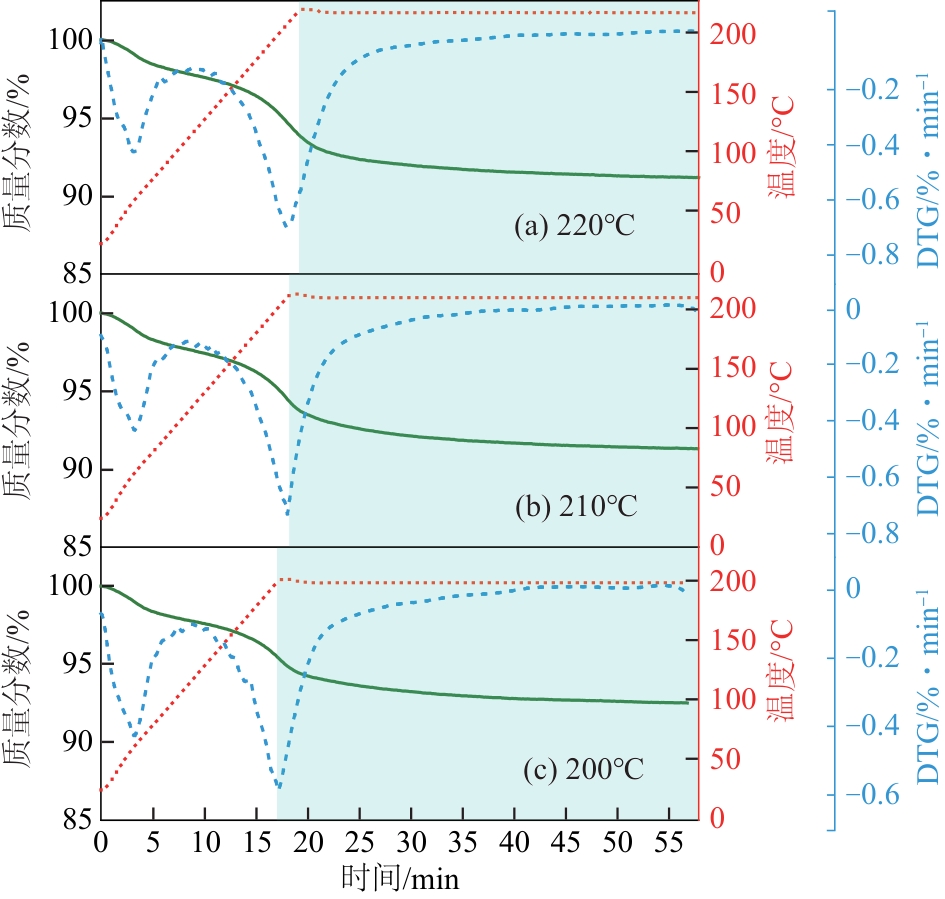
| 恒温 温度/℃ | 失重峰1 | 失重峰2 | 总失重 /% | ||
|---|---|---|---|---|---|
| 温度/℃ | 失重/% | 温度/℃ | 失重/% | ||
| 220 | 62 | 2.28 | 210 | 6.19 | 8.47 |
| 210 | 63 | 2.33 | 210 | 5.99 | 8.32 |
| 200 | 62 | 2.24 | 201 | 5.00 | 7.24 |
| 恒温 温度/℃ | 失重峰1 | 失重峰2 | 总失重 /% | ||
|---|---|---|---|---|---|
| 温度/℃ | 失重/% | 温度/℃ | 失重/% | ||
| 220 | 62 | 2.28 | 210 | 6.19 | 8.47 |
| 210 | 63 | 2.33 | 210 | 5.99 | 8.32 |
| 200 | 62 | 2.24 | 201 | 5.00 | 7.24 |
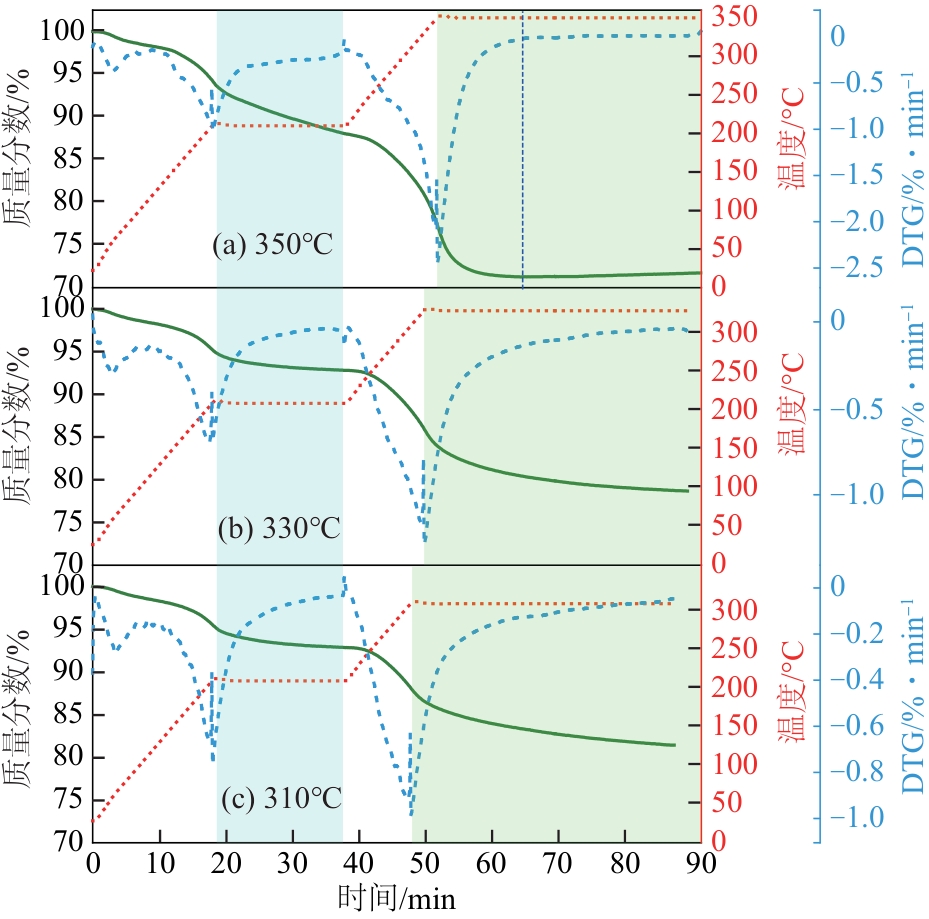
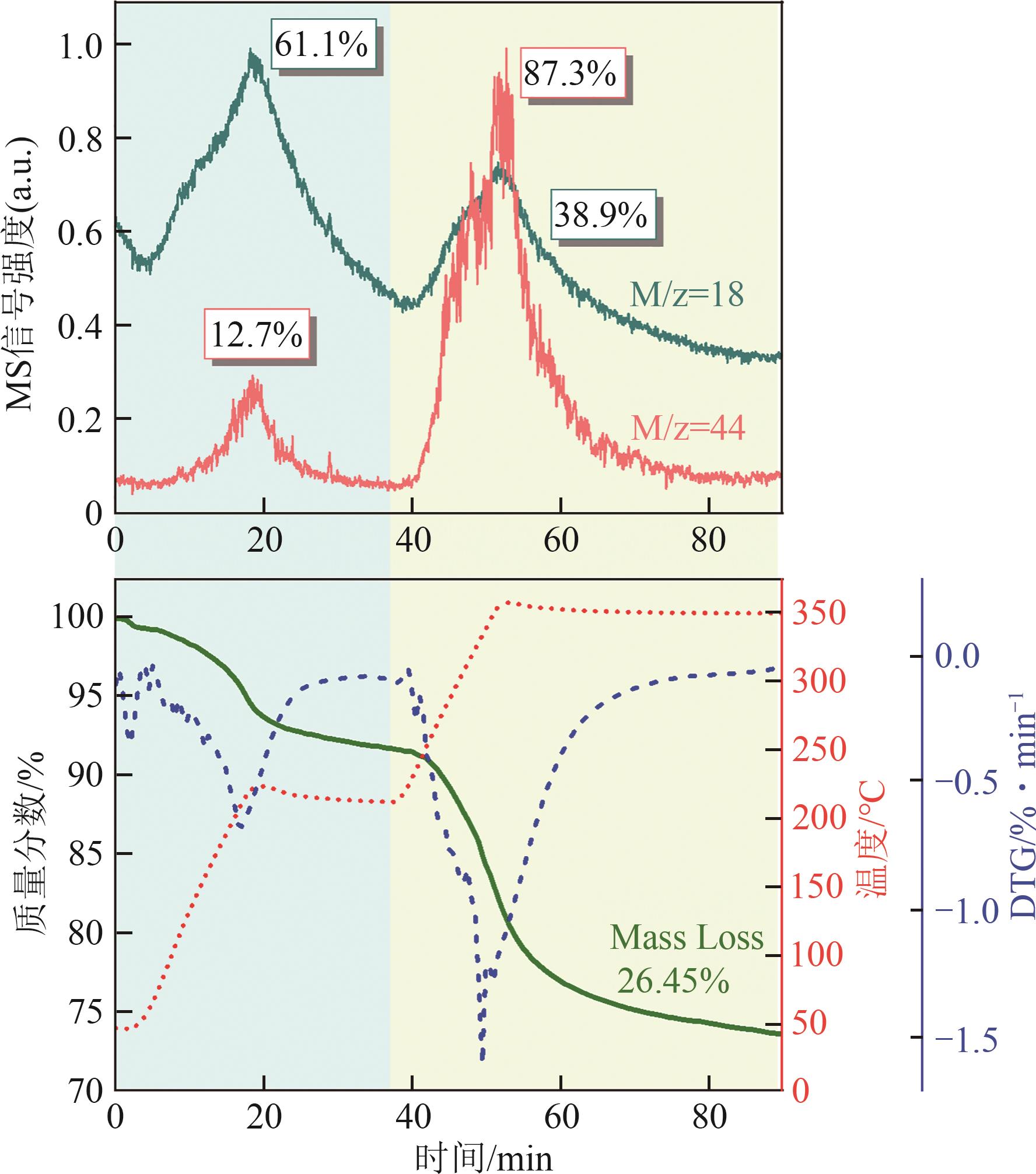
恒温温度 /℃ | 失重峰1 | 失重峰2 | 失重峰3 | 失重峰2+失重峰3总失重 /% | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 温度/℃ | 失重/% | 温度/℃ | 失重/% | 温度/℃ | 失重/% | |||||||
| 350 | 60.5 | 1.60 | 210 | 10.35 | 350 | 16.78 | 27.13 | |||||
| 330 | 58.6 | 1.63 | 210 | 5.53 | 330 | 14.12 | 19.65 | |||||
| 310 | 64.5 | 1.49 | 210 | 5.64 | 310 | 11.42 | 17.06 | |||||
恒温温度 /℃ | 失重峰1 | 失重峰2 | 失重峰3 | 失重峰2+失重峰3总失重 /% | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 温度/℃ | 失重/% | 温度/℃ | 失重/% | 温度/℃ | 失重/% | |||||||
| 350 | 60.5 | 1.60 | 210 | 10.35 | 350 | 16.78 | 27.13 | |||||
| 330 | 58.6 | 1.63 | 210 | 5.53 | 330 | 14.12 | 19.65 | |||||
| 310 | 64.5 | 1.49 | 210 | 5.64 | 310 | 11.42 | 17.06 | |||||
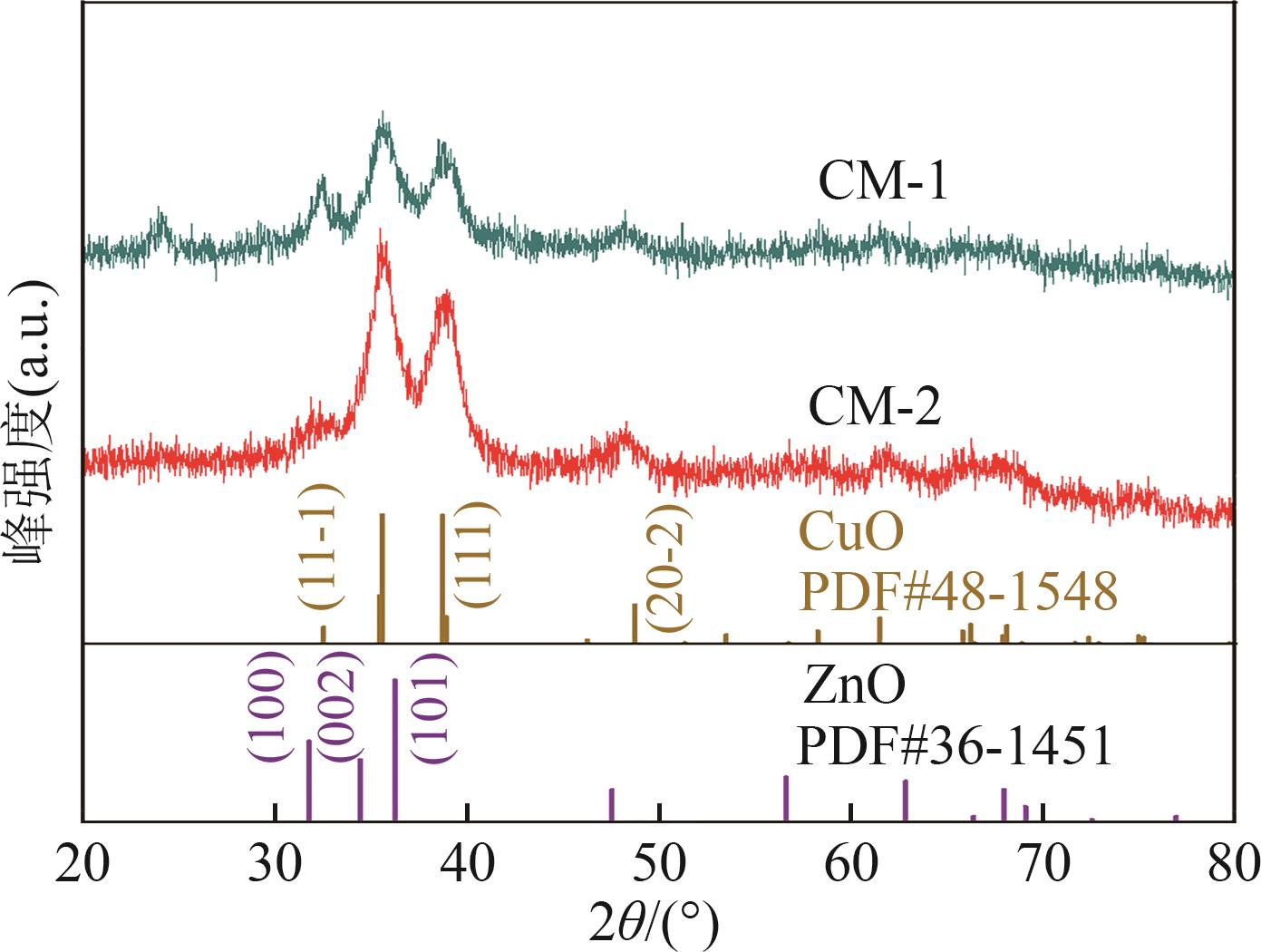
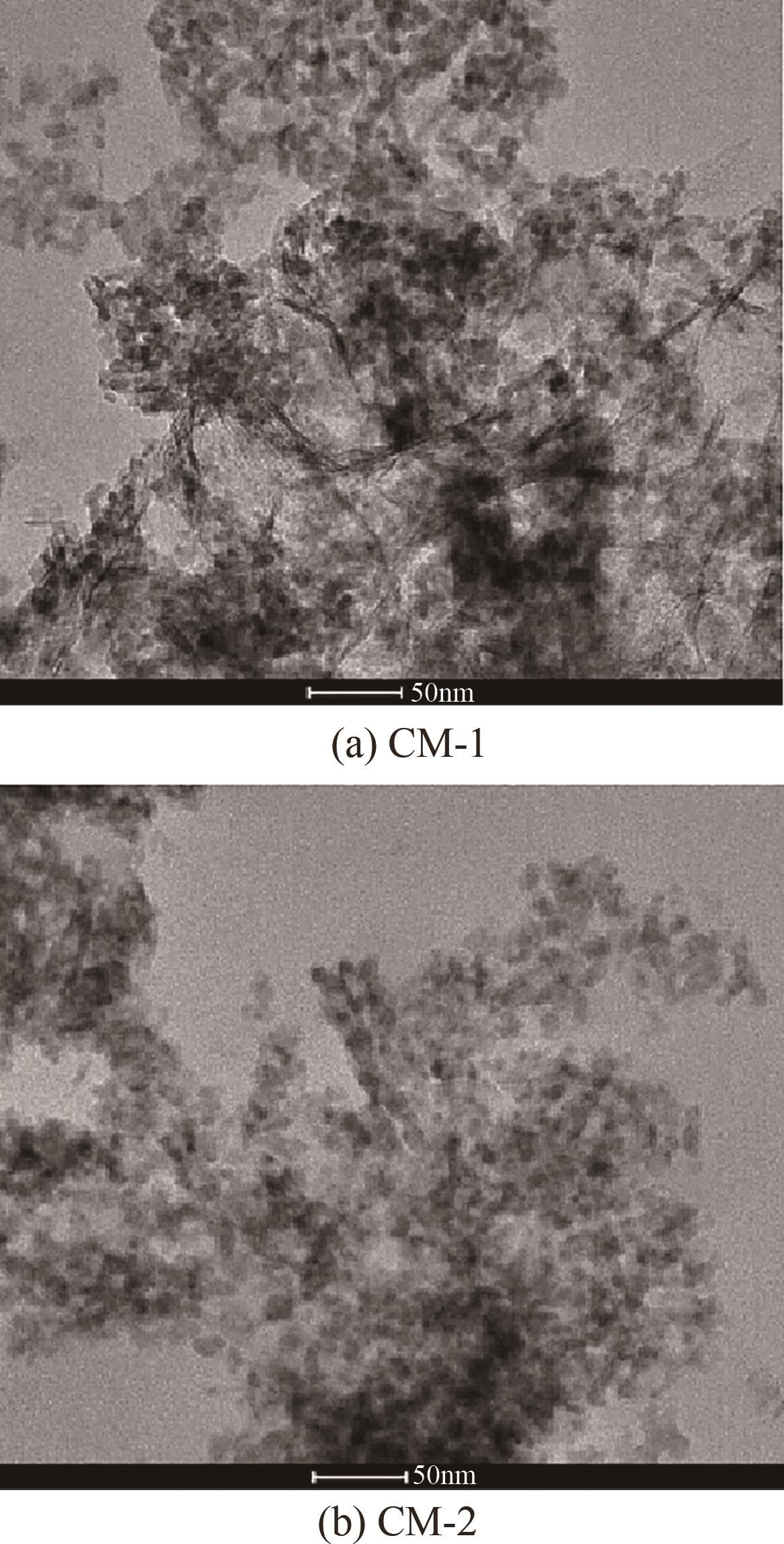
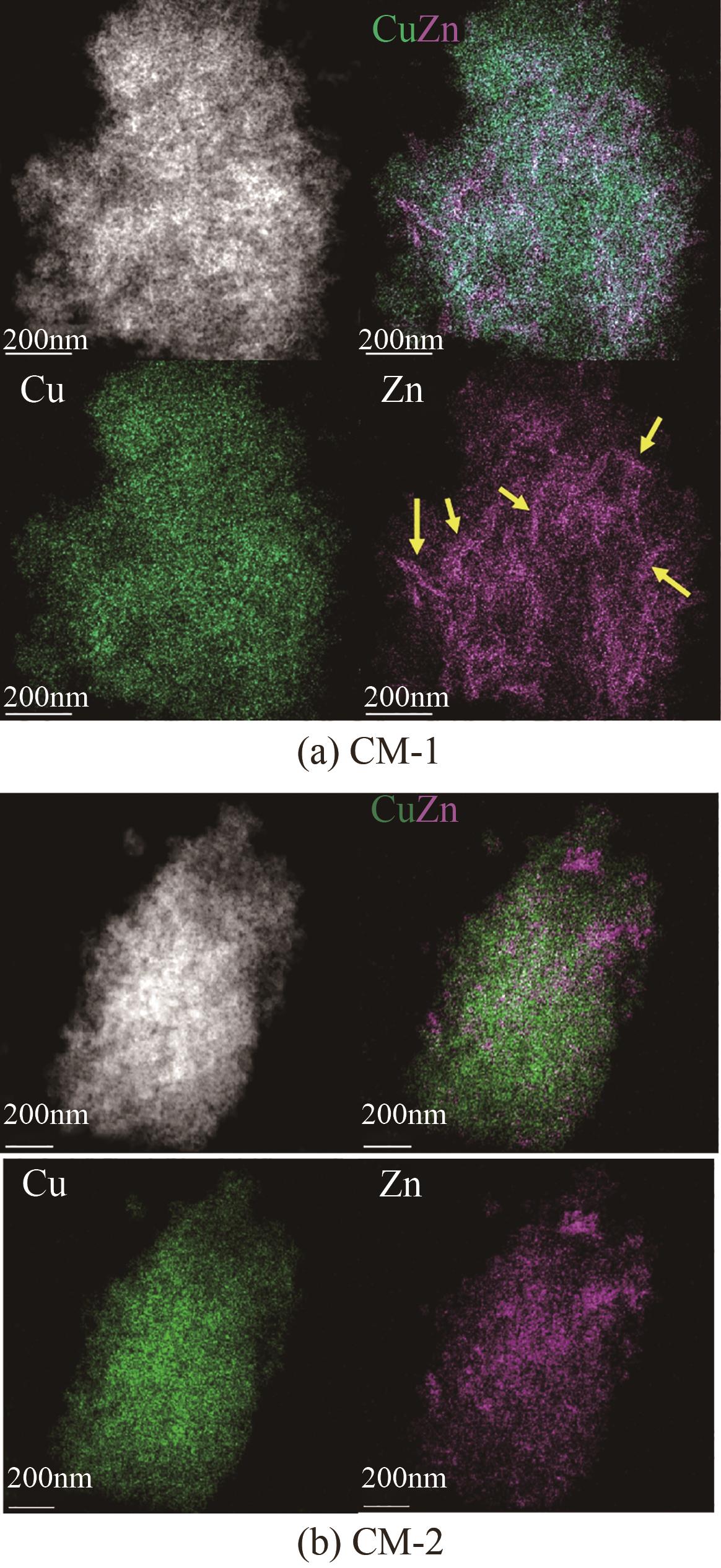
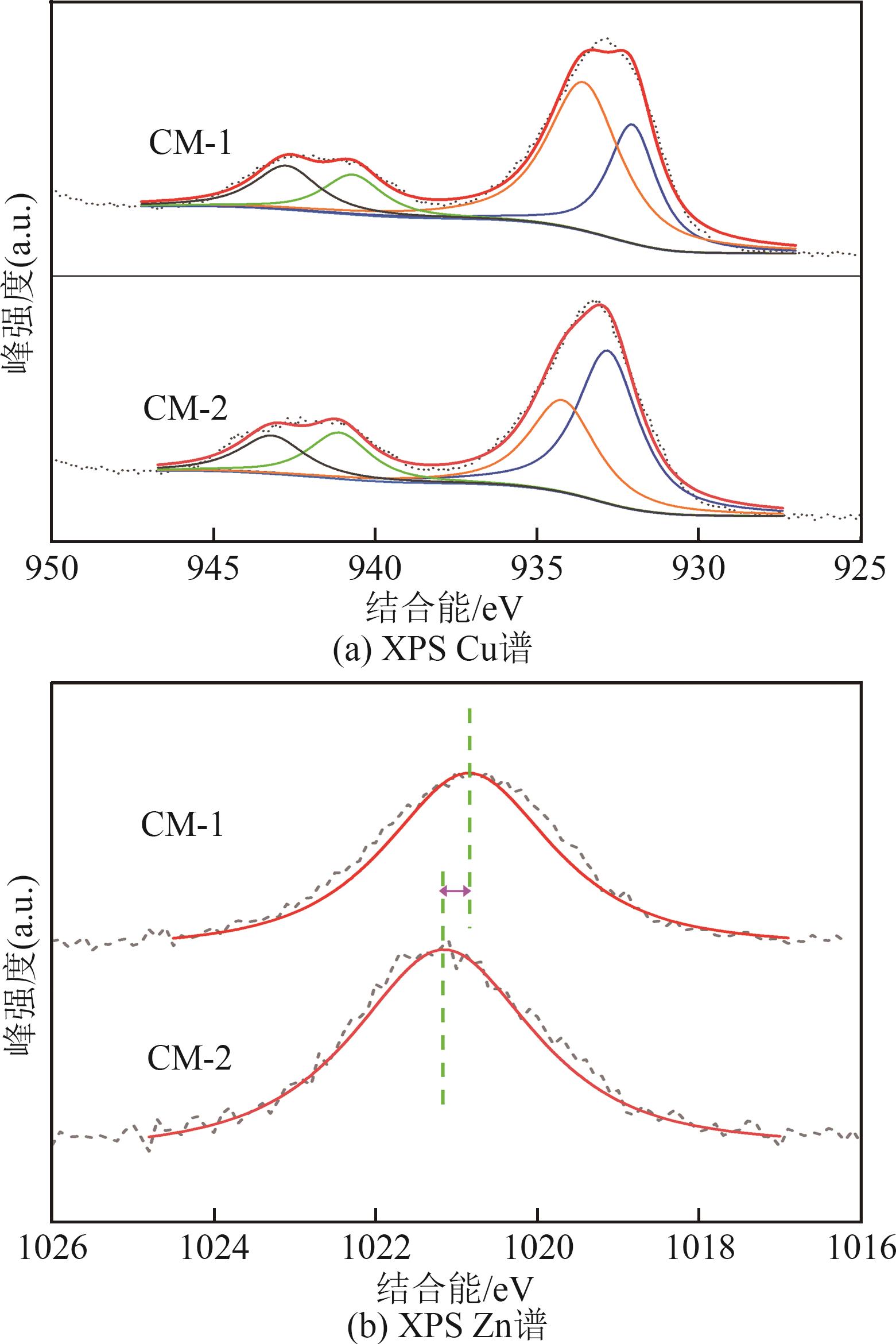
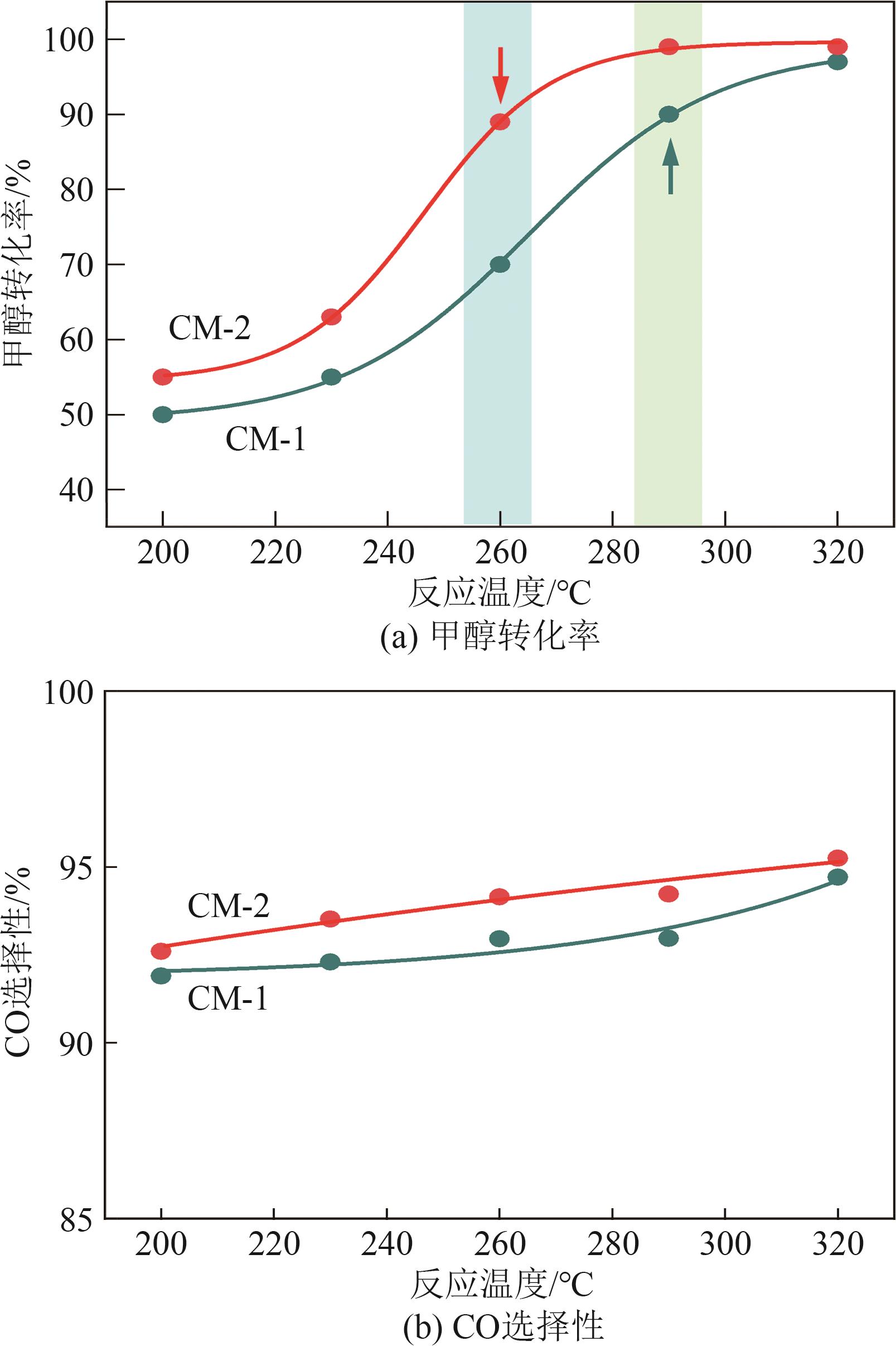
| 催化剂 | 晶粒度/nm | |
|---|---|---|
| CuO | ZnO | |
| CM-1 | 9.0 | 7.3 |
| CM-2 | 6.3 | 6.8 |
| 催化剂 | 晶粒度/nm | |
|---|---|---|
| CuO | ZnO | |
| CM-1 | 9.0 | 7.3 |
| CM-2 | 6.3 | 6.8 |
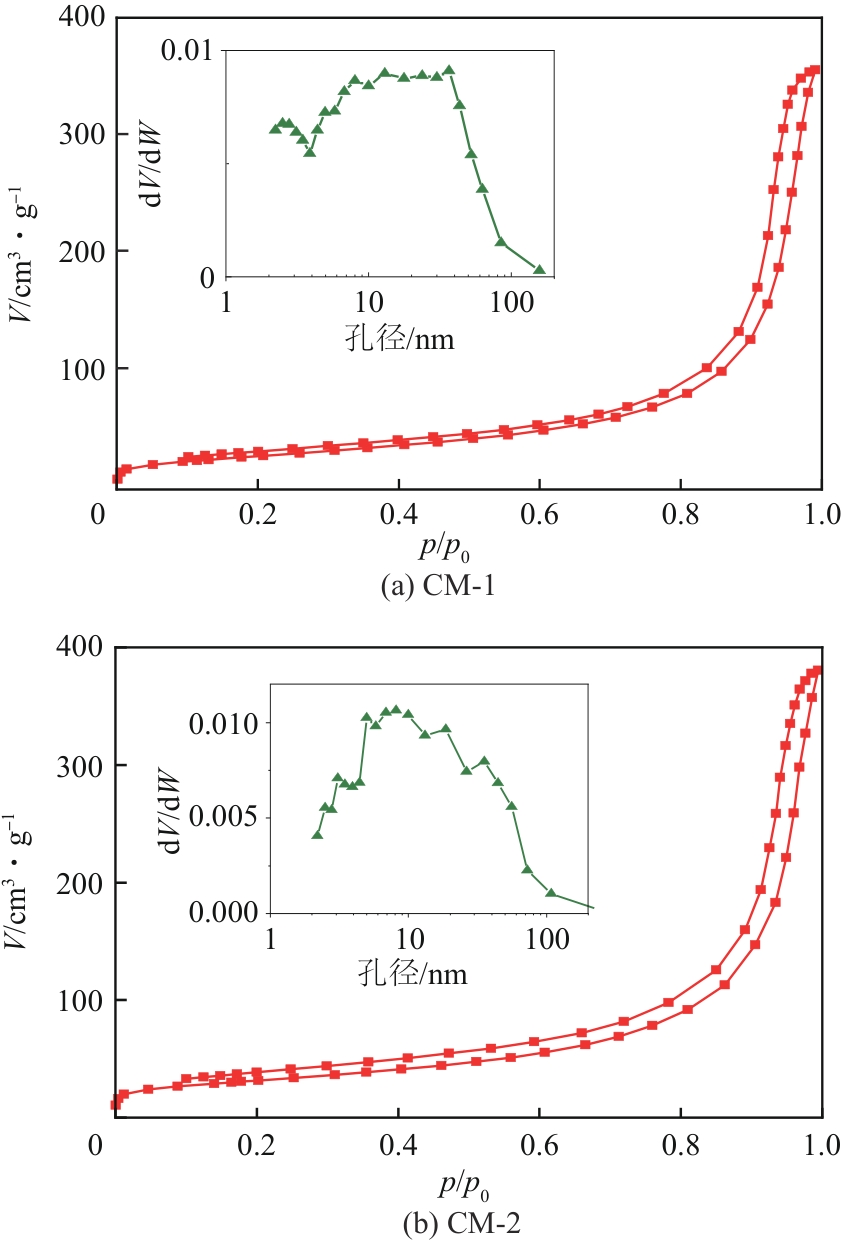
| 催化剂 | BET比表面积/m2·g-1 | 孔容/cm3·g-1 | 平均孔径/nm | 最可几孔径/nm |
|---|---|---|---|---|
| CM-1 | 102 | 0.56 | 20.6 | 36.5 |
| CM-2 | 113 | 0.60 | 20.7 | 8.1 |
| 催化剂 | BET比表面积/m2·g-1 | 孔容/cm3·g-1 | 平均孔径/nm | 最可几孔径/nm |
|---|---|---|---|---|
| CM-1 | 102 | 0.56 | 20.6 | 36.5 |
| CM-2 | 113 | 0.60 | 20.7 | 8.1 |
| 1 | BYUN Manhee, KIM Heehyang, LEE Hyunjun, et al. Conceptual design for methanol steam reforming in serial packed-bed reactors and membrane filters: Economic and environmental perspectives[J]. Energy, 2022, 241: 122516. |
| 2 | ANANDARAJAH Gabrial, MCDOWALL Will, EKINS Paul. Decarbonising road transport with hydrogen and electricity: Long term global technology learning scenarios[J]. International Journal of Hydrogen Energy, 2013, 38(8): 3419-3432. |
| 3 | NUNES Paula, OLIVEIRA Fabricio, HAMACHER Silvio, et al. Design of a hydrogen supply chain with uncertainty[J]. International Journal of Hydrogen Energy, 2015, 40(46): 16408-16418. |
| 4 | SIDDIQUI Osamah, DINCER Ibrahim. A well to pump life cycle environmental impact assessment of some hydrogen production routes[J]. International Journal of Hydrogen Energy, 2019, 44(12): 5773-5786. |
| 5 | ZHAO Jiaqi, SHI Run, LI Zhenhua, et al. How to make use of methanol in green catalytic hydrogen production[J]. Nano Select, 2020, 1(1): 12-29. |
| 6 | BALCOMBE Paul, SPEIRS Jamie, JOHNSON Erin, et al. The carbon credentials of hydrogen gas networks and supply chains[J]. Renewable and Sustainable Energy Reviews, 2018, 91: 1077-1088. |
| 7 | GARCIA Gabriel, ARRIOLA Emmanuel, CHEN Wei-Hsin, et al. A comprehensive review of hydrogen production from methanol thermochemical conversion for sustainability[J]. Energy, 2021, 217: 119384. |
| 8 | XIAO Ze, MENG Qingwei, YUAN Qingchun, et al. High-performance metal-base bifunctional catalysts (Ni x Mg y -MMO) for aqueous phase reforming of methanol to hydrogen[J]. Fuel, 2023, 350: 128808. |
| 9 | YANG Huanhuan, CHEN Yanyan, CUI Xiaojing, et al. A highly stable copper-based catalyst for clarifying the catalytic roles of Cu0 and Cu+ species in methanol dehydrogenation[J]. Angewandte Chemie International Edition, 2018, 57(7): 1836-1840. |
| 10 | MAURI Silvia, Gianluca D’OLIMPIO, GHICA Corneliu, et al. Hydrogen production mechanism in low-temperature methanol decomposition catalyzed by Ni3Sn4 intermetallic compound: A combined operando and density functional theory investigation[J]. The Journal of Physical Chemistry Letters, 2023, 14(5): 1334-1342. |
| 11 | BAGHERZADEH Seyed Behnam, HAGHIGHI Mohammad. Plasma-enhanced comparative hydrothermal and coprecipitation preparation of CuO/ZnO/Al2O3 nanocatalyst used in hydrogen production via methanol steam reforming[J]. Energy Conversion and Management, 2017, 142: 452-465. |
| 12 | XU Xinhai, SHUAI Kaipeng, XU Ben. Review on copper and palladium based catalysts for methanol steam reforming to produce hydrogen[J]. Catalysts, 2017, 7(6): 183. |
| 13 | HUANG Gang, LIAW Biing-Jye, JHANG Cheng-Jyun, et al. Steam reforming of methanol over CuO/ZnO/CeO2/ZrO2/Al2O3 catalysts[J]. Applied Catalysis A: General, 2009, 358(1): 7-12. |
| 14 | LI Guangjun, GU Chuantao, ZHU Wanbin, et al. Hydrogen production from methanol decomposition using Cu-Al spinel catalysts[J]. Journal of Cleaner Production, 2018, 183: 415-423. |
| 15 | LIU X Y, TOYIR J, DE LA PISCINA P R, et al. Hydrogen production from methanol steam reforming over Al2O3 and ZrO2-modified CuOZnOGa2O3 catalysts[J]. International Journal of Hydrogen Energy, 2017, 42(19): 13704-13711. |
| LIU Xianyun, TOYIR Jamil, RAMÍREZ DE LA PISCINA Pilar, et al. Hydrogen production from methanol steam reforming over Al2O3- and ZrO2-modified CuOZnOGa2O3 catalysts[J]. International Journal of Hydrogen Energy, 2017, 42(19): 13704-13711. | |
| 16 | MATTER Paul H, BRADEN Drew J, OZKAN Umit S. Steam reforming of methanol to H2 over nonreduced Zr-containing CuO/ZnO catalysts[J]. Journal of Catalysis, 2004, 223(2): 340-351. |
| 17 | TWIGG Martyn V, SPENCER Michael S. Deactivation of supported copper metal catalysts for hydrogenation reactions[J]. Applied Catalysis A: General, 2001, 212(1/2): 161-174. |
| 18 | MARTÍN Antonio J, MITCHELL Sharon, MONDELLI Cecilia, et al. Unifying views on catalyst deactivation[J]. Nature Catalysis, 2022, 5(10): 854-866. |
| 19 | Sandra SÁ, SILVA Hugo, Lúcia BRANDÃO, et al. Catalysts for methanol steam reforming—A review[J]. Applied Catalysis B: Environmental, 2010, 99(1/2): 43-57. |
| 20 | YANG Jun, ZHENG Hongyan, ZHU Yulei, et al. Effects of calcination temperature on performance of Cu-Zn-Al catalyst for synthesizing γ-butyrolactone and 2-methylfuran through the coupling of dehydrogenation and hydrogenation[J]. Catalysis Communications, 2004, 5(9): 505-510. |
| 21 | BEHRENS Malte, GIRGSDIES Frank. Structural effects of Cu/Zn substitution in the malachite-rosasite system[J]. Zeitschrift Für Anorganische und Allgemeine Chemie, 2010, 636(6): 919-927. |
| 22 | KLOKISHNER Sophia, BEHRENS Malte, Oleg REU, et al. Cation ordering in natural and synthetic (Cu1– x Zn x )2CO3(OH)2 and (Cu1– x Zn x )5(CO3)2(OH)6 [J]. The Journal of Physical Chemistry A, 2011, 115(35): 9954-9968. |
| 23 | WELLS A F. Malachite: re-examination of crystal structure[J]. Acta Crystallographica, 1951, 4(3): 200-204. |
| 24 | ZHANG Fan, XU Xiaoying, QIU Zhengpu, et al. Improved methanol synthesis performance of Cu/ZnO/Al2O3 catalyst by controlling its precursor structure[J]. Green Energy & Environment, 2022, 7(4): 772-781. |
| 25 | Stefanie KÜHL, TARASOV Andrey, ZANDER Stefan, et al. Cu-based catalyst resulting from a Cu, Zn, Al hydrotalcite-like compound: A microstructural, thermoanalytical, and In Situ XAS study[J]. Chemistry:A European Journal, 2014, 20(13): 3782-3792. |
| 26 | SCHUMANN Julia, LUNKENBEIN Thomas, TARASOV Andrey, et al. Synthesis and characterisation of a highly active Cu/ZnO: Al catalyst[J]. ChemCatChem, 2014, 6(10): 2889-2897. |
| 27 | BEMS Bettina, SCHUR Michael, DASSENOY Alina, et al. Relations between synthesis and microstructural properties of copper/zinc hydroxycarbonates[J]. Chemistry:A European Journal, 2003, 9(9): 2039-2052. |
| 28 | SING Kenneth. The use of nitrogen adsorption for the characterisation of porous materials[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2001, 187: 3-9. |
| 29 | WANG Zixiang, ZOU Ying, FANG Zhibin, et al. Partitioning of pore space in hydrogen-bonded organic frameworks for enhanced CO2 photoreduction[J]. Science China Materials, 2024, 67(6): 1846-1850. |
| 30 | ZHANG Haoqing, ZHANG Limin, et al. Controllable synthesis of hollow mesoporous ZSM-5 with improved catalytic performance for tetralin hydrocracking to light aromatics[J]. Industrial & Engineering Chemistry Research, 2025, 64(8): 4330-4341. |
| 31 | IVANOVA T M, MASLAKOV K I, SIDOROV A A, et al. XPS detection of unusual Cu( Ⅱ) to Cu( Ⅰ ) transition on the surface of complexes with redox-active ligands[J]. Journal of Electron Spectroscopy and Related Phenomena, 2020, 238: 146878. |
| 32 | DIVINS Núria J, KORDUS David, TIMOSHENKO Janis, et al. operando high-pressure investigation of size-controlled CuZn catalysts for the methanol synthesis reaction[J]. Nature Communications, 2021, 12(1): 1435. |
| [1] | LIU Wei, HOU Xuelan, YANG Guidong. Green hydrogen-ammonia cycle: Current status and perspective [J]. Chemical Industry and Engineering Progress, 2025, 44(5): 2625-2641. |
| [2] | DING Ajing, ZHOU Qiaoqiao, GU Xuehong. Catalytic gasification of poplar wood in a membrane reactor to produce clean syngas [J]. Chemical Industry and Engineering Progress, 2025, 44(5): 2716-2723. |
| [3] | WANG Shuyuan, YIN Lingling, GAO Zhihua, HUANG Wei. Effect of intercalated Cu proportion on the structure and catalytic performance of CuZnAl-LDHs catalysts [J]. Chemical Industry and Engineering Progress, 2025, 44(4): 2036-2044. |
| [4] | ZHANG Yiru, HAN Dongmei, MA Weifang. Research progress on iron-based composite bismuth oxyhalide magnetic materials for enhanced visible light catalytic treatment of refractory organic wastewater [J]. Chemical Industry and Engineering Progress, 2025, 44(4): 2258-2273. |
| [5] | CHEN Yuhang, LI Qiaoyan, LIANG Meisheng, SONG Tianyuan, WANG Yue, LI Simeng, ZHOU Yuxuan. Role of the Sn dopant on Cu/CeZrO2/γ-Al2O3 three-way catalyst: Enhancement of low-temperature activity and sulfur resistance [J]. Chemical Industry and Engineering Progress, 2025, 44(3): 1368-1377. |
| [6] | ZHANG Xin’er, PEI Liujun, ZHOU Yudie, JIN Kaili, WANG Jiping. Progress of TiO2-based photocatalysts for hydrogen production by water splitting with solar energy [J]. Chemical Industry and Engineering Progress, 2025, 44(3): 1298-1308. |
| [7] | LIU Junjie, WU Jianmin, SUN Qiwen, WANG Jiancheng, SUN Yan. Research of metallocene catalysts for linear α-olefins polymerization to obtain high molecular weight products [J]. Chemical Industry and Engineering Progress, 2025, 44(3): 1309-1322. |
| [8] | ZHU Guoyu, GE Qi, FU Mingli. Durability testing and life prediction of methanol reforming catalysts for hydrogen production [J]. Chemical Industry and Engineering Progress, 2025, 44(3): 1338-1346. |
| [9] | XIE Xinyao, WAN Fen, FU Xuanyu, FAN Yuting, CHEN Lingxiu, LI Peng. Catalytic performance and mechanism of CO2 electroreduction of Cu-Ag nanoclusters [J]. Chemical Industry and Engineering Progress, 2025, 44(3): 1387-1395. |
| [10] | ZUO Ji, LUO Li, XIE Yongkai, CHEN Wenyao, QIAN Gang, ZHOU Xinggui, DUAN Xuezhi. Effect of Cu catalyst particle size on methanol nonoxidative dehydrogenation to formaldehyde [J]. Chemical Industry and Engineering Progress, 2025, 44(3): 1347-1354. |
| [11] | BI Wentao, WANG Xuelin, QU Wei, WANG Congxin, TIAN Zhijian. Effect of Mg-modification on the catalytic performance of Pt/ZSM-22 with low Pt content in n-alkane hydroisomerization [J]. Chemical Industry and Engineering Progress, 2025, 44(3): 1355-1367. |
| [12] | ZHANG Maorun, SUN Weiru, MA Tianlin, XIN Zhiling. Anti-SO2 poisoning performance of Mo-modified MnCe/SiC in low-temperature SCR denitrification [J]. Chemical Industry and Engineering Progress, 2025, 44(3): 1378-1386. |
| [13] | ZHANG Qi, WANG Tao, ZHANG Xuebing, LI Weizhen, CHENG Meng, ZHANG Kui, LYU Yijun, MEN Zhuowu. Advances in Fe-based catalysts for conversion of syngas/CO2 to higher alcohols [J]. Chemical Industry and Engineering Progress, 2025, 44(3): 1323-1337. |
| [14] | TAO Jinquan, JIA Yijing, BAI Tianyu, YAO Rongpeng, HUANG Wenbin, CUI Yan, ZHOU Yasong, WEI Qiang. Synthesis and catalytic MTP performance of Silicalite-1 zeolite with low cost [J]. Chemical Industry and Engineering Progress, 2025, 44(3): 1550-1558. |
| [15] | LIU Jiangtao, PENG Chong, ZHANG Yongchun. Low-carbon olefins from CO2 hydrogenation over Zn-modulated Fe-based catalysts [J]. Chemical Industry and Engineering Progress, 2025, 44(3): 1396-1405. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||