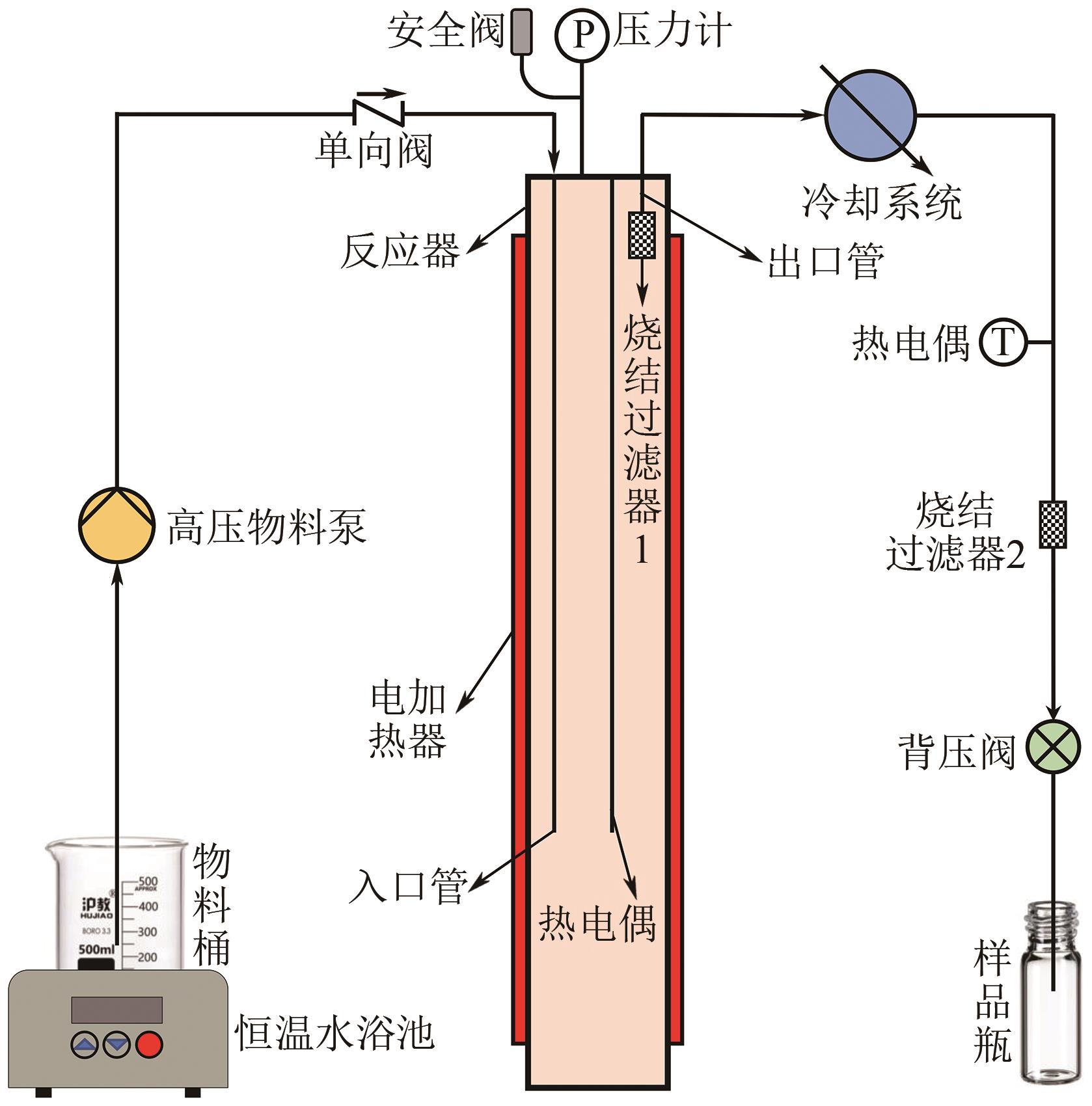
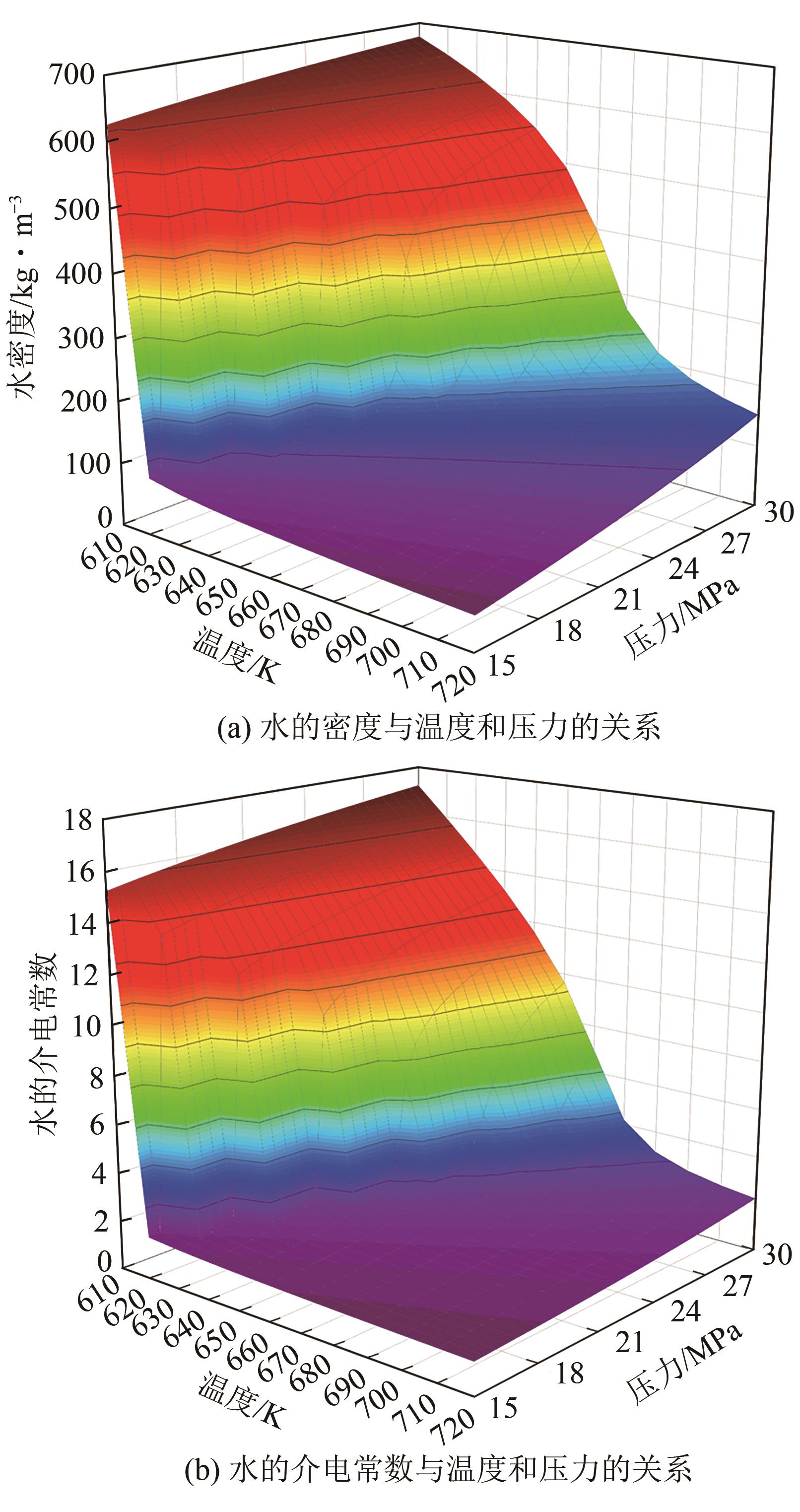
Chemical Industry and Engineering Progress ›› 2025, Vol. 44 ›› Issue (3): 1706-1715.DOI: 10.16085/j.issn.1000-6613.2024-0372
• Resources and environmental engineering • Previous Articles Next Articles
Dissolution characteristics and mechanisms of typical sulphates Na2SO4 and K2SO4 in sub-/supercritical water
FENG Peng( ), XU Donghai(
), XU Donghai( ), HE Bing, LIU Huanteng, YANG Lijie, WANG Pan, LIU Qingshan
), HE Bing, LIU Huanteng, YANG Lijie, WANG Pan, LIU Qingshan
- School of Energy and Power Engineering, Xi’an Jiaotong University, Xi’an 710049, Shaanxi, China
-
Received:2024-03-07Revised:2024-05-13Online:2025-04-15Published:2025-03-25 -
Contact:XU Donghai
亚/超临界水中典型硫酸盐Na2SO4和K2SO4的溶解特性及机理
冯鹏( ), 徐东海(
), 徐东海( ), 何冰, 刘欢腾, 杨立杰, 王攀, 刘青山
), 何冰, 刘欢腾, 杨立杰, 王攀, 刘青山
- 西安交通大学能源与动力工程学院,陕西 西安 710049
-
通讯作者:徐东海 -
作者简介:冯鹏(1994—),男,博士研究生,研究方向为亚/超临界水中无机盐的溶解和沉积特性及机理。E-mail:4120103172@stu.xjtu.edu.cn。 -
基金资助:国家自然科学基金(22078258);西安交通大学青年拔尖人才支持计划(ND6J018);中央高校基本科研业务费(xtr052022009);西安科学家和工程师团队建设项目(23KGDW0001-2023)
CLC Number:
Cite this article
FENG Peng, XU Donghai, HE Bing, LIU Huanteng, YANG Lijie, WANG Pan, LIU Qingshan. Dissolution characteristics and mechanisms of typical sulphates Na2SO4 and K2SO4 in sub-/supercritical water[J]. Chemical Industry and Engineering Progress, 2025, 44(3): 1706-1715.
冯鹏, 徐东海, 何冰, 刘欢腾, 杨立杰, 王攀, 刘青山. 亚/超临界水中典型硫酸盐Na2SO4和K2SO4的溶解特性及机理[J]. 化工进展, 2025, 44(3): 1706-1715.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2024-0372
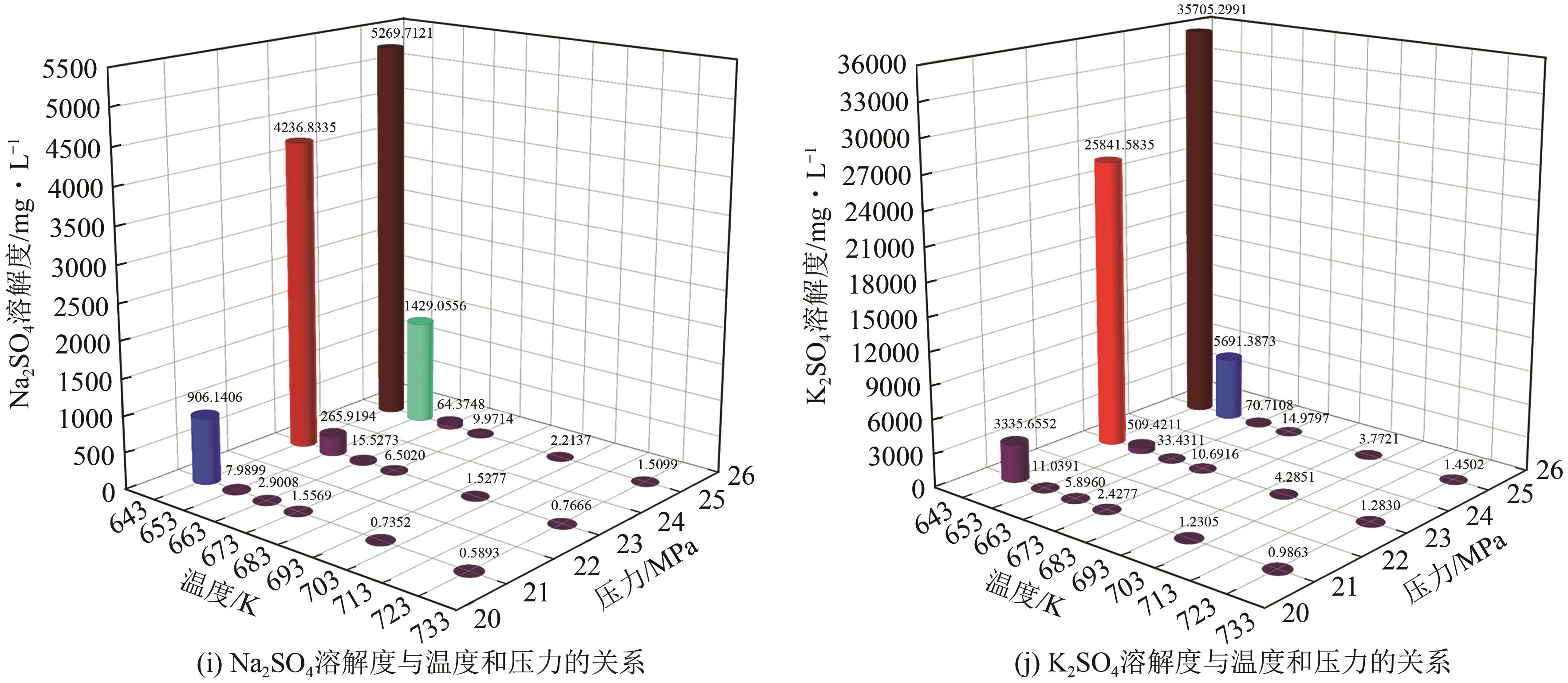
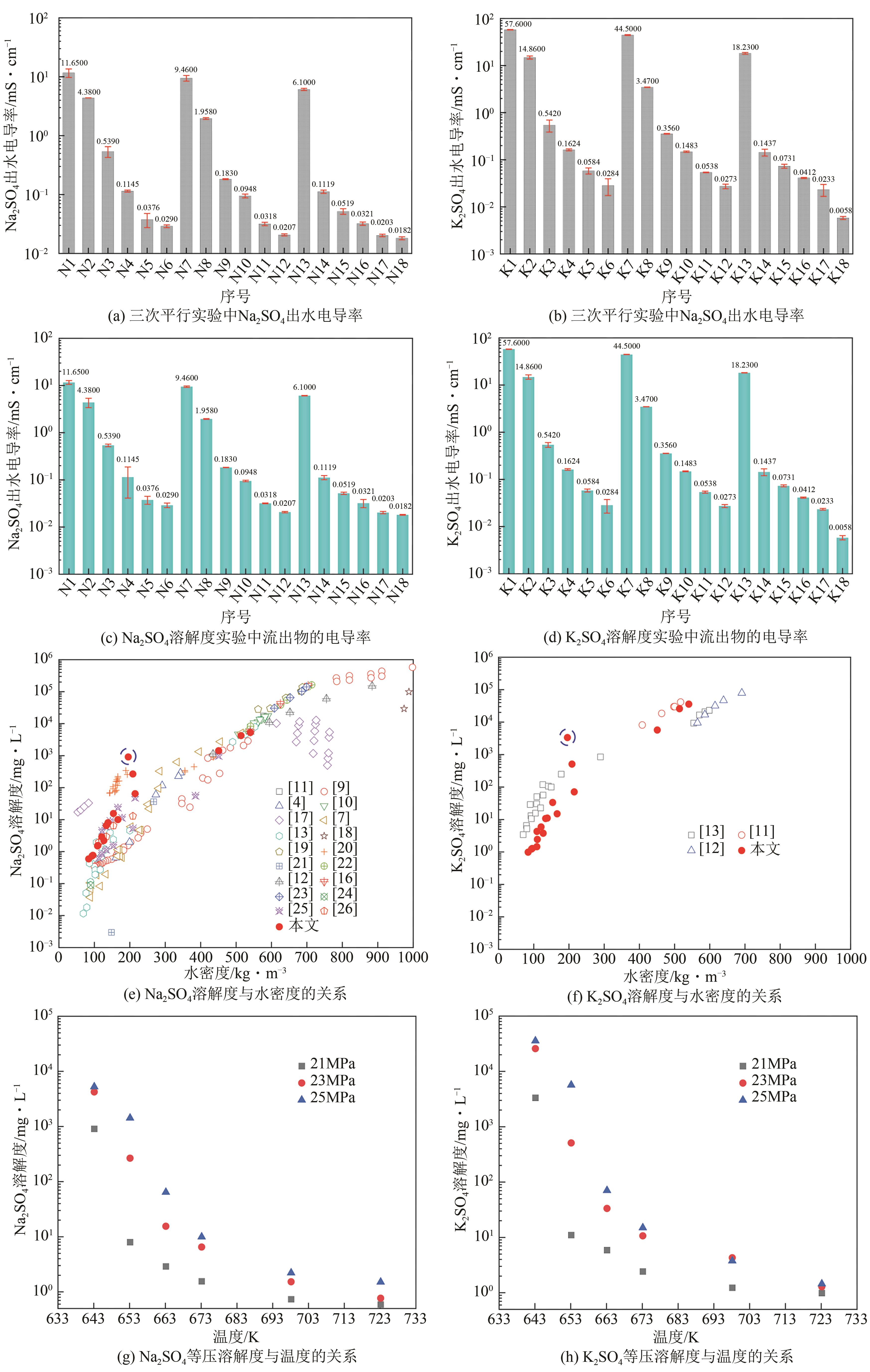
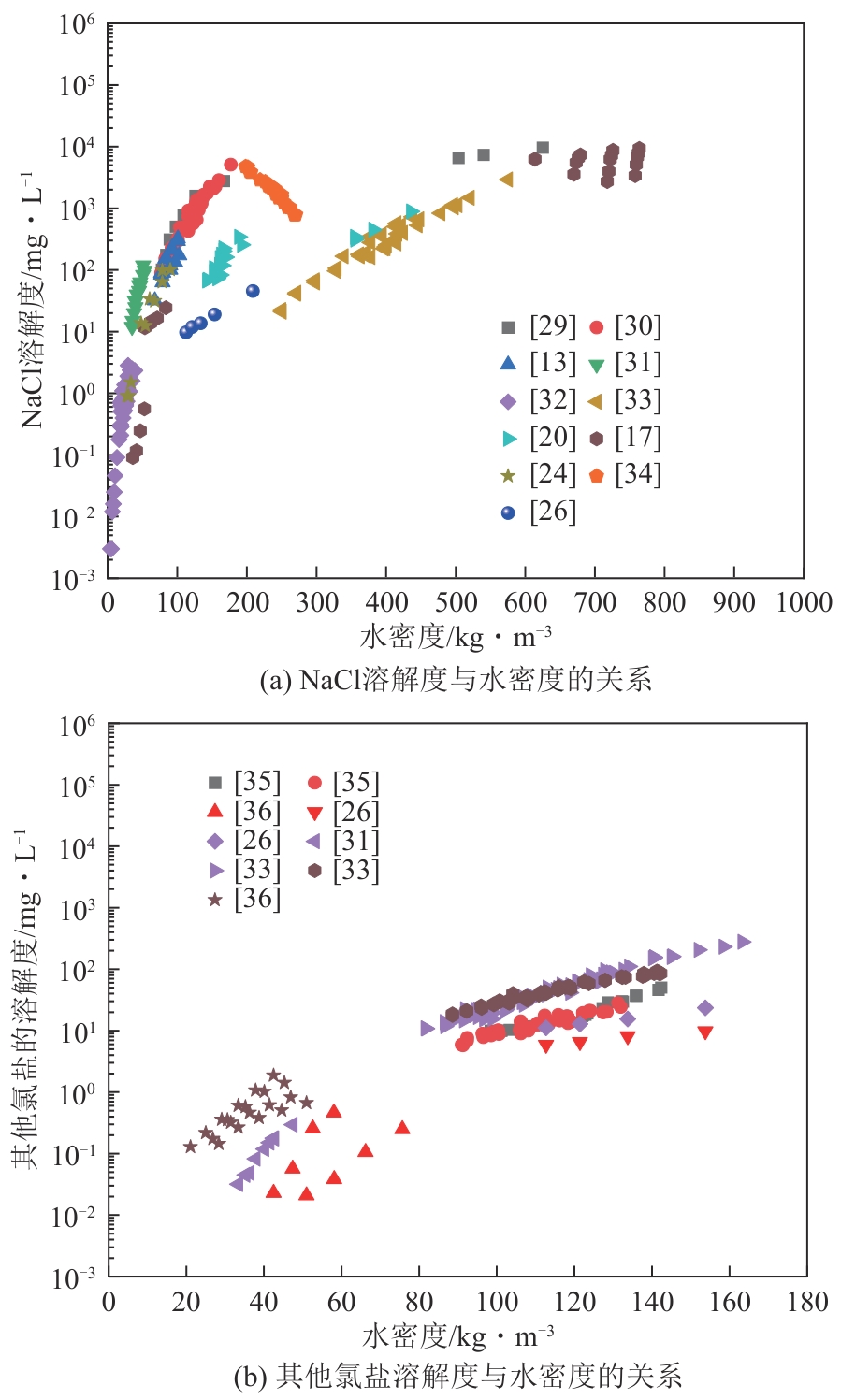
| 序号 | 配置溶液浓度/g·L-1 | 温度/K | 压力/MPa | 水密度/kg·m-3 | 流出物电导率/mS·cm-1 | 溶解度/mg·L-1 |
|---|---|---|---|---|---|---|
| N1 | 30 | 643.15±0.2 | 25±0.1 | 540.46 | 11.65000 | 5269.712 |
| N2 | 30 | 653.15±0.2 | 25±0.1 | 450.82 | 4.38000 | 1429.056 |
| N3 | 10 | 663.15±0.2 | 25±0.1 | 215.18 | 0.53900 | 64.375 |
| N4 | 10 | 673.15±0.2 | 25±0.1 | 166.54 | 0.11450 | 9.971 |
| N5 | 10 | 698.15±0.2 | 25±0.1 | 126.81 | 0.03760 | 2.214 |
| N6 | 10 | 723.15±0.2 | 25±0.1 | 108.98 | 0.02900 | 1.510 |
| N7 | 30 | 643.15±0.2 | 23±0.1 | 513.97 | 9.46000 | 4236.833 |
| N8 | 10 | 653.15±0.2 | 23±0.1 | 208.68 | 1.95800 | 265.920 |
| N9 | 10 | 663.15±0.2 | 23±0.1 | 153.75 | 0.18300 | 15.527 |
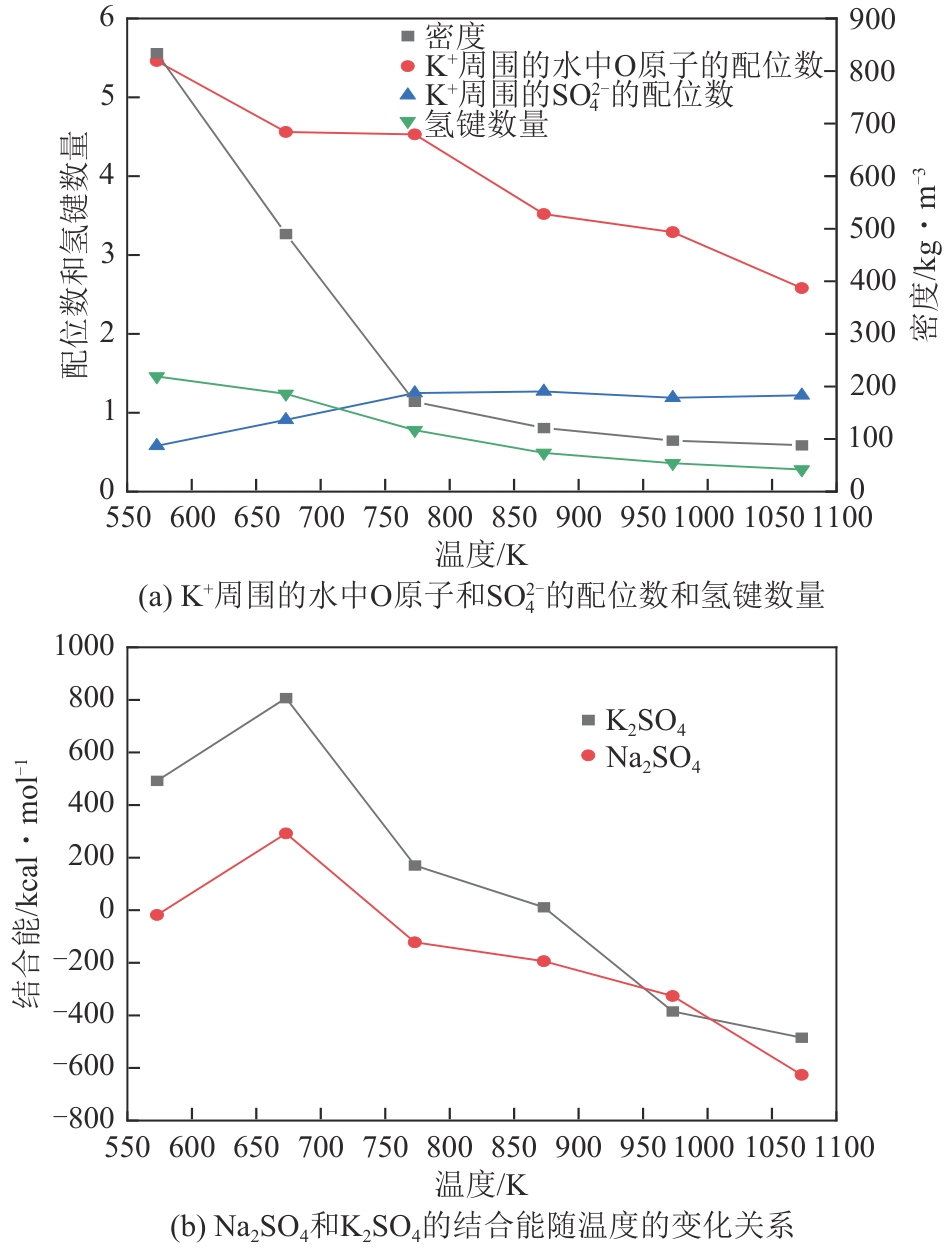
| N10 | 10 | 673.15±0.2 | 23±0.1 | 133.73 | 0.09480 | 6.502 |
| N11 | 10 | 698.15±0.2 | 23±0.1 | 109.14 | 0.03180 | 1.528 |
| N12 | 10 | 723.15±0.2 | 23±0.1 | 95.96 | 0.02070 | 0.767 |
| N13 | 10 | 643.15±0.2 | 21±0.1 | 195.73 | 6.10000 | 906.141 |
| N14 | 10 | 653.15±0.2 | 21±0.1 | 138.57 | 0.11190 | 7.990 |
| N15 | 10 | 663.15±0.2 | 21±0.1 | 120.96 | 0.05190 | 2.901 |
| N16 | 10 | 673.15±0.2 | 21±0.1 | 110.18 | 0.03210 | 1.557 |
| N17 | 10 | 698.15±0.2 | 21±0.1 | 93.97 | 0.02027 | 0.735 |
| N18 | 10 | 723.15±0.2 | 21±0.1 | 84.13 | 0.01815 | 0.590 |
| K1 | 80 | 643.15±0.2 | 25±0.1 | 540.46 | 57.60000 | 35705.299 |
| K2 | 30 | 653.15±0.2 | 25±0.1 | 450.82 | 14.86000 | 5691.387 |
| K3 | 10 | 663.15±0.2 | 25±0.1 | 215.18 | 0.54200 | 70.711 |
| K4 | 10 | 673.15±0.2 | 25±0.1 | 166.54 | 0.16240 | 14.980 |
| K5 | 10 | 698.15±0.2 | 25±0.1 | 126.81 | 0.05840 | 3.772 |
| K6 | 10 | 723.15±0.2 | 25±0.1 | 108.98 | 0.02840 | 1.450 |
| K7 | 80 | 643.15±0.2 | 23±0.1 | 513.97 | 44.50000 | 25841.584 |
| K8 | 30 | 653.15±0.2 | 23±0.1 | 208.68 | 3.47000 | 509.421 |
| K9 | 10 | 663.15±0.2 | 23±0.1 | 153.75 | 0.35600 | 33.431 |
| K10 | 10 | 673.15±0.2 | 23±0.1 | 133.73 | 0.14830 | 10.692 |
| K11 | 10 | 698.15±0.2 | 23±0.1 | 109.14 | 0.05380 | 4.285 |
| K12 | 10 | 723.15±0.2 | 23±0.1 | 95.96 | 0.02730 | 1.283 |
| K13 | 30 | 643.15±0.2 | 21±0.1 | 195.73 | 18.23000 | 3335.655 |
| K14 | 10 | 653.15±0.2 | 21±0.1 | 138.57 | 0.14370 | 11.039 |
| K15 | 10 | 663.15±0.2 | 21±0.1 | 120.96 | 0.07310 | 5.896 |
| K16 | 10 | 673.15±0.2 | 21±0.1 | 110.18 | 0.04120 | 2.428 |
| K17 | 10 | 698.15±0.2 | 21±0.1 | 93.97 | 0.02330 | 1.231 |
| K18 | 10 | 723.15±0.2 | 21±0.1 | 84.13 | 0.00583 | 0.986 |
| 序号 | 配置溶液浓度/g·L-1 | 温度/K | 压力/MPa | 水密度/kg·m-3 | 流出物电导率/mS·cm-1 | 溶解度/mg·L-1 |
|---|---|---|---|---|---|---|
| N1 | 30 | 643.15±0.2 | 25±0.1 | 540.46 | 11.65000 | 5269.712 |
| N2 | 30 | 653.15±0.2 | 25±0.1 | 450.82 | 4.38000 | 1429.056 |
| N3 | 10 | 663.15±0.2 | 25±0.1 | 215.18 | 0.53900 | 64.375 |
| N4 | 10 | 673.15±0.2 | 25±0.1 | 166.54 | 0.11450 | 9.971 |
| N5 | 10 | 698.15±0.2 | 25±0.1 | 126.81 | 0.03760 | 2.214 |
| N6 | 10 | 723.15±0.2 | 25±0.1 | 108.98 | 0.02900 | 1.510 |
| N7 | 30 | 643.15±0.2 | 23±0.1 | 513.97 | 9.46000 | 4236.833 |
| N8 | 10 | 653.15±0.2 | 23±0.1 | 208.68 | 1.95800 | 265.920 |
| N9 | 10 | 663.15±0.2 | 23±0.1 | 153.75 | 0.18300 | 15.527 |
| N10 | 10 | 673.15±0.2 | 23±0.1 | 133.73 | 0.09480 | 6.502 |
| N11 | 10 | 698.15±0.2 | 23±0.1 | 109.14 | 0.03180 | 1.528 |
| N12 | 10 | 723.15±0.2 | 23±0.1 | 95.96 | 0.02070 | 0.767 |
| N13 | 10 | 643.15±0.2 | 21±0.1 | 195.73 | 6.10000 | 906.141 |
| N14 | 10 | 653.15±0.2 | 21±0.1 | 138.57 | 0.11190 | 7.990 |
| N15 | 10 | 663.15±0.2 | 21±0.1 | 120.96 | 0.05190 | 2.901 |
| N16 | 10 | 673.15±0.2 | 21±0.1 | 110.18 | 0.03210 | 1.557 |
| N17 | 10 | 698.15±0.2 | 21±0.1 | 93.97 | 0.02027 | 0.735 |
| N18 | 10 | 723.15±0.2 | 21±0.1 | 84.13 | 0.01815 | 0.590 |
| K1 | 80 | 643.15±0.2 | 25±0.1 | 540.46 | 57.60000 | 35705.299 |
| K2 | 30 | 653.15±0.2 | 25±0.1 | 450.82 | 14.86000 | 5691.387 |
| K3 | 10 | 663.15±0.2 | 25±0.1 | 215.18 | 0.54200 | 70.711 |
| K4 | 10 | 673.15±0.2 | 25±0.1 | 166.54 | 0.16240 | 14.980 |
| K5 | 10 | 698.15±0.2 | 25±0.1 | 126.81 | 0.05840 | 3.772 |
| K6 | 10 | 723.15±0.2 | 25±0.1 | 108.98 | 0.02840 | 1.450 |
| K7 | 80 | 643.15±0.2 | 23±0.1 | 513.97 | 44.50000 | 25841.584 |
| K8 | 30 | 653.15±0.2 | 23±0.1 | 208.68 | 3.47000 | 509.421 |
| K9 | 10 | 663.15±0.2 | 23±0.1 | 153.75 | 0.35600 | 33.431 |
| K10 | 10 | 673.15±0.2 | 23±0.1 | 133.73 | 0.14830 | 10.692 |
| K11 | 10 | 698.15±0.2 | 23±0.1 | 109.14 | 0.05380 | 4.285 |
| K12 | 10 | 723.15±0.2 | 23±0.1 | 95.96 | 0.02730 | 1.283 |
| K13 | 30 | 643.15±0.2 | 21±0.1 | 195.73 | 18.23000 | 3335.655 |
| K14 | 10 | 653.15±0.2 | 21±0.1 | 138.57 | 0.14370 | 11.039 |
| K15 | 10 | 663.15±0.2 | 21±0.1 | 120.96 | 0.07310 | 5.896 |
| K16 | 10 | 673.15±0.2 | 21±0.1 | 110.18 | 0.04120 | 2.428 |
| K17 | 10 | 698.15±0.2 | 21±0.1 | 93.97 | 0.02330 | 1.231 |
| K18 | 10 | 723.15±0.2 | 21±0.1 | 84.13 | 0.00583 | 0.986 |
| 1 | FENG Peng, YANG Wanpeng, XU Donghai, et al. Characteristics, mechanisms and measurement methods of dissolution and deposition of inorganic salts in sub-/ supercritical water[J]. Water Research, 2022, 225: 119167. |
| 2 | XU Tiantian, WANG Shuzhong, LI Yanhui, et al. Review of the destruction of organic radioactive wastes by supercritical water oxidation[J]. Science of The Total Environment, 2021, 799: 149396. |
| 3 | CHEN Jingwei, MENG Tian, LENG Erwei, et al. Review on metal dissolution characteristics and harmful metals recovery from electronic wastes by supercritical water[J]. Journal of Hazardous Materials, 2022, 424: 127693. |
| 4 | KHAN M S, ROGAK S N. Solubility of Na2SO4, Na2CO3 and their mixture in supercritical water[J]. The Journal of Supercritical Fluids, 2004, 30(3): 359-373. |
| 5 | REIMER J, VOGEL F. Influence of anions and cations on the phase behavior of ternary salt solutions studied by high pressure differential scanning calorimetry[J]. The Journal of Supercritical Fluids, 2016, 109: 141-147. |
| 6 | ARMELLINI Fred J, TESTER Jefferson W, HONG Glenn T. Precipitation of sodium chloride and sodium sulfate in water from sub- to supercritical conditions: 150 to 550℃, 100 to 300bar[J]. The Journal of Supercritical Fluids, 1994, 7(3): 147-158. |
| 7 | ROGAK Steven N, TESHIMA Paul. Deposition of sodium sulfate in a heated flow of supercritical water[J]. AIChE Journal, 1999, 45(2): 240-247. |
| 8 | 向波涛, 王涛, 陈跖, 等. 超临界水中硫酸钠溶解度研究[J]. 化学工程, 2001, 29(1): 72-74, 6. |
| XIANG Botao, WANG Tao, CHEN Zhi, et al. The solubility of sodium sulfate in supercritical water[J]. Chemical Engineering (China), 2001, 29(1): 72-74. | |
| 9 | VOISIN T, ERRIGUIBLE A, PHILIPPOT G, et al. Investigation of the precipitation of Na2SO4 in supercritical water[J]. Chemical Engineering Science, 2017, 174: 268-276. |
| 10 | SHVEDOV Dmitri, TREMAINE Peter R. The solubility of sodium sulfate and the reduction of aqueous sulfate by magnetite under near-critical conditions[J]. Journal of Solution Chemistry, 2000, 29(10): 889-904. |
| 11 | HODES Marc, GRIFFITH Peter, SMITH Kenneth A, et al. Salt solubility and deposition in high temperature and pressure aqueous solutions[J]. AIChE Journal, 2004, 50(9): 2038-2049. |
| 12 | REIMER Joachim, VOGEL Frédéric. High pressure differential scanning calorimetry of the hydrothermal salt solutions K2SO4-Na2SO4-H2O and K2HPO4-H2O[J]. RSC Advances, 2013, 3(46): 24503-24508. |
| 13 | DING Xin, ZHANG Tian, ZHANG Shuai, et al. Experimental determination and modelling of the solubilities of sodium sulfate and potassium sulfate in sub- and supercritical water[J]. Fluid Phase Equilibria, 2019, 483: 31-51. |
| 14 | LI Xujun, SUN Jingli, WEI Xueying, et al. Molecular dynamics study with COMPASS II forcefield on nucleation and growth mechanism of sodium chloride in supercritical water[J]. The Journal of Supercritical Fluids, 2023, 202: 106053. |
| 15 | VALYASHKO V M. Phase equilibria of water-salt systems at high temperatures and pressures[M]// Aqueous systems at elevated temperatures and pressures. Amsterdam: Elsevier, 2004: 597-641. |
| 16 | LIU Bo, DING Xin, JIANG Zhao, et al. Research on the solubilities of sodium chloride and sodium sulfate under hydrothermal conditions[J]. Journal of Solution Chemistry, 2020, 49(9): 1186-1207. |
| 17 | ARMELLINI Fred J. Phase equilibria and precipitation phenomena of sodium chloride and sodium sulfate in sub- and supercritical water[D]. Cambridge, United States: Massachusetts Institute of Technology, 1993. |
| 18 | DIPIPPO Matthew M, SAKO Kentaro, TESTER Jefferson W. Ternary phase equilibria for the sodium chloride-sodium sulfate-water system at 200 and 250bar up to 400℃[J]. Fluid Phase Equilibria, 1999, 157(2): 229-255. |
| 19 | VOISIN T, ERRIGUIBLE A, AYMONIER C. Influence of Multiphasic systems on salt(s) solubility in supercritical water: The case of NaCl and NaCl-Na2SO4 [J]. The Journal of Supercritical Fluids, 2019, 152: 104567. |
| 20 | 李以圭, 陆九芳. 电解质溶液理论[M]. 北京: 清华大学出版社, 2005. |
| LI Yigui, LU Jiufang. Electrolyte solution theory[M]. Beijing: Tsinghua University Press, 2005. | |
| 21 | RAVICH M I, BOROVAYA F E. Phase equilibria in the sodium sulphate-water system at high temperatures and pressures[J]. Russian Journal of Inorganic Chemistry, 1964, 9: 520-532. |
| 22 | DIPIPPO M M. Phase behavior of inorganic salts in sub- and supercritical water[D]. Cambridge, United States: Massachusetts Institute of Technology, 1998. |
| 23 | ARMELLINI Fred J, TESTER Jefferson W. Solubility of sodium chloride and sulfate in sub- and supercritical water vapor from 450-550℃ and 100—250bar[J]. Fluid Phase Equilibria, 1993, 84: 123-142. |
| 24 | ZHU Qiao, LI Zhe, SONG Yafei, et al. Effects of superheated surface on the deposition behavior of Na2SO4 in supercritical water[J]. Processes, 2023, 11(6): 1779. |
| 25 | SONG Yafei, LI Zhe, ZHU Qiao, et al. Precipitation behavior of salts in supercritical water: Experiments and molecular dynamics simulations[J]. Processes, 2022, 10(2): 423. |
| 26 | RINCÓN J, CAMARILLO R, MARTÍN A. Solubility of aluminum sulfate in near-critical and supercritical water[J]. Journal of Chemical & Engineering Data, 2012, 57(7): 2084-2094. |
| 27 | ZHANG Yishu, WANG Shuzhong, GAO Ziliang, et al. Hydrothermal molten salt: A hydrothermal fluid in SCWO treatment of hypersaline wastewater[J]. Chemical Engineering Journal, 2021, 421: 129589. |
| 28 | LEUSBROCK Ingo, METZ Sybrand J, REXWINKEL Glenn, et al. Quantitative approaches for the description of solubilities of inorganic compounds in near-critical and supercritical water[J]. The Journal of Supercritical Fluids, 2008, 47(2): 117-127. |
| 29 | HIGASHI Hidenori, IWAI Yoshio, MATSUMOTO Kota, et al. Measurement and correlation for solubilities of alkali metal chlorides in water vapor at high temperature and pressure[J]. Fluid Phase Equilibria, 2005, 228: 547-551. |
| 30 | GALOBARDES Javier F, VAN HARE David R, ROGERS Lockhart B. Solubility of sodium chloride in dry steam[J]. Journal of Chemical & Engineering Data, 1981, 26(4): 363-366. |
| 31 | LEUSBROCK Ingo, METZ Sybrand J, REXWINKEL Glenn, et al. Solubility of 1:1 alkali nitrates and chlorides in near-critical and supercritical water[J]. Journal of Chemical & Engineering Data, 2009, 54(12): 3215-3223. |
| 32 | PITZER Kenneth S, BISCHOFF James L, ROSENBAUER Robert J. Critical behavior of dilute NaCl in H2O[J]. Chemical Physics Letters, 1987, 134(1): 60-63. |
| 33 | LEUSBROCK Ingo, METZ Sybrand J, REXWINKEL Glenn, et al. The solubility of magnesium chloride and calcium chloride in near-critical and supercritical water[J]. The Journal of Supercritical Fluids, 2010, 53(1/2/3): 17-24. |
| 34 | SHIMOYAMA Yusuke, HIGASHI Hidenori, TSUZAKI Seiya, et al. Effect of cation species on solubilities of metal chlorides in water vapor at high temperatures and pressures[J]. The Journal of Supercritical Fluids, 2009, 50(1): 1-5. |
| 35 | LI Xujun, QI Xingang, LU Libo, et al. Experimental and molecular dynamics simulation study on solubility characteristics of chloride and sulfate salts in supercritical water[J]. The Journal of Supercritical Fluids, 2024, 205: 106150. |
| 36 | VOISIN Thomas, ERRIGUIBLE Arnaud, BALLENGHIEN David, et al. Solubility of inorganic salts in sub- and supercritical hydrothermal environment: Application to SCWO processes[J]. The Journal of Supercritical Fluids, 2017, 120: 18-31. |
| 37 | YANG Wanpeng, XU Donghai, DIAO Yunfei, et al. Molecular dynamics simulations on K2SO4 nucleation in supercritical water[J]. Journal of Molecular Liquids, 2022, 367: 120565. |
| [1] | SHEN Yaozong, GUO Peimin, WANG Lei, KONG Lingbing, GUO Qing, XIE Yibin. Solubility and purification mechanism of AlF3 impurities in the HF system [J]. Chemical Industry and Engineering Progress, 2025, 44(2): 1157-1162. |
| [2] | GONG Decheng, SHEN Qian, ZHU Xianqing, HUANG Yun, XIA Ao, ZHANG Jingmiao, ZHU Xun, LIAO Qiang. Recent progress in the production of hydrogen-rich syngas via supercritical water gasification of microalgae [J]. Chemical Industry and Engineering Progress, 2024, 43(7): 3709-3728. |
| [3] | XIE Guoping, TAN Xuesong, LIU Peng, MIAO Changlin, XU Guangwen, ZHUANG Xinshu. Research progress of lignocellulosic pretreatment based on bio-based derived organic solvents [J]. Chemical Industry and Engineering Progress, 2024, 43(6): 3347-3358. |
| [4] | HU Hongyuan, ZHANG Yang, ZHANG Hedong, FAN Bingqiang, ZHENG Shili, TANG Jihai. Phase equilibrium of Na2SO4-NH3-CO2-H2O system in preparation of sodium bicarbonate from sodium sulfate [J]. Chemical Industry and Engineering Progress, 2024, 43(3): 1621-1629. |
| [5] | SANG Wei, TANG Jianfeng, HUA Yihuai, CHEN Jie, SUN Peiyuan, XU Yifei. Effects of physical solvent and amine properties on the performance of biphasic solvent [J]. Chemical Industry and Engineering Progress, 2023, 42(4): 2151-2159. |
| [6] | ZHAO Yi, YANG Zhen, WANG Jia, LI Jingwen, ZHENG Yu. Research progress on molecular dynamics simulation of self-healing behavior of asphalt binder [J]. Chemical Industry and Engineering Progress, 2023, 42(2): 803-813. |
| [7] |
DANG Xiao’e, ZHANG Ting.
Species distribution of Fe(Ⅲ) in Fe3+-C2O |
| [8] | SUN Xianhang, REN Zhu, ZHANG Guojun, SUN Yuan, FAN Kaifeng, HUANG Weiqiu. Study on the desorption mechanism of toluene in activated carbon under supercritical CO2 [J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 631-636. |
| [9] | GU Xubo, LIAO Chuanhua, WANG Changqing. Design optimization of supercritical water oxidation energy recovery system [J]. Chemical Industry and Engineering Progress, 2022, 41(9): 5094-5102. |
| [10] | MA Xu, ZOU Minggui, CUI Weiwei, FU Anran, LIAO Xiaolong, GONG Guifen. Synthesis and performance of a kind of water soluble negative electrode binder [J]. Chemical Industry and Engineering Progress, 2022, 41(6): 3138-3145. |
| [11] | ZHANG Shizhong, CHEN Zhanxiu, LIU Fengrui, PANG Runyu, WANG Qing. Molecular dynamics simulation of liquid boiling on nanostructured surfaces [J]. Chemical Industry and Engineering Progress, 2022, 41(5): 2311-2321. |
| [12] | TANG Jinqiong, KONG Yong, SHEN Xiaodong. Advances in the synthesis and application of the carbide-derived carbons [J]. Chemical Industry and Engineering Progress, 2022, 41(2): 791-802. |
| [13] | YANG Jin, YIN Yonggao, CHEN Wanhe, WANG Jingyuan, CHEN Jiufa. Preparation and performance optimization of phase change cold storage materials with sodium sulfate hydrate salt [J]. Chemical Industry and Engineering Progress, 2022, 41(11): 5977-5985. |
| [14] | YAO Desong, LIU Huang, CHEN Li, LI Ruijing, WANG Jian. Theoretical analysis of CO2 dissolution ability in ZIF-8/glycol-2-methylimidazole slurry [J]. Chemical Industry and Engineering Progress, 2021, 40(S2): 315-321. |
| [15] | Huayi JIANG, Yuanwang DUAN, Yulong WANG, Shaojie ZOU, Lanxin ZHANG, Jin LI, Bing WANG. Supercritical water gasification of oily sludge to produce hydrogen based on uniform design method [J]. Chemical Industry and Engineering Progress, 2020, 39(9): 3819-3825. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||