Chemical Industry and Engineering Progress ›› 2023, Vol. 42 ›› Issue (11): 6005-6014.DOI: 10.16085/j.issn.1000-6613.2022-2308
• Resources and environmental engineering • Previous Articles
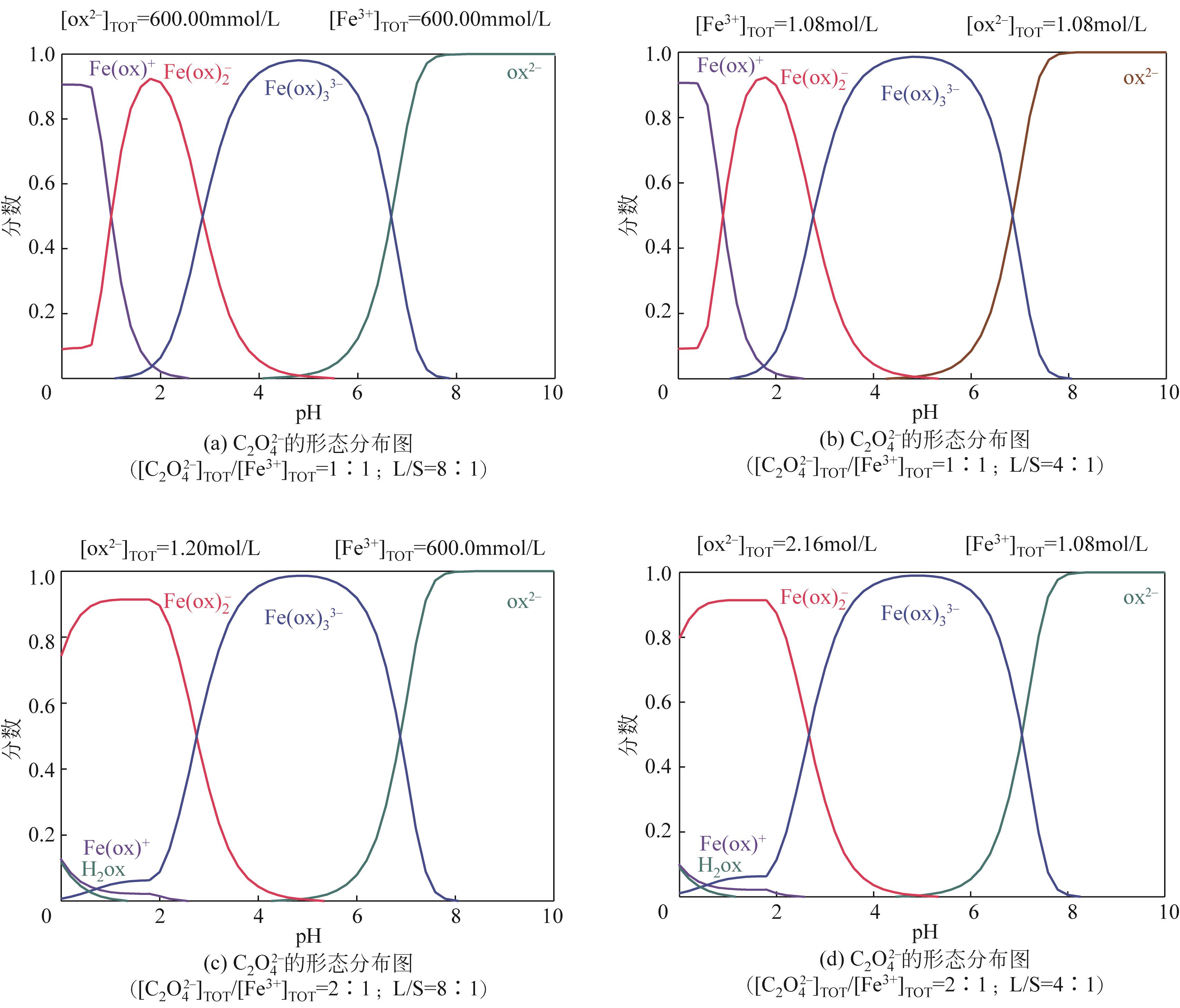
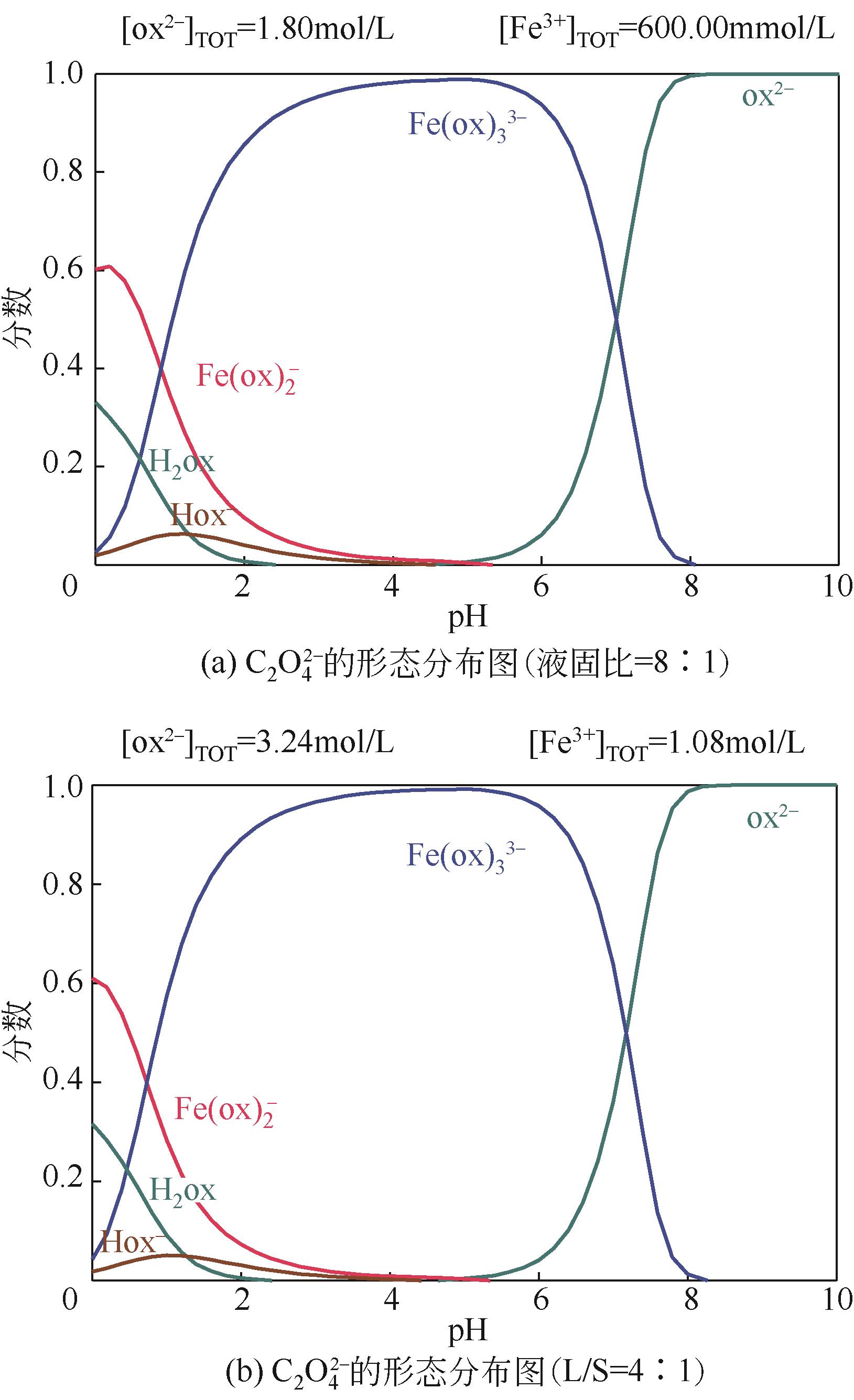
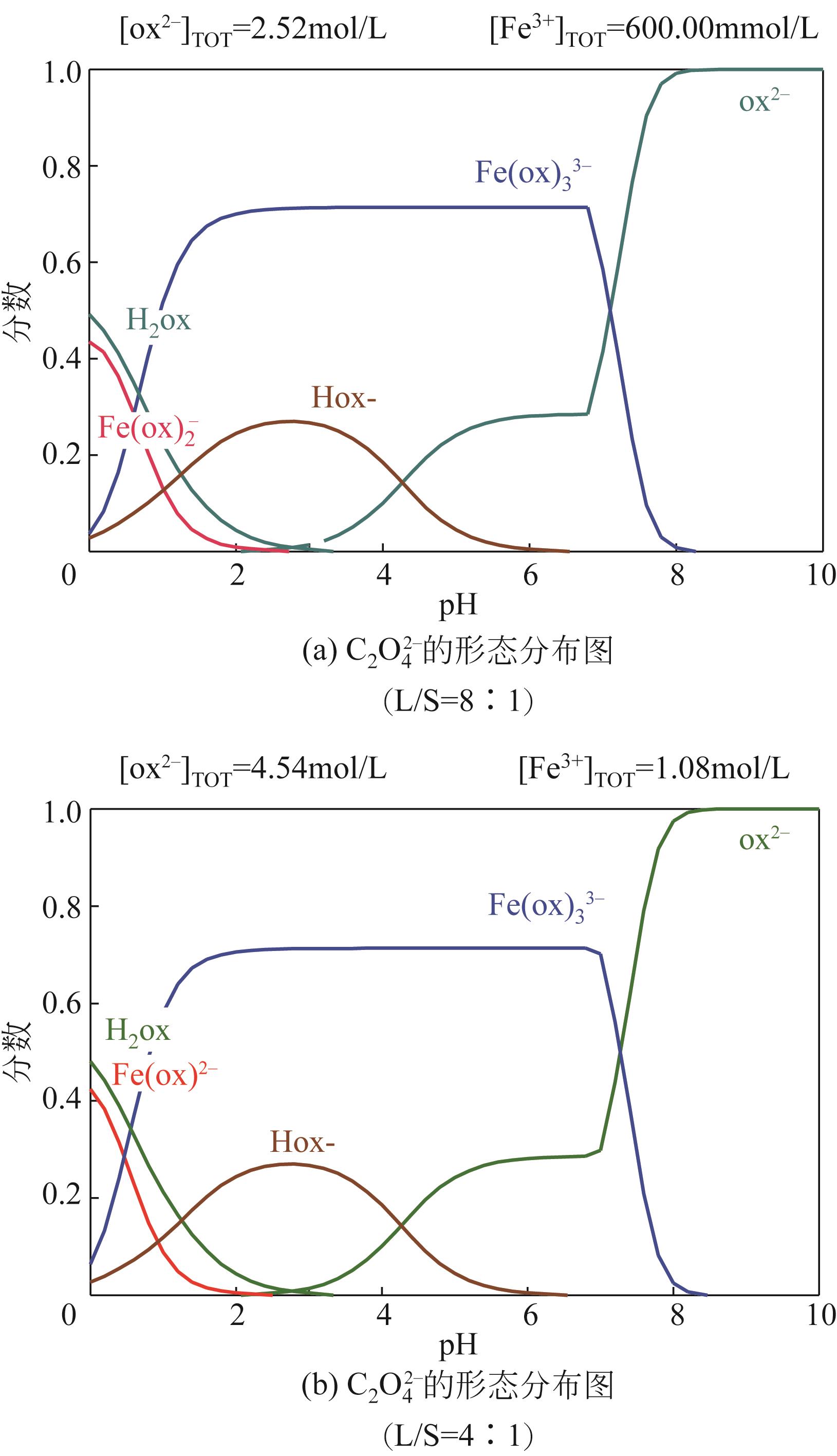
Species distribution of Fe(Ⅲ) in Fe3+-C2O
- 1.College of Metallurgical Engineering, Xi’an University of Architecture and Technology, Xi’an 710055, Shaanxi, China
2.Key Laboratory for Gold and Resources of Shaanxi Province, Xi’an 710055, Shaanxi, China
-
Received:2022-12-12Revised:2023-03-06Online:2023-12-15Published:2023-11-20 -
Contact:DANG Xiao’e
Fe3+-C2O
- 1.西安建筑科技大学冶金工程学院,陕西 西安 710055
2.陕西省黄金与资源重点实验室,陕西 西安 710055
-
通讯作者:党晓娥 -
作者简介:党晓娥(1971—),女,博士,研究方向为冶金二次资源综合利用及冶金新技术。E-mail:dxe2371@126.com。 -
基金资助:国家自然科学基金(51974221)
CLC Number:
Cite this article
DANG Xiao’e, ZHANG Ting. Species distribution of Fe(Ⅲ) in Fe3+-C2O
党晓娥, 张婷. Fe3+-C2O
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2022-2308
| 液固比 | 草酸与草酸铵摩尔比 | |||||||
|---|---|---|---|---|---|---|---|---|
| 2.5︰0.5 | 2︰1 | 1.5︰1.5 | 1︰2 | |||||
| pH | 铁浸出率 /% | pH | 铁浸出率 /% | pH | 铁浸出率 /% | pH | 铁浸出率 /% | |
| 4︰1 | 0.28 | 61.76 | 1.13 | 78.75 | 2.67 | 91.38 | 4.37 | 69.43 |
| 6︰1 | 0.36 | 66.03 | 1.25 | 78.25 | 2.76 | 89.73 | 4.66 | 67.40 |
| 8︰1 | 0.44 | 64.02 | 1.44 | 77.86 | 2.88 | 89.69 | 4.79 | 67.06 |
| 液固比 | 草酸与草酸铵摩尔比 | |||||||
|---|---|---|---|---|---|---|---|---|
| 2.5︰0.5 | 2︰1 | 1.5︰1.5 | 1︰2 | |||||
| pH | 铁浸出率 /% | pH | 铁浸出率 /% | pH | 铁浸出率 /% | pH | 铁浸出率 /% | |
| 4︰1 | 0.28 | 61.76 | 1.13 | 78.75 | 2.67 | 91.38 | 4.37 | 69.43 |
| 6︰1 | 0.36 | 66.03 | 1.25 | 78.25 | 2.76 | 89.73 | 4.66 | 67.40 |
| 8︰1 | 0.44 | 64.02 | 1.44 | 77.86 | 2.88 | 89.69 | 4.79 | 67.06 |
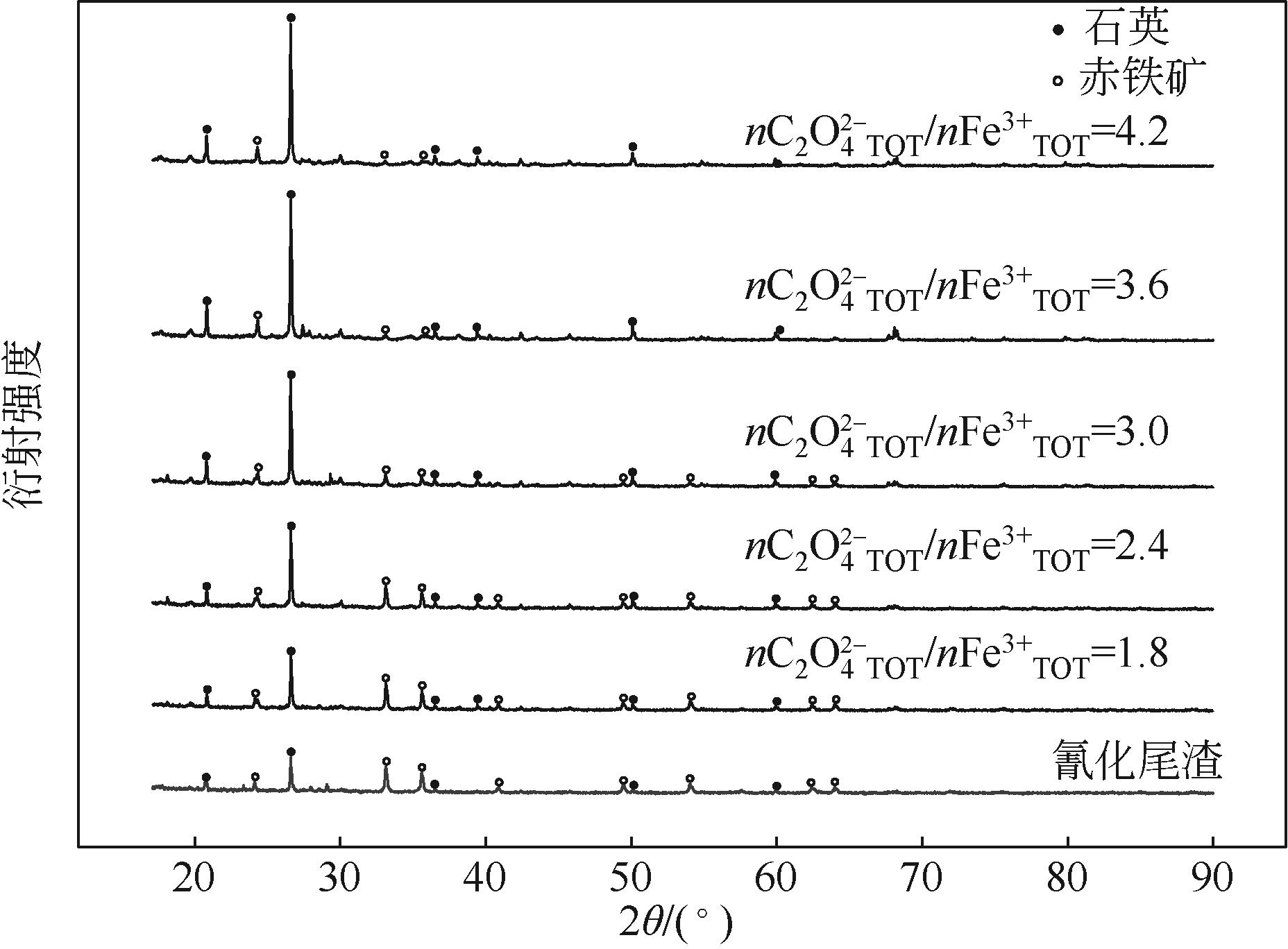
| C2O | 浸出pH | 除铁渣重/g | 渣含铁/% | 铁浸出率/% | 氰化前渣含金/g·t-1 | 氰化后渣含金/g·t-1 | 金浸出率/% |
|---|---|---|---|---|---|---|---|
| 0 | 200.00 | 30.10 | 13.03 | 9.53 | 26.86 | ||
| 1.8 | — | 152.41 | 16.50 | 58.09 | 14.23 | 9.83 | 30.92 |
| 2.4 | — | 141.25 | 11.43 | 73.09 | 16.55 | 9.69 | 41.45 |
| 3.0 | 2.77 | 125.02 | 6.30 | 86.87 | 19.66 | 9.48 | 51.78 |
| 3.6 | 1.94 | 105.62 | 3.70 | 93.49 | 23.54 | 9.56 | 59.39 |
| 4.2 | 1.92 | 101.37 | 1.90 | 96.79 | 24.83 | 9.66 | 61.10 |
| C2O | 浸出pH | 除铁渣重/g | 渣含铁/% | 铁浸出率/% | 氰化前渣含金/g·t-1 | 氰化后渣含金/g·t-1 | 金浸出率/% |
|---|---|---|---|---|---|---|---|
| 0 | 200.00 | 30.10 | 13.03 | 9.53 | 26.86 | ||
| 1.8 | — | 152.41 | 16.50 | 58.09 | 14.23 | 9.83 | 30.92 |
| 2.4 | — | 141.25 | 11.43 | 73.09 | 16.55 | 9.69 | 41.45 |
| 3.0 | 2.77 | 125.02 | 6.30 | 86.87 | 19.66 | 9.48 | 51.78 |
| 3.6 | 1.94 | 105.62 | 3.70 | 93.49 | 23.54 | 9.56 | 59.39 |
| 4.2 | 1.92 | 101.37 | 1.90 | 96.79 | 24.83 | 9.66 | 61.10 |
| 矿物种类 | 陕西两段焙烧 氰化尾渣 | 湖南郴州 硫酸烧渣 | 陕西某固废处理 中心氰化尾渣 | 山东两段焙烧 氰化尾渣 |
|---|---|---|---|---|
| 铁的浸出率 | 96.79 | 99.64 | 98.64 | 95.36 |
| 矿物种类 | 陕西两段焙烧 氰化尾渣 | 湖南郴州 硫酸烧渣 | 陕西某固废处理 中心氰化尾渣 | 山东两段焙烧 氰化尾渣 |
|---|---|---|---|---|
| 铁的浸出率 | 96.79 | 99.64 | 98.64 | 95.36 |
| 渣类型 | 元素 | 金含量/g·t-1 | 铁质量分数/% | 铜质量分数/% | 锌质量分数/% | 铅质量分数/% |
|---|---|---|---|---|---|---|
| 陕西两段焙烧氰化尾渣 | 除铁前 | 13.03 | 30.10 | 0.28 | 0.38 | 0.99 |
| 除铁后 | 25.39 | 2.20 | 0.51 | 0.34 | 1.91 | |
| 湖南郴州硫酸烧渣 | 除铁前 | 4.00 | 38.00 | 0.6 | 1.00 | 2.20 |
| 除铁后 | 11.23 | 1.45 | 1.59 | 2.67 | 5.02 | |
| 陕西某固废中心氰化渣 | 除铁前 | 2.19 | 35.66 | 0.02 | 0.04 | 0.29 |
| 除铁后 | 5.71 | 6.59 | 0.21 | 0.38 | 2.26 | |
| 山东两段焙烧氰化尾渣 | 除铁前 | 7.18 | 32.60 | 0.01 | 1.26 | 2.73 |
| 除铁后 | 15.71 | 3.26 | 0.03 | 3.41 | 4.59 |
| 渣类型 | 元素 | 金含量/g·t-1 | 铁质量分数/% | 铜质量分数/% | 锌质量分数/% | 铅质量分数/% |
|---|---|---|---|---|---|---|
| 陕西两段焙烧氰化尾渣 | 除铁前 | 13.03 | 30.10 | 0.28 | 0.38 | 0.99 |
| 除铁后 | 25.39 | 2.20 | 0.51 | 0.34 | 1.91 | |
| 湖南郴州硫酸烧渣 | 除铁前 | 4.00 | 38.00 | 0.6 | 1.00 | 2.20 |
| 除铁后 | 11.23 | 1.45 | 1.59 | 2.67 | 5.02 | |
| 陕西某固废中心氰化渣 | 除铁前 | 2.19 | 35.66 | 0.02 | 0.04 | 0.29 |
| 除铁后 | 5.71 | 6.59 | 0.21 | 0.38 | 2.26 | |
| 山东两段焙烧氰化尾渣 | 除铁前 | 7.18 | 32.60 | 0.01 | 1.26 | 2.73 |
| 除铁后 | 15.71 | 3.26 | 0.03 | 3.41 | 4.59 |
| 矿物名称 | 除铁前 | 除铁后 | 矿物名称 | 除铁前 | 除铁后 |
|---|---|---|---|---|---|
| 铁氧化物相 | 62.06 | 1.43 | 石英 | 17.46 | 43.34 |
| 闪锌矿 | 0.05 | 0.12 | 碳酸盐矿物 | 0.74 | 10.38 |
| 黄铜矿 | 0.15 | 0.79 | 方铅矿 | 0.23 | 0.43 |
| 斑铜矿 | 0.06 | 0.15 | 白云母 | 10.58 | 21.11 |
| 矿物名称 | 除铁前 | 除铁后 | 矿物名称 | 除铁前 | 除铁后 |
|---|---|---|---|---|---|
| 铁氧化物相 | 62.06 | 1.43 | 石英 | 17.46 | 43.34 |
| 闪锌矿 | 0.05 | 0.12 | 碳酸盐矿物 | 0.74 | 10.38 |
| 黄铜矿 | 0.15 | 0.79 | 方铅矿 | 0.23 | 0.43 |
| 斑铜矿 | 0.06 | 0.15 | 白云母 | 10.58 | 21.11 |
| 1 | 袁嘉声, 畅永锋, 郑春龙, 等. 氰化尾渣脱氰综述[J]. 中国有色金属学报, 2021, 31(6): 1568-1581. |
| YUAN Jiasheng, CHANG Yongfeng, ZHENG Chunlong, et al. Review on treatment technologies of cyanide tailing[J]. Journal of Chinese Nonferrous Metals, 2021, 31(6): 1568-1581. | |
| 2 | 阮书锋, 桑胜华, 李云, 等. 含金银硫酸烧渣综合利用研究-选择性脱铜[J]. 矿冶, 2017, 26(3): 46-49. |
| RUAN Shufeng, SANG Shenghua, LI Yun, et al. Study on selective copper removal for comprehensiveutilization of gold and silver bearing pyrite cinders[J]. Mining & Metallurgy, 2017, 26(3): 46-49. | |
| 3 | Alp I, Deveci H, Yazıcı E Y, et al. Potential use of pyrite cinders as raw material in cement production: Results of industrial scale trial operations[J]. Journal of Hazardous Materials, 2009, 166(1): 144-149. |
| 4 | 郑哗. 难处理金矿石预处理技术及应用现状[J]. 黄金, 2009, 30(1): 36-41. |
| ZHENG Hua. Pretreatment technique and its application of refractory gold ore[J]. Gold, 2009, 30(1): 36-41. | |
| 5 | HANG Hanquan, CHEN Guanhua, CAI Xiang, et al. The leaching behavior of copper and iron recovery from reduction roasting pyrite cinder[J]. Journal of Hazardous Materials, 2021, 420, 126561. |
| 6 | LI Haoyu, LONG Hailin, ZHANG Libo, et al. Effectiveness of microwave-assisted thermal treatment in the extraction of gold in cyanide tailings[J]. Journal of Hazardous Materials, 2020, 384: 121456. |
| 7 | LONG Hailin, LI Haoyu, PEI Jiannan, et al. Cleaner recovery of multiple valuable metals from cyanide tailings via chlorination roasting[J]. Separation Science and Technology, 2021, 56(12): 2113-2123. |
| 8 | WANG Weiwei, LI Zhengyao. Recovery and kinetics of gold and iron from cyanide tailings by one-step chlorination-reduction roasting. Minerals Engineering, 2020, 155(15): 1-4. |
| 9 | CHEN Dong, GUO Hongwei, XU Jifang, et al. Recovery of Iron from cyrite cinder containing non-ferrous metals using high-temperature chloridizing-reduction-magnetic separation[J]. Metallurgical and materials transactions b-process metallurgy and materials processing science, 2017, 48(2): 933-942. |
| 10 | 常耀超, 王云, 刘大学, 等. 氰化尾渣熔融氯化提金扩大试验[J]. 有色金属(冶炼部分), 2016(4): 43-45. |
| CHANG Yaochao, WANG Yun, LIU Daxue, et al. Expanding test on gold recovery from cyanide residue by molten chlorination[J]. Nonferrous Metals(Extractive Metallurgy), 2016(4): 43-45. | |
| 11 | 杨永斌, 曾冠武, 李骞, 等. 高硫砷金矿焙砂的硫酸熟化法预处理[J]. 中国有色金属学报, 2014, 24(9): 2380-2386. |
| YANG Yongbin, ZENG Guanwu, LI qian, et al. Pretreatment by sulfuric acid-curing of calcine roasting for gold ores with high sulfur and arsenic contents[J]. Journal of Chinese Nonferrous Metals, 2014, 24(9): 2380-2385. | |
| 12 | 郑雅杰,陈白珍. 硫铁矿烧渣的熟化及机理[J]. 中国有色金属学报, 2001,11(1) :144-147. |
| ZHENG Yajie, CHEN Baizhen. Maturation of pytite cinders and its mechanism[J]. Journal of Chinese Nonferrous Metals, 2001,11(1): 144-147. | |
| 13 | 马红周, 王丁丁, 王耀宁, 等 .硫酸熟化法浸出焙烧氰化尾渣中的铁[J]. 有色金属(冶炼部分), 2020, (10): 19-22. |
| MA Hongzhou, WANG Dingding, WANG Yaoning, et al. Leaching of iron from roasting cyanide tailings by sulfuric acid curing method[J].Nonferrous Metals(Extractive Metallurgy), 2020, (10): 19-22. | |
| 14 | 郑雅杰, 龚昶, 孙召明. 氰化尾渣还原焙烧酸浸提铁及氰化浸金新工艺[J]. 中国有色金属学报, 2014, 24(9): 2426-2433 |
| ZHENG Yajie, GONG Chang, SUN Zhaoming. New technology of iron extraction and gold recovery from cyanide tailings by cyanide tailings by cyanide process after reduction roasting and acid leaching[J]. The Chinese Journal of Nonferrous Metals, 2014, 24(9): 2426-2433. | |
| 15 | 刘志建, 金哲男, 王保仁,等. 黄金冶炼氰渣火法处理研究现状及展望[J]. 有色金属(冶炼部分), 2021(1): 84-88. |
| LIU Zhijian, JIN Zhenan, WANG Baoren, et al. Research status prospect of pyrometallurgical treatment of cyanide residue residue from gold smelting[J]. Nonferrous Metals(Extractive Metallurgy), 2021(1): 84-88. | |
| 16 | 张永峰, 武鑫. 两段焙烧工艺在黄金生产中的应用[J]. 中国有色冶金, 2010(5): 37-40. |
| ZHANG Yongfeng, WU Xin. Application of two-stage roasting process in gold production[J]. China Nonferrous Metallurgy, 2010(5): 37-40. | |
| 17 | 金程, 李登新. 硫酸烧渣还原浸取铁[J]. 有色金属(冶炼部分), 2012(1): 9-12. |
| JIN Cheng, LI Dengxin. Reductive leaching of iron from sulfate slag[J]. Nonferrous Metals(Extractive Metallurgy), 2012(1): 9-12. | |
| 18 | 王威, 高照国, 曹耀华, 等. 提高含砷金精矿二段焙砂金浸出率的研究[J]. 中国矿业, 2015, 24(7): 104-107. |
| WANG Wei, GAO Zhaoguo, CAO Yaohua, et al. The research on enhancing gold leaching rate from a arsenic-containing gold concentrate with two-stage roasting calcine[J]. China Mining Magazine, 2015, 24(7): 104-107. | |
| 19 | 杨保俊, 方晓宇, 王百年, 等. 草酸助剂强化酸浸法提取硫酸烧渣中的铁[J]. 化工进展, 2019, 38(3):1552-1560. |
| YANG Baojun, FANG Xiaoyu, WANG Bainian, et al. Extraction of iron from pyrite cinder via oxalic acid-intensified leaching method[J]. Chemical Industry and Engineering Progress, 2019, 38(3):1552-1560. |
| [1] | MA Yi, CAO Shiwei, WANG Jiajun, LIN Liqun, XING Yan, CAO Tengliang, LU Feng, ZHAO Zhenlun, ZHANG Zhijun. Research progress in recovery of spent cathode materials for lithium-ion batteries using deep eutectic solvents [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 219-232. |
| [2] | WANG Peng, ZHANG Yang, FAN Bingqiang, HE Dengbo, SHEN Changshuai, ZHANG Hedong, ZHENG Shili, ZOU Xing. Process and kinetics of hydrochloric acid leaching of high-carbon ferrochromium [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 510-517. |
| [3] | ZHU Chuanqiang, RU Jinbo, SUN Tingting, XIE Xingwang, LI Changming, GAO Shiqiu. Characteristics of selective non-catalytic reduction of NO x with solid polymer denitration agent [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4939-4946. |
| [4] | LI Weihua, YU Qianwen, YIN Junquan, WU Yinkai, SUN Yingjie, WANG Yan, WANG Huawei, YANG Yufei, LONG Yuyang, HUANG Qifei, GE Yanchen, HE Yiyang, ZHAO Lingyan. Leaching behavior of heavy metals from broken ton bags filled with fly ash in acid rain environment [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4917-4928. |
| [5] | MAO Shanjun, WANG Zhe, WANG Yong. Group recognition hydrogenation: From concept to application [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 3917-3922. |
| [6] | WANG Baoying, WANG Huangying, YAN Junying, WANG Yaoming, XU Tongwen. Research progress of polymer inclusion membrane in metal separation and recovery [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 3990-4004. |
| [7] | XIANG Yang, HUANG Xun, WEI Zidong. Recent progresses in the activity and selectivity improvement of electrocatalytic organic synthesis [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4005-4014. |
| [8] | WANG Yaogang, HAN Zishan, GAO Jiachen, WANG Xinyu, LI Siqi, YANG Quanhong, WENG Zhe. Strategies for regulating product selectivity of copper-based catalysts in electrochemical CO2 reduction [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4043-4057. |
| [9] | WANG Xiaohan, ZHOU Yasong, YU Zhiqing, WEI Qiang, SUN Jinxiao, JIANG Peng. Synthesis and hydrocracking performance of Y molecular sieves with different crystal sizes [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4283-4295. |
| [10] | LU Yang, ZHOU Jinsong, ZHOU Qixin, WANG Tang, LIU Zhuang, LI Bohao, ZHOU Lingtao. Leaching mechanism of Hg-absorption products on CeO2/TiO2 sorbentsin syngas [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3875-3883. |
| [11] | WANG Jiaxin, PAN Yong, XIONG Xinyi, WAN Xiaoyue, WANG Jianchao. Reaction process and hazards of dinitrotoluene preparation by one-step catalytic nitration of toluene [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3420-3430. |
| [12] | HAN Hengwen, HAN Wei, LI Mingfeng. Research progress in olefin hydration process and the catalysts [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3489-3500. |
| [13] | YIN Pengzhen, WU Qin, LI Hansheng. Advances in catalysts for liquid-phase selective oxidation of methyl aromatic hydrocarbons [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 2916-2943. |
| [14] | YUE Xin, LI Chunying, SUN Dao’an, LI Jiangwei, DU Yongmei, MA Hui, LYU Jian. Progress on heterogeneous catalysts for cyclopropanation of diazo compounds [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2390-2401. |
| [15] | WANG Keju, ZHAO Cheng, HU Xiaomei, YUN Junge, WEI Ninghan, JIANG Xueying, ZOU Yun, CHEN Zhihang. Research progress of low temperature catalytic oxidation of VOCs by metal oxides [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2402-2412. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||