Chemical Industry and Engineering Progress ›› 2024, Vol. 43 ›› Issue (12): 7004-7017.DOI: 10.16085/j.issn.1000-6613.2023-1973
• Resources and environmental engineering • Previous Articles
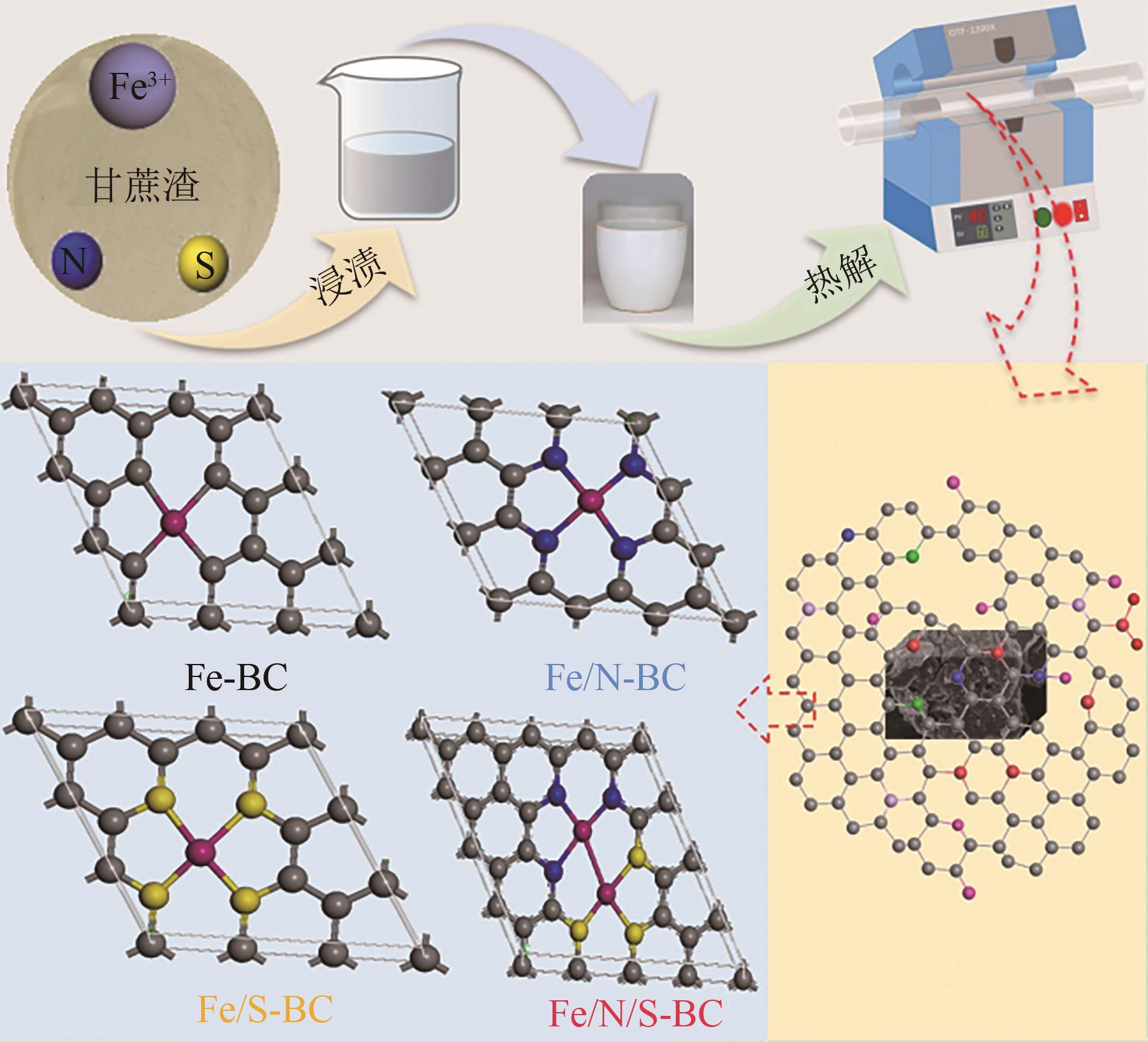
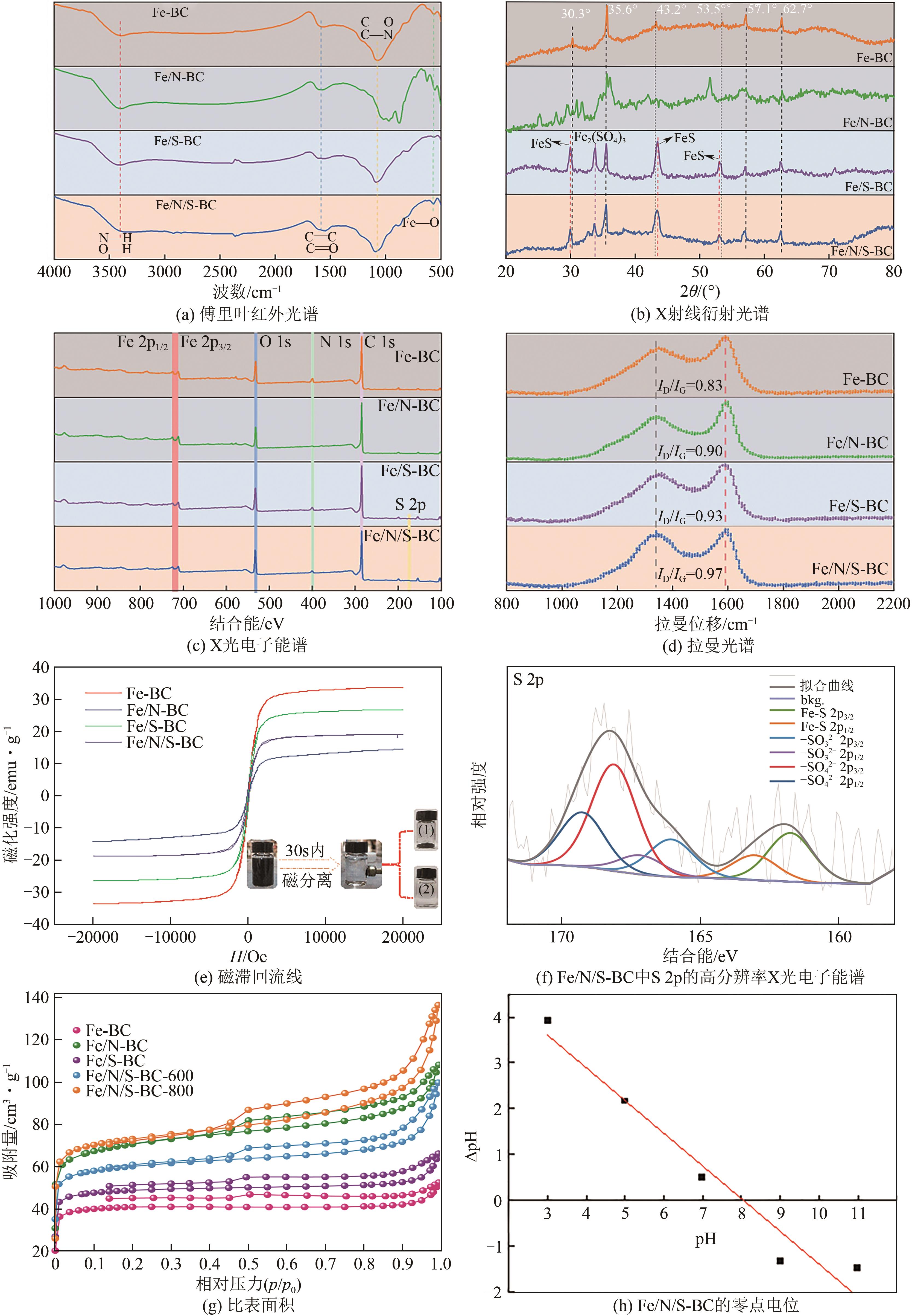
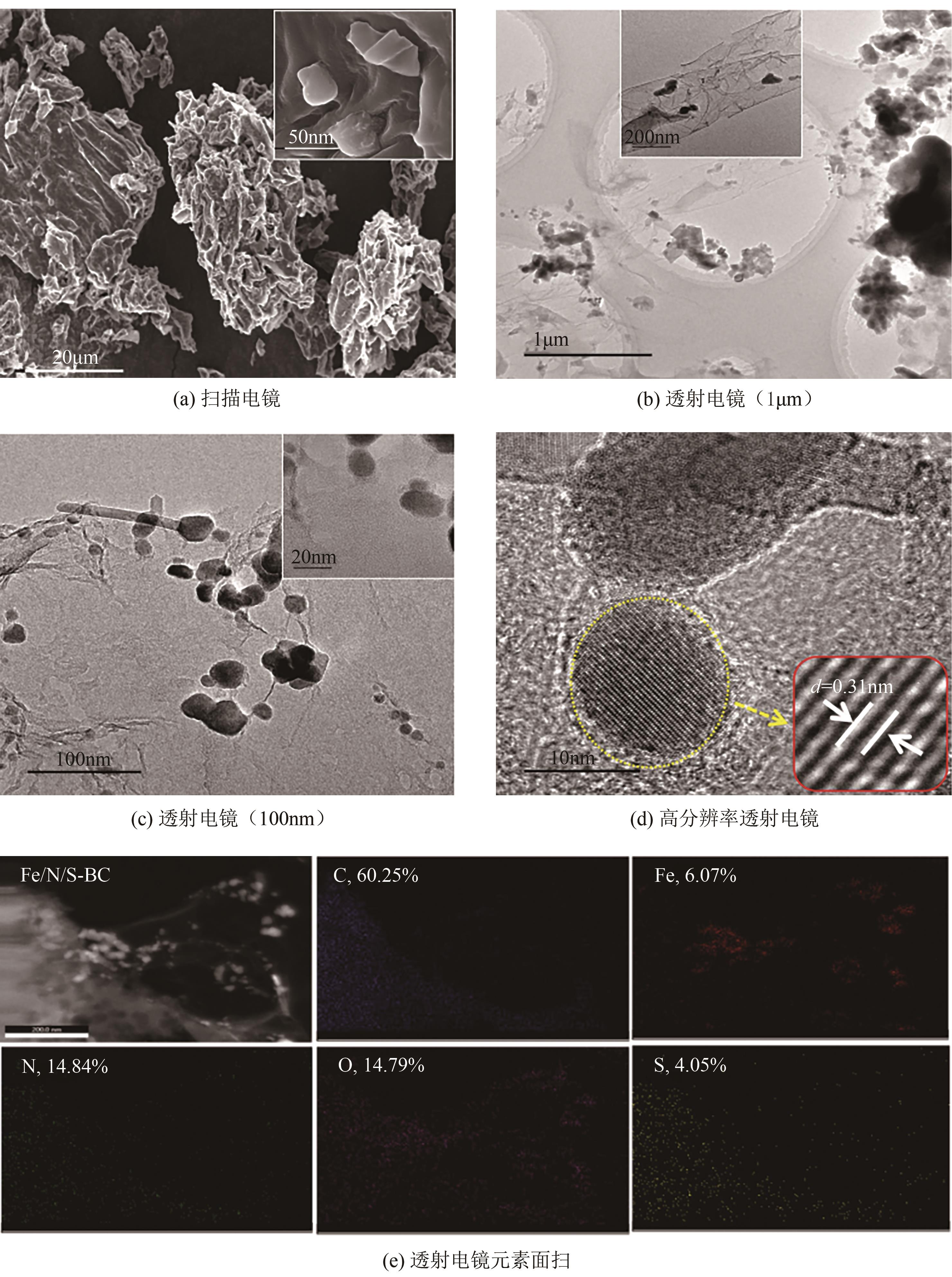
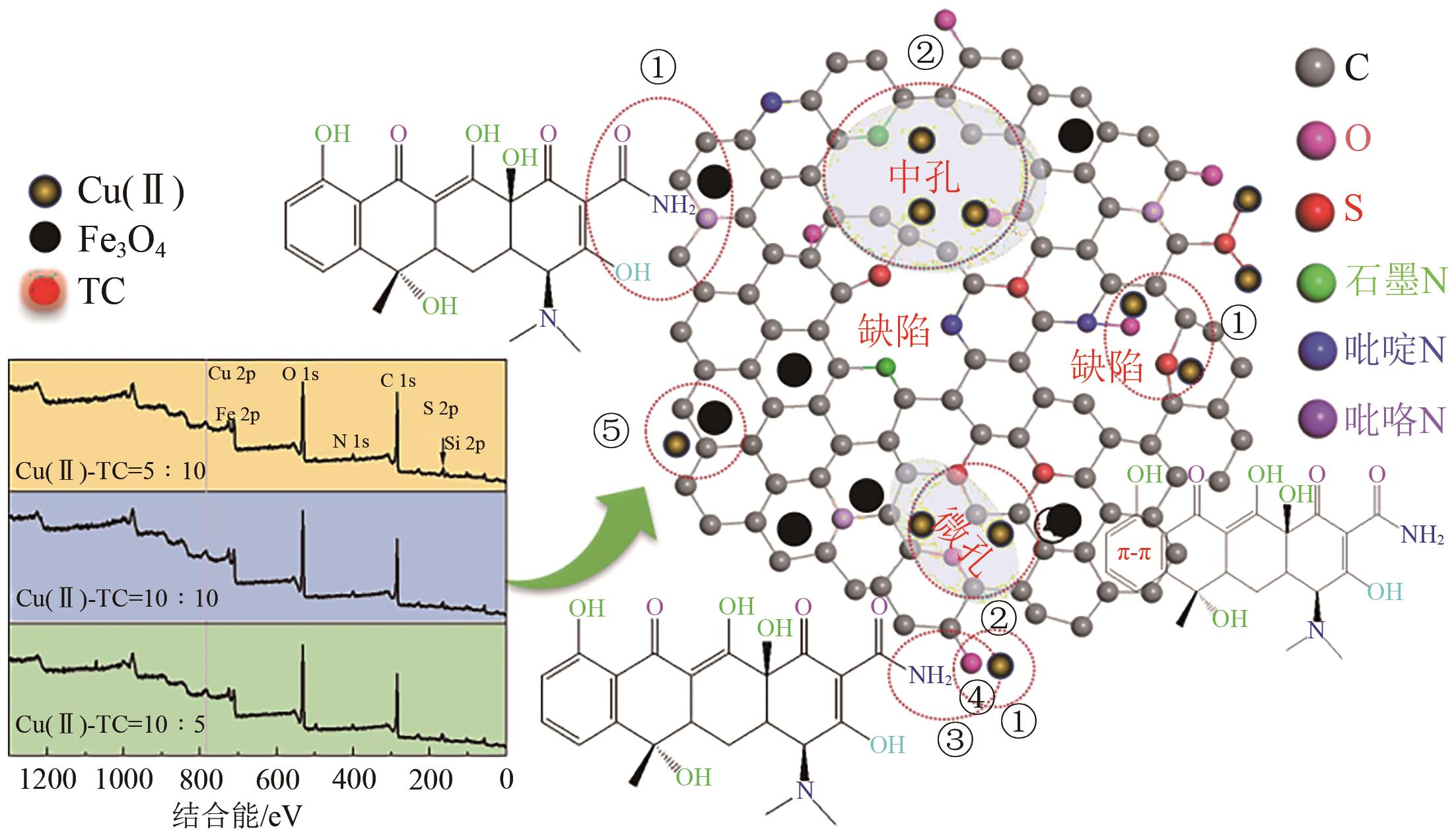
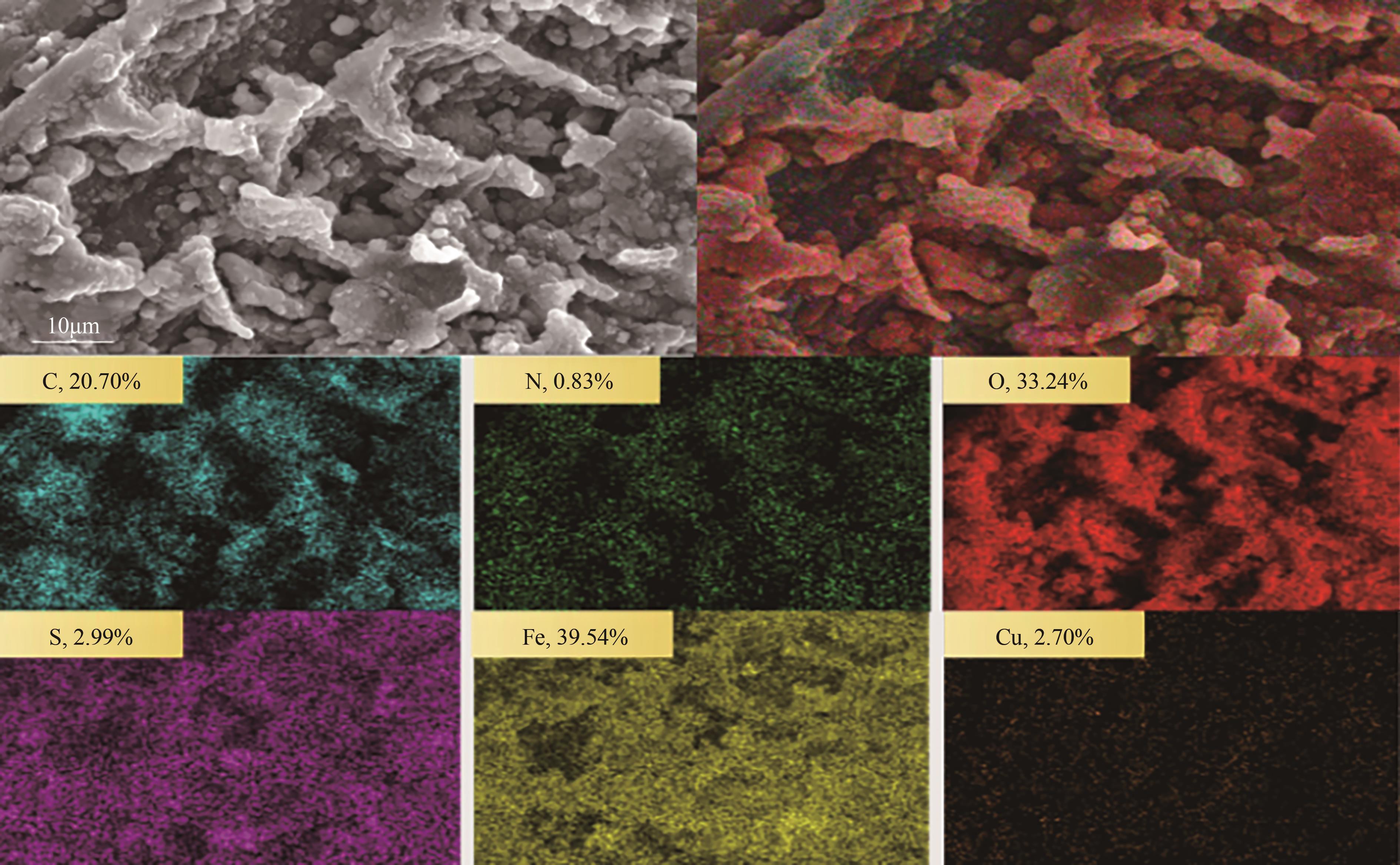
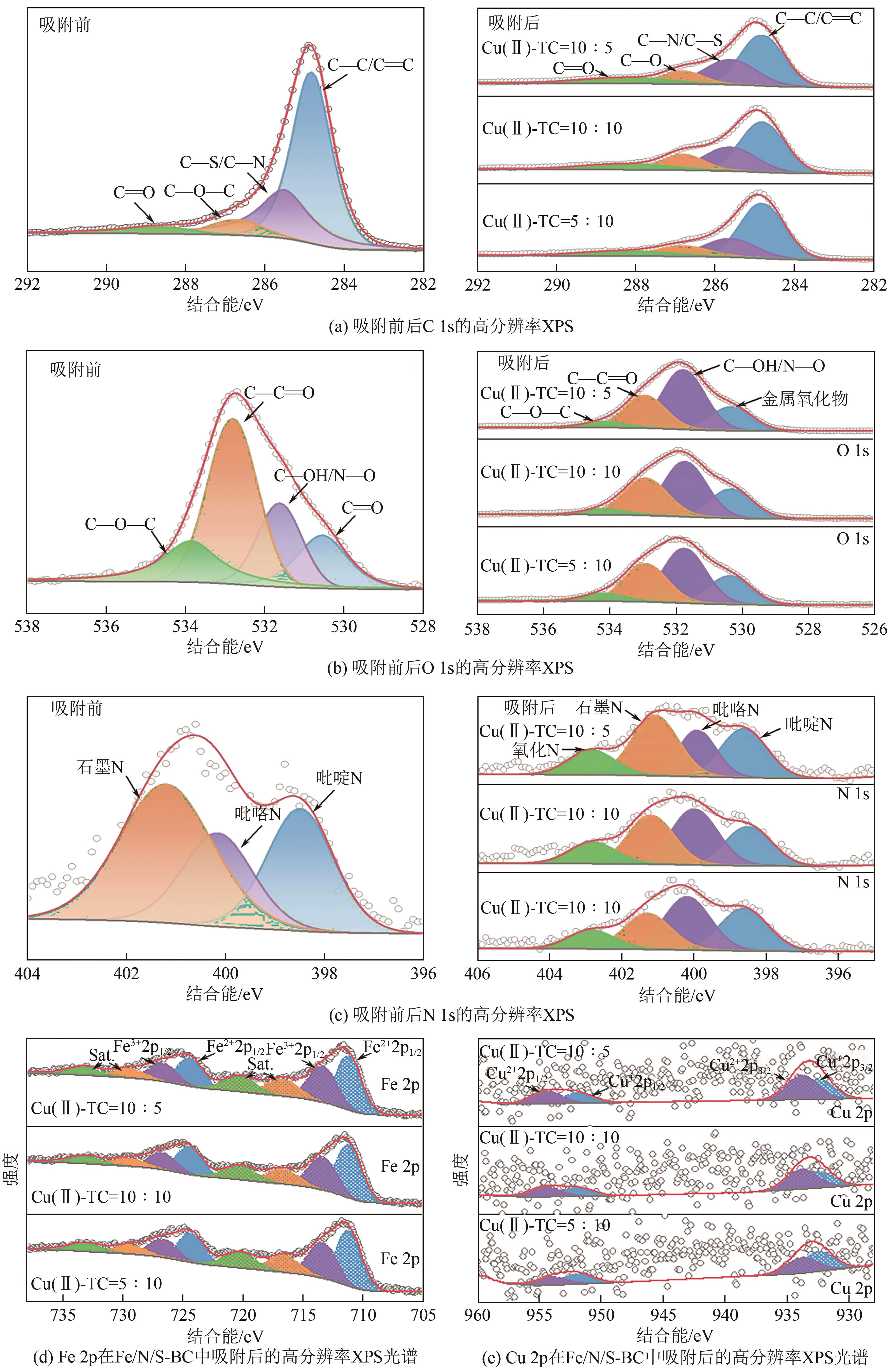
Synergistic adsorption mechanism of aqueous Cu(Ⅱ) and TC by N and S co-doped biochar
ZENG Xiangchu1,2,3( ), MO Zhenrong1,2, YIN Xiuju1,2(
), MO Zhenrong1,2, YIN Xiuju1,2( ), WU Zhe1,2(
), WU Zhe1,2( )
)
- 1.Guangxi Collaborative Innovation Center of Modern Sericulture and Silk, School of Chemistry and Bioengineering, Hechi University, Hechi 546300, Guangxi, China
2.Guangxi Key Laboratory of Sericulture Ecology and Applied Intelligent Technology, School of Chemistry and Bioengineering, Hechi University, Hechi 546300, Guangxi, China
3.Chemical Biology Center, Lishui Institute of Agriculture and Forestry Sciences, Lishui 323000, Zhejiang, China
-
Received:2023-11-13Revised:2024-02-20Online:2025-01-11Published:2024-12-15 -
Contact:YIN Xiuju, WU Zhe
N、S共掺杂磁性生物炭对水体Cu(Ⅱ)和四环素的协同吸附机制
曾湘楚1,2,3( ), 莫镇榕1,2, 银秀菊1,2(
), 莫镇榕1,2, 银秀菊1,2( ), 武哲1,2(
), 武哲1,2( )
)
- 1.广西现代蚕桑丝绸协同创新中心,河池学院化学与生物工程学院,广西 河池 546300
2.广西蚕桑生态学与智能化技术应用重点实验室,河池学院化学与生物工程学院,广西 河池 546300
3.化学生物中心,丽水市农林科学研究院,浙江 丽水 323000
-
通讯作者:银秀菊,武哲 -
作者简介:曾湘楚(1990—),男,博士,讲师,研究方向为环境功能材料。E-mail: xiangchuzeng@163.com。 -
基金资助:广西现代蚕桑丝绸协同创新中心基金(2023GXCSSC03);河池学院高层次人才科研启动项目(2019GCC008)
CLC Number:
Cite this article
ZENG Xiangchu, MO Zhenrong, YIN Xiuju, WU Zhe. Synergistic adsorption mechanism of aqueous Cu(Ⅱ) and TC by N and S co-doped biochar[J]. Chemical Industry and Engineering Progress, 2024, 43(12): 7004-7017.
曾湘楚, 莫镇榕, 银秀菊, 武哲. N、S共掺杂磁性生物炭对水体Cu(Ⅱ)和四环素的协同吸附机制[J]. 化工进展, 2024, 43(12): 7004-7017.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2023-1973
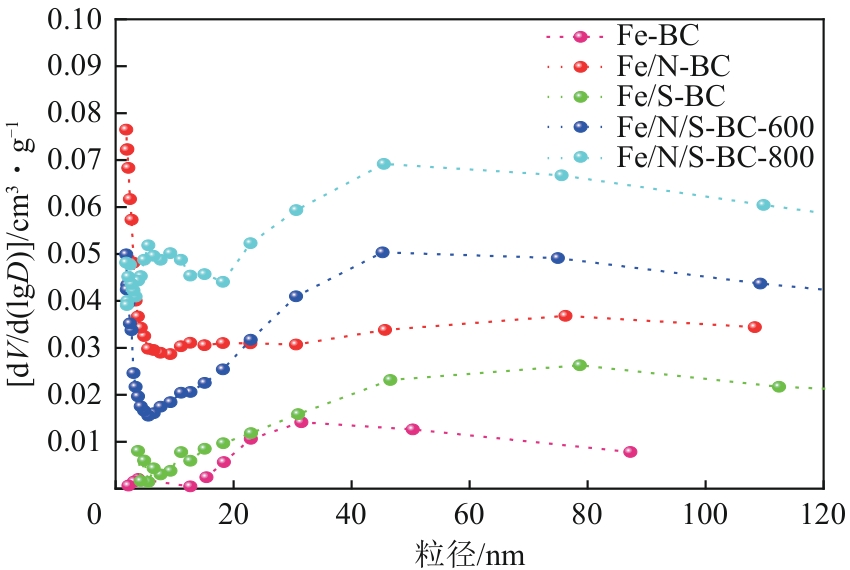
| 生物炭 | 比表面积/m2·g-1 | 孔径/nm | 孔容/cm3·g-1 |
|---|---|---|---|
| Fe-BC | 182.39 | 12.27 | 0.08 |
| Fe/N-BC | 320.46 | 6.16 | 0.17 |
| Fe/S-BC | 217.28 | 10.23 | 0.10 |
| Fe/N/S-BC-600 | 269.58 | 10.09 | 0.15 |
| Fe/N/S-BC-800 | 329.79 | 10.06 | 0.21 |
| 生物炭 | 比表面积/m2·g-1 | 孔径/nm | 孔容/cm3·g-1 |
|---|---|---|---|
| Fe-BC | 182.39 | 12.27 | 0.08 |
| Fe/N-BC | 320.46 | 6.16 | 0.17 |
| Fe/S-BC | 217.28 | 10.23 | 0.10 |
| Fe/N/S-BC-600 | 269.58 | 10.09 | 0.15 |
| Fe/N/S-BC-800 | 329.79 | 10.06 | 0.21 |
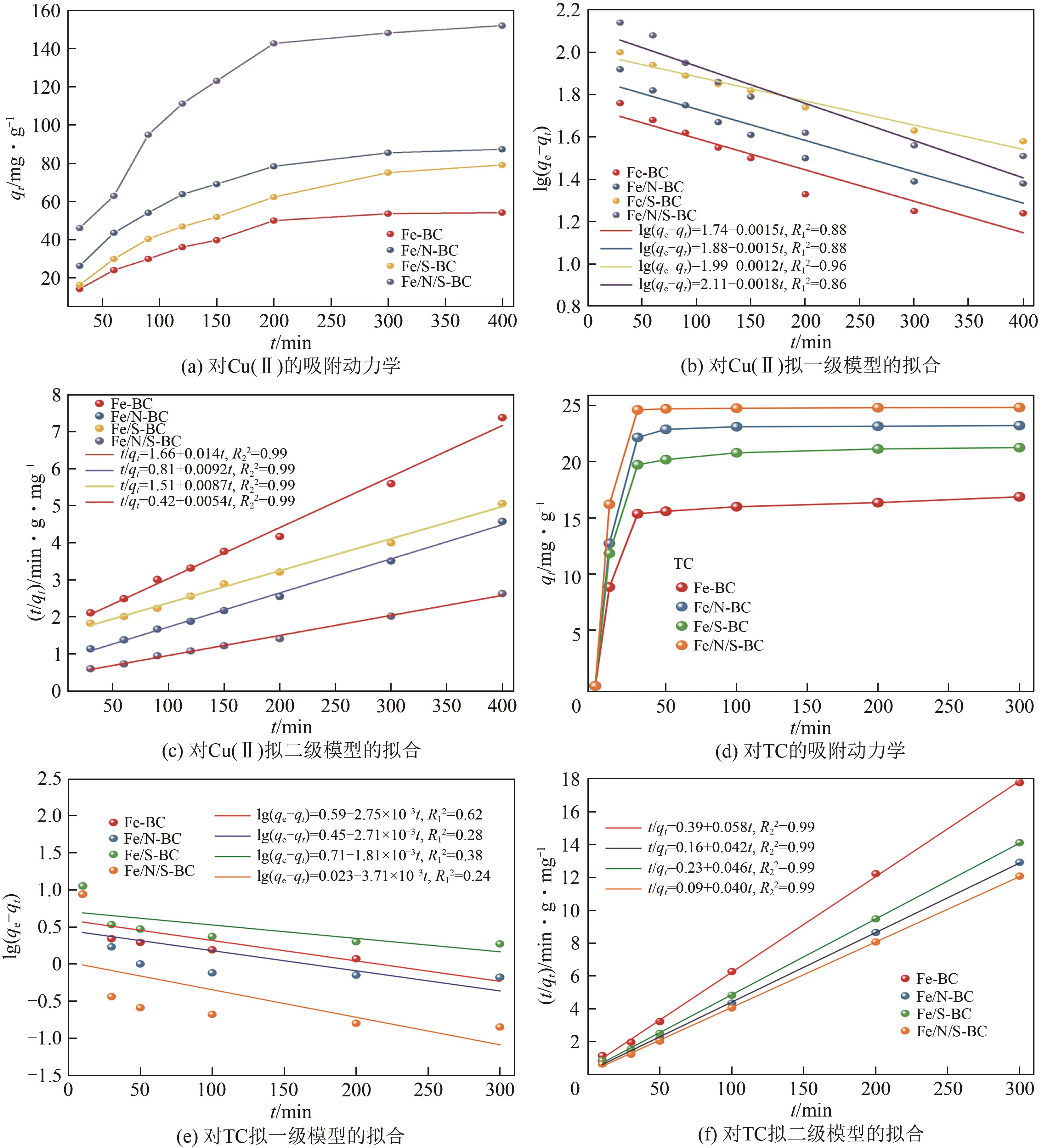
| 生物炭 | 污染物 | qe,exp/mg·g-1 | 拟一级模型 | 拟二级模型 | ||||
|---|---|---|---|---|---|---|---|---|
| qe,cal/mg·g-1 | k1 | R12 | qe,cal/mg·g-1 | k2 | R22 | |||
| Fe-BC | Cu(Ⅱ) | 75.31 | 54.53 | 3.45×10-3 | 0.88 | 71.42 | 1.18×10-4 | 0.99 |
| TC | 17.53 | 3.94 | 6.33×10-3 | 0.62 | 17.24 | 8.63×10-3 | 0.99 | |
| Fe/N-BC | Cu(Ⅱ) | 120.35 | 74.66 | 3.43×10-3 | 0.88 | 118.70 | 1.04×10-4 | 0.99 |
| TC | 23.87 | 2.85 | 6.24×10-3 | 0.28 | 23.81 | 1.10×10-2 | 0.99 | |
| Fe/S-BC | Cu(Ⅱ) | 115.31 | 99.87 | 2.76×10-3 | 0.96 | 114.94 | 5.01×10-5 | 0.99 |
| TC | 23.12 | 5.16 | 4.17×10-3 | 0.38 | 21.74 | 9.20×10-3 | 0.99 | |
| Fe/N/S-BC | Cu(Ⅱ) | 186.52 | 130.12 | 4.14×10-3 | 0.86 | 185.19 | 6.94×10-5 | 0.99 |
| TC | 24.97 | 1.06 | 8.54×10-3 | 0.24 | 25.00 | 1.78×10-2 | 0.99 | |
| 生物炭 | 污染物 | qe,exp/mg·g-1 | 拟一级模型 | 拟二级模型 | ||||
|---|---|---|---|---|---|---|---|---|
| qe,cal/mg·g-1 | k1 | R12 | qe,cal/mg·g-1 | k2 | R22 | |||
| Fe-BC | Cu(Ⅱ) | 75.31 | 54.53 | 3.45×10-3 | 0.88 | 71.42 | 1.18×10-4 | 0.99 |
| TC | 17.53 | 3.94 | 6.33×10-3 | 0.62 | 17.24 | 8.63×10-3 | 0.99 | |
| Fe/N-BC | Cu(Ⅱ) | 120.35 | 74.66 | 3.43×10-3 | 0.88 | 118.70 | 1.04×10-4 | 0.99 |
| TC | 23.87 | 2.85 | 6.24×10-3 | 0.28 | 23.81 | 1.10×10-2 | 0.99 | |
| Fe/S-BC | Cu(Ⅱ) | 115.31 | 99.87 | 2.76×10-3 | 0.96 | 114.94 | 5.01×10-5 | 0.99 |
| TC | 23.12 | 5.16 | 4.17×10-3 | 0.38 | 21.74 | 9.20×10-3 | 0.99 | |
| Fe/N/S-BC | Cu(Ⅱ) | 186.52 | 130.12 | 4.14×10-3 | 0.86 | 185.19 | 6.94×10-5 | 0.99 |
| TC | 24.97 | 1.06 | 8.54×10-3 | 0.24 | 25.00 | 1.78×10-2 | 0.99 | |
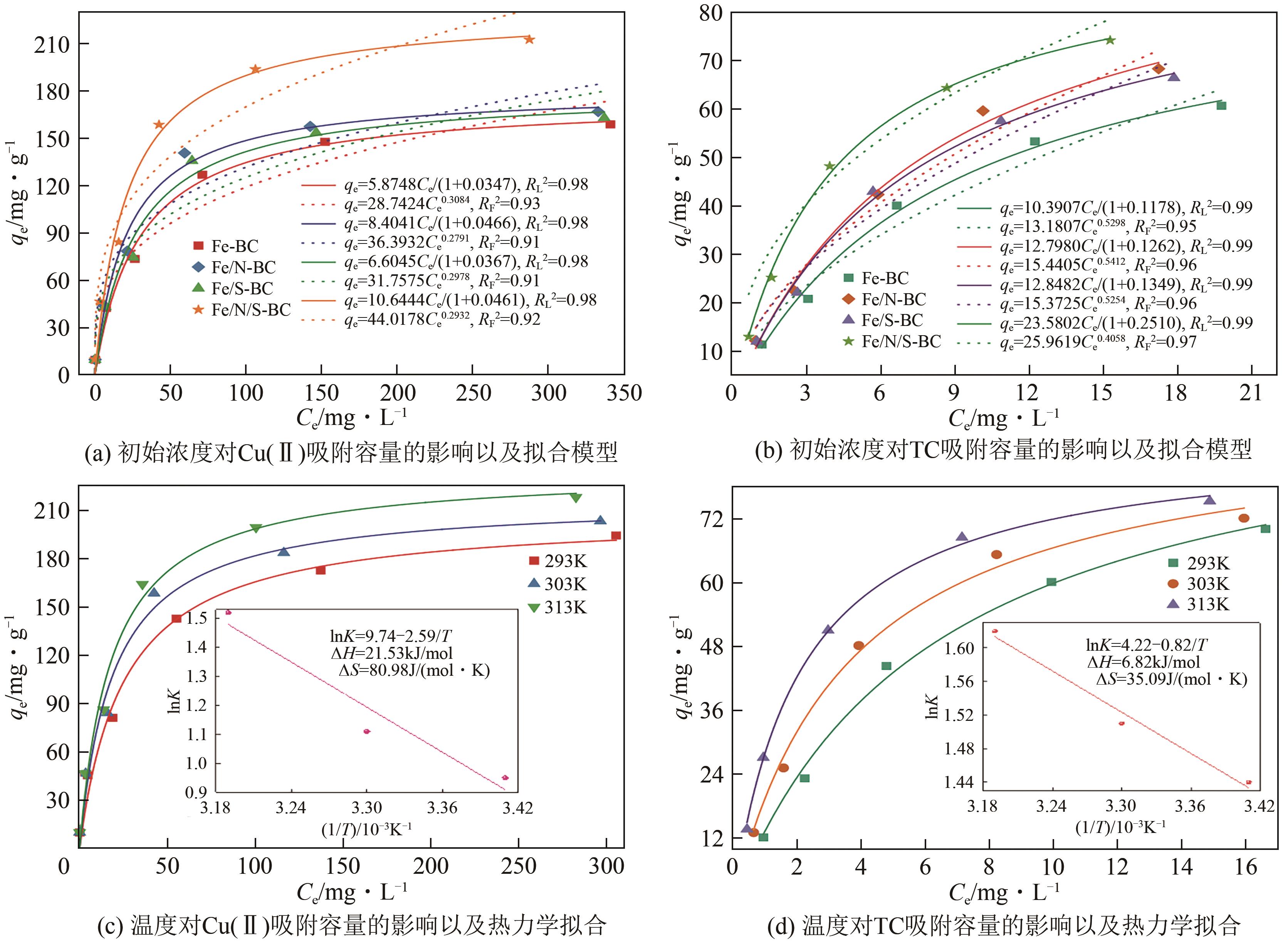
| 生物炭 | 污染物 | Langmuir模型 | Freundlich模型 | ||||
|---|---|---|---|---|---|---|---|
| qm,cal/mg·g-1 | kL | RL2 | kF | n | RF2 | ||
| Fe-BC | Cu(Ⅱ) | 174.47 | 0.03 | 0.98 | 28.75 | 3.24 | 0.93 |
| TC | 88.21 | 0.12 | 0.99 | 13.18 | 1.89 | 0.95 | |
| Fe/N-BC | Cu(Ⅱ) | 180.34 | 0.05 | 0.98 | 36.39 | 3.58 | 0.91 |
| TC | 101.41 | 0.13 | 0.99 | 0.96 | 1.85 | 0.96 | |
| Fe/S-BC | Cu(Ⅱ) | 180.01 | 0.04 | 0.98 | 31.76 | 3.36 | 0.91 |
| TC | 95.24 | 0.13 | 0.99 | 15.37 | 1.90 | 0.96 | |
| Fe/N/S-BC | Cu(Ⅱ) | 230.70 | 0.05 | 0.98 | 44.02 | 3.41 | 0.92 |
| TC | 93.63 | 0.25 | 0.99 | 25.96 | 2.46 | 0.97 | |
| 生物炭 | 污染物 | Langmuir模型 | Freundlich模型 | ||||
|---|---|---|---|---|---|---|---|
| qm,cal/mg·g-1 | kL | RL2 | kF | n | RF2 | ||
| Fe-BC | Cu(Ⅱ) | 174.47 | 0.03 | 0.98 | 28.75 | 3.24 | 0.93 |
| TC | 88.21 | 0.12 | 0.99 | 13.18 | 1.89 | 0.95 | |
| Fe/N-BC | Cu(Ⅱ) | 180.34 | 0.05 | 0.98 | 36.39 | 3.58 | 0.91 |
| TC | 101.41 | 0.13 | 0.99 | 0.96 | 1.85 | 0.96 | |
| Fe/S-BC | Cu(Ⅱ) | 180.01 | 0.04 | 0.98 | 31.76 | 3.36 | 0.91 |
| TC | 95.24 | 0.13 | 0.99 | 15.37 | 1.90 | 0.96 | |
| Fe/N/S-BC | Cu(Ⅱ) | 230.70 | 0.05 | 0.98 | 44.02 | 3.41 | 0.92 |
| TC | 93.63 | 0.25 | 0.99 | 25.96 | 2.46 | 0.97 | |
| 生物炭 | 污染物 | T/K | ΔG/kJ·mol-1 | ΔH/kJ·mol-1 | ΔS/kJ·mol-1·K-1 |
|---|---|---|---|---|---|
| Fe/N/S-BC | Cu(Ⅱ) | 293 | -2.31 | 21.53 | 80.98 |
| 303 | -2.80 | ||||
| 313 | -3.96 | ||||
| TC | 293 | -3.51 | 6.82 | 35.09 | |
| 303 | -3.80 | ||||
| 313 | -4.22 |
| 生物炭 | 污染物 | T/K | ΔG/kJ·mol-1 | ΔH/kJ·mol-1 | ΔS/kJ·mol-1·K-1 |
|---|---|---|---|---|---|
| Fe/N/S-BC | Cu(Ⅱ) | 293 | -2.31 | 21.53 | 80.98 |
| 303 | -2.80 | ||||
| 313 | -3.96 | ||||
| TC | 293 | -3.51 | 6.82 | 35.09 | |
| 303 | -3.80 | ||||
| 313 | -4.22 |
| 1 | ZENG Xiangchu, ZHANG Guanghua, ZHU Junfeng, et al. Adsorption of heavy metal ions in water by surface functionalized magnetic composites: A review[J]. Environmental Science: Water Research & Technology, 2022, 8(5): 907-925. |
| 2 | 曾湘楚, 张光华, 张万斌, 等. 希夫碱改性Fe3O4杂化材料的制备与表征[J]. 精细化工, 2021, 38(9): 1791-1797, 1807. |
| ZENG Xiangchu, ZHANG Guanghua, ZHANG Wanbin, et al. Preparation and characterization of Schiff base modified Fe3O4 hybrid material[J]. Fine Chemicals, 2021, 38(9): 1791-1797, 1807. | |
| 3 | YANG Yuxiao, ZHU Junfeng, ZENG Qingzhu, et al. Activation of persulfate by iron-loaded soybean straw biochar for efficient degradation of dye contaminants: Synthesis, performance, and mechanism[J]. Environmental Progress & Sustainable Energy, 2023, 42(5): e14190. |
| 4 | ZENG Xiangchu, ZHANG Guanghua, WEN Jia, et al. Simultaneous removal of aqueous same ionic type heavy metals and dyes by a magnetic chitosan/polyethyleneimine embedded hydrophobic sodium alginate composite: Performance, interaction and mechanism[J]. Chemosphere, 2023, 318: 137869. |
| 5 | YANG Yuxiao, ZHU Junfeng, ZENG Qingzhu, et al. Enhanced activation of peroxydisulfate by regulating pyrolysis temperature of biochar supported nZVI for the degradation of oxytetracycline[J]. Journal of the Taiwan Institute of Chemical Engineers, 2023, 145: 104775. |
| 6 | 彭程, 徐漪琳, 石钰婧, 等. 生物炭改性及其对除草剂污染水体和土壤修复的研究进展[J]. 化工进展, 2024, 43(2): 1069-1081. |
| PENG Cheng, XU Yilin, SHI Yujing, et al. Research progress on the biochar modification and its remediation of herbicide-contaminated water and soil[J]. Chemical Industry and Engineering Progress, 2024, 43(2): 1069-1081. | |
| 7 | FENG Zhuqing, YUAN Rongfang, WANG Fei, et al. Preparation of magnetic biochar and its application in catalytic degradation of organic pollutants: A review[J]. The Science of the Total Environment, 2021, 765: 142673. |
| 8 | ZENG Xiangchu, ZHU Junfeng, ZHANG Guanghua, et al. Molecular-level understanding on complexation-adsorption-degradation during the simultaneous removal of aqueous binary pollutants by magnetic composite aerogels[J]. Chemical Engineering Journal, 2023, 468: 143536. |
| 9 | 周春地, 阳婷, 闵熙泽, 等. 零价铁、铜改性生物炭及其对Cr(Ⅵ)吸附性能的影响[J]. 化工进展, 2020, 39(10): 4275-4282. |
| ZHOU Chundi, YANG Ting, MIN Xize, et al. Influence of zero valent iron and copper modified biochar on Cr(Ⅵ) adsorption[J]. Chemical Industry and Engineering Progress, 2020, 39(10): 4275-4282. | |
| 10 | HUANG Kuncheng, YANG Shuquan, LIU Xiaohao, et al. Adsorption of antibiotics from wastewater by cabbage-based N, P co-doped mesoporous carbon materials[J]. Journal of Cleaner Production, 2023, 391: 136174. |
| 11 | ZENG Xiangchu, ZHANG Guanghua, WU Zhe. Preparation and characterization of Schiff-base modified Fe3O4 hybrid material and its selective adsorption for aqueous Hg2+ [J]. Environmental Science and Pollution Research International, 2022, 29(20): 30324-30336. |
| 12 | REN Zhijun, WANG Zhanxin, Longyi LYU, et al. Fe-N complex biochar as a superior partner of sodium sulfide for methyl orange decolorization by combination of adsorption and reduction[J]. Journal of Environmental Management, 2022, 316: 115213. |
| 13 | CHOONG Zheng Yi, GASIM Mohamed Faisal, LIN Kun- Yi Andrew, et al. Unravelling the formation mechanism and performance of nitrogen, sulfur codoped biochar as peroxymonosulfate activator for gatifloxacin removal[J]. Chemical Engineering Journal, 2023, 451: 138958. |
| 14 | PANG Kangfeng, SUN Wei, YE Feng, et al. Sulfur-modified chitosan derived N, S-co-doped carbon as a bifunctional material for adsorption and catalytic degradation sulfamethoxazole by persulfate[J]. Journal of Hazardous Materials, 2022, 424: 127270. |
| 15 | LIU Yuyan, SOHI Saran P, LIU Siyuan, et al. Adsorption and reductive degradation of Cr(Ⅵ) and TCE by a simply synthesized zero valent iron magnetic biochar[J]. Journal of Environmental Management, 2019, 235: 276-281. |
| 16 | DUAN Xiaoguang, Kane O’DONNELL, SUN Hongqi, et al. Sulfur and nitrogen co-doped graphene for metal-free catalytic oxidation reactions[J]. Small, 2015, 11(25): 3036-3044. |
| 17 | GUO Yong, YAN Congcong, WANG Peifang, et al. Doping of carbon into boron nitride to get the increased adsorption ability for tetracycline from water by changing the pH of solution[J]. Chemical Engineering Journal, 2020, 387: 124136. |
| 18 | ZENG Xiangchu, ZHANG Guanghua, LI Xiuling, et al. Selective removal of aqueous Hg2+ by magnetic composites sulfur-containing on the hyper-branched surface: Characterization, performance and mechanism[J]. Journal of Environmental Management, 2023, 325: 116621. |
| 19 | MA Yongfei, LI Ming, LI Ping, et al. Hydrothermal synthesis of magnetic sludge biochar for tetracycline and ciprofloxacin adsorptive removal[J]. Bioresource Technology, 2021, 319: 124199. |
| 20 | DUAN Ran, MA Shuanglong, XU Shengjun, et al. Soybean straw biochar activating peroxydisulfate to simultaneously eliminate tetracycline and tetracycline resistance bacteria: Insights on the mechanism[J]. Water Research, 2022, 218: 118489. |
| 21 | ZHANG Ting, WU Shuang, LI Ning, et al. Applications of vacancy defect engineering in persulfate activation: Performance and internal mechanism[J]. Journal of Hazardous Materials, 2023, 449: 130971. |
| 22 | ZENG Xiangchu, ZHANG Guanghua, ZHU Junfeng. Selective adsorption of heavy metals from water by a hyper-branched magnetic composite material: Characterization, performance, and mechanism[J]. Journal of Environmental Management, 2022, 314: 114979. |
| 23 | CHEN Cheng, MA Tengfei, SHANG Yanan, et al. In-situ pyrolysis of Enteromorpha as carbocatalyst for catalytic removal of organic contaminants: Considering the intrinsic N/Fe in Enteromorpha and non-radical reaction[J]. Applied Catalysis B: Environmental, 2019, 250: 382-395. |
| 24 | LU Wenhui, LI Jinhua, SHENG Yanqing, et al. One-pot synthesis of magnetic iron oxide nanoparticle-multiwalled carbon nanotube composites for enhanced removal of Cr(Ⅵ) from aqueous solution[J]. Journal of Colloid and Interface Science, 2017, 505: 1134-1146. |
| 25 | GUO Furong, WANG Kangjie, LU Jiahua, et al. Activation of peroxymonosulfate by magnetic carbon supported Prussian blue nanocomposite for the degradation of organic contaminants with singlet oxygen and superoxide radicals[J]. Chemosphere, 2019, 218: 1071-1081. |
| 26 | LUO Yidan, WANG Yonghu, ZHU Yaowei, et al. Ball-milled bismuth oxychloride/biochar nanocomposites with rich oxygen vacancies for reactive red-120 adsorption in aqueous solution[J]. Biochar, 2022, 4(1): 21. |
| 27 | HAO Yongyong, MA Hongrui, WANG Qing, et al. Complexation behaviour and removal of organic-Cr(Ⅲ) complexes from the environment: A review[J]. Ecotoxicology and Environmental Safety, 2022, 240: 113676. |
| 28 | GUO Yaoping, ZENG Zequan, ZHU Youcai, et al. Catalytic oxidation of aqueous organic contaminants by persulfate activated with sulfur-doped hierarchically porous carbon derived from thiophene[J]. Applied Catalysis B: Environmental, 2018, 220: 635-644. |
| 29 | YAO Yunjin, LIAN Chao, WU Guodong, et al. Synthesis of “sea urchin” -like carbon nanotubes/porous carbon superstructures derived from waste biomass for treatment of various contaminants[J]. Applied Catalysis B: Environmental, 2017, 219: 563-571. |
| 30 | WEI Yan, MIAO Jie, GE Jianxin, et al. Ultrahigh peroxymonosulfate utilization efficiency over CuO nanosheets via heterogeneous Cu(Ⅲ) formation and preferential electron transfer during degradation of phenols[J]. Environmental Science & Technology, 2022, 56(12): 8984-8992. |
| 31 | YANG Xiaobing, ZENG Xiangchu, CHEN Hanchun, et al. Coupled homogeneous/heterogeneous Fenton-like system to enhance the synchronized decontamination of aqueous tetracycline and Salmonella typhi[J]. Chemical Engineering Journal, 2024, 483: 148697. |
| 32 | 曾湘楚, 黄红诚, 宋华, 等. 聚乙烯亚胺改性壳聚糖气凝胶对Cr(Ⅵ)和Cu(Ⅱ)的吸附及机理[J]. 华南师范大学学报(自然科学版), 2023, 55(5): 47-58. |
| ZENG Xiangchu, HUANG Hongcheng, SONG Hua, et al. Adsorption and mechanism of Cr(Ⅵ) and Cu(Ⅱ) by polyethyleneimine modified chitosan aerogel[J]. Journal of South China Normal University (Natural Science Edition), 2023, 55(5): 47-58. |
| [1] | HUANG Jun, ZHANG Yingjuan, LIN Yintong, WEI Xuechun, WU Yutong, WU Gaobo, MO Junlin, ZHAO Zhenxia, ZHAO Zhongxing. Preparation of silkworm excrement-based porous biocarbon and synergistic adsorption and slow-release performance for monosultap and dinotefuran [J]. Chemical Industry and Engineering Progress, 2024, 43(7): 3964-3971. |
| [2] | LIU Mengfan, WANG Huawei, WANG Yanan, ZHANG Yanru, JIANG Xutong, SUN Yingjie. Efficiency and mechanism of Bio-FeMnCeO x activated PMS for degradation of tetracycline [J]. Chemical Industry and Engineering Progress, 2024, 43(6): 3492-3502. |
| [3] | XU Shiqi, ZHU Ying, CHEN Ninghua, LU Caimei, JIANG Luying, WANG Junhui, QIN Yuelong, ZHANG Hanbing. Effect of environmental factors on the photocatalytic degradation behavior of tetracycline in water [J]. Chemical Industry and Engineering Progress, 2024, 43(1): 551-559. |
| [4] | KE Yuxin, ZHU Xiaoli, SI Shaocheng, ZHANG Ting, WANG Junqiang, ZHANG Ziye. Adsorbent derived from spent bleaching earth for the synergistic removal of tetracycline and copper in wastewater [J]. Chemical Industry and Engineering Progress, 2023, 42(11): 5981-5992. |
| [5] | HUANG Jiaqi, GE Yuanyuan, LI Zhili, WANG Yipin, CUI Xuemin. Preparation of biochar/geopolymer composite film and its removal of tetracycline [J]. Chemical Industry and Engineering Progress, 2022, 41(1): 427-434. |
| [6] | TANG Rui, ZHANG Hanbing, LU Caimei, LIU Kun, WANG Zhongkai, YU Sishan, TONG Zhangfa, JI Junrong. Adsorption of ciprofloxacin and tetracycline by organically modified magnetic bentonite [J]. Chemical Industry and Engineering Progress, 2021, 40(11): 6235-6245. |
| [7] | CAO Lili, JIANG Shanqing, LING Zeyu, WANG Chuqiao, XU Xia, WANG Liping. Properties and mechanisms of tetracycline photocatalytic degradation by hydrothermal synthesis Ag-SrTiO3 in visible-light [J]. Chemical Industry and Engineering Progress, 2018, 37(11): 4500-4508. |
| [8] | YE Linjing, AN Xiaoying, JIANG Yunjie, YAN Chao, GUAN Weisheng. Preparation of ZnO/CdS composite photocatalyst and its degradability on tetracycline antibiotic [J]. Chemical Industry and Engineering Progree, 2015, 34(11): 3944-3950. |
| [9] | HUANG Shenglin, HE Shi, WEI Xin, XUE Gang, GAO Pin. Pollution characteristics of tetracycline residues and tetracycline resistance genes in sewage treatment plants:A review [J]. Chemical Industry and Engineering Progree, 2015, 34(06): 1779-1785. |
| [10] | CHENG Chen, YAN Zheng. Degradation of oxytetracycline in wastewater by multi-frequency ultrasonic [J]. Chemical Industry and Engineering Progree, 2015, 34(04): 1143-1146,1164. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||