Chemical Industry and Engineering Progress ›› 2024, Vol. 43 ›› Issue (1): 551-559.DOI: 10.16085/j.issn.1000-6613.2023-0217
• Resources and environmental engineering • Previous Articles
Effect of environmental factors on the photocatalytic degradation behavior of tetracycline in water
XU Shiqi1( ), ZHU Ying1, CHEN Ninghua2, LU Caimei1, JIANG Luying1, WANG Junhui1, QIN Yuelong2(
), ZHU Ying1, CHEN Ninghua2, LU Caimei1, JIANG Luying1, WANG Junhui1, QIN Yuelong2( ), ZHANG Hanbing1
), ZHANG Hanbing1
- 1.School of Resources, Environment and Materials, Guangxi University, Nanning 530004, Guangxi, China
2.Scientific Research Academy of Guangxi Environmental Protection, Nanning 530022, Guangxi, China
-
Received:2023-02-17Revised:2023-05-04Online:2024-02-05Published:2024-01-20 -
Contact:QIN Yuelong
环境因素对水体中四环素光催化降解行为的影响
徐诗琪1( ), 朱颖1, 陈宁华2, 陆彩妹1, 江露莹1, 王俊辉1, 覃岳隆2(
), 朱颖1, 陈宁华2, 陆彩妹1, 江露莹1, 王俊辉1, 覃岳隆2( ), 张寒冰1
), 张寒冰1
- 1.广西大学资源环境与材料学院,广西 南宁 530004
2.广西环境科学保护研究院,广西 南宁 530022
-
通讯作者:覃岳隆 -
作者简介:徐诗琪(1999—),女,硕士研究生,研究方向为水文学及水资源。E-mail:myy163052@163.com。 -
基金资助:国家自然科学基金地区项目(52263029);广西自然科学基金(2020GXNSFAA297036);广西自然科学基金(2021GXNSFAA220035);广西石化资源加工及过程强化技术重点实验室(2022Z005)
CLC Number:
Cite this article
XU Shiqi, ZHU Ying, CHEN Ninghua, LU Caimei, JIANG Luying, WANG Junhui, QIN Yuelong, ZHANG Hanbing. Effect of environmental factors on the photocatalytic degradation behavior of tetracycline in water[J]. Chemical Industry and Engineering Progress, 2024, 43(1): 551-559.
徐诗琪, 朱颖, 陈宁华, 陆彩妹, 江露莹, 王俊辉, 覃岳隆, 张寒冰. 环境因素对水体中四环素光催化降解行为的影响[J]. 化工进展, 2024, 43(1): 551-559.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2023-0217
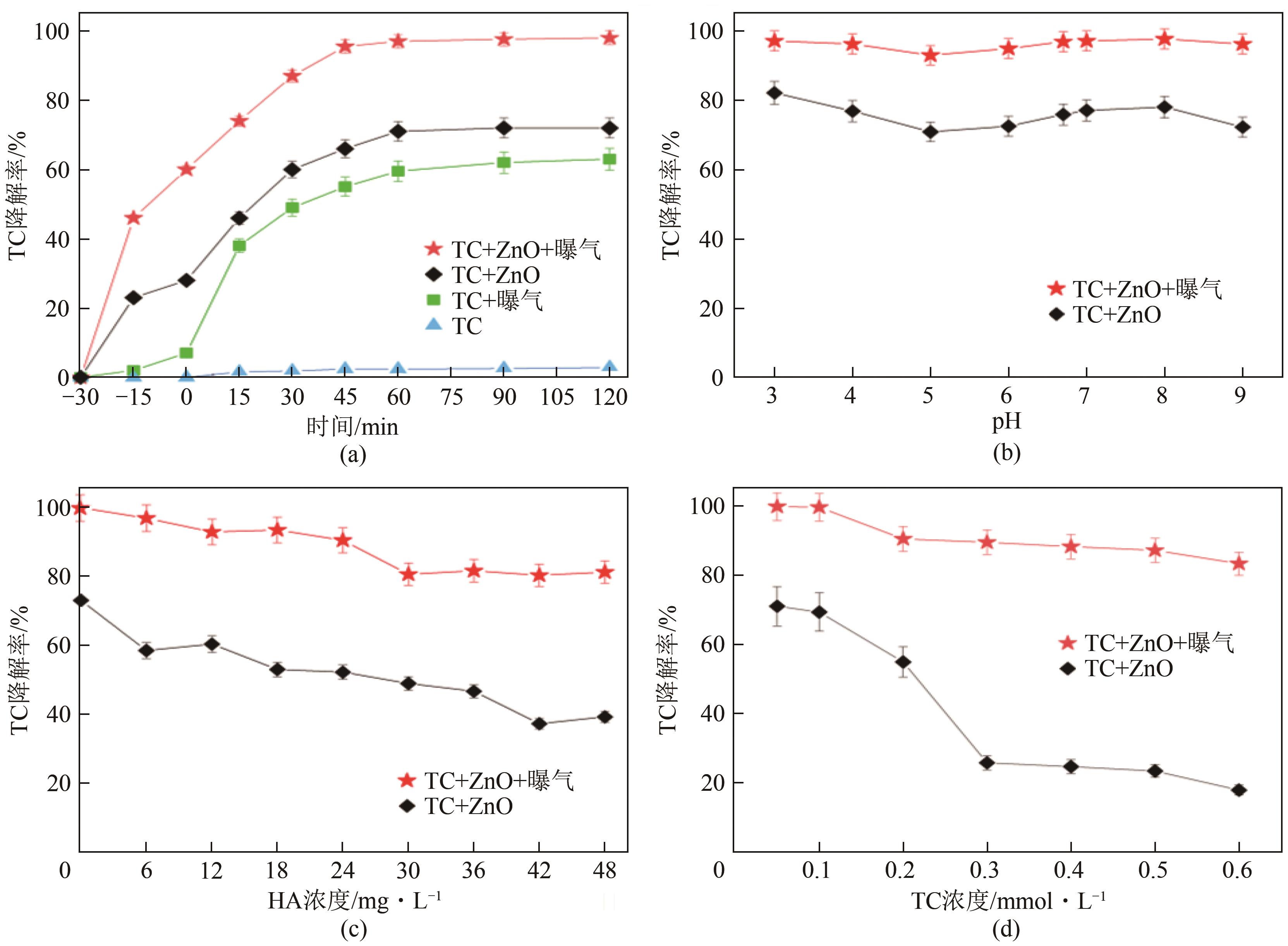
| 水平 | 曝气 | Cu(Ⅱ)浓度/mmol·L-1 | 自然光 |
|---|---|---|---|
| 1 | 无 | 0 | 无 |
| 2 | 曝气 | 0.15 | 照射 |
| 水平 | 曝气 | Cu(Ⅱ)浓度/mmol·L-1 | 自然光 |
|---|---|---|---|
| 1 | 无 | 0 | 无 |
| 2 | 曝气 | 0.15 | 照射 |
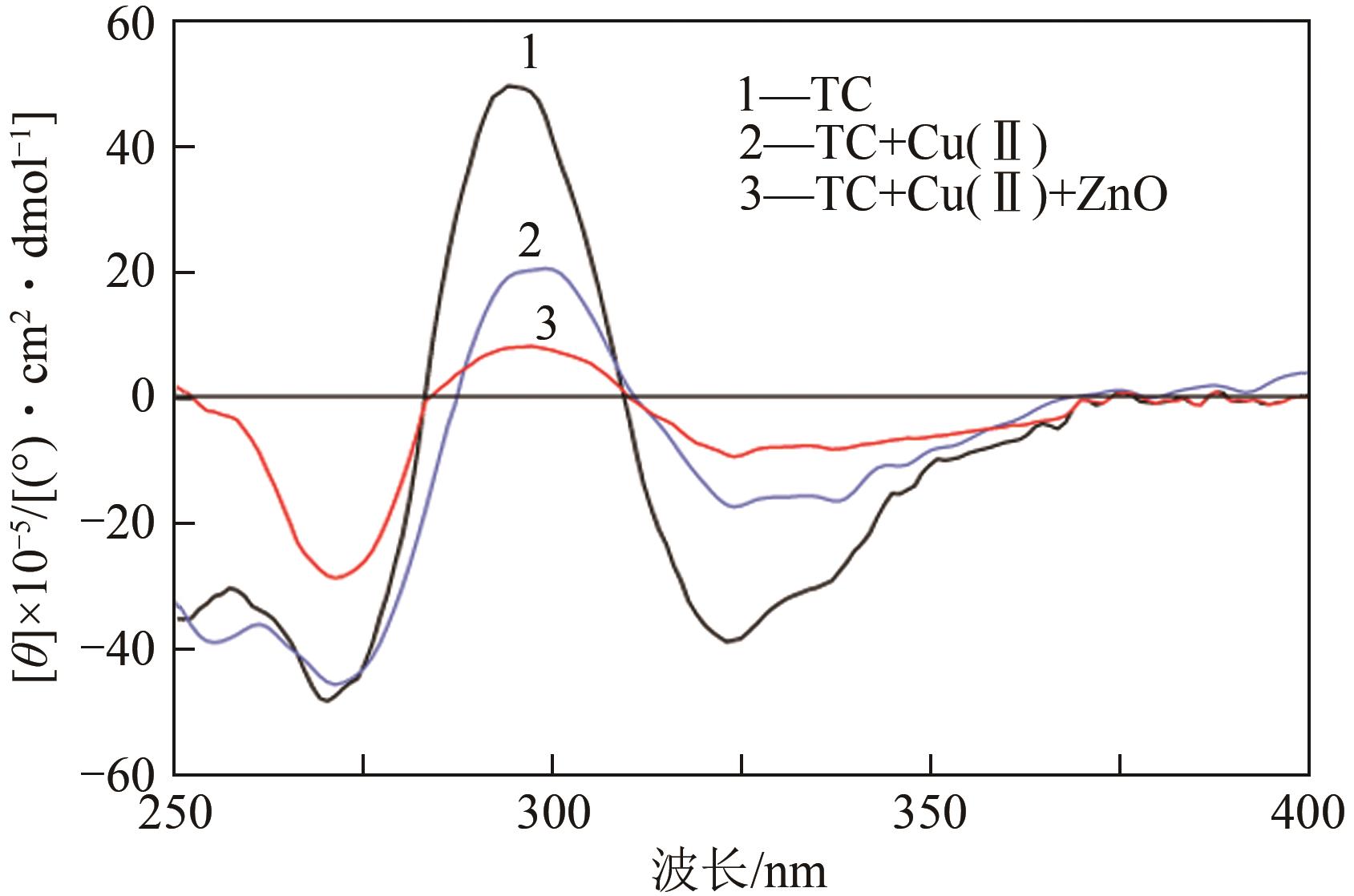
| 样品 | 最大带宽 | 最小带宽 | ||
|---|---|---|---|---|
| λ/nm | [θ]×10‒5/(°)·cm2·dmol-1 | λ/nm | [θ]×10‒5/(°)·cm2·dmol-1 | |
| TC | 295 | 49.8 | 325 | 38.8 |
| TC‒Cu(Ⅱ) | 300 | 20.6 | 338 | 16.3 |
| TC‒Cu(Ⅱ)‒ZnO | 307 | 2.4 | 338 | 7.1 |
| 样品 | 最大带宽 | 最小带宽 | ||
|---|---|---|---|---|
| λ/nm | [θ]×10‒5/(°)·cm2·dmol-1 | λ/nm | [θ]×10‒5/(°)·cm2·dmol-1 | |
| TC | 295 | 49.8 | 325 | 38.8 |
| TC‒Cu(Ⅱ) | 300 | 20.6 | 338 | 16.3 |
| TC‒Cu(Ⅱ)‒ZnO | 307 | 2.4 | 338 | 7.1 |
| 编号 | 曝气 | Cu(Ⅱ)浓度 | 自然光 | 降解平衡 时间/min | 降解平衡时 降解率/% |
|---|---|---|---|---|---|
| 1 | 2 | 1 | 1 | 120 | 99 |
| 2 | 1 | 2 | 1 | 90 | 91 |
| 3 | 1 | 1 | 2 | 120 | 86 |
| 4 | 2 | 2 | 1 | 90 | 99 |
| 5 | 2 | 1 | 2 | 90 | 99 |
| 6 | 1 | 2 | 2 | 90 | 94 |
| 7 | 2 | 2 | 2 | 75 | 99 |
| 编号 | 曝气 | Cu(Ⅱ)浓度 | 自然光 | 降解平衡 时间/min | 降解平衡时 降解率/% |
|---|---|---|---|---|---|
| 1 | 2 | 1 | 1 | 120 | 99 |
| 2 | 1 | 2 | 1 | 90 | 91 |
| 3 | 1 | 1 | 2 | 120 | 86 |
| 4 | 2 | 2 | 1 | 90 | 99 |
| 5 | 2 | 1 | 2 | 90 | 99 |
| 6 | 1 | 2 | 2 | 90 | 94 |
| 7 | 2 | 2 | 2 | 75 | 99 |
| 反应条件 | 拟合方程 | 回归系数R2 | 动力学常数k/min-1 |
|---|---|---|---|
| TC | y=0.110x-0.101 | 0.979 | 0.110 |
| TC+Cu(Ⅱ) | y=0.489x+0.430 | 0.951 | 0.489 |
| TC+曝气 | y=0.769x-1.057 | 0.960 | 0.769 |
| TC+自然光 | y=0.286x-0.130 | 0.944 | 0.286 |
| 反应条件 | 拟合方程 | 回归系数R2 | 动力学常数k/min-1 |
|---|---|---|---|
| TC | y=0.110x-0.101 | 0.979 | 0.110 |
| TC+Cu(Ⅱ) | y=0.489x+0.430 | 0.951 | 0.489 |
| TC+曝气 | y=0.769x-1.057 | 0.960 | 0.769 |
| TC+自然光 | y=0.286x-0.130 | 0.944 | 0.286 |
| 1 | 汤睿, 张寒冰, 陆彩妹, 等. 有机磁性膨润土对环丙沙星和四环素的吸附性能[J]. 化工进展, 2021, 40(11): 6235-6245. |
| TANG Rui, ZHANG Hanbing, LU Caimei, et al. Adsorption of ciprofloxacin and tetracycline by organically modified magnetic bentonite[J]. Chemical Industry and Engineering Progress, 2021, 40(11): 6235-6245. | |
| 2 | JIANG Liushan, XIE Yu, HE Fan, et al. Facile synthesis of GO as middle carrier modified flower-like BiOBr and C3N4 nanosheets for simultaneous treatment of chromium(Ⅵ) and tetracycline[J]. Chinese Chemical Letters, 2021, 32(7): 2187-2191. |
| 3 | ZHANG Yanan, ZHAO Yangguo, YANG Dexiang, et al. Insight into the removal of tetracycline-resistant bacteria and resistance genes from mariculture wastewater by ultraviolet/persulfate advanced oxidation process[J]. Journal of Hazardous Materials Advances, 2022, 7: 100129. |
| 4 | 郭新兴, 刘建国, 王鹏, 等. 电化学沉积法制备ZIF-8及其对四环素的吸附[J]. 环境化学, 2020, 39(3): 581-592. |
| GUO Xinxing, LIU Jianguo, WANG Peng, et al. Electrochemical synthesis of ZIF-8 for adsorption of tetracycline[J]. Environmental Chemistry, 2020, 39(3): 581-592. | |
| 5 | 张畅滕, 申乾宏, 郑素华, 等. 三维阶层结构BiOBr/Bi2WO6多孔微球制备及其对盐酸四环素的可见光催化降解性能[J]. 材料科学与工程学报, 2022, 40(4): 567-574. |
| ZHANG Changteng, SHEN Qianhong, ZHENG Suhua, et al. Preparation of three-dimensional hierarchical BiOBr/Bi2WO6 porous microsphere and its visible-light photocatalytic degradation for tetracycline hydrochloride[J]. Journal of Materials Science and Engineering, 2022, 40(4): 567-574. | |
| 6 | 石宇, 杨晓婷, 兰贵红, 等. MnO x 掺杂纳米石墨阴极的制备及其对盐酸四环素的降解[J]. 精细化工, 2022, 39(4): 798-805. |
| SHI Yu, YANG Xiaoting, LAN Guihong, et al. Preparation of nano graphite cathode doped with MnO x and its degradation for tetracycline hydrochloride[J]. Fine Chemicals, 2022, 39(4): 798-805. | |
| 7 | 李佳泽, 吴宝利, 刘富荣, 等. BAF工艺深度处理四环素类制药废水研究[J]. 中国给水排水, 2022, 38(5): 24-31. |
| LI Jiaze, WU Baoli, LIU Furong, et al. Treatment of tetracyclines antibiotics pharmaceutical wastewater by BAF process[J]. China Water & Wastewater, 2022, 38(5): 24-31. | |
| 8 | 陈杰, 李明明, 刘治刚, 等. Fe3O4@TiO2核壳微球吸附-光催化联合去除四环素性能[J]. 化学研究与应用, 2022, 34(8): 1803-1812. |
| CHEN Jie, LI Mingming, LIU Zhigang, et al. Removal of tetracycline by Fe3O4@TiO2 core-shell microsphere combined with adsorption-photocatalysis[J]. Chemical Research and Application, 2022, 34(8): 1803-1812. | |
| 9 | 宋继梅, 朱婉蓉, 杨捷, 等. Ag/AgBr/BiOBr的制备及其快速光催化降解四环素[J]. 安徽大学学报(自然科学版), 2022, 46(5): 71-82. |
| SONG Jimei, ZHU Wanrong, YANG Jie, et al. Preparation of Ag/AgBr/BiOBr and their prominent photocatalytic activity for the degradation of tetracycline under visible light[J]. Journal of Anhui University (Natural Science Edition), 2022, 46(5): 71-82. | |
| 10 | 仇思, 李小明, 罗琨, 等. g-C3N4/Ag3PO4/CNT对亚甲基蓝和四环素光催化的降解[J]. 环境化学, 2022, 41(7): 2414-2424. |
| CHOU Si, LI Xiaoming, LUO Kun, et al. Study on the photocatalytic degradation performance of g-C3N4/Ag3PO4/CNT on methylene blue and tetracycline[J]. Environmental Chemistry, 2022, 41(7): 2414-2424. | |
| 11 | 邓郁蓉, 欧阳卓智, 杨琛, 等. 四环素在水体中的自然光解作用机制[J]. 环境科学学报, 2022, 42(9): 40-50. |
| DENG Yurong, OUYANG Zhuozhi, YANG Chen, et al. Photolysis of tetracycline in aqueous environment[J]. Acta Scientiae Circumstantiae, 2022, 42(9): 40-50. | |
| 12 | ZOUHEIR M, TANJI K, NAVIO J A, et al. Effective photocatalytic conversion of formic acid using iron, copper and sulphate doped TiO2 [J]. Journal of Central South University, 2022, 29(11): 3592-3607. |
| 13 | HU Xueli, LU Peng, FU Min, et al. Activating the photocatalytic activity of insulator Barium silicate: A liquid-phase alkalized tetracycline photosensitizer and its self-destruction[J]. Chemical Engineering Journal, 2023, 454: 140281. |
| 14 | 刘怡璇, 林跃朝, 马伟芳. 可见光催化降解水中卤代有机污染物的研究进展[J]. 化工进展, 2022, 41(S1): 571-579. |
| LIU Yixuan, LIN Yuechao, MA Weifang. Research progress on degradation of halogenated organic contaminants in water by visible light photocatalysis[J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 571-579. | |
| 15 | 乔波, 欧阳紫菱, 郭丽, 等. 曝气量对PCMR处理腐殖酸废水中膜污染控制研究[J]. 水处理技术, 2022, 48(5): 81-84. |
| QIAO Bo, OUYANG Ziling, GUO Li, et al. Study on the control of membrane fouling in humic acid wastewater treated by PCMR with aeration volume[J]. Technology of Water Treatment, 2022, 48(5): 81-84. | |
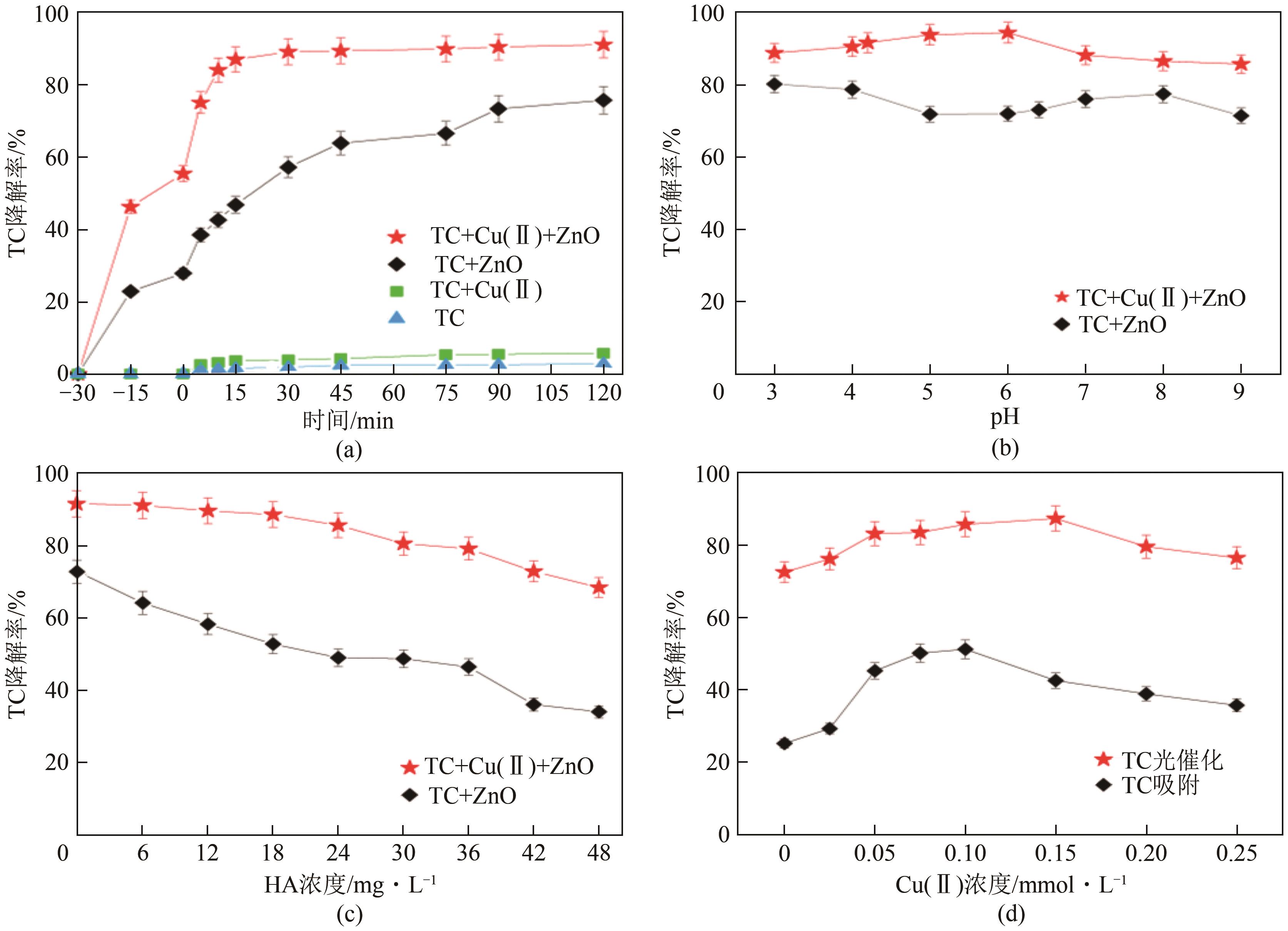
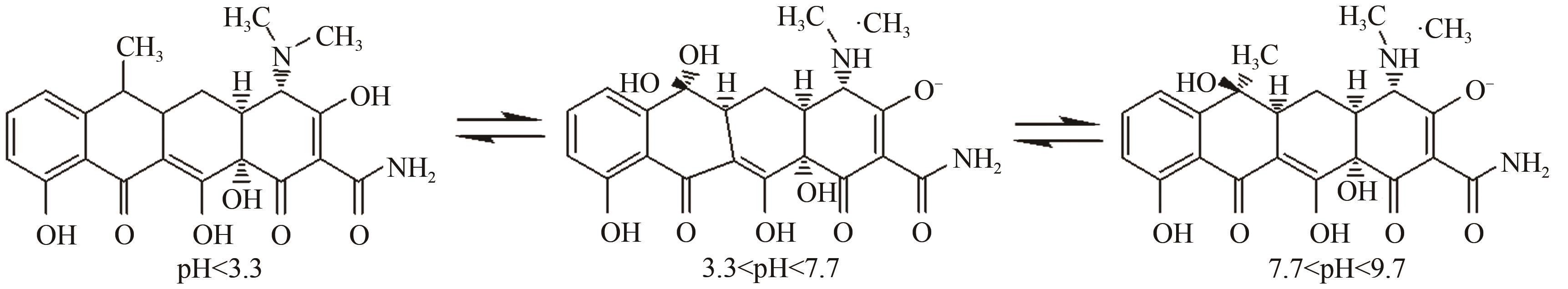
| 16 | ZHU Ying, LIU Kun, MUHAMMAD Y, et al. Effects of divalent copper on tetracycline degradation and the proposed transformation pathway[J]. Environmental Science and Pollution Research, 2020, 27(5): 5155-5167. |
| 17 | GOPAL G, NATARAJAN C, MUKHERJEE A. Synergistic removal of tetracycline and copper(Ⅱ) by in-situ B-Fe/Ni nanocomposite—A novel and an environmentally sustainable green nanomaterial[J]. Environmental Technology & Innovation, 2022, 25: 102187. |
| 18 | AI Yuejie, ZHAO Chaofeng, SUN Lu, et al. Coagulation mechanisms of humic acid in metal ions solution under different pH conditions: A molecular dynamics simulation[J]. Science of the Total Environment, 2020, 702: 135072. |
| 19 | MUÑOZ-FLORES P, POON P S, ANIA C O, et al. Performance of a C-containing Cu-based photocatalyst for the degradation of tartrazine: Comparison of performance in a slurry and CPC photoreactor under artificial and natural solar light[J]. Journal of Colloid and Interface Science, 2022, 623: 646-659. |
| 20 | FILIPE O M S, SANTOS E B H, OTERO Marta, et al. Photodegradation of metoprolol in the presence of aquatic fulvic acids. Kinetic studies, degradation pathways and role of singlet oxygen, OH radicals and fulvic acids triplet states[J]. Journal of Hazardous Materials, 2020, 385: 121523. |
| 21 | HUANG Hua, NIU Zhirui, SHI Ruru, et al. Thermal oxidation activation of hydrochar for tetracycline adsorption: The role of oxygen concentration and temperature[J]. Bioresource Technology, 2020, 306: 123096. |
| 22 | YANG Bo, WANG Chengjin, CHENG Xin, et al. Interactions between the antibiotic tetracycline and humic acid: Examination of the binding sites, and effects of complexation on the oxidation of tetracycline[J]. Water Research, 2021, 202: 117379. |
| 23 | 潘柳疏, 焦春霖, 熊建华, 等. 蔗渣纤维基碳球负载TiO2复合材料的制备表征及对四环素的去除研究[J]. 水处理技术, 2022, 48(5): 85-89. |
| PAN Liushu, JIAO Chunlin, XIONG Jianhua, et al. Preparation, characterization and tetracycline removal of bagasse cellulose carbon spheres supported nano-TiO2 composites[J]. Technology of Water Treatment, 2022, 48(5): 85-89. | |
| 24 | CAI Ying, SHEN Shihao, FAN Jinhong. Enhanced degradation of tetracycline by Cu(Ⅱ) complexation in the FeS/sulfite system[J]. Journal of Hazardous Materials, 2022, 421: 126673. |
| 25 | 饶涵, 马永梅, 李思悦. NaYF4: Yb, Tm@TiO2复合催化剂光催化降解盐酸四环素[J]. 功能材料, 2022, 53(3): 3011-3019. |
| RAO Han, MA Yongmei, LI Siyue. Photocatalytic degradation of tetracycline hydrochloride by NaYF4: Yb, Tm@TiO2 composite catalyst[J]. Journal of Functional Materials, 2022, 53(3): 3011-3019. | |
| 26 | 段毅, 邹烨, 周书葵, 等. 过渡金属单原子催化剂活化H2O2/PMS/PDS降解有机污染物的研究进展[J]. 化工进展, 2022, 41(8): 4147-4158. |
| DUAN Yi, ZOU Ye, ZHOU Shukui, et al. Progress in the degradation of organic pollutants by H2O2/PMS/PDS activated by transition metal single-atom catalysts[J]. Chemical Industry and Engineering Progress, 2022, 41(8): 4147-4158. | |
| 27 | GONG Yinan, WANG Ying, TANG Miaomiao, et al. A two-step process coupling photocatalysis with adsorption to treat tetracycline-Copper(Ⅱ) hybrid wastewaters[J]. Journal of Water Process Engineering, 2022, 47: 102710. |
| 28 | 黄翔峰, 熊永娇, 彭开铭, 等. 金属离子络合对抗生素去除特性的影响研究进展[J]. 环境化学, 2016, 35(1): 133-140. |
| HUANG Xiangfeng, XIONG Yongjiao, PENG Kaiming, et al. The progress of antibiotics removal performance under the complexion effect of metal ions[J]. Environmental Chemistry, 2016, 35(1): 133-140. | |
| 29 | JIANG Chenxiao, CHEN Hanlin, ZHANG Yilue, et al. Complexation Electrodialysis as a general method to simultaneously treat wastewaters with metal and organic matter[J]. Chemical Engineering Journal, 2018, 348: 952-959. |
| 30 | FATIMA B, SIDDIQUI S I, RAJOR H K, et al. Photocatalytic removal of organic dye using green synthesized zinc oxide coupled cadmium tungstate nanocomposite under natural solar light irradiation[J]. Environmental Research, 2023, 216: 114534. |
| 31 | CIONTI C, PARGOLETTI E, FALLETTA E, et al. Combining pH-triggered adsorption and photocatalysis for the remediation of complex water matrices[J]. Journal of Environmental Chemical Engineering, 2022, 10(5): 108468. |
| 32 | 陈佳昆, 汤健, 夏恒, 等. 城市固废炉排炉焚烧过程二𫫇英排放浓度数值仿真[J]. 化工进展, 2023, 42(2): 1061-1072. |
| CHEN Jiakun, TANG Jian, XIA Heng, et al. Numerical simulation of dioxin emission concentration in grate furnace incineration processes for municipal solid waste[J]. Chemical Industry and Engineering Progress, 2023, 42(2): 1061-1072. | |
| 33 | VILLARREAL R C, LUQUE-MORALES M, CHINCHILLAS-CHINCHILLAS M J, et al. Langmuir-Hinshelwood-Hougen-Watson model for the study of photodegradation properties of zinc oxide semiconductor nanoparticles synthetized by Peumus boldus [J]. Results in Physics, 2022, 36: 105421. |
| 34 | 蒋柱武, 史安童, 沈俊宏. Cu-ZnO/g-C3N4复合材料可见光催化降解环丙沙星效率及机理研究[J]. 材料导报, 2022, 36(20): 84-90. |
| JIANG Zhuwu, SHI Antong, SHEN Junhong. Study on efficiency and mechanism of visible-light photocatalytic degradation of ciprofloxacin by using Cu-ZnO/g-C3N4 composite[J]. Materials Reports, 2022, 36(20): 84-90. |
| [1] | LUO Fen, YANG Xiaoqi, DUAN Fanglin, LI Xiaojiang, WU Liang, XU Tongwen. Recent advances in the bipolar membrane and its applications [J]. Chemical Industry and Engineering Progress, 2024, 43(1): 145-163. |
| [2] | PEI Wenyi, CHEN Ziyang, ZHAO Meng, JIANG Hong, CHEN Rizhi. Effect of pre-wetting on preparation and gas distribution performance of Janus ceramic membrane [J]. Chemical Industry and Engineering Progress, 2024, 43(1): 353-363. |
| [3] | WANG Shang, YAO Yao, WANG Jia, DONG Didi, CHANG Ganggang. Hierarchical porous carbon supported CoP derived from CoZn-MOF and its hydrogen evolution properties [J]. Chemical Industry and Engineering Progress, 2024, 43(1): 447-454. |
| [4] | WANG Bo, ZHANG Chang’an, ZHAO Limin, YUAN Jun, SONG Yongyi. Industrial wastewater treatment technology based on boron-doped diamond electrodes:A review [J]. Chemical Industry and Engineering Progress, 2024, 43(1): 501-513. |
| [5] | DAI Hongjing, MA Xuehu, WANG Sifang. Adsorption technology and materials for the treatment of low and intermediate level radioactive wastewater [J]. Chemical Industry and Engineering Progress, 2024, 43(1): 529-540. |
| [6] | WANG Ying, HAN Yunping, LI Lin, LI Yanbo, LI Huili, YAN Changren, LI Caixia. Research status and future prospects of the emission characteristics of virus aerosols in urban wastewater treatment plants [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 439-446. |
| [7] | XU Chunshu, YAO Qingda, LIANG Yongxian, ZHOU Hualong. Research progress on functionalization strategies of covalent organic frame materials and its adsorption properties for Hg(Ⅱ) and Cr(Ⅵ) [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 461-478. |
| [8] | ZHAO Jingchao, TAN Ming. Effect of surfactants on the reduction of industrial saline wastewater by electrodialysis [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 529-535. |
| [9] | WANG Chen, BAI Haoliang, KANG Xue. Performance study of high power UV-LED heat dissipation and nano-TiO2 photocatalytic acid red 26 coupling system [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4905-4916. |
| [10] | WANG Qi, KOU Lihong, WANG Guanyu, WANG Jikun, LIU Min, LI Lanting, WANG Hao. Molecular recognition of dissolved organic matter in bio-treated effluent of coking wastewater [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4984-4993. |
| [11] | SHI Tianxi, SHI Yonghui, WU Xinying, ZHANG Yihao, QIN Zhe, ZHAO Chunxia, LU Da. Effects of Fe2+ on the performance of Anammox EGSB reactor [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 5003-5010. |
| [12] | WANG Haoran, YIN Quanyu, FANG Ming, HOU Jianlin, LI Jun, HE Bin, ZHANG Mingyue. Optimization of near critical-water treatment process of tobacco stems [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 5019-5027. |
| [13] | LI Xuejia, LI Peng, LI Zhixia, JIN Dunshang, GUO Qiang, SONG Xufeng, SONG Peng, PENG Yuelian. Experimental comparation on anti-scaling and anti-wetting ability of hydrophilic and hydrophobic modified membranes [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4458-4464. |
| [14] | HUANG Yufei, LI Ziyi, HUANG Yangqiang, JIN Bo, LUO Xiao, LIANG Zhiwu. Research progress on catalysts for photocatalytic CO2 and CH4 reforming [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4247-4263. |
| [15] | XI Yonglan, WANG Chengcheng, YE Xiaomei, LIU Yang, JIA Zhaoyan, CAO Chunhui, HAN Ting, ZHANG Yingpeng, TIAN Yu. Research progress on the application of micro/nano bubbles in anaerobic digestion [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4414-4423. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||