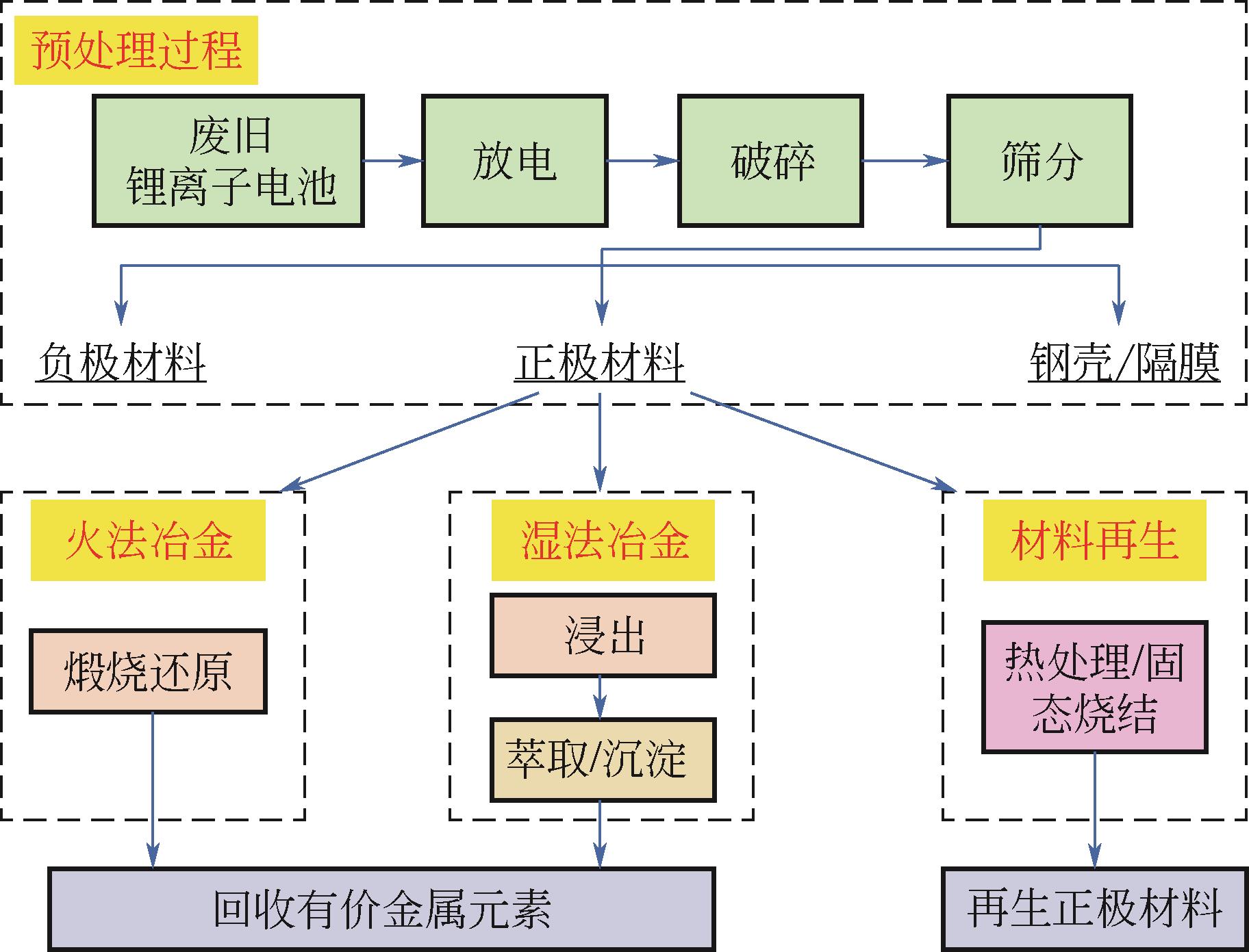
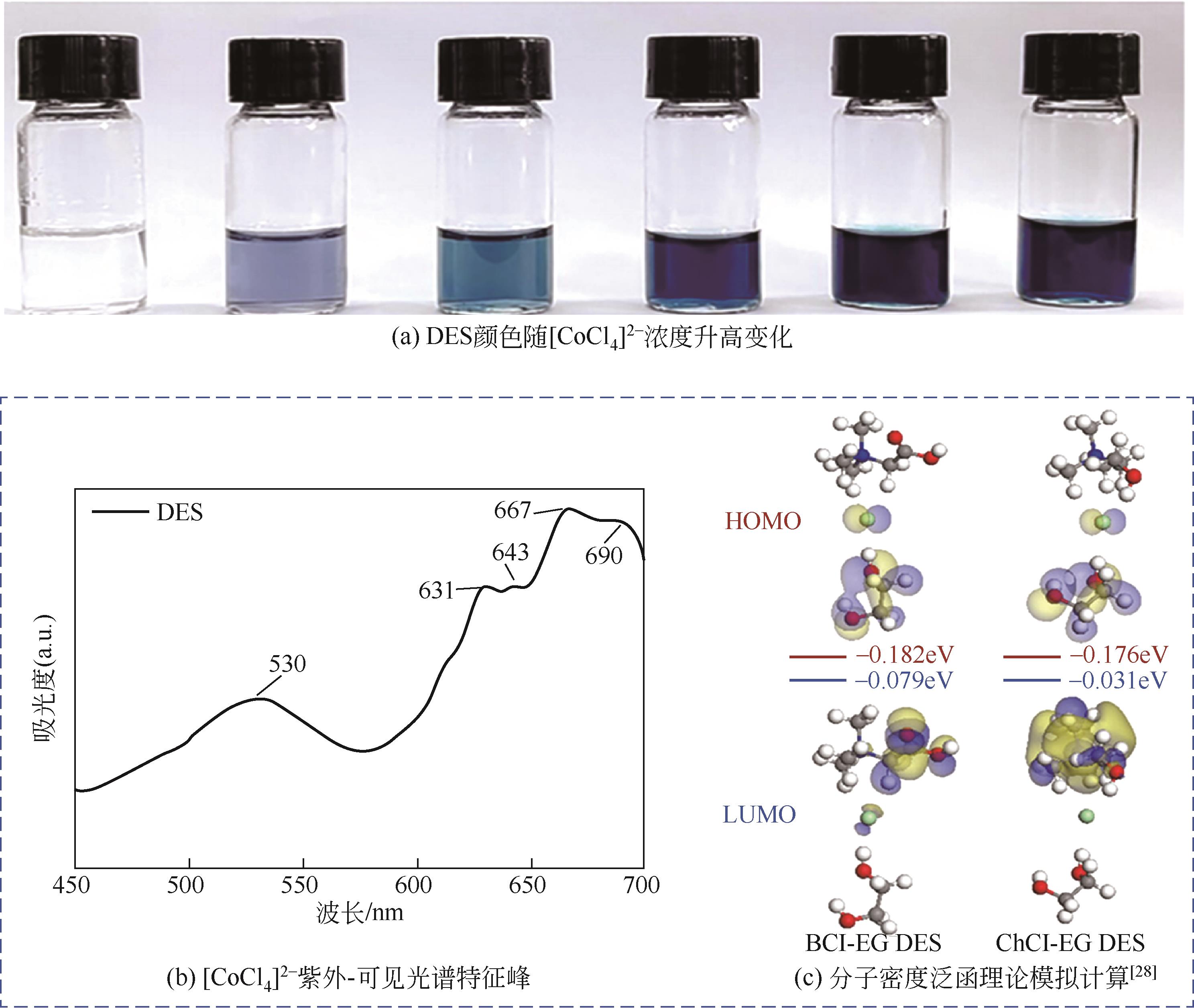
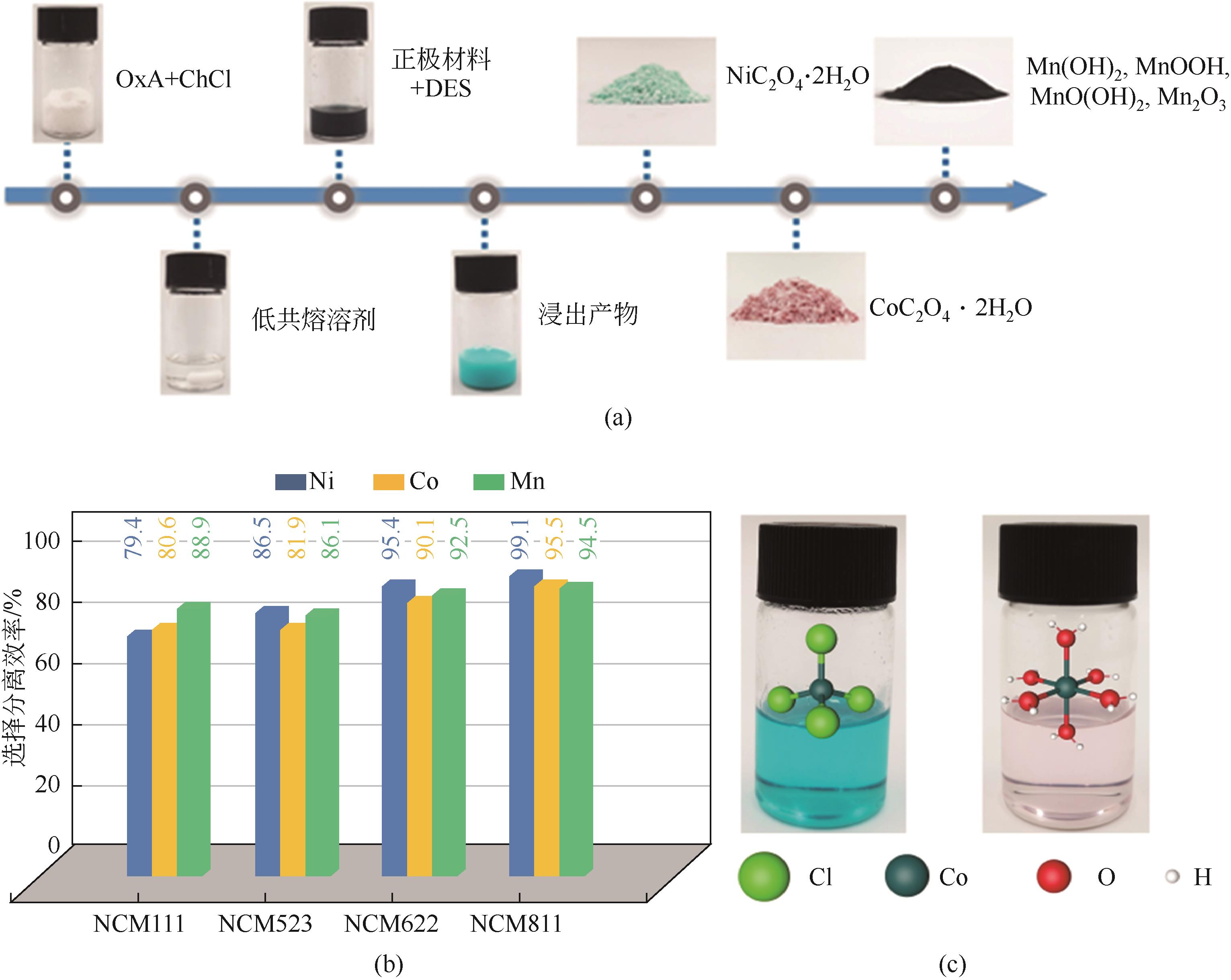
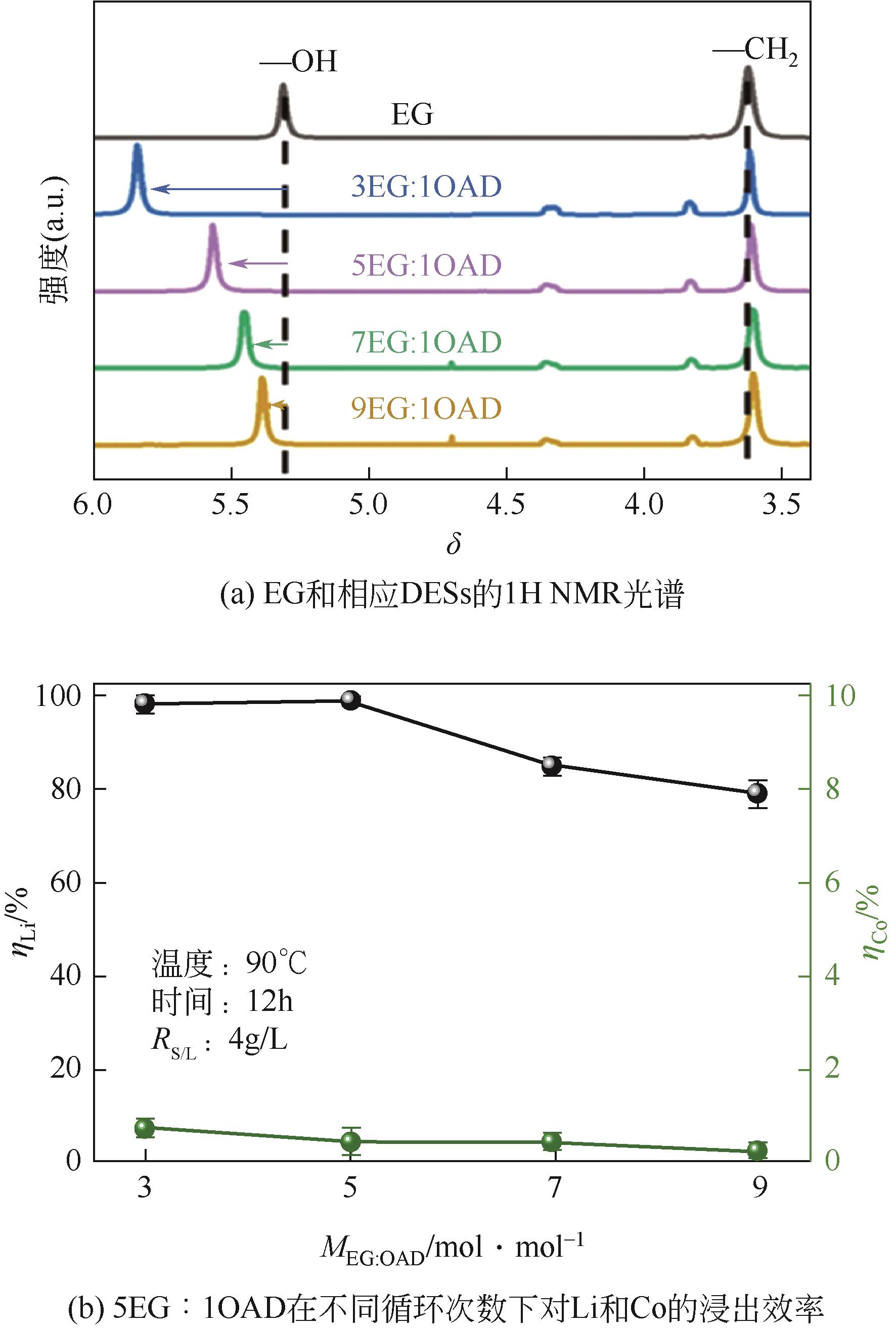
Chemical Industry and Engineering Progress ›› 2023, Vol. 42 ›› Issue (S1): 219-232.DOI: 10.16085/j.issn.1000-6613.2023-0633
• Energy processes and technology • Previous Articles Next Articles
Research progress in recovery of spent cathode materials for lithium-ion batteries using deep eutectic solvents
MA Yi( ), CAO Shiwei, WANG Jiajun, LIN Liqun, XING Yan, CAO Tengliang, LU Feng, ZHAO Zhenlun, ZHANG Zhijun
), CAO Shiwei, WANG Jiajun, LIN Liqun, XING Yan, CAO Tengliang, LU Feng, ZHAO Zhenlun, ZHANG Zhijun
- Cangzhou Risun Chemical Co. , Ltd. , Cangzhou 061000, Hebei, China
-
Received:2023-04-09Revised:2023-07-17Online:2023-11-30Published:2023-10-25 -
Contact:MA Yi
低共熔溶剂回收废旧锂离子电池正极材料的研究进展
马伊( ), 曹世伟, 王家骏, 林立群, 邢延, 曹腾良, 卢峰, 赵振伦, 张志军
), 曹世伟, 王家骏, 林立群, 邢延, 曹腾良, 卢峰, 赵振伦, 张志军
- 沧州旭阳化工有限公司,河北 沧州 100070
-
通讯作者:马伊 -
作者简介:马伊(1982—),男,博士,高级工程师,研究方向为新能源材料与高分子材料。E-mail:mayi@risun.com。
CLC Number:
Cite this article
MA Yi, CAO Shiwei, WANG Jiajun, LIN Liqun, XING Yan, CAO Tengliang, LU Feng, ZHAO Zhenlun, ZHANG Zhijun. Research progress in recovery of spent cathode materials for lithium-ion batteries using deep eutectic solvents[J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 219-232.
马伊, 曹世伟, 王家骏, 林立群, 邢延, 曹腾良, 卢峰, 赵振伦, 张志军. 低共熔溶剂回收废旧锂离子电池正极材料的研究进展[J]. 化工进展, 2023, 42(S1): 219-232.
share this article
Add to citation manager EndNote|Ris|BibTeX
URL: https://hgjz.cip.com.cn/EN/10.16085/j.issn.1000-6613.2023-0633
| DES | 液固比 | T/℃ | t/h | 浸出效果 | 参考文献 |
|---|---|---|---|---|---|
| ChCl∶Gly比为1∶2 | 2.5g/L | 180 | 2 | Co:47.59%,Li:50.4% | [ |
| ChCl∶LAA比为2∶1.6 | 1∶25 | 50 | 1 | Ni、Co、Mn>96% | |
| ChCl∶BSA∶EtOH比为1∶1∶2 | 20g/L | 90 | 2 | Co:98%,Li:99% | [ |
| ChCl∶MAL∶PTSA | 20g/L | 100 | 24 | Co:98.61%,Li:98.78% | [ |
| ChCl∶Urea∶EG比为1∶1∶2 | 9.69g/L | 100 | 72 | Co:1.61%,Li:92.82%,Ni:0.72%,Mn:0.42% | [ |
| TA(20g/L)∶CH3COOH(1mol/L) | 20g/L | 80 | 2 | Co:94%,Li:99% | [ |
| EG∶CA比为2.5∶1 | 15g/L | 95 | 10 | Co:96.2%,Li:99.1%,Ni:97.6%,Mn:98.3% | [ |
| PEG∶PSA比为1∶1 | 20g/L | 100 | 24 | Co:99.5% | [ |
| ChCl∶LA比为2∶1 | 70 | 24 | Li、Ni、Co、Mn为100% | [ | |
| ChCl∶CA比为2∶1, 35% H2O | 20g/L | 40 | 1 | Co>98% | [ |
| BCl∶EG比为1∶5 | 25g/kg | 140 | 0.17 | Li、Ni、Co、Mn>99% | [ |
| ChCl∶EG | 12.5g/L | 180 | 240 | Li:91.63%,Co:92.52%,Ni:94.92%,Mn:95.47% | [ |
| ChCl∶OA∶2H2O | 20g/L | 100 | 0.17 | Li:99.05%,Co:99.21% | [ |
| EG∶SAD比为12∶1 | 40g/L | 110 | 6 | Li:98.3%,Co:93.5%,Ni:99.1%,Mn:100% | [ |
| DES | 液固比 | T/℃ | t/h | 浸出效果 | 参考文献 |
|---|---|---|---|---|---|
| ChCl∶Gly比为1∶2 | 2.5g/L | 180 | 2 | Co:47.59%,Li:50.4% | [ |
| ChCl∶LAA比为2∶1.6 | 1∶25 | 50 | 1 | Ni、Co、Mn>96% | |
| ChCl∶BSA∶EtOH比为1∶1∶2 | 20g/L | 90 | 2 | Co:98%,Li:99% | [ |
| ChCl∶MAL∶PTSA | 20g/L | 100 | 24 | Co:98.61%,Li:98.78% | [ |
| ChCl∶Urea∶EG比为1∶1∶2 | 9.69g/L | 100 | 72 | Co:1.61%,Li:92.82%,Ni:0.72%,Mn:0.42% | [ |
| TA(20g/L)∶CH3COOH(1mol/L) | 20g/L | 80 | 2 | Co:94%,Li:99% | [ |
| EG∶CA比为2.5∶1 | 15g/L | 95 | 10 | Co:96.2%,Li:99.1%,Ni:97.6%,Mn:98.3% | [ |
| PEG∶PSA比为1∶1 | 20g/L | 100 | 24 | Co:99.5% | [ |
| ChCl∶LA比为2∶1 | 70 | 24 | Li、Ni、Co、Mn为100% | [ | |
| ChCl∶CA比为2∶1, 35% H2O | 20g/L | 40 | 1 | Co>98% | [ |
| BCl∶EG比为1∶5 | 25g/kg | 140 | 0.17 | Li、Ni、Co、Mn>99% | [ |
| ChCl∶EG | 12.5g/L | 180 | 240 | Li:91.63%,Co:92.52%,Ni:94.92%,Mn:95.47% | [ |
| ChCl∶OA∶2H2O | 20g/L | 100 | 0.17 | Li:99.05%,Co:99.21% | [ |
| EG∶SAD比为12∶1 | 40g/L | 110 | 6 | Li:98.3%,Co:93.5%,Ni:99.1%,Mn:100% | [ |
| 1 | HOU Caixia, WANG Jiajun, CHENG Huan, et al. Hyper-coal as binder for coking: Macroscopic caking property, microstructure basis and extraction temperature optimization index[J]. International Journal of Coal Preparation and Utilization, 2023, 43(3): 520-537. |
| 2 | 卢奇秀. 中汽协: 我国新能源汽车产销连续8年全球第一[N]. 中国能源报, 2023-01-16(11). |
| 3 | 国务院办公厅. 国务院办公厅关于印发新能源汽车产业发展规划(2021—2035年)的通知[J]. 中华人民共和国国务院公报, 2020(31): 16-23. |
| The State Council of the People’s Republic of China. Notice of the general office of the state council on printing and distributing the development plan of new energy automobile industry (2021—2035)[J]. Gazette of the State Council of the People’s Republic of China, 2020(31): 16-23. | |
| 4 | XU Panpan, TAN Darren H S, JIAO Binglei, et al. A materials perspective on direct recycling of lithium-ion batteries: Principles, challenges and opportunities[J]. Advanced Functional Materials, 2023, 33(14): 2213168. |
| 5 | WANG Mengmeng, LIU Kang, YU Jiadong, et al. Challenges in recycling spent lithium-ion batteries: Spotlight on polyvinylidene fluoride removal[J]. Global Challenges, 2023, 7(3): 2200237. |
| 6 | BAI Xue, JIANG Zengyan, SUN Yanzhi, et al. Clean universal solid-state recovery method of waste lithium-ion battery ternary positive materials and their electrochemical properties[J]. ACS Sustainable Chemistry & Engineering, 2023, 11(9): 3673-3686. |
| 7 | YOON Juhee, HAN Geonhee, CHO Sungmin, et al. Microbial-copolyester-based eco-friendly binder for lithium-ion battery electrodes[J]. ACS Applied Polymer Materials, 2023, 5(2): 1199-1207. |
| 8 | TRAN M K, RODRIGUES M F, KATO K, et al. Deep eutectic solvents for cathode recycling of Li-ion batteries[J]. Nature Energy, 2019, 4(4): 339-345. |
| 9 | 李文昌, 李建威, 谢桂青, 等. 中国关键矿产现状、研究内容与资源战略分析[J]. 地学前缘, 2022, 29(1): 1-13. |
| LI Wenchang, LI Jianwei, XIE Guiqing, et al. Critical minerals in China: Current status, research focus and resource strategic analysis[J]. Earth Science Frontiers, 2022, 29(1): 1-13. | |
| 10 | ZHU Ahui, BIAN Xinyu, HAN Weijiang, et al. The application of deep eutectic solvents in lithium-ion battery recycling: A comprehensive review[J]. Resources, Conservation and Recycling, 2023, 188: 106690. |
| 11 | 程明强, 汝娟坚, 华一新, 等. 低共熔溶剂在废旧锂离子电池正极材料回收中的研究进展[J]. 化工进展, 2022, 41(6): 3293-3305. |
| CHENG Mingqiang, RU Juanjian, HUA Yixin, et al. Progress of deep eutectic solvents in recovery of cathode materials from spent lithium ion batteries[J]. Chemical Industry and Engineering Progress, 2022, 41(6): 3293-3305. | |
| 12 | WANG Ronghao, ZHANG Yuhao, SUN Kaiwen, et al. Emerging green technologies for recovery and reuse of spent lithium-ion batteries — A review[J]. Journal of Materials Chemistry A, 2022, 10(33): 17053-17076. |
| 13 | QIAO Yu, ZHAO Huaping, SHEN Yonglong, et al. Recycling of graphite anode from spent lithium-ion batteries: Advances and perspectives[J]. EcoMat, 2023, 5(4): e12321. |
| 14 | MANTHIRAM A, GOODENOUGH J B. Layered lithium cobalt oxide cathodes[J]. Nature Energy, 2021, 6(3): 323. |
| 15 | YAN Pengfei, ZHENG Jianming, CHEN Tianwu, et al. Coupling of electrochemically triggered thermal and mechanical effects to aggravate failure in a layered cathode[J]. Nature Communications, 2018, 9: 2437. |
| 16 | 颜群轩, 罗碧云, 陈嘉鑫, 等. 废旧磷酸铁锂电池可持续回收技术研究进展[J/OL]. 矿冶工程, 2023. . |
| YAN Qunxuan, LUO Biyun, CHEN Jiaxin, et al. Progress in sustainable recycling of spent LiFePO4 batteries[J]. Mining and Metallurgical engineering, 2023. . | |
| 17 | CHEN Zuhang, DENG Yelin, LI Honglei, et al. An efficient regrouping method of retired lithium-ion iron phosphate batteries based on incremental capacity curve feature extraction for echelon utilization[J]. Journal of Energy Storage, 2022, 56: 105917. |
| 18 | DU Kaidi, Edison Huixiang ANG, WU Xinglong, et al. Progresses in sustainable recycling technology of spent lithium-ion batteries[J]. Energy & Environmental Materials, 2022, 5(4): 1012-1036. |
| 19 | 徐政和, 刘振达, 王树宾, 等. 湿法回收废旧锂离子电池有价金属的研究进展[J]. 中国矿业大学学报, 2022, 51(3): 454-465. |
| XU Zhenghe, LIU Zhenda, WANG Shubin, et al. Review on hydrometallurgical recovery of valuable metals from spent lithium-ion batteries[J]. Journal of China University of Mining & Technology, 2022, 51(3): 454-465. | |
| 20 | ABBOTT A P, GLEN C, DAVIES D L, et al. Novel solvent properties of choline chloride/urea mixtures[J]. Chemical Communications, 2003(1): 70-71. |
| 21 | Gregorio GARCÍA, ATILHAN Mert, APARICIO Santiago. The impact of charges in force field parameterization for molecular dynamics simulations of deep eutectic solvents[J]. Journal of Molecular Liquids, 2015, 211: 506-514. |
| 22 | Kudłak BŁAŻEJ, KATARZYNA Owczarek, JACEK Namieśnik. Selected issues related to the toxicity of ionic liquids and deep eutectic solvents—A review[J]. Environmental Science and Pollution Research, 2015, 22(16): 11975-11992. |
| 23 | JURIĆ T, UKA D, HOLLÓ B B, et al. Comprehensive physicochemical evaluation of choline chloride-based natural deep eutectic solvents[J]. Journal of Molecular Liquids, 2021, 343: 116968. |
| 24 | 马文君, 张旭, 刘孟顺, 等. 新兴湿法退役锂电池正极材料回收技术研究进展[J/OL]. 化工进展, 2023, DOI:10.16085/j.issn.1000-6613.2023-0547 . |
| MA Wenjun, ZHANG Xu, LIU Mengshun, et al. Research progress of novel hydrometallurgy in recycling cathode materials from spent lithium-ion batteries[J/OL]. Chemical Industry and Engineering Progress, 2023, DOI: 10.16085/j.issn.1000-6613.2023-0547 . | |
| 25 | CHEN Xiangping, XU Bao, ZHOU Tao, et al. Separation and recovery of metal values from leaching liquor of mixed-type of spent lithium-ion batteries[J]. Separation and Purification Technology, 2015, 144: 197-205. |
| 26 | JOULIÉ M, LAUCOURNET R, BILLY E. Hydrometallurgical process for the recovery of high value metals from spent lithium nickel cobalt aluminum oxide based lithium-ion batteries[J]. Journal of Power Sources, 2014, 247: 551-555. |
| 27 | FAN Yuxin, KONG Yuelin, JIANG Pinxian, et al. Development and challenges of deep eutectic solvents for cathode recycling of end-of-life lithium-ion batteries[J]. Chemical Engineering Journal, 2023, 463: 142278. |
| 28 | LUO Yi, YIN Chengzhe, Leming OU, et al. Highly efficient dissolution of the cathode materials of spent Ni-Co-Mn lithium batteries using deep eutectic solvents[J]. Green Chemistry, 2022, 24(17): 6562-6570. |
| 29 | WANG Kang, HU Tianyou, SHI Penghui, et al. Efficient recovery of value metals from spent lithium-ion batteries by combining deep eutectic solvents and coextraction[J]. ACS Sustainable Chemistry & Engineering, 2022, 10(3): 1149-1159. |
| 30 | SCHIAVI P G, ALTIMARI P, BRANCHI M, et al. Selective recovery of cobalt from mixed lithium ion battery wastes using deep eutectic solvent[J]. Chemical Engineering Journal, 2021, 417: 129249. |
| 31 | CHEN Yu, LU Yanhong, LIU Zhenghui, et al. Efficient dissolution of lithium-ion batteries cathode LiCoO2 by polyethylene glycol-based deep eutectic solvents at mild temperature[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(31): 11713-11720. |
| 32 | CHEN Linlin, CHAO Yanhong, LI Xiaowei, et al. Engineering a tandem leaching system for the highly selective recycling of valuable metals from spent Li-ion batteries[J]. Green Chemistry, 2021, 23(5): 2177-2184. |
| 33 | LI Taibai, XIONG Yige, YAN Xiaohui, et al. Closed-loop cobalt recycling from spent lithium-ion batteries based on a deep eutectic solvent (DES) with easy solvent recovery[J]. Journal of Energy Chemistry, 2022, 72: 532-538. |
| 34 | LU Qingqiang, CHEN Linlin, LI Xiaowei, et al. Sustainable and convenient recovery of valuable metals from spent Li-ion batteries by a one-pot extraction process[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(41): 13851-13861. |
| 35 | MORINA Riccardo, CALLEGARI Daniele, MERLI Daniele, et al. Cathode active material recycling from spent lithium batteries: A green (circular) approach based on deep eutectic solvents[J]. ChemSusChem, 2022, 15(2): e202102080. |
| 36 | ROLDÁN-RUIZ M J, FERRER M L, GUTIÉRREZ M C, et al. Highly efficient p-toluenesulfonic acid-based deep-eutectic solvents for cathode recycling of Li-ion batteries[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(14): 5437-5445. |
| 37 | THOMPSON D L, PATELI I M, LEI C, et al. Separation of nickel from cobalt and manganese in lithium ion batteries using deep eutectic solvents[J]. Green Chemistry, 2022, 24(12): 4877-4886. |
| 38 | LU Bing, DU Rong, WANG Gang, et al. High-efficiency leaching of valuable metals from waste Li-ion batteries using deep eutectic solvents[J]. Environmental Research, 2022, 212: 113286. |
| 39 | PRASETYO E, MURYANTA W A, ANGGRAINI A G, et al. Tannic acid as a novel and green leaching reagent for cobalt and lithium recycling from spent lithium-ion batteries[J]. Journal of Material Cycles and Waste Management, 2022, 24(3): 927-938. |
| 40 | ABBOTT A P, CAPPER G, DAVIES D L, et al. Solubility of metal oxides in deep eutectic solvents based on choline chloride[J]. Journal of Chemical & Engineering Data, 2006, 51(4): 1280-1282. |
| 41 | TIAN Yurun, CHEN Wenjun, ZHANG Baolong, et al. A weak acidic and strong coordinated deep eutectic solvent for recycling of cathode from spent lithium-ion batteries[J]. ChemSusChem, 2022, 15(16): e202200524. |
| 42 | CHANG Xin, FAN Min, GU Chaofan, et al. Selective extraction of transition metals from spent LiNi x Co y Mn1- x- y O2 cathode via regulation of coordination environment[J]. Angewandte Chemie International Edition, 2022, 61(24): e202202558. |
| 43 | TANG Shujie, FENG Jiali, SU Runchang, et al. New bifunctional deep-eutectic solvent for in situ selective extraction of valuable metals from spent lithium batteries[J]. ACS Sustainable Chemistry & Engineering, 2022, 10(26): 8423-8432. |
| 44 | TANG Shujie, ZHANG Mei, GUO Min. A novel deep-eutectic solvent with strong coordination ability and low viscosity for efficient extraction of valuable metals from spent lithium-ion batteries[J]. ACS Sustainable Chemistry & Engineering, 2022, 10(2): 975-985. |
| 45 | CHEN Yu, WANG Yanlong, BAI Yue, et al. Mild and efficient recovery of lithium-ion battery cathode material by deep eutectic solvents with natural and cheap components[J]. Green Chemical Engineering, 2022. . |
| 46 | CHEN Yu, WANG Yanlong, BAI Yue, et al. Significant improvement in dissolving lithium-ion battery cathodes using novel deep eutectic solvents at low temperature[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(38): 12940-12948. |
| 47 | HUANG Fengyu, LI Taibai, YAN Xiaohui, et al. Ternary deep eutectic solvent (DES) with a regulated rate-determining step for efficient recycling of lithium cobalt oxide[J]. ACS Omega, 2022, 7(13): 11452-11459. |
| 48 | WANG Shubin, ZHANG Zuotai, LU Zhouguang, et al. A novel method for screening deep eutectic solvent to recycle the cathode of Li-ion batteries[J]. Green Chemistry, 2020, 22(14): 4473-4482. |
| 49 | WANG Hongmin, LI Mengran, GARG Sahil, et al. Cobalt electrochemical recovery from lithium cobalt oxides in deep eutectic choline Chloride+Urea solvents[J]. ChemSusChem, 2021, 14(14): 2972-2983. |
| 50 | SURIYANARAYANAN S, BABU M P, MURUGAN R, et al. Highly efficient recovery and recycling of cobalt from spent lithium-ion batteries using an N-methylurea-acetamide nonionic deep eutectic solvent[J]. ACS Omega, 2023, 8(7): 6959-6967. |
| 51 | Sylvain DÉSILETS, BROUSSEAU Patrick, CHAMBERLAND Daniel, et al. Analyses of the thermal decomposition of urea nitrate at high temperature[J]. Thermochimica Acta, 2011, 521(1/2): 59-65. |
| 52 | NAND Peeters, KOEN Binnemans, Riaño SOFÍA. Solvometallurgical recovery of cobalt from lithium-ion battery cathode materials using deep-eutectic solvents[J]. Green Chemistry, 2020, 22(13): 4210-4221. |
| 53 | XU Zhiwen, SHAO Huaishuang, ZHAO Qinxin, et al. Use of microwave-assisted deep eutectic solvents to recycle lithium manganese oxide from Li-ion batteries[J]. JOM, 2021, 73(7): 2104-2110. |
| 54 | LIU Mengshun, MA Wenjun, ZHANG Xu, et al. Recycling lithium and cobalt from LIBs using microwave-assisted deep eutectic solvent leaching technology at low-temperature[J]. Materials Chemistry and Physics, 2022, 289: 126466. |
| 55 | MA Wenjun, LIU Mengshun, ZHANG Xu, et al. An efficient and precipitant-free approach to selectively recover lithium cobalt oxide made for cathode materials using a microwave-assisted deep eutectic solvent[J]. Energy & Fuels, 2023, 37(1): 724-734. |
| 56 | WANG Mengmeng, LIU Kang, XU Zibo, et al. Selective extraction of critical metals from spent lithium-ion batteries[J]. Environmental Science & Technology, 2023, 57(9): 3940-3950. |
| 57 | LIAO Yanshun, GONG Shanshan, WANG Guange, et al. A novel ternary deep eutectic solvent for efficient recovery of critical metals from spent lithium-ion batteries under mild conditions[J]. Journal of Environmental Chemical Engineering, 2022, 10(6): 108627. |
| 58 | JAFARI M, SHAFAIE S Z, ABDOLLAHI H, et al. A green approach for selective ionometallurgical separation of lithium from spent Li-ion batteries by deep eutectic solvent (DES): Process optimization and kinetics modeling[J]. Mineral Processing and Extractive Metallurgy Review, 2023, 44(3): 218-230. |
| 59 | ZENG Guisheng, YAO Junxia, LIU Chunli, et al. Simultaneous recycling of critical metals and aluminum foil from waste LiNi1/3Co1/3Mn1/3O2 cathode via ethylene glycol-citric acid system[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(48): 16133-16142. |
| 60 | 李丽, 姚莹, 郁亚娟. 锂离子电池回收与资源化技术[M]. 北京: 科学出版社, 2021. |
| LI Li, YAO Ying, YU Yajuan. Sustainable recycling and resource utilization technology of lithium ion batteries[M]. Beijing: Science Press, 2021. | |
| 61 | NAYAKA G P, ZHANG Yingjie, DONG Peng, et al. An environmental friendly attempt to recycle the spent Li-ion battery cathode through organic acid leaching[J]. Journal of Environmental Chemical Engineering, 2019, 7(1): 102854. |
| 62 | PATELI I M, DANA T, ALABDULLAH S S, et al. The effect of pH and hydrogen bond donor on the dissolution of metal oxides in deep eutectic solvents[J]. Green Chemistry, 2020, 22(16): 5476-5486. |
| 63 | ABBOTT A P, COLLINS J, DALRYMPLE I, et al. Processing of electric arc furnace dust using deep eutectic solvents[J]. Australian Journal of Chemistry, 2009, 62(4): 341. |
| 64 | POURGHASEMI H A, NADERI R, KOWSARI E, et al. Corrosion behavior of mild steel in H2SO4 solution with 1, 4-di[1′-methylene-3′-methyl imidazolium bromide]-benzene as an ionic liquid[J]. Corrosion Science, 2016, 107: 96-106. |
| 65 | WANG Yuqing, AN Ning, WEN Lei, et al. Recent progress on the recycling technology of Li-ion batteries[J]. Journal of Energy Chemistry, 2021, 55: 391-419. |
| 66 | NAND Peeters, KWINTEN Janssens, DE VOS Dirk, et al. Choline chloride-ethylene glycol based deep-eutectic solvents as lixiviants for cobalt recovery from lithium-ion battery cathode materials: Are these solvents really green in high-temperature processes?[J]. Green Chemistry, 2022, 24(17): 6685-6695. |
| 67 | AMPHLETT J T, CHOI S. The effect of increasing water content on transition metal speciation in deep eutectic solvents[J]. Journal of Molecular Liquids, 2021, 332: 115845. |
| 68 | MENG Qi, ZHANG Yingjie, DONG Peng. A combined process for cobalt recovering and cathode material regeneration from spent LiCoO2 batteries: Process optimization and kinetics aspects[J]. Waste Management, 2018, 71: 372-380. |
| 69 | HE Lipo, SUN Shuying, SONG Xingfu, et al. Leaching process for recovering valuable metals from the LiNi1/3Co1/3Mn1/3O2 cathode of lithium-ion batteries[J]. Waste Management, 2017, 64: 171-181. |
| 70 | KUMARI A, JHA M K, PATHAK D D. An innovative environmental process for the treatment of scrap Nd-Fe-B magnets[J]. Journal of Environmental Management, 2020, 273: 111063. |
| 71 | JHA M K, KUMARI A, JHA A K, et al. Recovery of lithium and cobalt from waste lithium ion batteries of mobile phone[J]. Waste Management, 2013, 33(9): 1890-1897. |
| 72 | WU Caibin, LI Bensheng, YUAN Chengfang, et al. Recycling valuable metals from spent lithium-ion batteries by ammonium sulfite-reduction ammonia leaching[J]. Waste Management, 2019, 93: 153-161. |
| 73 | ALHASHIM S H, BHATTACHARYYA S, TROMER R, et al. Mechanistic study of lithium-ion battery cathode recycling using deep eutectic solvents[J]. ACS Sustainable Chemistry & Engineering, 2023, 11(18): 6914-6922. |
| [1] | WANG Peng, ZHANG Yang, FAN Bingqiang, HE Dengbo, SHEN Changshuai, ZHANG Hedong, ZHENG Shili, ZOU Xing. Process and kinetics of hydrochloric acid leaching of high-carbon ferrochromium [J]. Chemical Industry and Engineering Progress, 2023, 42(S1): 510-517. |
| [2] | LI Weihua, YU Qianwen, YIN Junquan, WU Yinkai, SUN Yingjie, WANG Yan, WANG Huawei, YANG Yufei, LONG Yuyang, HUANG Qifei, GE Yanchen, HE Yiyang, ZHAO Lingyan. Leaching behavior of heavy metals from broken ton bags filled with fly ash in acid rain environment [J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4917-4928. |
| [3] | WANG Baoying, WANG Huangying, YAN Junying, WANG Yaoming, XU Tongwen. Research progress of polymer inclusion membrane in metal separation and recovery [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 3990-4004. |
| [4] | LYU Jie, HUANG Chong, FENG Ziping, HU Yafei, SONG Wenji. Performance and control system of gas engine heat pump based on waste heat recovery [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4182-4192. |
| [5] | HU Yafei, FENG Ziping, TIAN Jiayao, SONG Wenji. Waste heat recovery performance of an air-source gas engine-driven heat pump system in multi-heating operation modes [J]. Chemical Industry and Engineering Progress, 2023, 42(8): 4204-4211. |
| [6] | LU Yang, ZHOU Jinsong, ZHOU Qixin, WANG Tang, LIU Zhuang, LI Bohao, ZHOU Lingtao. Leaching mechanism of Hg-absorption products on CeO2/TiO2 sorbentsin syngas [J]. Chemical Industry and Engineering Progress, 2023, 42(7): 3875-3883. |
| [7] | HOU Dianbao, HE Maoyong, CHEN Yugang, YANG Haiyun, LI Haimin. Application analysis of resource allocation optimization and circular economy in development and utilization of potassium resources [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 3197-3208. |
| [8] | ZENG Tianxu, ZHANG Yongxian, YAN Yuan, LIU Hong, MA Jiao, DANG Hongzhong, WU Xinbo, LI Weiwei, CHEN Yongzhi. Effects of hydroxylamine on the activity and kinetic parameters of nitrifying bacteria [J]. Chemical Industry and Engineering Progress, 2023, 42(6): 3272-3280. |
| [9] | LIU Yulong, YAO Junhu, SHU Chuangchuang, SHE Yuehui. Biosynthesis and EOR application of magnetic Fe3O4 NPs [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2464-2474. |
| [10] | LI Weihua, WU Yinkai, SUN Yingjie, YIN Junquan, XIN Mingxue, ZHAO Youjie. Progress on evaluation methods for toxic leaching of heavy metals from MSW incineration fly ash [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2666-2677. |
| [11] | LI Huahua, LI Yihang, JIN Beichen, LI Longxin, CHENG Shao’an. Research progress of Anammox bio-electrochemical coupling wastewater treatment system [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2678-2690. |
| [12] | WANG Hao, HUO Jinda, QU Guorui, YANG Jiaqi, ZHOU Shiwei, LI Bo, WEI Yonggang. Research progress of positive electrode material recycling technology for retired lithium batteries [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2702-2716. |
| [13] | CHANG Zhankun, ZHANG Chi, SU Bingqin, ZHANG Congzheng, WANG Jian, QUAN Xiaohui. Effect of H2S gaseous substrate on sludge bioleaching treatment efficiency [J]. Chemical Industry and Engineering Progress, 2023, 42(5): 2733-2743. |
| [14] | HU Yafei, FENG Ziping, TIAN Jiayao, HUANG Chong, SONG Wenji. Energy saving simulation and operation economic analysis of fuel driven non-electric heat pump systems [J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1217-1227. |
| [15] | CHEN Shaoqin, HU Ling, LEI Tianya, WANG Rong, SHU Jiancheng, CHEN Mengjun. Mechanical activation for zinc enhanced leaching from zinc calcine [J]. Chemical Industry and Engineering Progress, 2023, 42(3): 1649-1658. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||